ABSTRACT
The infratemporal fossa approach described by Fisch overcame most of the factors that had previously prevented the total removal of tympanojugular paragangliomas (TJP). The remaining problem has been infiltration of the internal carotid artery (ICA) for which there has been no entirely satisfactory solution. At the least, severe encasement risks the possibility of an arterial rupture at surgery. In order to reduce this risk, preoperative endovascular interventions have been employed—mainly balloon occlusion, with or without arterial bypass. Recently, intra-arterial stents to reinforce the encased segment of the ICA have been introduced. This study evaluates our experience with 20 patients affected by TJP in which the ICA has been subjected to preoperative interventions. Ten patients underwent a preoperative balloon occlusion and the other 10 patients had their ICAs reinforced with stents. Problems that arose during embolization necessitated that one patient with a stent required ligation of their ICA. No other problems were encountered during endovascular treatment or surgical resection. In one patient with a stent, it was impossible to establish a cleavage plane between their recurrent tumour and the ICA. These early results are encouraging and suggest that intra-arterial stents have a part to play in the surgical management of large TJPs.
Keywords: Paraganglioma, glomus tumor, internal carotid artery, balloon occlusion, stent
Advances in lateral skull base surgery over the last few decades have made it possible to remove tumors formerly considered inoperable. The development of the infratemporal fossa type A approach by Fisch in 19781 has become the cornerstone in the treatment of tympanojugular paragangliomas (TJPs) as a result of anterior rerouting of the facial nerve. This single maneuver made it possible to gain direct access to the jugular foramen and the petrous carotid artery, hence allowing gross total removal of these aggressive lesions.
Once the problem of the facial nerve was solved, the focus of the surgeon's attention shifted to the internal carotid artery (ICA). The presence of the ICA within the operative field, often infiltrated by tumor, is today the main factor that influences operability and our ability to achieve a gross total removal. Carotid involvement by TJPs usually starts at the level of the posterolateral surface of the vertical segment, the closest area to the jugular bulb, where the tumor develops. Fortunately, this is also where carotid manipulation appears to be easier and safer than elsewhere because after facial nerve rerouting the surgeon has complete and direct control of the artery within the surgical field. Limited involvement of the ICA at this level usually does not require preoperative endovascular therapy. In experienced hands, the ICA can be managed at surgery without excessive risks. It is always possible to control the artery proximally into the neck and distally at the level of the genu. The unobstructed view of this arterial segment makes it feasible to repair small accidental tears of the arterial wall.
In the case of “complex” TJPs, which are usually characterized by more aggressive ICA involvement, manipulation of the artery, in our opinion, exposes the patient to extreme danger; the only reasonable option without adequate preoperative treatment is incomplete tumor removal. Some of these tumors, especially in young patients, are very aggressive and partial removal can be counterproductive. This provided the impetus to develop and introduce endovascular techniques to manage the ICA and therefore help the surgeon remove the tumor completely with minimal morbidity and mortality. These techniques include preoperative permanent balloon occlusion (PBO) of the ICA,2,3,4,5 external-internal carotid artery bypass followed by PBO,6 and, more recently, reinforcement with stents.7,8,9,10 None of these procedures are without shortcomings; as a consequence, the selection of ICA management in every single patient has to be balanced against the morbidity that may result. A careful preoperative evaluation is necessary that takes into consideration not only the degree of ICA involvement but also the anatomical integrity of the circle of Willis, previous surgery or radiotherapy, and the patient's age and general condition.
This study illustrates the present attitude of the Gruppo Otologico in the management of the ICA in TJP surgery on the basis of all the patients treated from 1989 onwards.
MATERIALS AND METHODS
A retrospective review was conducted of 20 patients from a cohort of 88 patients with TJP who underwent skull base surgery at the Gruppo Otologico from 1988 to 2006. This group of patients had required preoperative interventional techniques directed to secure the ICA. The study included 8 women and 12 men whose ages ranged from 20 to 66 years (mean, 45 years). The tumors were staged using the Fisch system11 on the basis of computed tomography (CT) and magnetic resonance imaging (MRI) data. Six patients had multiple tumors. One patient had received previous radiotherapy and six patients had previously undergone surgery.
The CT images were reviewed to evaluate the extent of erosion of the vertical and horizontal portions of the petrous ICA canal. Magnetic resonance imaging results were reviewed to ascertain the presence of a tumor at the petrous apex and to determine the degree of encasement of the ICA at the junction between the distal cervical and the vertical petrosal portions of the artery. To quantify encasement, the circumference of the ICA was subdivided into four quadrants—each of 90 degrees: posterior, lateral, anterior, and medial. To evaluate encasement of the horizontal petrosal segment of the ICA, sagittal views were particularly helpful and identified those with tumors present along the anterior, superior, posterior, and inferior walls of the petrous carotid canal.
The angiographic data were reassessed to detect vascular supply from ICA branches and scrutinized for signs of infiltration of the ICA walls, such as irregularities and stenosis of the arterial lumen. In addition, the functional integrity of the circle of Willis was checked using Matas' and Alcock's tests. Matas' test was performed by injecting contrast into the contralateral ICA while the ipsilateral common carotid artery was compressed. Rapid and complete filling of the anterior and middle cerebral arteries on the compressed side was taken to indicate a patent anterior communicating arterial system. Alcock's test was performed by injecting contrast into the dominant vertebral artery while the ipsilateral common carotid artery was compressed. Rapid and complete filling of the middle cerebral artery on the compressed side was taken to indicate a patent posterior communicating arterial system. Patients were only scheduled for a balloon occlusion test (BOT) if there was good cross-filling from at least one of the two communicating systems. A PBO would be undertaken if the patient tolerated the BOT and good angiographic data demonstrated cross-flow.
The BOT-PBO procedure was performed under local anesthesia with mild sedation and systemic heparinization. A bilateral femoral approach was employed in which an 8F guiding catheter was inserted into one femoral artery and positioned in the ICA to be occluded. The contralateral femoral artery puncture was used for the angiographic evaluation. To permanently occlude the ICA, the GVB 16 balloon mounted on a CIF catheter (Minyvasis, Gennevilliers, France) was employed. The first balloon was usually placed into the cavernous segment of the ICA just proximal to the origin of the opthalmic artery. After balloon inflation, occlusion of the ICA was confirmed angiographically by injection of contrast into the guiding catheter, followed by confirmatory angiography to establish that adequate cross-flow was achieved, with special attention to the symmetry of the arterial, capillary, and venous phases on either side. If this was the case, the first balloon was detached and the PBO completed by inserting and detaching two other balloons in the petrous and proximal cervical segments of the ICA, respectively. If balloon occlusion was not tolerated, the balloon was deflated immediately. In most cases this would be apparent very quickly, in the first few minutes after carotid occlusion. In some cases the patient tolerated the test but showed some asymmetry (> 1 second) in the capillary and venous phases of the angiogram. In these patients, angiography was repeated a few minutes later. If the appearances were more symmetric, we proceeded to PBO. If not, the balloon was deflated and the patient was scheduled for bypass. After PBO, the patient was monitored for 24 hours in an intensive care unit.
Reinforcement with stents was performed under general anesthesia as a separate procedure some time after diagnostic angiography. Three different types of self-expanding nitinol stents have been employed: Xpert Stent System (Abbott Laboratories Vascular Enterprises, Dublin, Ireland), Neuroform 3 (Boston Scientific, Fremont, CA), and LEO (Balt Extrusion, Montmorency, France). To reduce the risk of thromboembolic complications, antiplatelet therapy was commenced 1 week before the stent insertion using a combination of ticlopidine (250 mg × 2 day−1) and aspirin (100 mg/d−1), or clopidogrel (75 mg/d) and aspirin (100 mg/d−1). This therapeutic regimen was administrated for a minimum of 30 days after stenting and then reduced to single-drug treatment with aspirin only.
The interval between insertion of a stent and surgery varied between 1 and 3 months. Antiplatelet therapy was suspended 1 week before surgery and resumed 1 week afterward. Low molecular weight heparin was given during the intervening period.
The infratemporal fossa type A approach was used in all cases, either alone or in combination with a translabyrinthine approach. Surgery was staged in four patients because of large intradural components to diminish the risk of cerebrospinal fluid leakage into the neck. The second stage was performed a few months later through the same route but without reopening the neck.
The surgical results have been studied in terms of three main points: surgical control of the ICA, extent of tumor resection, and vascular complications during or after surgery.
RESULTS
Data on patients treated with PBO are given in Table 1 and on patients who underwent endovascular reinforcement with a stent are given in Table 2.
Table 1.
Patients Treated through Permanent Balloon Occlusion
| Patient | Sex | Age | C Class | Previous Treatment | Year | ICA supply | Encasement (MRI) | Encasement (Angiography) | Circle of Willis Compensation | Surgical Removal | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICA, internal carotid artery; MRI, magnetic resonance imaging; M, male; A, effective compensation through anterior communicating artery; P, effective compensation through posterior communicating artery; F, female; mht, meningohypophyseal trunk; ilt, inferolateral trunk; v, vidian trunk; a, ineffective compensation through anterior communicating artery; ct, caroticotympanic trunk; p, ineffective compensation through posterior communicating artery; vps, ventriculoperitoneal shunt. | |||||||||||
| 1 | M | 28 | C3 | Radiotherapy | 1989 | / | 270° | Stenosis | A P | Total | |
| 2 | F | 53 | C3 | None | 1995 | mht, ilt | 270° | / | A P | Total | ICA aneurysm |
| 3 | F | 28 | C3 | Surgery | 1996 | v | 270° | / | a P | Total | |
| 4 | M | 20 | C3 | None | 1999 | ct, mht | 270° | / | A P | Subtotal | Death for recurrence |
| 5 | M | 31 | C4 | Surgery + bypass | 1999 | mht | 360° | Stenosis | A P | Total | |
| 6 | M | 32 | C3 | Surgery | 2000 | ct, mht | 270° | Stenosis | A p | Total | |
| 7 | F | 43 | C3 | Surgery | 2000 | / | 360° | / | A P | Total | |
| 8 | F | 66 | C3 | None | 2005 | v, mht | 360° | Stenosis | A | Total | |
| 9 | M | 61 | C4 | Surgery + bypass | 2005 | mht | 360° | / | a P | / | vps, death |
| 10 | F | 51 | C4 | Surgery | 2005 | ct, v, mht | 360° | Stenosis | A P | / | vps |
| 11* | M | 26 | C3 | Surgery | 2006 | ilt, mht | 360° | / | A P | Total | |
Same patient as number 9 in Table 2.
Table 2.
Patients Treated through Reinforcement with Stent
| Patient | Sex | Age | C Class | Previous Treatment | Multiple Tumors | Year | ICA Supply | Encasement (MRI) | Encasement (angiography) | Stent (cervical) | Stent (petrous) | Surgical Removal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICA, internal carotid artery; MRI, magnetic resonance imaging; M, male; vao, vertebral artery occlusion; mht, meningohypophyseal trunk; ilt, inferolateral trunk; Xp, Xpert stent; Subtotal, not related to ICA; F, female; ct, caroticotympanic trunk; v, vidian trunk; NF, Neuroform 3 stent; ccao, contralateral carotid artery occlusion; Subtotal (ICA), related to ICA; PBO, permanent balloon occlusion; wfs, waiting for surgery. | ||||||||||||
| 1 | M | 50 | C3 | Surgery, vao | No | 2004 | mht, ilt | 360° | Stenosis | Xp | Xp | Subtotal |
| 2 | F | 40 | C4 | Surgery | No | 2004 | ct, v, mht | 360° | Stenosis | Xp | Xp | Subtotal |
| 3 | M | 35 | C3 | / | Yes | 2004 | ct, mht | 180° | Stenosis | Xp | Xp | Total |
| 4 | F | 32 | C3 | / | Yes | 2005 | / | 360° | Stenosis | Xp | NF | Total |
| 5 | M | 40 | C3 | Surgery, ccao | Yes | 2005 | / | 270° | Stenosis | Xp | Xp | Total |
| 6 | M | 33 | C2 | Surgery | Yes | 2005 | / | 270° | Stenosis | Xp | NF | Total |
| 7 | F | 49 | C2 | / | No | 2005 | ct | 180° | Stenosis | NF | NF | Total |
| 8 | M | 53 | C3 | Surgery | Yes | 2005 | mht | 270° | / | LEO | LEO | Subtotal (ICA) |
| 9* | M | 26 | C3 | Surgery | No | 2006 | ilt, mht | 360° | / | Xp | NF (2 stents) | Total after PBO |
| 10 | M | 46 | C2 | Surgery | Yes | 2006 | mht | 180° | / | Xp | NF (2 stents) | wfs |
Same patient as number 11 in Table 1.
Endovascular Treatment Results
PERMANENT BALLOON OCCLUSION OF THE INTERNAL CAROTID ARTERY GROUP
Eleven patients underwent PBO of the ICA without complications (Figs. 1, 2, and 3). Eight of these patients underwent surgery subsequently. One patient deteriorated and died from intracranial hypertension 1 week before the scheduled operation. Another patient, at risk of intracranial hemorrhage from a huge arteriovenous shunt in the basal cerebral and posterior fossa veins, underwent PBO to reduce that shunt.
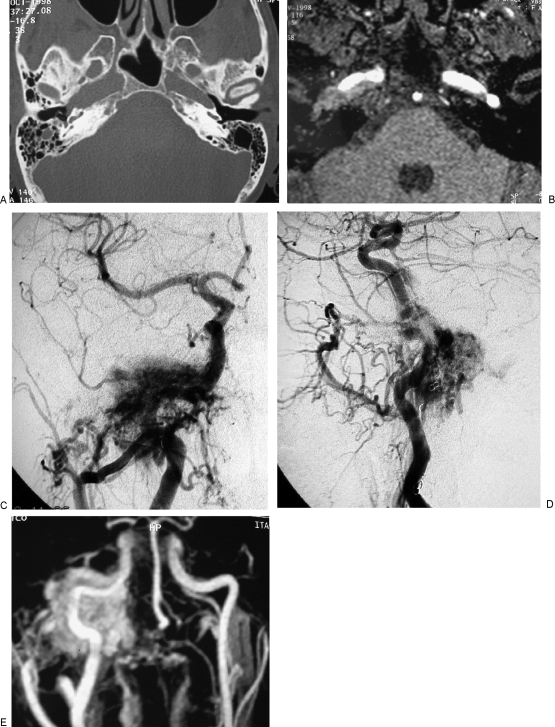
Figure 1.
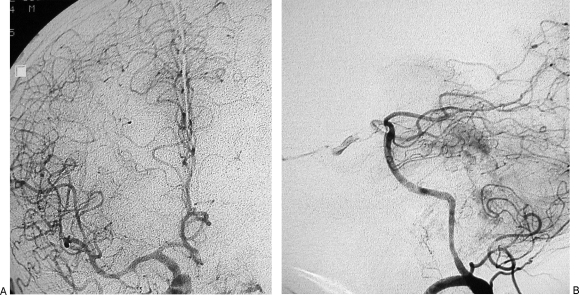
Permanent balloon occlusion (PBO) of internal carotid artery (ICA) in a class C4 recurrent jugular paraganglioma (Table 1, case 5). (A) Axial high-resolution computed tomography (CT) shows enlargement of horizontal petrous segment of right ICA. (B) Axial postgadolinium partition of time-of-flight magnetic resonance (MR) sequence shows extension of the tumor along the horizontal petrous segment of ICA. (C) Right common carotid artery injection in anteroposterior view is shown. (D) Right common carotid artery injection in laterolateral view is shown. (E) MR angiography with maximum intensity projection reconstruction shows the relationship between ICA and vascular blush.
Figure 2.
Same case as Fig. 1. (A,B) Left internal carotid artery injection during balloon occlusion test (BOT) shows patency of anterior communicating artery and temporal symmetry of arterial and venous phases between left and right hemispheres. Less opacification occurs in the vascular bed of right middle cerebral artery due to unopacified blood coming from posterior circulation. (C,D) Left vertebral artery injection during BOT shows patency of posterior communicating artery and symmetry of arterial and venous phases between posterior vascular territory and right middle cerebral artery vascular territory.
Figure 3.
Same case as Figs. 1 and 2. (A) Left vertebral artery injection was administered after placement of detachable balloon in cavernous segment of internal carotid artery (ICA). (B) Second balloon was inserted in petrous and distal cervical segment of ICA after detachment of the balloon in cavernous segment of ICA. (C) A third balloon that was positioned and detached in proximal cervical segment of ICA is seen. (D) Injection of right common carotid artery, in laterolateral view, shows complete devascularization of the tumoral mass after balloon occlusion of ICA and embolization of external carotid artery branches.
A superficial temporal artery–middle cerebral artery bypass before PBO had been performed at another center (Table 1, case 5) that subsequently blocked. It had been a precautionary measure only as the patient had tolerated the BOT and then underwent uneventful PBO.
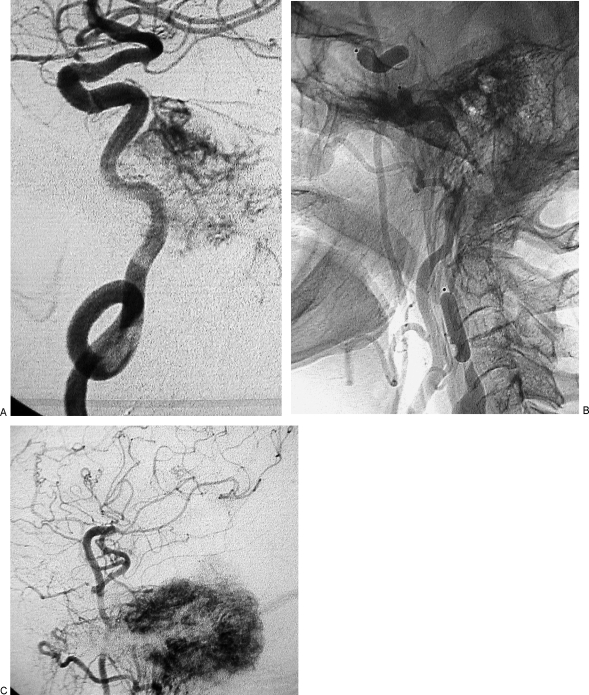
In another patient, a radial artery graft bypass before PBO was performed in the Neurosurgical Department of the University of Parma because of an incomplete circle of Willis (Table 1, case 9). One month later the patient underwent an uneventful PBO of the ICA (Figs. 4, 5, and 6) but, as previously stated, died 1 week before surgery.
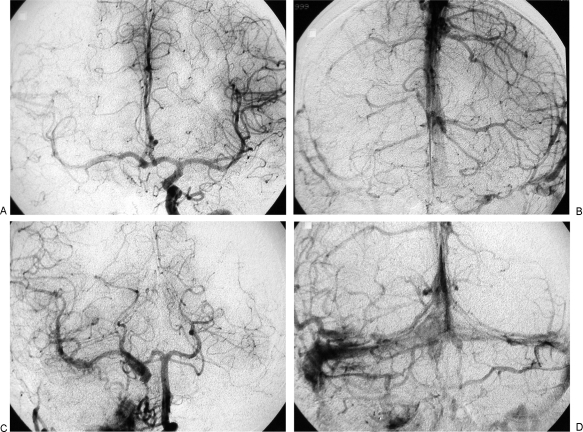
Figure 4.
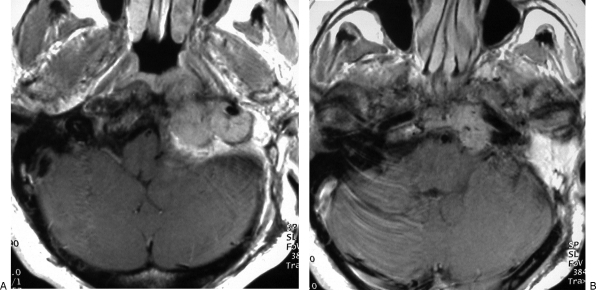
Permanent balloon occlusion (PBO) of internal carotid artery (ICA) in class C4 recurrent jugular paraganglioma after bypass (Table 1, case 9). (A,B) Axial postgadolinium T1-weighted image shows extension of tumor along horizontal segment of left ICA, anterior foramen lacerum, and Meckel's cave. Intracranial intradural extension in the left cerebellopontine angle, with compression of the middle cerebellar peduncle and contralateral displacement of the pons and fourth ventricle, is also evident.
Figure 5.
Same case as Fig. 4. (A) Right carotid artery injection in frontal view, during balloon occlusion test (BOT) of left internal carotid artery (ICA), shows absence of cross-flow. (B) Left vertebral artery injection, lateral view, during BOT of left ICA shows an hypoplastic posterior communicating artery, with slight opacification of the supraclinoid portion of left ICA and absence of flow in left middle cerebral artery vascular territory.
Figure 6.
Same case as Figs. 4 and 5. (A) Left internal carotid artery (ICA) injection, lateral view, shows tumoral vascular supply coming from the meningohypophyseal trunk and stenosis of the petrosal horizontal segment; coiling of midcervical portion of ICA, which precludes insertion of a stent, is also evident. (B) Left common carotid artery injection, lateral view cervical level, shows three detachable balloons in place and patency of the bypass. (C) Left common carotid artery injection, lateral view at cranial level, shows patency of bypass, intracranial anastomosis with middle cerebral artery, revascularization of supraclinoid portion of left ICA and tumoral blush coming from branches of external carotid artery.
A ventriculoperitoneal shunt was placed in two patients (Table 1, cases 9 and 10) because of intracranial hypertension. In one case (case 10) the shunt is working well while in the other (case 9) it was unsuccessful and the patient died.
REINFORCEMENT WITH STENTS OF THE INTERNAL CAROTID ARTERY GROUP
Ten patients underwent reinforcement with stents without clinical complications. In four cases, an Xpert stent was inserted in the cervical and petrosal segments of the ICA without difficulty (Figs. 7 and 8). One patient developed vasospasm, which was treated with intra-arterial vasodilators. In two patients, it was impossible to insert an Xpert stent in the petrous segment of the ICA. Instead, a softer Neuroform 3 stent was inserted with the Xpert stent placed in the cervical segment. In one case, where significant kinking of the cervical portion of the ICA occurred, we decided to use the softer Neuroform 3 stent for both the cervical and petrous segments of the ICA. In another patient, a 6 × 60 mm LEO stent was inserted because it had a larger diameter and was more appropriate. In the last two patients, we inserted two Neuroform 3 stents in the precavernous (in patient 9) and petrosal (in patient 10) segments and an Xpert stent in the distal cervical segment of the ICA, with partial overlap. One of them had already been operated on three times in another center. After insertion of the stent in this patient, it was not possible to perform a conventional embolization because of the previous ligation of the external carotid artery. Consequently, direct embolization of the tumor was required. Subsequent angiography revealed that this was not effective. Therefore, after BOT confirmed collateral flow, the patient underwent PBO.
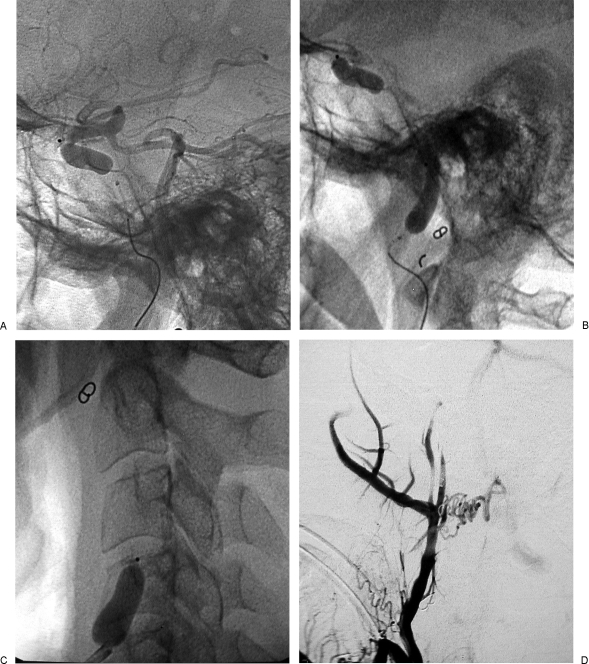
Figure 7.
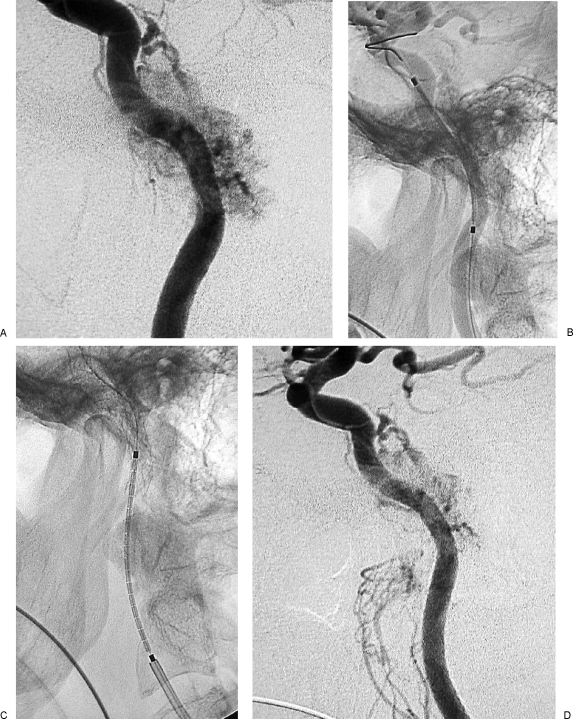
Reinforcement with stent of internal carotid artery (ICA) in class C3 recurrent temporal paraganglioma (Table 2, case 1). (A,B) Axial postgadolinium T1-weighted image shows complete encasement of distal cervical portion of left ICA and extension along horizontal petrous segment of ICA, toward the anterior foramen lacerum.
Figure 8.
Same case as Fig. 7. (A) Left internal carotid artery (ICA) injection, lateral view, shows tumoral supply from meningohypophyseal trunk and stenosis of distal cervical and petrosal vertical segments of ICA. (B) Left ICA, lateral view, shows insertion of Xpert stent in petrous segment of ICA. (C) Left ICA, lateral view, shows insertion of Xpert stent in the distal cervical segment, with 10 mm overlapping with the first stent. (D) Left ICA injection, lateral view, after insertion of two Xpert stents, shows disappearance of the previously seen stenosis and slight reduction of the tumoral blush.
Surgical Treatment Results
PERMANENT BALLOON OCCLUSION OF THE INTERNAL CAROTID ARTERY GROUP
In the group of patients treated preoperatively by means of PBO, there were no complications or significant findings during the surgery, which was uneventful in every case.
REINFORCEMENT WITH STENTS OF THE ICA GROUP
It was not possible to establish a plane of dissection between the artery and recurrent tumor (Table 2, case 8) in one patient who had ICA reinforcement with stents. Perhaps this was because the patient had already undergone dissection of the ICA at the time of his first operation, and the recurrent lesion was engulfed by scar tissue. This patient had not tolerated the BOT.
DISCUSSION
Extensive involvement of the bony petrous carotid canal and of the wall of the ICA by TJP—together with conspicuous blood supply from the petrous and cavernous branches of the ICA—signals high intraoperative risk. These features should influence the decision-making process. Al-Mefty and Teixeira12 suggested that it was possible to identify a plane of dissection between the tumor and the ICA using a microscope even when the tumor encased the ICA or received a blood supply from it. They had found it unnecessary to sacrifice or reconstruct the ICA. This opinion is not shared by other very experienced surgeons who have reported intraoperative injury to the ICA. In such cases, the surgeon had temporarily occluded the ICA to repair the vessel wall13,14 or had to sacrifice it the ICA,2,14 fortunately without complications. But this has not always been the case, and in some other series major complications like stroke3 and death15,16 have been reported as a direct result of ICA sacrifice.
A few surgeons14,16 have advocated partial resection of tumors when the ICA is encased. Although this policy seems entirely reasonable in older patients, it is hard to justify for the young who have a much longer life expectancy and in whom these tumors are usually more aggressive. It is for this reason that alternative forms of carotid artery management have been developed.
At this point and in the context of advances in the preoperative management of the ICA, it is worth reconsidering the rationale of our treatment protocol. We obtain high-resolution CT, high-field MRI, and intra-arterial digital subtraction angiography in all patients. These investigations allow us to make an accurate diagnosis and stage the tumor. We use the Fisch classification11 of staging, in which tumors are segregated into classes C1, C2, C3, or C4 according to their relationship to the carotid canal. This is best evaluated by a combination of CT and MRI data.
Aggressive bone infiltration, which is confirmed by the characteristic pattern of bony erosion and gadolinium enhancement along the medial wall of the carotid canal, is a particular feature of TJP. Sometimes it is extremely difficult to distinguish between normal and infiltrated bone in both the preoperative scan and during surgery. Every effort has to be made to detect infiltration and to avoid leaving tumor around the ICA. Drilling down the carotid canal and dissection of the arterial wall in class C3 and C4 tumors are particularly demanding because there is little hope of controlling the distal ICA if it is damaged.
We also integrate the Moret classification into our preoperative assessment.17 This scheme categorizes TJP from an angiographic point of view into four compartments: inferomedial, posterolateral, superior, and anterior. The ICA is contained in the anterior compartment, together with the anterior tympanic cavity, the petrous apex, and the cavernous sinus. In C1 and C2 tumors, the anterior compartment is usually supplied by the anterior tympanic branch of the internal maxillary artery and/or the caroticotympanic branch of the ICA. In C3 and C4 tumors, further vascular supply comes from cavernous branches of the ascending pharyngeal artery and sometimes from the meningohypophyseal and inferolateral trunks of the ICA. Some C3 and C4 tumors have a dominant blood supply from branches of the ICA, which results in increased intraoperative blood loss. This makes resection much more difficult, particularly in that part that is close to the artery. However, these branches are usually thin and angulated, which precludes selective microcatheterization and embolization. The solution is a more aggressive form of preoperative treatment.
Evaluation of encasement of the ICA is best undertaken by MRI and angiography. Narrowing and irregularities of the arterial lumen are strongly suggestive of infiltration of the ICA wall. Vascular encasement by TJP is particularly common at the distal cervical and vertical segments in the petrous bone. In class C1 and C2 tumors, such involvement is at the level of posterior and lateral surfaces of the ICA, the closest area to the jugular bulb where the tumor develops. In class C3 and C4 tumors, major encasement of the ICA is usually found at the inferomedial wall of the horizontal petrous segment—the most difficult to be manipulated.
If extensive involvement of the ICA is found, some form of preoperative treatment should be taken into consideration, to lower the risk of an intraoperative arterial tear. Our indications for presurgical management of the ICA are:
Encasement of the distal cervical and petrosal vertical segments of the ICA between 270 and 360 degrees, as shown by CT and MRI in the axial plane
Evidence of stenosis and irregularities of the arterial lumen of the distal cervical and petrosal segments of the ICA as determined by angiography
Class C3 and C4 TJP
Extensive blood supply from ICA branches as seen on angiography
Previous surgery18 with ICA manipulation and/or previous radiotherapy
In these situations, we consider preoperative PBO, external-internal carotid artery bypass followed by PBO and reinforcement with stents.
Preoperative Permanent Balloon Occlusion of the Internal Carotid Artery
Permanent balloon occlusion was employed by Fisch and coworkers2,3 and to a lesser extent by other surgeons.4,19,20,21
The goals of PBO are to:
Facilitate radical tumor removal
Enable safe mobilization of pericarotid tumor during surgery without risk of uncontrollable hemorrhage from a laceration
Devascularize pericarotid tumor
Even though we rarely employ PBO nowadays as a result of the introduction of reinforcement with stents, PBO still represents the safest option available in selected cases, especially for those in which reinforcement with stents is not feasible. However, permanent occlusion and removal of the ICA should not be entered into lightly because of the potential morbidity related to the procedure. Careful selection is vital because not all patients can tolerate the procedure. A battery of tests is used in preparation for PBO, none of which is foolproof and each has its pros and cons.23,24,25,26,27,28,29 At our center, the patient tolerance for PBO is assessed using angiography as previously described. In our hands, this technique proved to be extremely safe and quite simple to perform; our results have been confirmed by the experience of others.30,31,32 The timing of PBO also plays an important role in determining the final outcome. An interval of 3 to 4 weeks between the occlusion and surgery is usually advocated because surgery under general anesthesia causes hypotension with subsequent hemodynamic insufficiency. This delay allows adaptation of the intracranial vasculature to the altered hemodynamics and reduces the chances of complications.5
From a surgical point of view, PBO of the ICA allows safe removal of the lesion without any limitation. There are some concerns about using PBO, especially in young patients, because of the risk of subsequent intracranial aneurysms.33 For this reason, careful monitoring of the patient's intracranial vasculature at 5 and 10 years is advisable. Another clinical situation in which extreme caution must be exercised is that of bilateral paragangliomas, a condition in which both carotid arteries are at risk.
Bypass–Permanent Balloon Occlusion Procedure
Bypass of the ICA has always been considered the only option in cases of inadequate collateral circulation. Regardless of the technique used, this procedure is a major surgical operation and carries a relatively high risk of stenosis, thromboembolism, occlusion, and anastomosis site blowout. In the present series, one patient underwent a temporal artery–middle cerebral artery bypass that resulted in occlusion. Fortunately, the patient tolerated a BOT and PBO without any problem. Another patient underwent an uneventful radial artery/external carotid artery/middle cerebral artery bypass followed by PBO because of inadequacy of the circle of Willis and presence of a coiled and kinked cervical ICA that precluded reinforcement with stents. Unfortunately, the patient died from intracranial hypertension a few days before the planned surgical intervention. Nevertheless, the high-flow bypass followed by PBO still remains an option in selected cases, and with proper timing it should be less risky than direct surgical reconstruction of the ICA. It gives time for the cerebral circulation to adapt and does not expose the patient to a prolonged intervention often under unstable hemodynamic conditions.
Reinforcement with Stents of the Internal Carotid Artery
The recent introduction of preoperative reinforcement with stents represents a significant advance in the treatment of patients who are at risk of damage to the ICA. Stent insertion reinforces the artery and allows more aggressive carotid dissection while reducing the possibility of intraoperative injury to the artery.7,8,9,10 To prevent stent-induced thrombosis,22 double antiplatelet therapy must be instituted 1 week before stent insertion. In our practice, patients are started on oral ticlopidine, 250 mg twice a day, with 100 mg of aspirin daily 1 week before insertion of the stent. This combination is continued for 1 month after the procedure, after which single-agent therapy with aspirin is continued indefinitely. In case of ticlopidine intolerance, clopidogrel, 75 mg a day, is administrated. The only exception to this regimen is during the perioperative period when we rely on low molecular weight heparin only.
At present, we consider the Xpert stent the most suitable for reinforcement of both the cervical and intratemporal portions of the ICA because of its diameter (4 or 5 mm) and length (20, 30, or 40 mm). Each stent is carefully selected and tailored to the individual patient. Difficulties are encountered during stent positioning at the bend between the vertical and horizontal portions of the carotid canal and in arteries that are coiled or kinked in the neck. In such situations, a softer and more flexible stent must be chosen. It would be a mistake to force the more rigid stent because a dissection of the ICA wall might be precipitated. With very tortuous ICAs, a PBO still represents the safest option. The timing of reinforcement with stents also plays an important role; an interval of at least 4 to 6 weeks has been advocated10 between stenting and surgery, to allow the formation of a stabilized neointimal lining on the luminal surface of the stent.34 One very special situation is worth mention: the presence of an important blood supply from the ICA. In this circumstance, a bare stent is unable to reduce the vascular supply to the tumor. Use of PBO, preoperative embolization with particles during temporary balloon occlusion of the ICA, or insertion of covered stents could represent an alternative solution.
There are a few case reports of insertion of a balloon-expandable, covered stent in hypervascular tumors8,9 and self-expandable covered stents in pseudoaneurysms.35,36 Currently, covered stents have several theoretical disadvantages—such as increased thrombogenicity, rigidity, and greater difficulty in positioning at arterial angles—compared with bare stents. From a surgical point of view, preoperative stent insertion allows the skull base surgeon to perform ICA dissection with a significant reduction of the surgical risk. To reduce the possibility of injuring the ICA at the stent-tumor border, we believe that at least 10 mm of tumor-free vessel wall should be reinforced with the stent, both proximally and distally. To achieve this, it might be necessary to insert two or even three stents.
In the presence of an intraluminal stent, the surgeon is usually able to establish a cleavage plane on the external surface of the stent, removing all the involved portion of the arterial wall. At the same time, the consistency of the metallic net of the stent represents a safe protection against accidental rupture. This is particularly true when working at the level of the carotid genu and the horizontal segment of the petrous ICA. In this region, room to mobilize the artery is reduced and direct control of the medial wall is particularly demanding, increasing the difficulty and the risk of surgery. Although surgical dissection in the presence of the Xpert stent has appeared more comfortable, even in the presence of softer stents like Neuroform and LEO, it has been possible without any surgical problem. Dissection usually starts in the neck well away from the tumor, where it is easier to find the correct cleavage plane, and proceeds distally. The anteromedial wall of the artery is considered the most difficult because direct visualization requires bony decompression and anterior displacement of the intrapetrous segment of the ICA. The unsolved problem still remains: The medial wall of the ICA at the level of the anterior foramen lacerum, until now has been unreachable by surgery.
The long-term assessment of patency of the stented ICA is performed by ultrasound; although our series had no cases of in-stent stenosis or occlusion. We recognize, however, that a much longer period of follow-up is necessary for definitive evaluation. Nevertheless, absence of atherosclerotic plaques in our cases is a good omen for good results in the long term.
Some concerns still exist in terms of the radicalness of surgery after reinforcement with stents. Although gross total removal has been achieved in our cases, there is still time for these patients to develop recurrence. Finally, we do have some worries about the use of reinforcement with stents in previously irradiated patients in whom the ICA may be more fragile, or in patients with recurrence that have already been operated on with some form of carotid dissection. In these, scar tissue engulfing the ICA makes it extremely difficult to find the correct cleavage plane with consequent risks of damage to the ICA or incomplete dissection.
CONCLUSIONS
Despite of the benign nature of paragangliomas from a histopathological point of view and of reports documenting their slow growth rate, a significant number of TJPs are very aggressive. Indeed, a few seem to become even more aggressive after subtotal resection. Notwithstanding several reports of good results following radiotherapy, the principal argument against the use of radiation is that in some tumor continues to grow. For these unfortunate patients, the morbidity of salvage surgery in grossly increased. Although surgery is considered the treatment of choice in simple TJPs, some doubts exist with regard to complex TJPs. In these, gross total removal requires ICA manipulation, which can be extremely dangerous in terms of spasm, thrombosis, and rupture in absence of adequate preoperative preparation. The recent introduction of reinforcement with stents has significantly helped ICA management. Stents allow the surgeon to perform an aggressive anatomic dissection of the artery with minimal risk. Our initial experience suggests that the procedure is almost risk free in the short term. In selected cases in which reinforcement with stents does not seem feasible, PBO of the ICA alone or preceded by high-flow external carotid–middle cerebral artery bypass is a viable option. We do not claim that these techniques make all tumors amenable to surgery; a few patients will always be inoperable and, sadly, some patients will die as a result of extensive and progressive disease.
ACKNOWLEDGMENTS
This article was supported by a grant from AINOT (Associazione Italiana Neuro-otologica).
REFERENCES
- Fisch U. The infratemporal fossa approach to tumors of the temporal bone and base of the skull. J Laryngol Otol. 1978;92:949–967. doi: 10.1017/s0022215100086382. [DOI] [PubMed] [Google Scholar]
- Andrews J C, Valavanis A, Fisch U. Management of the internal carotid artery in surgery of the skull base. Laryngoscope. 1989;99:1224–1229. doi: 10.1288/00005537-198912000-00003. [DOI] [PubMed] [Google Scholar]
- Zane R S, Aeschbacher P, Moll C, Fisch U. Carotid occlusion without reconstruction: a safe surgical option in selected patients. Am J Otol. 1995;16:353–359. [PubMed] [Google Scholar]
- Sanna M, Piazza P, Ditrapani G, Agarwal M. Management of the internal carotid artery in tumors of the lateral skull base: preoperative permanent balloon occlusion without reconstruction. Otol Neurotol. 2004;25:998–1005. doi: 10.1097/00129492-200411000-00023. [DOI] [PubMed] [Google Scholar]
- Christoforidis G, Valavanis A. Balloon occlusion of internal carotid artery. Neurointerventionist. 2000;2:95–102. [Google Scholar]
- Urken M L, Biller H F, Haimov M. Intratemporal carotid artery bypass in resection of a base of skull tumor. Laryngoscope. 1985;95:1472–1477. doi: 10.1288/00005537-198512000-00007. [DOI] [PubMed] [Google Scholar]
- Sanna M, Khrais T, Menozi R, Piaza P. Surgical removal of jugular paragangliomas after stenting of the intratemporal internal carotid artery: a preliminary report. Laryngoscope. 2006;116:742–746. doi: 10.1097/01.mlg.0000205199.61105.cb. [DOI] [PubMed] [Google Scholar]
- Cohen J E, Spektor S, Valarezo J, Fellig Y, Umansky F. Endolymphatic sac tumor: staged endovascular-neurosurgical approach. Neurol Res. 2003;25:237–240. doi: 10.1179/016164103101201436. [DOI] [PubMed] [Google Scholar]
- Cohen J E, Ferrario A, Ceratto R, Miranda C, Lylyk P. Covered stent as an innovative tool for tumor devascularization and endovascular arterial reconstruction. Neurol Res. 2003;25:169–172. doi: 10.1179/016164103101201148. [DOI] [PubMed] [Google Scholar]
- Nussbaum E S, Levine S C, Hamlar D, Madison M T. Carotid stenting and “extarterectomy” in the management of head and neck cancer involving the internal carotid artery: technical cases report. Neurosurgery. 2000;47:981–984. doi: 10.1097/00006123-200010000-00041. [DOI] [PubMed] [Google Scholar]
- Fisch U, Mattox D. Microsurgery of the Skull Base. Stuttgart: Thieme; 1988. pp. 136–281.
- Al-Mefty O, Teixeira A. Complex tumors of the glomus jugulare: criteria, treatment, and outcome. J Neurosurg. 2002;97:1356–1366. doi: 10.3171/jns.2002.97.6.1356. [DOI] [PubMed] [Google Scholar]
- Patel S J, Sekhar L N, Cass S P, Hirsch B E. Combined approach for resection of extensive glomus jugulare tumors. J Neurosurg. 1994;80:1026–1038. doi: 10.3171/jns.1994.80.6.1026. [DOI] [PubMed] [Google Scholar]
- Witiak D G, Pensak M L. Limitations to mobilizing the internal carotid artery. Ann Otol Rhinol Laryngol. 2002;111:343–348. doi: 10.1177/000348940211100411. [DOI] [PubMed] [Google Scholar]
- Leonetti J P, Smith P G, Grubb R L. The perioperative management of the petrous carotid artery in contemporary surgery of the skull base. Otolaryngol Head Neck Surg. 1990;103:46–51. doi: 10.1177/019459989010300107. [DOI] [PubMed] [Google Scholar]
- Jackson C G, McGrew B M, Forest J A, Netterville J L, Hampf C F, Glasscock M E., III Lateral skull base surgery for glomus tumors: long-term control. Otol Neurotol. 2001;22:377–382. doi: 10.1097/00129492-200105000-00018. [DOI] [PubMed] [Google Scholar]
- Moret J, Lasjaunias P, Théron J. Vascular compartments and territories of tympano-jugular glomic tumors. J Belge Radiol. 1980;63:321–337. [PubMed] [Google Scholar]
- Sanna M, De Donato G, Piazza P, Falcioni M. Revision glomus tumor surgery. Otolaryngol Clin North Am. 2006;39:763–782. doi: 10.1016/j.otc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Sanna M, Jain Y, De Donato G, Rohit , Lauda L, Taibah A. Management of jugular paragangliomas: the Gruppo Otologico experience. Otol Neurotol. 2004;25:797–804. doi: 10.1097/00129492-200409000-00025. [DOI] [PubMed] [Google Scholar]
- Sanna M, De Donato G, Russo A, Khrais T H. In: Wiet RJ, editor. Ear and Temporal Bone Surgery: Minimizing Risks and Complications. New York: Thieme; 2006. Middle ear and skull base glomus tumors: tympanic and tympanojugular paragangliomas. pp. 221–233.
- Pareschi R, Righini S, Destito D, Raucci A F, Colombo S. Surgery of glomus jugulare tumors. Skull Base. 2003;13:149–157. doi: 10.1055/s-2003-43325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Sohrab S, Tselie A. Carotid stent thrombosis: report of 2 fatal cases. Stroke. 2001;32:2700–2702. [PubMed] [Google Scholar]
- Standard S C, Ahuja A, Guterman L R, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453–1458. [PMC free article] [PubMed] [Google Scholar]
- Mathews D, Walker B S, Purdy P D, et al. Brain blood flow SPECT in temporary balloon occlusion of carotid and intracerebral arteries. J Nucl Med. 1993;34:1239–1243. [PubMed] [Google Scholar]
- Barker D W, Jungreis C A, Horton J A, Pentheny S, Lemley T. Balloon test occlusion of the internal carotid artery: change in stump pressure over 15 minutes and its correlation with xenon Ct cerebral blood flow. AJNR Am J Neuroradiol. 1993;14:587–590. [PMC free article] [PubMed] [Google Scholar]
- Brunberg J A, Frey K A, Horton J A, Deveikis J P, Ross D A, Koeppe R A. H2O positron emission tomography determination of cerebral blood flow during test occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1994;15:725–732. [PMC free article] [PubMed] [Google Scholar]
- Mathis J M, Barr J D, Jungreis C A, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749–754. [PMC free article] [PubMed] [Google Scholar]
- Niimi Y, Berenstein A, Setton A, Kupersmith M J. Occlusion of the internal carotid artery based on a simple tolerance test. Interventional Neuroradiol. 1996;2:289–296. doi: 10.1177/159101999600200408. [DOI] [PubMed] [Google Scholar]
- Giller C A, Mathews D, Walker B, Purdy P, Roseland A M. Prediction of tolerance to carotid artery occlusion using transcranial Doppler ultrasound. J Neurosurg. 1994;81:15–19. doi: 10.3171/jns.1994.81.1.0015. [DOI] [PubMed] [Google Scholar]
- Vazquez Añon V, Aymard A, Gobin Y P, et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring and results. Neuroradiology. 1992;34:245–251. doi: 10.1007/BF00596347. [DOI] [PubMed] [Google Scholar]
- Van Rooij W J, Sluzewski M, Slob M J, Rinkel G J. Predictive value of angiographic testing for tolerance of therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005;26:175–178. [PMC free article] [PubMed] [Google Scholar]
- Abud D G, Spelle L, Piotin M, Mounayer C, Vanzin J R, Moret J. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005;26:2602–2609. [PMC free article] [PubMed] [Google Scholar]
- Timperman P E, Tomsick T A, Tew J M, van Loveren H R. Aneurism formation after carotid occlusion. AJNR Am J Neuroradiol. 1995;16:329–331. [PMC free article] [PubMed] [Google Scholar]
- Toma N, Matsushima S, Murao K, et al. Histopathological findings in a human carotid artery after stent implantation: case report. J Neurosurg. 2003;98:199–204. doi: 10.3171/jns.2003.98.1.0199. [DOI] [PubMed] [Google Scholar]
- Alexander M J, Smith T P, Tucci D L. Treatment of an iatrogenic carotid artery pseudoaneurysm with Symbiot covered stent: technical case report. Neurosurgery. 2002;50:658–662. doi: 10.1097/00006123-200203000-00047. [DOI] [PubMed] [Google Scholar]
- Auyeung K M, Lui W M, Chow L CK, Chan F L. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol. 2003;24:1449–1452. [PMC free article] [PubMed] [Google Scholar]