Abstract
Background and objectives: Renal transplantation is increasingly performed in elderly patients, and the incidence of benign prostatic hyperplasia (BPH) increases with age. Anuric males on dialysis may have occult BPH and not develop obstructive symptoms until urine flow is restored after transplantation. If left untreated, BPH poses a risk for numerous complications, including acute urinary retention (AUR), recurrent urinary tract infections (UTI), and renal failure. The authors hypothesized that incident BPH after renal transplantation would adversely affect allograft survival.
Design, setting, participants, & measurements: Medicare claims for BPH, AUR, UTI, and prostate resection procedures (transurethral resection of the prostate; TURP) were assessed in a retrospective cohort of 23,622 adult male Medicare primary renal transplant recipients in the United States Renal Data System database who received transplants from 1 January 2000 to 31 July 2005 and followed through 31 December 2005.
Results: The 3-yr incidence of BPH post-transplant was 9.7%. The incidences of AUR, UTI, and TURP after BPH diagnosis (up to 3 yr posttransplant) were 10.3%, 6.5%, and 7.3% respectively, and each was significantly associated with BPH. Cox regression analysis showed that recipient age per year, later year of transplant, and dialysis vintage were associated with incident BPH. Using Cox nonproportional hazards regression, BPH was significantly associated with renal allograft loss (including death).
Conclusions: BPH is common in males after renal transplant and is independently associated with AUR, UTI, and graft loss. It is unknown whether treatment of BPH, either medical or surgical, attenuates these risks.
Renal transplantation is increasingly performed in elderly patients. According to the 2006 United States Renal Data System (USRDS) Annual Report, the proportion of renal transplant recipients over age 60 increased from 10.4% in 1994 to 20.7% in 2004 (1). The incidence of benign prostatic hyperplasia (BPH) increases with age, and more than 50% of men have histologic evidence of BPH by age 60 (2). Older males on dialysis may be oliguric/anuric and not have lower urinary tract symptoms (LUTS); thus, BPH may be occult and underdiagnosed in this population. Some cases of urinary obstruction are detected by pretransplant urologic screening, but many do not develop LUTS until urine flow is restored after transplantation.
There are several reports of bladder outlet obstruction from posttransplant BPH, and many of these patients eventually required surgical procedures such as transurethral resection of the prostate (TURP) to alleviate urinary obstruction (3,4,5,6,7). Because BPH may accelerate the progression of renal disease in other processes (8), we hypothesized that incident BPH diagnosed after renal transplantation would adversely affect renal allograft survival. To date, there are no studies on the epidemiology of BPH and its complications after renal transplantation.
Materials and Methods
Patients and Sources
This study used the USRDS database, which incorporates extensive baseline and follow-up demographic and clinical data on all patients involved in the Medicare ESRD program in the United States. The variables included in the USRDS standard analysis files, as well as methods and validation studies, are published and listed at the USRDS website, under Researcher's Guide to the USRDS Database’, Section E. Males over age 18 who underwent renal transplantation between 1 January 2000 and 31 July 2005 and had Medicare primary insurance (parts A&B) were analyzed.
Outcome Variables
The outcome variables were based on Institutional (IC) and Physician Supplier (PS) claims reported to Medicare from 1 January 1999 to 31 December 2005. Claims were identified by International Classification of Diseases-9th Revision Diagnosis and Procedure Codes (ICD9). Primary outcome variables were BPH, AUR, UTI, and TURP (Appendix A), with allograft loss (including death) as a secondary outcome variable. We assessed the earliest Medicare claim for BPH (both IC and PS) after transplantation and excluded patients who had pretransplant claims. Claims for AUR, UTI, and TURP were assessed both after date of transplant and after the date of BPH diagnosis. Two or more claims were required for PS and one for IC, as per previous reports.
Appendix A.
Diagnostic Codes Used to Define Outomes
| Diagnosis/event | Medicare claims used | Diagnostic codes useda |
|---|---|---|
| Benign prostatic hyperplasia | Institutional claims and Physician supplier claims | 600, 600.0, 600.2, 600.9 |
| Transurethral resection of the prostate (and other prostate procedures) | Institutional claims | 60.0, 60.2x |
| Urinary tract infection | Institutional claims | 590, 590.1, 590.2, 590.8, 590.9, 595, 595.89, 595.9 |
| Acute urinary retention | Institutional claims | 788.2x |
| Obstructive cause of ESRD (DGNL USRDS codes) | Not applicable | 3007 (chronic pyelonephritis), 3030 (acquired obstructive uropathy), 3052 (chronic obstructive uropathy) |
All ICD-9 codes except DGNL codes used for Obstructive cause of ESRD. ESRD, end stage renal disease; DGNL, diagnosis at listing; USRDS, United States Renal Data System.
Survival Times
Time to BPH was calculated as the time from transplant until the first Medicare claim for BPH, with recipients censored at death, loss to follow-up, or the end of the study period (December 31, 2005). Time to first claim for AUR, UTI, and TURP were calculated from BPH diagnosis date, with recipients censored for death, loss to follow-up, or end of study period. Time to Medicare claims were censored at 3 yr because Medicare coverage ends 3 yr after kidney transplantation unless a patient maintains coverage as a result of disability or age, which would lead to nonrandom censoring beyond 3 yr.
Independent Variables
Patient characteristics were those at the date of transplant, with the exception of comorbidity data from CMS Form 2728, which was obtained at the first treatment for end stage renal disease (ESRD), whether dialysis or transplant. The pretransplant duration of dialysis (dialysis vintage) was defined as the time from first recorded dialysis treatment until the date of transplantation. Other variables assessed include donor and recipient age, race, sex, induction/maintenance immunosuppressants, graft loss, delayed graft function (DGF), human leukocyte antigen (HLA) match status, panel reactive antibody (PRA), cold-ischemic time (CIT), expanded donor criteria (ECD), donation after cardiac death (DCD), and donor type. Data from CMS form 2728 included information on cardiovascular comorbid conditions including diabetes mellitus (DM), ischemic heart disease (IHD), congestive heart failure (CHF), peripheral vascular disease (PVD), and tobacco use.
Statistical Analyses
All analyses were performed using SPSS 12.0 ™ (SPSS, Chicago, IL). Files were merged and converted to SPSS files using DBMS/Copy (Conceptual Software, Houston, TX). Univariate analysis was performed with χ2 testing for categorical variables (Fisher's exact test used for violations of Cochran's assumptions) and t test for continuous variables (Mann–Whitney test used for non-normally distributed variables), respectively. Statistical significance was defined as P < 0.05.
To evaluate for possible prevalent cases of BPH, we used Kaplan-Meier analysis to plot time to BPH for patients who had prior evidence of obstructive ESRD as shown by diagnosis at listing codes (which are causes of ESRD recorded at transplant listing, Appendix A) in the USRDS database. The log-rank test was used for bivariate significance testing. Kaplan–Meier analysis was also used to plot time to first AUR, UTI, or TURP for patients who had diagnosis of BPH. For index patients, time to AUR, UTI, or TURP started at the date of BPH claim, and for the remainder, time started at date of transplant. Otherwise, patients were censored at death, loss to follow-up, or end of study period.
The independent associations between patient factors and outcomes (BPH, AUR, UTI, and TURP) were examined using multivariate analysis with forward stepwise Cox regression (likelihood ratio method). Variables with P < 0.10 tested in univariate analysis were entered into multivariate analysis as covariates, because of the possibility of negative confounding. Variables thought to have a known clinical association with outcomes were also introduced into multivariate models even if univariate P values were >0.10, in accordance with established principles of model development.
The association between renal allograft survival and BPH as a time-dependent variable was assessed with Cox nonproportional hazards regression. Variables found to be independently associated with BPH in the above Cox regression were included in the model, as were factors known to be independently associated with allograft loss.
Results
We identified 42,403 Medicare primary renal transplant recipients who underwent surgery between 1 January 2000 and 31 July 2005, of whom 25,383 were male. There were 1761 patients with prior Medicare claims for BPH. Among the 23,622 male transplant patients without prior evidence of BPH, there were 2292 individual cases of BPH with a 3-yr incidence of 9.7%. Mean time to BPH diagnosis was 1.09 ± 0.87 yr, and most (52.0%) were diagnosed within the 1st year posttransplant.
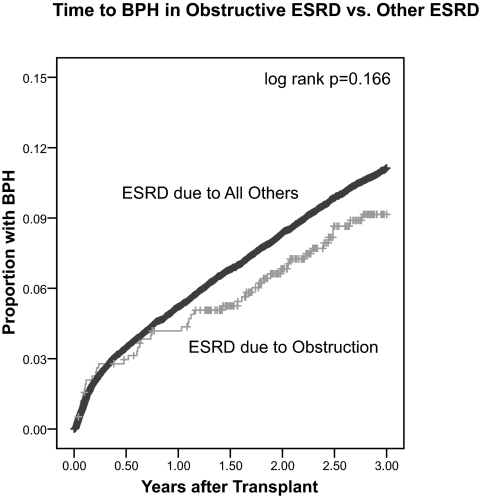
In the USRDS database, there were no ESRD cases attributed to BPH (primary disease code 600z). However, there were 576 cases of ESRD presumably related to urinary obstruction in that the patients’ diagnosis at listing codes were chronic pyelonephritis, acquired obstructive uropathy, and chronic obstructive uropathy. BPH is the most frequent form of urinary obstruction in men, so it is possible that these represent prevalent BPH cases, although univariate comparison with BPH was nonsignificant (Table 1). If these cases of obstructive ESRD are excluded, the 3-yr incidence is unchanged at 9.7%. Time to BPH diagnosis was evaluated by comparing these obstructive ESRD causes with all others, and there was no significant difference (Figure 1).
Table 1.
Baseline characteristics of the study sample
| Characteristics | N (%) or mean (SD) | BPH (%) | No BPH (%) | p |
|---|---|---|---|---|
| Total | 23,622 | 2292 (9.7) | 21,330 (90.3) | |
| Transplant recipient | ||||
| recipient age (years) | 48.24 (15.23) | 60.48 (10.04) | 46.92 (15.10) | <0.001 |
| black race | 6653 (28.2) | 555 (24.2) | 6098 (28.6) | <0.001 |
| years on dialysis prior to transplant | 3.31 (2.74) | 3.34 (2.62) | 3.31 (2.75) | 0.575 |
| Peak PRA >20% | 1798 (7.6) | 166 (7.2) | 1632 (7.7) | 0.072 |
| Comorbid conditions | ||||
| obstructive cause of ESRDa | 576 (2.4) | 48 (2.1) | 528 (2.5) | 0.286 |
| diabetes mellitus | 6066 (25.7) | 709 (30.9) | 5357 (25.1) | <0.001 |
| congestive heart failure | 2421 (10.2) | 322 (14.0) | 2099 (9.8) | <0.001 |
| ischemic heart disease | 2193 (9.3) | 350 (15.3) | 1843 (8.6) | <0.001 |
| peripheral vascular disease | 1548 (6.6) | 206 (9.0) | 1342 (6.3) | <0.001 |
| hypertension | 16,545 (70.0) | 1691 (73.8) | 14,854 (69.6) | <0.001 |
| tobacco use | 1081 (4.6) | 80 (3.5) | 1001 (4.7) | 0.010 |
| Transplant donor | ||||
| age >50 yr | 5186 (22.0) | 703 (30.7) | 4483 (21.0) | <0.001 |
| black race | 3113 (13.2) | 258 (11.3) | 2855 (13.4) | 0.004 |
| deceased | 16,831 (71.3) | 1728 (75.4) | 15,103 (70.8) | <0.001 |
| cold ischemic time >24 h | 3625 (15.3) | 432 (18.8) | 3193 (15.0) | <0.001 |
| donation after cardiac death | 688 (2.9) | 65 (2.8) | 623 (2.9) | 0.890 |
| expanded criteria donorb | 2423 (10.3) | 391 (17.1) | 2032 (9.5) | <0.001 |
| delayed graft functionc | 4853 (20.5) | 504 (22.0) | 4349 (20.4) | 0.072 |
| Year of transplant | <0.001 | |||
| 2000 to 2001 | 8056 (34.1) | 701 (30.6) | 7355 (34.5) | |
| 2002 to 2003 | 8610 (36.4) | 1001 (43.7) | 7609 (35.7) | |
| 2004 to 2005 | 6,956 (29.4) | 590 (25.7) | 6366 (29.8) | |
| Induction immunosuppression | 19,409 (82.2) | 1903 (83.0) | 17,506 (82.1) | 0.263 |
| Discharge Immunosuppression | ||||
| Tacrolimus | 14,165 (60.0) | 1302 (56.8) | 12,863 (60.3) | 0.001 |
| Cyclosporine (Neoral®) | 5892 (24.9) | 609 (26.6) | 5283 (24.8) | 0.060 |
| Mycophenolate | 18,534 (78.5) | 1826 (79.7) | 16,708 (78.3) | 0.142 |
| Azathioprine | 560 (2.4) | 48 (2.1) | 512 (2.4) | 0.385 |
| Sirolimus | 3395 (14.4) | 304 (13.3) | 3091 (14.5) | 0.118 |
| Number of HLA mismatches | 0.180 | |||
| 0 to 2 | 5308 (22.8) | 514 (22.7) | 4794 (22.8) | |
| 3 to 4 | 9592 (41.3) | 899 (39.7) | 8693 (41.4) | |
| 5 to 6 | 8350 (35.9) | 851 (37.6) | 7499 (35.7) |
Data given as the number (% of total) or mean ± one standard deviation (SD). BPH, benign prostatic hyperplasia; PRA, panel reactive antibody; ESRD, end stage renal disease; HLA, human leukocyte antigen.
Obstructive cause of ESRD: USRDS diagnosis at listing (DGNL) codes for either chronic pyelonephritis (3007), acquired obstructive uropathy (3030), or chronic obstructive uropathy (3052)
Expanded criteria donor: donor age >50 yr with history of two of the following (stroke, hypertension, creatinine >1.5 mg/dl) or donor age >60 yr
Delayed graft function: need for dialysis within the 1st wk after transplant
Figure 1.
Kaplan-Meier 1− survival plot for time (in years) to Medicare claim for benign prostatic hyperplasia (BPH) in patients with ESRD associated with urinary obstruction (chronic pyelonephritis, acquired obstructive uropathy, chronic obstructive uropathy) versus all other causes of ESRD (log rank P = 0.166) censored for death, loss to follow-up, or the end of the study period (31 December 2005).
On univariate analysis, patients diagnosed with BPH were older and less likely to be of black race. They were more likely to have medical comorbid disease (DM, CHF, IHD, PVD, HTN) and less likely to use tobacco. Other transplant factors that were more frequent in BPH patients were ECD, deceased donor, donor age >50, and CIT >24 h. Use of immunosuppressant medications was also slightly different in that BPH patients were more likely to be discharged with cyclosporine (Neoral®) and less likely to be discharged with tacrolimus (Table 1).
According to result of Cox regression analysis (adjusting for recipient age, recipient black race, PRA >20%, dialysis vintage, DM, CHF, IHD, PVD, tobacco use, HLA matching, donor age >50, donor black race, CRT, ECD, DCD, DGF, CIT >24 h, year of transplant, and induction/discharge immunosuppression), only recipient age per year (AHR 1.08 [95% CI 1.08 to 1.09]; P < 0.001), later year of transplant (AHR 1.05 [95% CI 1.03 to 1.08]; P < 0.001), and longer dialysis vintage (AHR 1.03 [95% CI 1.02 to 1.04]; P = 0.009) were significantly associated with BPH. Cox nonproportional hazards regression (adjusting for factors listed above for Cox regression analysis) BPH was associated with increased risk of graft loss (AHR 1.36 [95% CI 1.24 to 1.49]; P < 0.001). This association remained significant when graft loss was censored for death (AHR 1.17 [95% CI 1.02 to 1.37]; P = 0.047), although with a wider confidence interval and larger P value, which are likely due to the short follow-up time after BPH diagnosis in this cohort (necessitated by 3-yr Medicare window posttransplant) and the reduction in the number of outcomes as a result of excluding death with function.
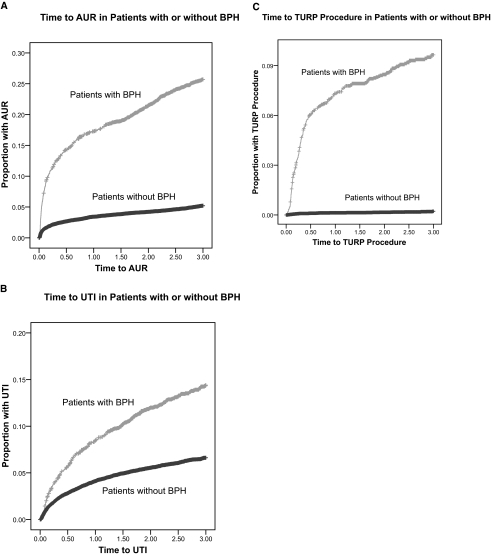
Among patients with BPH, 236 (10.3%) were later diagnosed with AUR, and 150 (6.5%) were later diagnosed with UTI at a mean of 0.39 ± 0.62 yr and 0.52 ± 0.61 yr, respectively after BPH diagnosis. There were 252 TURP procedures during the study period, and 168 (66.7%) of these patients had a prior diagnosis of BPH. Among patients with BPH, the 168 (7.3%) TURP procedures were performed at a mean of 0.06 ± 0.16 yr after diagnosis. As outcome variables, AUR, UTI, and TURP procedures were all also associated with increased recipient age (Table 2). Results of univariate analysis showed that BPH was associated with increased risk of AUR (AHR 6.48 [95% CI 5.78 to 7.27]; P < 0.001), UTI (AHR 2.42 [95% CI 2.13 to 2.77]; P < 0.001), and TURP (AHR 52.65 [95% CI 37.57 to 73.79]; P < 0.001). Kaplan-Meier time to event plots are shown in Figure 2A-C, which demonstrate increased proportions of AUR, UTI, and TURP in patients with BPH compared with those without. Log-rank test result is given, but Cox nonproportional hazards regression was used to evaluate for statistical significance given different time origins for patients with and without BPH. As a time-dependent variable, BPH was significantly associated with AUR, UTI, and TURP (Figure 2A-C).
Table 2.
Cox regression analysis of factors associated with BPH, AUR, UTI, and TURP after renal transplantation
| Variable | AHR | 95% CI | p |
|---|---|---|---|
| BPH | |||
| recipient age | 1.081 | 1.077 to 1.085 | <0.001 |
| dialysis vintagea | 1.033 | 1.018 to 1.049 | <0.001 |
| transplant year | 1.054 | 1.025 to 1.083 | <0.001 |
| AUR (after BPH) | |||
| recipient age | 1.089 | 1.076 to 1.102 | <0.001 |
| dialysis vintage | 1.077 | 1.036 to 1.119 | <0.001 |
| UTI (after BPH) | |||
| recipient age | 1.076 | 1.060 to 1.091 | <0.001 |
| dialysis vintage | 1.091 | 1.044 to 1.141 | <0.001 |
| TURP (after BPH) | |||
| recipient age | 1.087 | 1.072 to 1.103 | <0.001 |
| dialysis vintage | 1.101 | 1.057 to 1.147 | <0.001 |
AHR, adjusted hazard ratio; CI, confidence interval; BPH, benign prostatic hyperplasia; AUR, acute urinary retention; UTI, urinary tract infection; TURP, transurethral resection of the prostate.
Dialysis vintage: transplant date minus date of first dialysis
Figure 2.
(A) Kaplan-Meier 1− survival plot for time (in years) to Medicare claim for acute urinary retention (AUR) in patients with prior diagnosis of BPH versus those without a diagnosis of BPH censored for death, loss to follow-up, or the end of the study period (31 December 2005). For patients with BPH, time starts at first Medicare claim for BPH. For patients without BPH, time starts at date of transplant (log-rank P < 0.001). As a time-dependent variable in a Cox nonproportional hazards regression analysis, BPH was also associated with AUR (AHR 4.91 [95% CI 4.21 to 5.72]; < 0.001). (B) Similar Kaplan-Meier plot for time to urinary tract infection (UTI) (log rank P = 0.202). As a time-dependent variable in a Cox nonproportional hazards regression analysis, BPH was also associated with UTI (AHR 2.62 [95% CI 2.19 to 3.13]; P < 0.001). (C) Similar Kaplan-Meier plot for time to transurethral resection of the prostate (TURP) procedure (log rank P < 0.001). As a time-dependent variable in a Cox nonproportional hazards regression analysis, BPH was also associated with TURP (AHR 63.01 [95% CI 46.69 to 85.02]; P < 0.001).
Discussion
There is no reason to suspect that the actual incidence of BPH should be greater in the renal transplant population. However, the 3-yr incidence of 9.7% in this study is elevated compared with those from longitudinal studies of the general population such as the Olmsted County study, in which the 10-yr corrected cumulative incidence was 0.85% (9). However, several smaller observational posttransplant studies have yielded similar incidences to those found in our study (3,4,10,11) Presumably, several prevalent patients may be mislabeled as “incident” cases in oliguric/anuric ESRD patients once urine output is restored after transplantation, and based on the average dialysis vintage in this cohort, BPH diagnosis by LUTS could be delayed for several years. In addition, it would seem reasonable that the observed incidence may be higher in transplant patients given the increased medical surveillance in this population.
BPH was significantly associated with renal allograft loss in this study. There is evidence from longitudinal studies that BPH is a progressive disease, (9,12) and that if left untreated, BPH poses a risk for numerous complications, including AUR, recurrent UTIs, hydronephrosis, and renal failure. (13,14) BPH is known to be associated with UTI risk, and UTI are frequently present upon BPH diagnosis. (3,13) In the renal transplant population, UTI have been shown to be an independent risk factor for graft loss, (15) and were noted in 6.5% of the BPH patients in this study. AUR was noted in 10.3% of patients with BPH in this cohort. This frequency is slightly elevated compared with other studies, reporting cumulative incidences of 1% to 7% over 2 to 5 yr of follow-up. (9,16,17,18,19)
Prostate-related surgeries (TURP) were noted in 7.3% of patients with BPH. This frequency is similar to that reported in the general population (5% to 10%) (9,16,18,19). Of note, there were 84 (33.3%) TURP procedures performed in patients without prior diagnosis of BPH, presumably because they had prostate cancer. As a result of database limitations, we were unable to assess medical therapies for BPH. There are no reports of medical therapy use in the renal transplant literature, but, presumably, this would be the first-line therapy as it is in the general population (9). However, medical therapy does add additional drugs to the lifelong drug regimen that already exists in transplant patients, (6) and there is one report of pretransplant TURP in a patient who refused medical therapy (20).
Regarding surgical therapy, the literature is conflicting as to whether or not it is better to perform TURP procedures before or after transplantation. If performed before transplant while the patient is still oliguric/anuric, bladder neck contracture or urethral scarring may develop (10). However, if performed after transplant, one study reported a 25% incidence of major perioperative complications including death (5). In other series, patients who underwent TURP posttransplant had ureteral complications, with deaths reported from suprapubic catheter urosepsis (4,6). Given the risk of infection with immunosuppressants and possible prolonged catheterization post-TURP, several authors recommend performing TURP 6 to 8 wk pretransplant. (10,11) However, this is not possible with deceased donor kidneys, and other authors have advocated that TURP can be performed safely posttransplant (14).
Evaluation for urologic abnormalities including BPH before transplant listing is generally regarded as necessary, although programs utilize different methods of screening. At a minimum, it has been recommended that this evaluation include a history, examination, urinalysis, urine culture, and upper tract imaging, (14,20,21) for men in whom outlet obstruction is suspected; uroflometry combined with a postvoid residual volume is an excellent objective screening test (14). Once a patient is listed for transplant, BPH surveillance is not standard practice, although one author recommends annual assessment for patients on the waiting list (10). On the basis of incident BPH cases in this study, both recipient age and dialysis vintage should be taken into account, and screening should commence at approximately age 50.
Limitations
BPH is technically a histologic diagnosis, but is frequently used interchangeably with prostatism, a clinical syndrome of LUTS resulting from urethral obstruction at the level of the prostate. It is impossible to determine whether the Medicare claims used in the paper reflect the histologic diagnosis or the clinical syndrome, but it has become standard for investigators to use surrogate clinical measures (e.g., LUTS) to diagnose BPH (22).
Although the date of first claim is used to define the onset of diagnosis, it is possible that BPH could have been diagnosed previously, before their Medicare eligibility, and thus could represent prevalent cases. We attempted to exclude prevalent BPH by assessing claims for at least 1 yr pretransplant and excluding patients whose first BPH claim was before the transplant date. To account for ascertainment bias, we repeated this analysis using only patients who had Medicare coverage for at least 1 yr pretransplant as a sensitivity analysis, and the 3 yr incidence of BPH remained elevated compared with the general population at 10.9%.
As above, this may not be a true incidence of posttransplant BPH and may be a manifestation of deferred diagnosis while patients were oliguric/anuric and did not experience LUTS. If this is the case, one would expect a peak of BPH cases after transplantation, followed by a return to levels consistent with those of the general population. We did not observe this, but perhaps this would be evident with a longer follow-up period.
There could be surveillance bias in this study regarding the BPH-related complications of AUR and UTI. When evaluating these as complications of BPH, we included only patients diagnosed with AUR/UTI after BPH. However, clinically, these can be diagnosed concurrently or even before formal BPH diagnosis, which could lead to misclassification. For example, a male transplant patient could have two to three UTIs before a urologic workup yields a diagnosis of BPH.
There could also be misclassification with the diagnosis of AUR in this study in that there could be other causes of urinary retention. For example, progressive vesical dysfunction caused by decreased diuresis has been noted to cause bladder outlet obstruction in male patients on dialysis (23). Information regarding medical therapy for BPH is not available in the USRDS database, so, unlike the controlled studies mentioned in this article, we cannot comment on the effect of medical therapy for these conditions.
The association of later year of transplant is likely an era effect reflecting the older age of recipients in later years. In addition, the significant associations between BPH and ECD, CRT, CIT, immunosuppression regimens, and donor age were likely confounded by recipient age because these were no longer significant on the adjusted analysis. The lack of association of black race with BPH on univariate analysis was likely confounded by the younger age of black recipients.
Controversy exists as to whether BPH is truly associated with CKD. For example, in the MTOPS study, no men developed renal insufficiency as a result of BPH during a mean follow-up of 4.5 yr. In addition, UTI were rare and the authors suggested that LUTS and BPH were not clinically significant risk factors for UTI (16). However, the unique susceptibility of the transplanted kidney (denervation, reflux with abnormal ureteral valves, immunosuppression) may influence this situation similarly to posttransplant nephrolithiasis (24). Although we have demonstrated an independent association of BPH and AUR, UTI, and allograft loss, we cannot comment on the specific mechanism of graft loss. Limitations specific to the methods of USRDS database research have been described previously (25).
Conclusions
In summary, incident BPH in males after renal transplant is common and is associated with AUR, UTI, and graft loss. Given these risks, older male patients (especially over age 50 yr) should be screened for BPH before listing, periodically while on the waiting list, and after transplantation, especially in the 1st yr. However, it is unknown whether treatment, either medical or surgical, attenuates the risk of graft loss or urologic complications associated with BPH. Controversy exists as to whether surgical correction is more advantageous if performed before or after transplant, and there is no literature on medical therapy for BPH in this population. Given the above surgical risks in transplant patients, medical therapy would presumably be the first-line treatment, started promptly on detection of urinary obstruction. A randomized trial of medical therapy for BPH related urinary obstruction is needed for both renal transplant recipients and patients on the waiting list.
Disclosures
None.
Acknowledgments
These data were presented as a poster on 1 June 2008 at the American Transplant Congress 2008 Meeting in Toronto, Canada and later published as an abstract (American Journal of Transplantation 8(s2): 450. 2008)
Published online ahead of print. Publication date available at www.cjasn.org.
Disclaimer: The views expressed in this paper are those of the authors and do not reflect the official policy of the National Institutes of Health, the Department of Army, the Department of Defense, or the United States government.
References
- 1.U.S. Renal Data System, USRDS 2006 Annual Data Report: Atlas of end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2006
- 2.Berry SJ, Coffey DS, Walsh PC, Ewing LL: The development of human benign prostatic hyperplasia with age. J Urol 132: 474, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Dorsam J, Wiesel M, Mohring K, Pomer S, Kalble T, Staehler G: Transurethral incision of the prostate following renal transplantation. J Urol 153: 1499–1501, 1995 [PubMed] [Google Scholar]
- 4.Streeter EH, Little DM, Cranston DW, Morris PJ: The Urological Complications of Renal Transplantation: a series of 1535 patients. BJU International 90: 627–634, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Reinberg Y, Manivel JC, Sidi AA, Ercole CJ: Transurethral resection of the prostate immediately after renal transplantation. Urology 39: 319, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Shoskes DA, Hanbury D, Cranston D, Morris PJ: Urological complications in 1,000 consecutive renal transplant recipients. J Urol 153: 18–21, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Schiff M Jr., Weiss RM, Kraus P, Lytton B: Acute urinary retention following renal transplantation. Urology 1: 108–110, 1973 [DOI] [PubMed] [Google Scholar]
- 8.Rule AD, Lieber MM, Jacobsen SJ: Is benign prostatic hyperplasia a risk factor for chronic renal failure? J Urol Mar; 173: 691–696, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Sarma AV, Jacobson DJ, McGree ME, Roberts RO, Lieber MM, Jacobsen SJ: A population based study of incidence and treatment of benign prostatic hyperplasia among residents of Olmsted County, Minnesota: 1987 to 1997 J Urol 173: 2048–2053, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Shenasky JH: Renal transplantation in patients with urologic abnormalities. J Urol 115: 490, 1976 [DOI] [PubMed] [Google Scholar]
- 11.Kabler RL, Cerny JC: Pre-transplant urologic investigation and treatment of end stage renal disease. J Urol 129: 475, 1983 [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick JM: The natural history of benign prostatic hyperplasia. BJU Int 97[Suppl 2]: 3–6, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sacks SH, Aparicio SA, Bevan A, Oliver DO, Will EJ, Davison AM: Late renal failure due to prostatic outflow obstruction: a preventable disease. BMJ 298: 156–1599, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power RE, Hickey DP, Little DM: Urological evaluation prior to renal transplantation. Transplant Proc 36: 2962–2967, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Abbott KC, Swanson SJ, Richter ER, Bohen EM, Agodoa LY, Peters TG, Barbour G, Lipnick R, Cruess DF: Late urinary tract infection after renal transplantation in the United States. Am J Kidney Dis Aug; 44: 353–362, 2004 [DOI] [PubMed] [Google Scholar]
- 16.McConnel JD, Roehrborn CG, Bautista OM et al. for the Medial Therapy of Prostatic Symptoms (MTOPS) Research Group: The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 349: 2387–2398, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Meigs JB, Barry MJ, Giovannucci E, Rimm EB, Stampfer MJ, Kawachi I: Incidence rates and risk factors for acute urinary retention: The Health Professionals Follow Up Study. J Urol 162: 376–382, 1999 [PubMed] [Google Scholar]
- 18.McConnell JD, Bruskewitz R, Walsh P et al: The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 338: 557–563, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Roehrborn CG for the ALTESS study group: Alfuzosin 10mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: Results of a 2-year placebo-controlled study: BJU Int 97: 734–741, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Glazier DB, Whang MI, Geffner SR, Lyman NW, Friedman GS, Viscuso R, Jacobs MG, Mulgaonkar SP: Evaluation of voiding cystourethrography prior to renal transplantation. Transplantation Dec 27; 62(12): 1762–1765, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Jefferson RH, Burns JR: Urological evaluation of adult renal transplant recipients. J Urol 153(3 Pt 1): 615–618, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen SJ, Girman CJ, Lieber MM: The natural history of benign prostatic hyperplasia. Urology 58: 5, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Menendez V, Cofan F, Talbot-Wright R, Ricart MJ, Gutierrez R, Carretero P: Urodynamic evaluation in simultaneous insulin-dependent diabetes mellitus and end stage renal disease. J Urol 155(6): 2001–2004, 1996 [PubMed] [Google Scholar]
- 24.Abbott KC, Schenkman N, Swanson SJ, Agodoa LY: Hospitalized nephrolithiasis after renal transplantation in the United States. Am J Transplant Apr; 3(4): 465–470, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Abbott KC, Bucci JR, Cruess D, Taylor AJ, Agodoa LY: Graft loss and acute coronary syndromes after renal transplantation in the United States. J Am Soc Nephrol 13: 2560–2569, 2002 [DOI] [PubMed] [Google Scholar]