Abstract
Background: Coronary calcification (CAC) is found in early stages of CKD. Pulse pressure (PP) predicts CAC in dialysis patients. This study evaluates the accuracy of PP in predicting CAC in patients not yet on dialysis (CKD patients).
Methods: CKD patients (n = 388) underwent coronary calcium score (CAC score) and abdominal x-ray (n = 128) for estimating aorta calcification (AAC). Biochemistry and PP were measured every 3 and 6 months in patients with stage 4 to 5 and 2 to 3 CKD, respectively. The accuracy of PP and AAC was assessed by receiver operating characteristics analysis.
Results: PP correlated with CAC score in the whole cohort and in patients with stages 2 to 3 and stages 4 to 5 CKD. PP >60 mmHg predicted CAC score >0 (OR: 2.14; P < 0.001), ≥100 (OR: 2.92; P < 0.001), ≥400 (OR: 6.17; P < 0.001) after multivariable adjustment. Area under the curve (AUC) was 0.626 for CAC score >0, 0.676 for score >100, and 0.746 for score >400. PP >60 mmHg reduced the rate of event-free survival. AAC was found in 58% of patients and correlated with CAC score. AUC was 0.628 for CAC score >0, 0.652 for score >100, 0.831 for score >400.
Conclusion: PP may identify CKD patients with subclinical CAC who need further evaluation. Accuracy of PP and AAC is nearly similar in predicting CAC. High PP indicates vessel wall alterations leading to adverse outcome.
Coronary artery calcification (CAC) is present even in asymptomatic patients with stage 2 to 5 of chronic kidney disease (CKD) who are not yet on dialysis (1–3). The disease progresses rapidly and is associated with fatal and nonfatal cardiovascular events (4,5). It is likely that in CKD patients, who frequently die from coronary heart disease before dialysis initiation, CAC is an important risk factor for cardiovascular events as in patients on dialysis (ESRD patients) (6–9). However, unanswered questions are (1) how to distinguish CKD patients who may have CAC and need further cardiovascular tests and early therapeutic intervention and (2) what procedure should be used for the preliminary screening.
The numbers of patients with stages 2 to 5 of CKD are increasing worldwide (10,11), and CAC is found in less than half of this population (1–3). In addition, gold standard procedures such as electron beam or multislice computed tomography (EBCT or MSCT) are not suitable for screening large population (12–14); EBCT is available in only few nephrology units and is expensive, MSCT is time consuming and exposes patients to large radiation doses. Therefore, in CKD patients, it is important to find simple and inexpensive tests for the preliminary screening. Standard radiographs, echocardiography, and pulse pressure may predict the presence of CAC in ESRD patients (15–18).
The aim of the present study was to evaluate the diagnostic accuracy of pulse pressure in predicting the presence of CAC in CKD patients. To our knowledge, no data are available on this issue.
Materials and Methods
This is a retrospective study carried out in consecutive outpatients who had been evaluated for presence of CAC from March 2002 to September 2006 at our institution after providing their written informed consent. Protocol for screening had been approved by our internal ethical board. The cohort was represented by 420 patients who had been selected on the basis of the following inclusion criteria: no symptoms of heart failure or coronary artery disease, no history of myocardial infarction and/or coronary bypass surgery or angioplasty, no stroke, no current arrhythmia, and no rapidly progressive renal disease. From the database of the initial cohort, we selected the files of 388 patients on the basis of the following further inclusion criteria: age > 18 yr, stage 2 to 5 CKD (not yet on dialysis), minimum 6 month follow-up in our clinic before the MSCT. Recruited patients were followed until the end of the study (June 2007) or occurrence of an event.
Physical examination was performed and routine blood chemistry; lipid profile; and serum concentrations of calcium (corrected for serum albumin), phosphorus, intact parathyroid hormone (i-PTH), homocysteine, and high-sensitivity C-reactive protein (hs-CRP) were assessed every 3 and 6 months in patients with stage 4 to 5 and 2 to 3 CKD, respectively. Biochemical determinations obtained 3 and 6 months apart were averaged. GFR was measured as 24-h creatinine clearance. Normal ranges for serum calcium, phosphorus, and i-PTH were established on the basis of the stage of CKD according to Kidney Disease Outcome Quality Initiative guidelines. i-PTH was assayed by a chemiluminescent immunometric method (Diagnostic Products Corporation, Los Angeles, CA; normal values in general population: 10 to 75 pg/ml; 10 to 75 ng/L).
In each patient, BP was measured by a manual sphygmomanometer after 5 min in a seated position; the mean of three consecutive readings, taken 1 min apart, was recorded. Pulse pressure was calculated as the difference between peak systolic and trough diastolic arterial BP. In patients with stage 4 to 5 CKD, readings recorded 3 months apart before MSCT were averaged and used for statistical analysis; the reading closest to MSCT was used in other patients. According to guidelines of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, hypertension was defined as systolic BP (SBP) ≥140 mm and/or diastolic BP (DBP) ≥90 mmHg (19). Patients who used insulin or oral hypoglycemic drugs on a regular basis were considered diabetic.
The rates of occurrence of sudden death, cardiovascular events, and onset of dialysis were collected. The following cardiovascular events were recorded: myocardial infarction, cerebrovascular accident, coronary bypass graft, percutaneous coronary angioplasty, peripheral artery bypass, amputation, abdominal aortic aneurism repair, and carotid endoarterectomy.
CAC score was assessed by MSCT (2). Calcific lesions in the abdominal aorta (AAC) were evaluated by lateral lumbar x-ray and graded by semiquantitative scoring system (20). Score of patients who had undergone the radiograph of the abdomen within 12-mo period (before or after) MSCT was recorded.
The correlation between the continuous variables (pulse pressure, CAC score, and abdominal aorta calcification score) was investigated using the Spearman rank coefficient (rho) because they were not normally distributed. The categorical variables were compared using the chi-square test.
Receiver operating characteristics (ROC) analyses were used to assess the diagnostic accuracy of pulse pressure for detecting CAC score >0, ≥ 100, and ≥ 400. The optimal cut-off for each value of CAC score was the corresponding value of pulse pressure that gave percent sensitivity and specificity closest to the point of a perfect marker (sensitivity and specificity of 100%). ROC analyses were also performed for AAC.
Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for coronary calcification at the different score levels and at the value of pulse pressure ≥60 mmHg. Adjustment was made for relevant covariates that had been found significant (P < 0.05) in univariate analysis.
Kaplan–Meier survival analysis was done using composite censoring variables: dialysis initiation, nonfatal cardiovascular disease, and death. Survival curves were generated by data collected from patients without coronary calcifications as well as from patients with CAC score >100 or >400, who did or did not have pulse pressure ≥60 mmHg. The survival probability at various time points was compared using the log-rank test.
Data were expressed as mean ± SD unless otherwise indicated. A two-sided P value of < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using NCSS 2004 statistical software (NCSS, Kaysville, UT).
Results
The mean duration of follow-up was 24.4 ± 12.7 mo (median: 24.5 mo) from the assessment of CAC score up to the end of the study or occurrence of an event.
Enrolled patients had glomerulonephritis (27%), diabetic nephropathy (16%), ischemic nephropathy (10%), interstitial nephropathy (3%), and unknown renal diseases (44%).
Duration of diabetes was 157 ± 113 mo. Hypertension was present in 322 patients (83%), with a mean duration of 95 ± 88 mo. BP was on target in 111 (40%) patients. Of treated but uncontrolled hypertensive patients, 92% had diastolic BP ranging from 80 to 95 mmHg. Mean number of antihypertensive medications was 2.08 ± 0.9. A single antihypertensive medication (calcium channel blocker, converting enzyme inhibitors, angiotensin II receptor antagonist) was prescribed to 28% of patients. The association of converting enzyme inhibitors or angiotensin II receptor antagonists with calcium channel blocker was the most frequent (45%). Other demographic, clinical and laboratory characteristics are summarized in Table 1.
Table 1.
Demographic, clinical and laboratory findings of subjects (N = 388)
| Characteristic | Value |
|---|---|
| Male gender (%) | 77.8 |
| Age (yrs) | 52.6 ± 12.7 |
| Diabetes, type II (%) | 15.8 |
| GFR (mL/s) | 1.05 ± 0.67 (64 ± 40 ml/min) |
| Systolic blood pressure (mmHg) | 134.3 ± 16.5 |
| Diastolic blood pressure (mmHg) | 80.2 ± 7.8 |
| Pulse pressure (mmHg) | 53.9 ± 15.9 |
| i-PTH (ng/L) | 106.8 ± 126.8 (106.8 ± 126.8 pg/ml) |
| Serum calcium (mmol/L) | 2.37 ± 0.14 (9.52 ± 0.57 mg/dl) |
| Serum calcium (mmol/L) | 2.57 ± 0.14 (10.3 ± 0.57 mg/dl) |
| Serum phosphorus (mmol/L) | 1.26 ± 0.29 (3.90 ± 0.91 mg/dl) |
| Ca × P product (mg2/dl2) | 39.9 ± 8.59 |
| LDL-C (mmol/L) | 3.04 ± 1.10 (117.6 ± 42.6 mg/dl) |
| Homocysteine (μmol/L) | 182.7 ± 169.4 (24.7 ± 22.9 nmol/ml) |
| Fibrinogen (μmol/L) | 10.8 ± 3.3 (367 ± 112.1 mg/dl) |
| HsCRP (mg/L) | 0.49 ± 0.77 |
Data are expressed as mean ± standard deviation. Gender and diabetes mellitus are reported as proportion of population. GFR, glomerular filtration rate; hs-CRP, high-sensitivity C reactive protein; i-PTH, intact parathyroid hormone; LDL-C, low-density lipoprotein-cholesterol; Ca × P, calcium–phosphorus product.
Derangement of mineral metabolism was not significantly different among patients with and without CAC (Table 2). On the basis of CKD stages, serum concentration of i-PTH and phosphorus above the upper normal limit was found in 39.5% and 19.3% of patients, respectively; concentrations below the lower normal limit were found in 7.9% and 5.4% of patients, respectively. Patients treated with oral calcitriol (n. 20; 5%) had GFR ranging from 10 to 32 ml/min and were equally distributed between patients with (6%) and without (4%) CAC. The use of phosphate binders was not different among patients with and without CAC, nor was the use of antihypertensive drug regimens.
Table 2.
Clinical and laboratory characteristics of patients with and without coronary calcification (CAC)
| Characteristic | Without CAC | With CAC | P |
|---|---|---|---|
| No. of patients (%) | 224 (57.7) | 164 (42.3) | |
| Male gender (%) | 72.3 | 85.4 | 0.0022 |
| Age > 65 yr old (%) | 4.9 | 29.9 | 0.0000 |
| Diabetes mellitus (%) | 6.8 | 22.8 | 0.0001 |
| GFR<60 ml/min (%) | 44.5 | 61.6 | 0.0009 |
| Hypertension (%) | 78.6 | 92.0 | 0.0004 |
| Pulse pressure ≥75th percentile (%) | 31.4 | 50.7 | 0.0003 |
| Fibrinogen ≥75th percentile (%) | 19.0 | 32.7 | 0.01 |
| hs-CRP ≥ 75th percentile (%) | 23.8 | 28.9 | NS |
| i-PTH above upper normal limit (%) | 39.6 | 39.3 | NS |
| Calcium above upper normal limit (%) | 38.1 | 43.2 | NS |
| Phosphorus above upper normal limit (%) | 22.3 | 16.3 | NS |
| Ca × P product above 55 mg2/dL2 (%) | 5.7 | 4.9 | NS |
| LDL-C above upper normal limit (%) | 67.6 | 58.2 | NS |
| Homocysteine ≥75th percentile (%) | 24.8 | 25.3 | NS |
Fibrinogen 75th percentile value = 418 mg/dl; hs- CRP 75th percentile value = 0.4 mg/L; homocysteine 75th percentile value = 28.4 mg/L. Upper normal limit for i-PTH: 60 pg/ml for GFR ≥ 60 ml/min, 70 pg/ml for GFR range 59-30 ml/min, 110 pg/ml for GFR range 29-15 ml/min, and 300 pg/ml for GFR <15 ml/min. Upper normal limit for serum phosphorus: 4.6 mg/dl for GFR ≥15 ml/min and 5.5 mg/dl for GFR<15 ml/min. Upper normal limit for serum calcium:10.5 mg/dl for GFR ≥15 ml/min and 9.5 mg/dl for GFR <15 ml/min. LDL-C upper normal limit:100 mg/dl. GFR, glomerular filtration rate (as creatinine clearance); hs-CRP, high-sensitivity C-reactive protein; i-PTH, intact parathyroid hormone; Ca × P product, calcium–phosphorus product; LDL-C, low density lipoprotein-cholesterol.
CAC was found in 164 patients (42.3%); CAC score ranged from 3 to 3303 Agatston units. CAC score >100 and >400 was found in 64.6% and 26.2% of patients, respectively. Patients with CAC were more likely to be male and over 65 yr old, and to have GFR <60 ml/min, diabetes, hypertension, and hyperfibrinogenemia. On the contrary, no significant difference was observed for hs-CRP, LDL-C, and homocysteine.
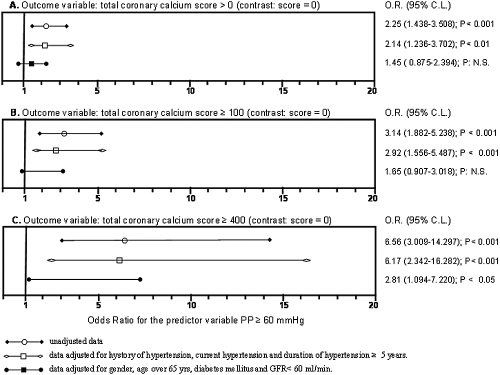
In the entire cohort of patients, a significant positive correlation between pulse pressure and CAC score was observed (ρ = 0.28; P < 0.0001); the correlation remained significant but at lower level (P < 0.048) after adjusting for age, duration of hypertension, and diabetes. The correlation was confirmed in patients with stage 2 to 3 CKD (ρ = 0.30, P < 0.0002) as well as in those with stage 4 to 5 CKD (ρ = 0.26, P < 0.007). The cut-off value of pulse pressure ≥ 60 mmHg was chosen because it was more frequently observed in patients with CAC than in those without CAC (50.7% versus 31.4%, P = 0.001) and was the best cut-off value to discriminate patients with CAC, as shown by ROC curve (as reported below). Unadjusted and adjusted ORs to estimate the risk of CAC in patients with pulse pressure ≥60 mmHg, using patients without CAC as a reference group, are shown in Figure 1. Pulse pressure ≥60 mmHg predicted CAC with score >0 (OR: 2.25; P < 0.001), CAC with score ≥100 (OR: 3.14; P < 0.001), and CAC with score ≥400 (OR: 6.56; P < 0.001) in univariate analysis. Pulse pressure ≥60 mmHg was a significant predictor of CAC with score >0 (OR: 2.14; P < 0.001), ≥100 (OR: 2.92; P < 0.001), and ≥400 (OR: 6.17; P < 0.001) after adjusting for hypertension and duration of hypertension over 5 years. Finally, pulse pressure ≥60 mmHg predicted CAC with score ≥400 (OR: 2.81; P < 0.05) after adjusting for gender, age >65 yr, diabetes mellitus, and GFR <60 ml/min. Thus, both in univariate and multivariate analysis, OR increased in parallel with CAC score values.
Figure 1.
Plot of unadjusted and adjusted odds ratios to estimate the risk of having coronary calcification in patients with pulse pressure ≥60 mmHg, assuming patients without CAC as reference group. Panel A illustrates the odds ratios and the 95% confidence limits (95% CL) for the predictor variable Pulse Pressure ≥60 mmHg, considering as dichotomic outcome variable coronary calcium score >0. Panels B and C indicate calcium score ≥100 and ≥400, respectively.
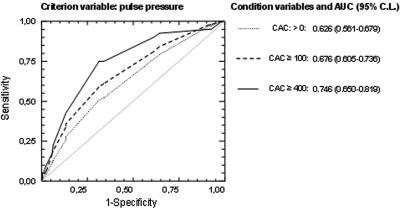
The areas under the curve (AUCs) for pulse pressure criteria to determine the presence or absence of CAC, CAC score ≥100 and ≥400 versus absence of CAC are shown in Figure 2. The values of AUC were 0.626 for presence of CAC, 0.676 for CAC score ≥100, 0.746 for CAC score ≥400. Each area was significantly different (P < 0.001) from AUC = 0.5; this value indicates a noninformative test. Thus, the value of AUC found in our patients with CAC score ≥400 may be regarded as a moderately informative test (21). The best cut-off value of pulse pressure was 60 mmHg for all of the AUCs, and the corresponding values of sensitivity and specificity were 51% and 69% for the presence or absence of CAC, 59% and 69% for CAC score ≥100, and 75% and 69% for CAC score ≥400, respectively.
Figure 2.
Receiver operating characteristic (ROC) curves for the criterion variable represented by pulse pressure and three condition variables represented by different scores of coronary artery calcifications (CAC); each variable has the absence of CAC as contrast. The dotted line represents the condition variable calcium score 0, the dashed and solid lines represent calcium score ≥100 and calcium score ≥400, respectively. AUC represents area under the curve. Numbers in brackets are upper and lower 95% confidence limits (95% CL).
Abdominal x-ray was performed in 128 patients, and AAC was found in 58% of them. The mean score was 4.81 ± 4.3. Compared with patients without AAC, those with AAC had significantly longer duration of diabetes (P < 0.01); no differences were found in lipid profile, serum concentrations of calcium, phosphorus, i-PTH, homocysteine, hs-CRP, or antihypertensive medication regimen. In these patients, a positive correlation between AAC and CAC score was observed (ρ = 0.32; P < 0.0002). In addition, the values of AUC for AAC criterion for the presence or absence of CAC, CAC score ≥100 and ≥400 versus absence of CAC were 0.628 for presence of CAC, 0.652 for CAC score ≥100, and 0.831 for CAC score ≥400.
During the observation time, 113 events were recorded: 77 dialysis initiations, 22 nonfatal cardiovascular events, and 14 deaths.
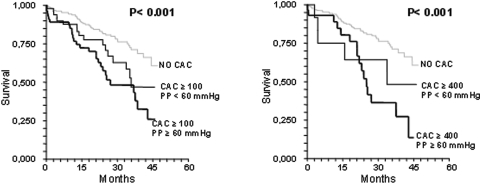
The Kaplan-Meyer survival curves in patients without and with CAC, with or without pulse pressure ≥60 mmHg, are illustrated in Figure 3. Patients without CAC showed better survival rate. Patients with CAC score ≥100 and ≥400 had a similar survival rate. Patients with CAC score ≥100 or ≥400 and concomitant pulse pressure ≥60 mmHg showed the worst survival rate (log-rank P < 0.001). The risk for an event remained higher (RR = 2.02; 1.007 to 4.040; P < 0.03) in patients with CAC >0 and pulse pressure ≥60 mmHg after adjusting for age, gender, underlying renal disease, GFR, hemoglobin, and 24-h urinary protein excretion.
Figure 3.
Kaplan-Meyer survival curves. The left panel shows curves of patients with no CAC and CAC ≥100, with and without Pulse Pressure ≥60 mmHg respectively; the right panel shows survival curves of patients with no CAC and CAC ≥400 with and without Pulse Pressure ≥60 mmHg. The logrank probability level is represented by P.
Excluding initiation of dialysis, 36 events were recorded: 22 non fatal and 14 fatal events (sudden death or death following acute myocardial infarction). Thirty-three out of 36 events occurred in patients with CAC (nonfatal: 95%; fatal: 86%).
Discussion
This study was undertaken on the basis of the following points: (1) CAC may be regarded as index of advanced atherosclerotic process and as strong predictor of cardiovascular events (7,22); (2) traditional and uraemia-related risk factors do not predict the presence of CAC (23,24); (3) rates of stages 2 to 5 CKD are increasing worldwide (10,25); (4) CAC is found even in early stages of CKD and rapidly progresses (2,4,5); and (5) gold standard procedures in assessing presence and extent of CAC such as EBCT and MSCT are not suitable for screening large populations.
It should be clinically relevant to develop an accurate test for identifying patients with subclinical CAC to submit to further evaluation. This stepped strategy would recognize patients with CAC in advance and allow early therapeutic choice before coronary heart disease become symptomatic (24,26). In ESRD patients, standard radiographs, echocardiography, and pulse pressure have been found to be suitable procedures for predicting the presence of CAC (15–18).
Aging, long-lasting hypertension, diabetes, loss of elastic tissue in the vessel wall, and progressive arterial calcification are major factors responsible for vascular stiffness; as a consequence, the capacity of vessels to dampen the increases of arterial pressure during ventricular systole markedly decreases and, in turn, the pulse wave propagation velocity increases. The most evident hemodynamic consequence is increased pulse pressure and pulse wave velocity that cause left ventricular hypertrophy and reduce the filling of coronary arteries during the diastole. In ESRD patients the increased pulse pressure and pulse wave velocity are strongly correlated with arterial calcification and cardiovascular events (22,27–29). For instance, neither hypertension nor systolic BP were associated with the presence of coronary artery disease, whereas diastolic BP was inversely related to the presence of coronary artery disease, decreasing the OR for coronary disease by 16% for every 10 mmHg increase in diastolic BP (6). In the present study, a positive correlation was found between pulse pressure and CAC score in the entire cohort of patients, even after data were adjusted for age, diabetes, hypertension, and duration of diabetes and hypertension. In addition, the correlation remained significant in patients with both early (2 to 3) and advanced (4 to 5) stages of CKD, suggesting that pulse pressure and CAC may be related within a width range of GFRs. However, even a powerful statistical association may have no clinical relevance.
The OR estimating the risk for CAC in patients with pulse pressure ≥60 mmHg increased in parallel with CAC score (i.e. from score >0 up to score ≥400) in univariate and multivariate analysis. Of note, in multivariate analysis, the relationship between pulse pressure and CAC was more pronounced for score ≥400. Finally, after adjusting data for age over 65 yr, presence of diabetes mellitus, and GFR <60 ml/min, the relationship remained significant and the OR increased.
In ESRD patients, several studies have evaluated the relationship between pulse pressure and vascular calcifications, with contrasting results (17,22,30,31). In one study, significant positive association was observed between increasing values of pulse pressure and the number of calcified peripheral arteries assessed by a high-resolution B-mode echo-tracking system. In the other studies, pulse pressure did not correlate to the extent of CAC evaluated with EBCT and showed fair discriminatory value and low accuracy in predicting CAC; thus, it was excluded from the cardiovascular calcification index for predicting the presence of CAC. However, these interesting findings were observed in nonwhite ESRD-patients with mean dialysis vintage of 4.2 yr; almost half had diabetes, and many had heart failure and history of cerebro- and cardiovascular diseases. Importantly, timing of BP measurements and their relation to dialysis session was not reported; in ESRD patients, arterial BP greatly fluctuates depending on hydration status.
ROC curve and AUC indicated that pulse pressure ≥60 mmHg was the best cut-off point to predict CAC in our patients. Interestingly, in CKD patients, it has been observed that pulse pressure ≥60 mmHg is involved in diastolic dysfunction, left ventricular remodelling or hypertrophy, and cardiovascular disease progression, and is strongly correlated with pulse wave velocity (32–34). However, an association between pulse pressure and the presence of CAC has never been evaluated.
In this study, the achieved sensitivity (percentage of true positive patients) was 51%, 59%, and 75% for score >0, score ≥100, and score ≥400, respectively; whereas specificity (percentage of true negative patients) was 69% for each level of CAC score. The highest sensitivity and specificity (75% and 69%, respectively), as well as the largest AUC, were found in the presence of pulse pressure ≥60 mmHg and CAC score ≥400. Thus, pulse pressure ≥60 mmHg may predict the presence of CAC score ≥400 in 75% of patients (true positive patients) and may exclude it in 69% of patients (true negative patients). As a result, 31% of patients might be erroneously diagnosed as “calcified” (false positive patients), and 25% of patients might have missed the diagnosis (false negative patients). We are aware that false positive test may result in further unnecessary diagnostic procedures, but false negative tests may have even more detrimental effects, such as delay in diagnosis and care and consequent worse prognosis, especially in high-risk patients. Patients with CKD are at a high level of cardiovascular risk even when they are asymptomatic. In a review on accredited screening techniques for cardiovascular diseases (such as resting electrocardiogram and exercise treadmill testing), the median false negative rate in detecting asymptomatic subjects at high cardiovascular risk for coronary heart disease was 40% (34). In addition, the sensitivity and specificity of the artery intimal-media thickness, assessed by B-mode ultrasound, to correctly identify asymptomatic subjects with CAC score >400 were 60% and 75%, respectively (35,36).
AAC was found in 58% of 128 patients in whom this determination was available. The latter finding indicates that the abdominal calcification process starts in early stages of CKD, as has been found for coronary calcification (1–3). The mean AAC score was similar to that reported in ESRD patients (30); the prevalence rate was also similar (A. Bellasi, Medical Manager, Genzyme SpA, Modena, Italy, personal communication, September 2008). The absence of a difference between CKD and ESRDpatients is probably due to selection bias.
Positive correlation was observed between AAC and CAC scores. Interestingly, the AUC for AAC score was lower than that of pulse pressure in discriminating the presence/absence of CAC and CAC score >100, but higher in discriminating CAC score >400. This suggests that pulse pressure may be superior to AAC in predicting mild CAC, but not severe CAC. These findings are the first available for CKD patients. In ESRD patients, AAC score showed a good correlation with CAC assessed by EBCT (30).
Ascertaining the survival rate was not aim of this study; however, some interesting findings are worthy of few comments. Patients without CAC had better survival rate; survival of patients with score ≥100 was not different compared with that of patients with score ≥400, underlining that the risk for an outcome is evident even at low score. Pulse pressure ≥60 mmHg markedly impaired the survival in patients with score ≥400.
Limitations of the Study
Survival curves were done on the basis of composite outcomes, inclusive of initiation of dialysis that is not strictly dependent on arterial calcification. However, experimental studies have shown that arterial calcification may accelerate the progression of renal diseases (37,38). The low incidence of events observed during the study (despite the large cohort of CKD patients evaluated) did not allow additional survival evaluations. The presence of abdominal aorta calcification was not ascertained in all patients who had undergone MSCT. In some patients, a single value of pulse pressure was available for statistical analysis (as has been the case in many studies in ESRD patients). Despite these limitations, this is the first work to evaluate the usefulness of pulse pressure and AAC in predicting the presence of CAC in CKD patients.
Conclusions
The data of the present study suggest that pulse pressure may identify patients with subclinical CAC who likely need further evaluation for early therapeutic choice before coronary heart disease becomes symptomatic. High pulse pressure may indicate the presence of vessel wall alterations that lead to adverse outcome.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Fox CS, Larson MG, Keyes MJ, Levy D, Clouse ME, Culleuton B, O’Donnel CJ: Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: The Framingham Heart Study. Kidney Int 66: 2017–2021, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kramer H, Toto R, Peshock R, Cooper R, Victor R: Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J Am Soc Nephrol 16: 507–513, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, Brancaccio D, Cozzolino M, Biondi ML, Andreucci VE: Progression of coronary artery calcification in predialysis patients. Am J Nephrol 27: 152–158, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE: The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72: 1255–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Stack AG, Bloembergen WE: Prevalence and clinical correlation of coronary artery disease among new dialysis patients in the United States: A cross-sectional study. J Am Soc Nephrol 12: 1516–1523, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Raggi P, Bellasi A, Kooienga L, Spigel DM: Mortality effects of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Keith D, Nicholls G, Guillon C, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, Wang H: The burden of kidney disease: Improving global outcomes. Kidney Int 66: 1310–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lamiere N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives–a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–249, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lamiere N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Goodman GW, London G: Vascular calcification in chronic kidney disease. Am J Kidney Dis 43: 572–579, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Goncalves M, Negrao AP: A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant 19: 1480–1488, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Muntner P, Ferramosca E, Bellasi A, Block GA, Raggi P: Development of a cardiovascular calcification index using simple imaging tools in haemodialysis patients. Nephrol Dial Transplant 22: 508–514, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Mayer B, Lieb W, Radke PW, Gotz A, Fischer M, Bassler A, Doehring LC, Aherrahrou Z, Liptau H Erdmann J, Holmer S, Hense HW, Hengstenberg C, Schunkert H: Association between arterial pressure and coronary artery calcification. J Hypertens 25: 1731–1738, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL jr, Jones DW, Matherson BJ, Opanil S, Wright JT jr, Roccella EJ: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 132: 245–250, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Swets JA. Measuring the Accuracy of Diagnostic Systems. www.sciencemag.org 5 September, 2007 [DOI] [PubMed]
- 22.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Cozzolino M, Brancaccio D, Gallieni M, Slatopolsky E: Pathogenesis of vascular calcification in chronic kidney disease. Kidney Int 68: 429–436, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Best PJM, Reddan DN, Berger PB, Szczech LA, McCullough PA, Callif RM: Cardiovascular disease and chronic kidney disease: Insights and an update. Am Heart J 148: 230–232, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lamiere N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2090, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Shaw LJ, Blumenthal RS, Raggi P. Screening asymptomatic low-risk individuals for coronary heart disease: Issues and controversies. J Nuclear Cardiol 11(4): 382–387, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Davies MR,, Hruska KA: Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int 60: 472–479, 2001 [DOI] [PubMed] [Google Scholar]
- 28.London GM, Guérin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 15: 2959–2964, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, Raggi P Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int 70: 1623–1628, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Raggi P, Bellasi A, Ferramosca E, Islam T, Muntner P, Block GA Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 71: 802–807, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Levin A, MD, Djurdjev O, Barrett B, Burgess E, Carlisle E, Ethier J, Jindal K, Mendelssohn D, Tobe S, Singer J, Thompson C: Cardiovascular disease in patients with chronic kidney disease: Getting to the heart of the matter. Am J Kidney Dis 38: 1398–1407, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Wang MC, Tsai WC, Chen JY, Huang JJ: Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 45: 494–501, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Essig M, Escoubet B, de Zuttere D, Blanchet F, Arnoult F, Dupuis E, Michel C, Mignon F, Mentre F, Clerici C. Vrtovsnik F: Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant 23: 239–248, 2008 [DOI] [PubMed] [Google Scholar]
- 35.US Preventive Services Task Force. Screening for coronary heart disease: Recommendation statement. Ann Intern Med 140: 569–572, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Barrett-Connor E, Laughlin GA, Connor C: Coronary artery calcium versus intima-media thickness as a measure of cardiovascular disease among asymptomatic adults (from the Rancho Bernardo Study). Am J Cardiol 99: 227–231, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Nagano N, Miyata S, Obana S: Sevelamer hydrochloride, a calcium-free phosphate binder, inhibits parathyroid cell proliferation in partially nephrectomized rats. Nephrol Dial Transplant 18: 81–85, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Cozzolino M, Dusso SA, Liapis H, Finch J, LU Y, Burke SK, Slatopolsky E. The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am Soc Nephrol 13: 2299–2308, 2002 [DOI] [PubMed] [Google Scholar]