Abstract
Background and objectives: Assessments of iron stores by serum iron saturation ratio (ISAT) and ferritin are used to direct anemia therapy in chronic kidney disease (CKD) and are associated with clinical outcomes in patients on dialysis. The association of ISAT and ferritin with outcomes in patients with nondialysis-dependent CKD (NDD-CKD) has not been studied.
Design, setting, participants, & measurements: All-cause mortality and progression of CKD [slopes of estimated GFR (eGFR)] were examined in 453 men with NDD-CKD. Mortality and the composite of mortality and ESRD were studied in Cox models. Slopes of eGFR were examined in mixed-effects models.
Results: Lower ISAT was associated with higher mortality; adjusted hazard ratio [95% confidence interval (CI)] with ISAT of <12%, 13 to 17%, and >23% versus 18 to 23%; 1.40 (0.99 to 1.98), 1.20 (0.82 to 1.76), and 0.97 (0.67 to 1.41), P = 0.025 for trend. ISAT was also associated with steeper slopes of eGFR (one log-unit higher ISAT associated with a slope of −0.89 ml/min/1.73m2 /yr (95% CI: −1.75, −0.02, P = 0.044). Serum ferritin level showed no significant association with outcomes overall, but a trend for higher mortality was observed in patients with a serum ferritin level >250 ng/ml.
Conclusions: Higher ISAT is associated with lower mortality and with more progressive CKD. Clinical trials are needed to examine if correction of low iron levels can improve mortality without affecting kidney function in NDD-CKD.
Iron is an essential trace element which plays an important role in the catalysis of oxidative reactions and in the transport of soluble gases (1). Assessment of iron stores is done routinely in everyday nephrology practice (2), through measurement of total iron saturation (ISAT; also known as transferrin saturation) and serum ferritin (3).
Iron deficiency is common in patients with nondialysis-dependent chronic kidney disease (NDD-CKD) (4,5), but it is unclear to what extent levels of ISAT and ferritin are associated with outcomes in this population. Examining hard endpoints is especially important given the concern that exceeding certain limits of ISAT or ferritin may be deleterious through excess iron deposition and induction of oxidative stress (6–9). We examined the associations of these iron markers with all-cause mortality, the composite of predialysis mortality or ESRD, and the slopes of estimated GFR (eGFR) in 453 men with moderate and advanced NDD-CKD.
Materials and Methods
The study population consisted of 1012 patients evaluated for NDD-CKD at Salem Veteran Affairs Medical Center (VAMC) between January 1, 1990, and June 30, 2005, and followed until April 1, 2007. Four hundred and sixty-one patients (46%) had both ISAT and ferritin measurements available for analyses. Five women and three patients whose race was not white or black were excluded; the final study population consisted of 453 patients.
Baseline characteristics were recorded retrospectively as measured at the time when iron stores were assessed, as detailed elsewhere (10,11). Use of commonly prescribed medications [aspirin, angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), statins, and activated vitamin D products] was assessed over the entire follow-up period and used as surrogate markers for quality of clinical care. Serum creatinine levels were recorded longitudinally for estimation of slopes. GFR was estimated using the abbreviated equation developed for the Modification of Diet in Renal Disease Study (12). Serum iron, transferrin, and ferritin were measured in a single laboratory. Serum iron was measured by a timed endpoint method [reference range: 49 to 181 μg/dl, coefficient of variation (CV): 4%], and transferrin was measured by a turbidimetric method (180 to 329 mg/dl, CV: 2.5%), both by using a Beckman Coulter LXI autoanalyzer (Beckman, Fullerton, California). Serum ferritin was measured by an immunoenzymatic method (22 to 322 ng/ml, CV: 5.5%) using a Beckman Coulter Access2 autoanalyzer (Beckman, Fullerton, California). ISAT was calculated as serum iron/total iron binding capacity (TIBC) × 100%.
Statistical Analyses
Both serum ISAT and ferritin showed skewed distributions; hence, they were transformed to their natural logarithms. Because 90% of patients had an ISAT of <30% with normal distribution in this range, all analyses were performed on the entire sample and then repeated separately on the subset with ISAT <30%. Because the level of ISAT can be affected by changes in both serum iron and TIBC, analyses were repeated using separately these two variables as predictors. Missing data points for comorbidity index (0.3% missing), 24-h urine protein (3% missing) and white blood cell count (WBC, 3% missing) were imputed using linear regression with all other patient characteristics serving as independent variables. ISAT and ferritin were analyzed both as log-transformed continuous variables and as categorical measures after dividing them into quartiles. Patients were also grouped based on the cutoff levels of ISAT ≥15% (approximately corresponding to the median value of ISAT and also the level regarded as a threshold of absolute iron deficiency (13,14)) and ferritin ≥100 ng/ml [approximately corresponding to the median value of serum ferritin and the lower limit of desirable therapeutic target in CKD patients treated with erythropoiesis stimulating agents (ESAs) according to Kidney Disease Outcomes Quality Initiative guidelines (2)].
The starting time for survival analysis was the date of the first encounter in the Nephrology Clinic at Salem VAMC. Outcome measures were all-cause mortality and the composite of predialysis all-cause mortality or ESRD. Patients were considered lost to follow-up if no contact was documented with them for more than 6 mo, and they were censored at the date of the last documented contact. Cox models were used to adjust for the effect of confounders. Selection of variables to be included in multivariable models was performed by a multivariable regression spline function using backward elimination of weak predictors using a closed test approach (15). Age, race, comorbidity index, eGFR, albumin, hemoglobin, and WBC were forced in the models. Nonlinear associations were examined by including polynomial terms and by using restricted cubic splines; analyses were restricted to values above the fifth and below the 95th percentile of the predictor variable to maintain the stability of the spline models.
Progression of CKD was assessed by examining slopes of eGFR in mixed-effects models allowing for a random intercept and slope. The change in eGFR from baseline until death, start of dialysis, or loss of follow-up (whichever occurred first) was studied by building a multilevel model for change (16).
Subgroup analyses were performed in groups divided by median values of eGFR, albumin, hemoglobin, comorbidity index, WBC, and proteinuria. P values of less than 0.05 were considered significant. Statistical analyses were performed using STATA statistical software version 10 (STATA Corporation, College Station, Texas). The Research and Development Committee at the Salem VAMC approved the study protocol.
Results
The mean age of the cohort was 69 ± 10 yr, 26% were black, and the mean eGFR was 33 ± 14 ml/min/1.73m2. Two-hundred fifty-nine patients died [mortality rate: 156/1000 patient-years, 95% confidence interval (CI): 138 to 176] and 125 patients initiated dialysis (dialysis initiation rate: 94/1000 patient-years, 95% CI: 79 to 112) during a median follow-up of 3 yr. Eight patients were lost to follow-up; their characteristics were not significantly different (data not shown).
ISAT
The frequency distribution of serum ISAT is shown in Figure 1. Baseline characteristics in patients divided by quartiles of ISAT are shown in Table 1. Patients with higher ISAT were more likely to be diabetic, but less likely to have cardiovascular disease, had higher hemoglobin and ferritin, and lower comorbidity index and WBC. Serum iron concentrations were higher, but TIBC levels were lower in patients with higher ISAT. The frequency of medication use was identical in different ISAT quartiles except for ACEI/ARB, which were less likely to be prescribed to patients in the lowest ISAT quartile.
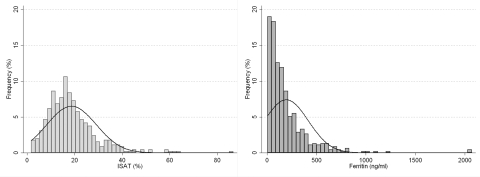
Figure 1.
Frequency distribution of iron saturation and serum ferritin concentration in 453 patients with moderate and advanced nondialysis-dependent chronic kidney disease.
Table 1.
Baseline characteristics in subgroups divided by quartiles of total iron saturationa
| Variable | ISAT (%)
|
P for linear trend | |||
|---|---|---|---|---|---|
| <12 (n = 118) | 12 to 17 (n = 114) | 17.1 to 23 (n = 109) | >23 (n = 112) | ||
| Age (yr) | 70.9 ± 9.0 | 66.4 ± 10.2 | 69.9 ± 9.4 | 68.3 ± 10.8 | 0.29 |
| Race (% black) | 28 (24) | 25 (22) | 30 (27) | 34 (30) | 0.16 |
| DM | 60 (51) | 74 (65) | 62 (57) | 75 (67) | 0.047 |
| CVD | 84 (71) | 75 (66) | 65 (60) | 64 (57) | 0.017 |
| Charlson index | 3.1 ± 1.9 | 2.8 ± 1.9 | 2.5 ± 1.8 | 2.6 ± 1.5 | 0.029 |
| SBP (mmHg) | 153 ± 27 | 150 ± 27 | 157 ± 31 | 153 ± 16 | 0.5 |
| DBP (mmHg) | 72 ± 15 | 74 ± 15 | 73 ± 16 | 76 ± 16 | 0.076 |
| Estimated GFR (ml/min/1.73m2) | 32.1 ± 14.6 | 31.7 ± 12.5 | 33.0 ± 11.9 | 35.1 ± 15.2 | 0.068 |
| K/DOQI stage (% 2/3/4/5) | 7/44/41/8 | 3/51/38/8 | 3/54/40/3 | 5/60/28/7 | 0.07 |
| Hemoglobin (g/dl) | 11.3 ± 1.7 | 11.9 ± 1.5 | 11.7 ± 1.5 | 12.0 ± 1.6 | 0.001 |
| Serum albumin (g/dl) | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.4 | 3.6 ± 0.6 | 0.18 |
| WBC (1000/mm3) | 7.7 ± 2.5 | 7.4 ± 2.4 | 7.3 ± 2.2 | 7.1 ± 2.3 | 0.04 |
| Serum ferritin (ng/ml) | 85 (67 to 106) | 108 (91 to 128) | 133 (114 to 156) | 167 (140 to 199) | <0.001 |
| Serum iron (mcg/dl) | 28 ± 10 | 49 ± 10 | 62 ± 14 | 94 ± 35 | <0.001 |
| TIBC (mcg/dl) | 341 ± 88 | 335 ± 63 | 315 ± 65 | 293 ± 74 | <0.001 |
| Proteinuria (g/24 h) | 833 (625 to 1110) | 886 (659 to 1192) | 841 (635 to 1115) | 1119 (825 to 1519) | 0.2 |
| ASA use | 75 (64) | 72 (63) | 65 (60) | 64 (57) | 0.26 |
| ACEI/ARB use | 80 (68) | 92 (81) | 86 (79) | 91 (81) | 0.027 |
| Statin use | 60 (51) | 76 (67) | 68 (62) | 72 (64) | 0.07 |
| Calcitriol use | 36 (30) | 43 (38) | 44 (40) | 38 (34) | 0.5 |
Data are presented as means ± SD, number (%), or geometric means ± 95% confidence intervals. Comparisons were made by χ2 test for trend.
DM, diabetes mellitus; CVD, cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; K/DOQI, Kidney Disease Outcomes Quality Initiative; WBC, white blood cell count; TIBC, total iron binding capacity; ASA, aspirin; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
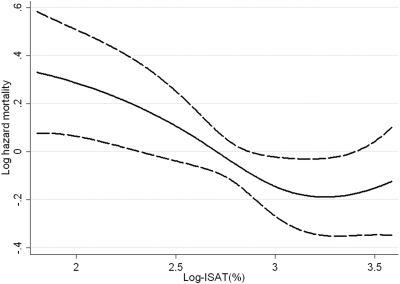
After adjustment for age, race, comorbidity index, eGFR, hemoglobin, albumin, and WBC, compared with patients with ISAT of 18 to 23%, patients with ISAT of <12%, 13 to 17%, and >23% had hazard ratios (95% CI) of all-cause mortality of 1.40 (0.99 to 1.98), 1.20 (0.82 to 1.76), and 0.97 (0.67 to 1.41), P for trend was 0.025. Lower log-ISAT was associated with higher mortality both overall (Figure 2) and in patients with ISAT <30% (data not shown). There was no interaction with comorbidity index, eGFR, hemoglobin, serum albumin, WBC, or proteinuria. The association between serum iron and mortality was similar to that seen for ISAT; however, TIBC was not associated with mortality. ISAT was not associated with the composite outcome of predialysis mortality or ESRD (data not shown).
Figure 2.
Multivariable adjusted log-hazards of all-cause mortality associated with levels of natural-log-transformed iron saturation. Model adjusted for age, race, Charlson comorbidity index, estimated GFR (eGFR), serum albumin, blood hemoglobin, and white blood cell count (WBC).
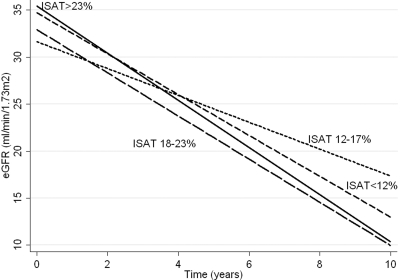
In mixed-effects models examining slopes of eGFR, each log-unit higher ISAT was associated with a 1.1 ml/min/1.73m2/yr lower (i.e., worse) slope of eGFR (95% CI: −1.99, −0.21, P = 0.015). This association remained significant after adjustment for age, race, comorbidity index, diabetes mellitus, blood pressure, hemoglobin, albumin, WBC, proteinuria, and ACEI/ARB (−0.89 [−1.75, −0.02], P = 0.044). The slopes appeared steepest in patients with ISAT in the highest quartile (>23%), as shown in Figure 3. Excluding patients with ISAT >30% from analyses rendered the association between ISAT and eGFR slopes nonsignificant; a 10% higher ISAT was associated with a 0.38 ml/min/1.73m2/yr lower slope of eGFR (95% CI: −1.14, 0.34), P = 0.3. An interaction with albumin and WBC was present, in that the associations were only significant in subgroups with lower albumin and with higher WBC. Both higher serum iron and lower TIBC were associated with a trend toward steeper slopes of eGFR.
Figure 3.
Predicted slopes of eGFR in groups of patients divided by quartiles of iron saturation in a mixed-effect model adjusted for age, race, Charlson comorbidity index, diabetes mellitus, systolic and diastolic blood pressure, serum albumin, blood hemoglobin, WBC, proteinuria, and use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers.
Serum Ferritin
Serum ferritin displayed skewed distribution, as shown in Figure 1. Baseline characteristics in patients divided by quartiles of serum ferritin are shown in Table 2. Patients with higher ferritin were younger, more likely to be black, less likely to have cardiovascular disease, and had lower serum albumin and higher proteinuria. ISAT levels were higher and TIBC levels were lower in patients with higher ferritin; serum iron, hemoglobin, and eGFR, on the other hand, were not significantly different across ferritin quartiles. The frequency of medication use was similar across different ferritin quartiles.
Table 2.
Baseline characteristics in subgroups divided by quartiles of serum ferritin concentrationa
| Characteristic | Serum Ferritin (ng/ml)
|
P for linear trend | |||
|---|---|---|---|---|---|
| <60 (n = 112) | 60 to 130 (n = 116) | 131 to 250 (n = 111) | >250 (n = 114) | ||
| Age (yr) | 72.1 ± 8.3 | 68.5 ± 10.0 | 67.2 ± 9.4 | 67.7 ± 11.4 | 0.001 |
| Race (% black) | 19 (17) | 28 (24) | 28 (25) | 42 (37) | 0.001 |
| DM | 63 (56) | 69 (59) | 64 (58) | 75 (66) | 0.19 |
| CVD | 80 (71) | 71 (61) | 73 (66) | 64 (56) | 0.04 |
| Charlson index | 2.8 ± 1.8 | 2.9 ± 1.8 | 2.6 ± 1.9 | 2.7 ± 1.8 | 0.6 |
| SBP (mmHg) | 152 ± 30 | 153 ± 26 | 155 ± 28 | 152 ± 27 | 0.8 |
| DBP (mmHg) | 71 ± 16 | 73 ± 16 | 76 ± 15 | 74 ± 15 | 0.065 |
| Estimated GFR (ml/min/1.73m2) | 34.7 ± 14.1 | 32.4 ± 12.5 | 31.9 ± 13.2 | 32.9 ± 14.9 | 0.3 |
| K/DOQI stage (% 2/3/4/5) | 7/50/40/3 | 2/53/38/7 | 3/53/35/9 | 5/53/33/9 | 0.4 |
| Hemoglobin (g/dl) | 11.9 ± 1.6 | 11.8 ± 1.5 | 11.8 ± 1.5 | 11.5 ± 1.8 | 0.1 |
| Serum albumin (g/dl) | 3.6 ± 0.4 | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.5 | 0.07 |
| WBC (1000/mm3) | 7.3 ± 2.3 | 7.5 ± 2.5 | 7.5 ± 2.2 | 7.3 ± 2.5 | 0.9 |
| Total iron saturation (%) | 15.5 ± 10.7 | 17.6 ± 8.2 | 20.2 ± 8.7 | 21.5 ± 12.5 | <0.001 |
| Serum iron (mcg/dl) | 55 ± 34 | 57 ± 29 | 61 ± 26 | 59 ± 36 | 0.18 |
| TIBC (mcg/dl) | 368 ± 70 | 326 ± 63 | 313 ± 73 | 279 ± 69 | <0.001 |
| Proteinuria (g/24 h) | 565 (418 to 764) | 885 (681 to 1150) | 1001 (732 to 1369) | 1384 (1053 to 1819) | <0.001 |
| ASA use | 70 (62) | 71 (61) | 61 (55) | 74 (65) | 0.9 |
| ACEI/ARB use | 82 (73) | 95 (82) | 85 (77) | 87 (76) | 0.8 |
| Statin use | 71 (63) | 69 (59) | 70 (63) | 66 (58) | 0.5 |
| Calcitriol use | 44 (39) | 36 (31) | 46 (41) | 35 (31) | 0.4 |
Data presented as means ± SD, number (percent), or geometric means ± 95% confidence intervals. Comparisons were made by χ2 test for trend.
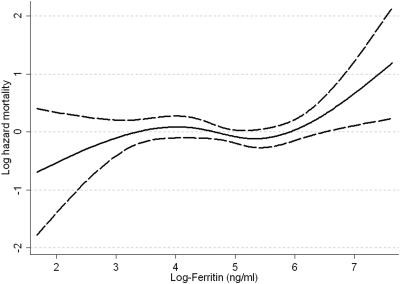
After adjustment for age, race, comorbidity index, cardiovascular disease, eGFR, hemoglobin, albumin, WBC, and proteinuria, relative to patients with ferritin of <60 ng/ml, those with ferritin levels of 60 to 130, 131 to 250, and >250 ng/ml had a hazard ratio (95% CI) of mortality of 1.33 (0.93 to 1.90), 0.87 (0.60 to 1.28), and 1.42 (0.98 to 2.05), P = 0.3 for trend. The association between ferritin and mortality was nonlinear (Figure 4; joint significance of polynomial terms of log-ferritin: P = 0.06) with a trend toward higher mortality seen in patients with ferritin >250 ng/ml (corresponding to log-ferritin of >5.5 ng/ml). The association between ferritin and mortality showed a similar pattern in the subgroup of patients with eGFR <30 ml/min/1.73m2 and with a comorbidity index >2, but not in those with eGFR ≥30 ml/min/1.73m2 and with a comorbidity index ≤2. There were no other interactions. The association between ferritin and the combined endpoint was similar to associations with all-cause mortality. There was also no significant association between ferritin and the slope of eGFR: one log-unit higher ferritin level was associated with a multivariable adjusted slope (95% CI) of −0.19 ml/min/1.73m2/yr (−0.66, 0.27), P = 0.4.
Figure 4.
Multivariable adjusted log-hazards of all-cause mortality associated with levels of natural-log-transformed serum ferritin concentration. Model adjusted for age, race, Charlson comorbidity index, cardiovascular disease, eGFR, serum albumin, blood hemoglobin, WBC, and proteinuria.
Concomitant ISAT and Ferritin
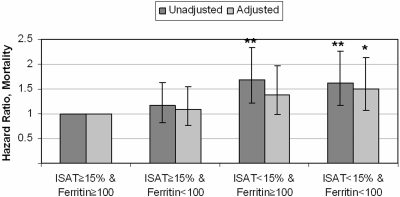
As shown in Figure 5, compared with the group with ISAT ≥15% and ferritin ≥100 ng/ml, a higher mortality was associated with ISAT <15% and ferritin ≥100 ng/ml, and with ISAT <15% and ferritin <100 ng/ml. Mortality was not significantly different in patients with ISAT ≥15% and ferritin <100 ng/ml. These associations were similar in all examined subgroups. There was no significant difference in the composite outcome of predialysis mortality or ESRD and in the slopes of eGFR between the four categories (data not shown).
Figure 5.
Multivariable adjusted hazard ratios of all-cause mortality in groups of patients divided by concomitant levels of iron saturation and serum ferritin concentration. The group with iron saturation of ≥15% and serum ferritin of ≥100 ng/ml served as a reference. Model adjusted for age, race, Charlson comorbidity index, eGFR, serum albumin, blood hemoglobin, and WBC.
Discussion
We found higher all-cause mortality associated with lower serum ISAT levels, independent of serum ferritin in NDD-CKD patients. Because mortality was similar in the two highest quartiles of ISAT, we applied cubic splines to more precisely characterize a potential nonlinear association and found higher mortality with ISAT levels below approximately 25% (log-ISAT of 3.25, Figure 1). A trend for higher mortality was observed in patients with serum ferritin values >250 ng/ml, especially in those with more advanced stages of CKD. The slopes of eGFR were steeper in patients with higher ISAT, but this finding was restricted to subgroups of patients with lower serum albumin or higher WBC, indicating an interaction with malnutrition and inflammation.
Serum ISAT and ferritin concentrations are often applied in clinical practice as markers of bodily iron stores, but their sensitivity and specificity as diagnostic tools have been shown to be limited (3,17,18). Both serum ferritin and the two components of ISAT (serum iron concentration and TIBC) are affected by numerous non-iron-related physiologic and pathologic conditions (19,20). Inflammation especially can result in higher serum ferritin levels (21–23). The complexity of interpreting ISAT and ferritin is illustrated in the baseline characteristics of our patients with different levels of these markers: higher ISAT was associated with higher serum iron and hemoglobin concentrations (indicating higher iron stores), but also with lower TIBC (indicating potentially the confounding presence of malnutrition and/or inflammation; Table 1). Similarly, higher ferritin concentrations were associated with significantly higher ISAT levels, but this association was solely due to the lower TIBC associated with higher ferritin levels (Table 2), suggesting that inflammation may be a more important determinant of higher serum ferritin concentrations, and questioning the role of elevated ferritin as a useful marker of adequate or elevated iron stores. These considerations could explain the higher mortality seen with higher ferritin, which could be ascribed to the confounding effect of chronic inflammation.
The higher sensitivity of a low ISAT as a diagnostic marker of iron deficiency (3,17) makes it more likely that the robust association between low ISAT and higher mortality in our study could be related to lower iron stores. Iron-containing enzymes participate in catalysis of oxidation reactions and in transport of several soluble gases, and adequate iron stores are indispensable for erythropoiesis, making an association between lower iron stores and higher mortality physiologically plausible. To the best of our knowledge, our study is the first one to examine the association between markers of iron stores and mortality in NDD-CKD. However, more detailed observational studies and prospective clinical trials will be needed to fully characterize the nature of such an association.
We also found more significant progression of CKD (steeper slopes of eGFR) in patients with higher ISAT. These results seemed to be driven mostly by the small fraction of our patient population with ISAT >30%; the smaller number of patients in this ISAT range and the uncertainty of the meaning of a higher ISAT in them (vide supra) make the interpretation of our finding difficult. Possible mechanisms whereby truly higher iron levels could be deleterious to the kidney function include the engenderment of oxidative stress and parenchymal iron deposition with subsequent tissue damage, (24,25) but one would expect to also find an effect on mortality from such a mechanism, which we did not. Conversely, the lower TIBC seen in patients with higher ISAT, the fact that the association between higher ISAT and progression of CKD was limited to subgroups with lower albumin and higher WBC, and that lower TIBC in itself showed a trend toward an association with a steeper slope of eGFR indicates that chronic inflammation (which has been linked to more significant progression of CKD in earlier studies (26,27)) could also have affected these outcomes. The concept that higher serum iron levels may be linked to lower mortality, but also more progression of kidney disease in patients with NDD-CKD, will have to be tested in prospective clinical trials.
Several limitations of our study need to be mentioned. The historical and observational nature of the study allows us to establish associations, but not causal relationships, between iron levels and outcomes. Our study sample was limited to male patients from a single medical center; hence, our results may not apply to the larger population with NDD-CKD. The enrollment of patients over an extended period of time in our study makes it possible that changing medical practices could have affected patient outcomes differently based on the time of enrollment. Similarly, the different baseline comorbidity characteristics of patients with different levels of iron markers could induce a bias related to different medical indications and hence influence outcomes independent of iron stores. We addressed these concerns by assessing the use of several medical interventions that are commonly applied in patients with CKD and that have undergone secular changes during the past 2 decades (such as ACEIs/ARBs, statins, or activated vitamin D products) and found no differences that could have indicated substantial discrepancies in the medical care delivered to patients with different levels of iron markers. We used single baseline values of the predictor, which could have biased our results because of the inherent variability of the parameters used. The lack of data on subsequent use of iron supplementation or erythropoiesis stimulating agents in our study population makes it possible that the outcomes associated with iron levels could be due to medications used in response to such levels. Lower iron levels have been associated with higher mortality independent of iron administration in dialysis patients (in whom iron products are more commonly used than in NDD-CKD), (28,29) but the results of such studies cannot be extrapolated to our patient population.
Conclusions
Lower serum ISAT level is associated with higher mortality, and higher ISAT with more pronounced progression of kidney disease in NDD-CKD, but the latter association was only observed in patients who had evidence of malnutrition or inflammation. The association between serum ferritin and mortality appears weak and limited to a subset of patients in whom the utility of serum ferritin as a sensitive marker of iron stores is questionable. Because serum ISAT and ferritin levels are often used to make decisions about iron replacement therapy, the clinical and public health implications of our findings may be quite significant, indicating potential benefits (lower mortality), although an acceleration of CKD progression was also noted. It is paramount that prospective clinical trials of iron supplementation are undertaken with proper safeguards in place to delineate the boundaries of benefit and safety with such therapy in patients with NDD-CKD.
Disclosures
C.P.K. and K.K.Z. have received consultant fees and/or honoraria from AMAG, Amgen and Watson.
Acknowledgments
Parts of this material were presented at the American Society of Nephrology Renal Week 2008, November 4 to 9, in Philadelphia, Pennsylvania. This study is supported by grant 1R01DK078106-01 for C.P.K. and K.K.Z.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rouault TA: How mammals acquire and distribute iron needed for oxygen-based metabolism. PLoS Biology 1: E79, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KDOQI, National Kidney Foundation. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis 47: S16, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Hoffken B, Wunsch H, Fink H, Kleiner M, Luft FC: Diagnosis of iron deficiency anemia in renal failure patients during the post-erythropoietin era. Am J Kidney Dis 26: 292–299, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Gotloib L, Silverberg D, Fudin R, Shostak A: Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol 19: 161–167, 2006 [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Curhan GC: Iron status and hemoglobin level in chronic renal insufficiency. J Am Soc Nephrol 13: 2783–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Balducci L: Anemia, cancer, and aging. Cancer Control 10: 478–486, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Kehrer JP: The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149: 43–50, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Papanikolaou G, Pantopoulos K: Iron metabolism and toxicity. Toxicol Appl Pharmacol 202: 199–211, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Samson FE, Nelson SR: The aging brain, metals and oxygen free radicals. Cell Mol Biol (Noisy-le-grand) 46: 699–707, 2000 [PubMed] [Google Scholar]
- 10.Kovesdy CP, Trivedi BK, Anderson JE: Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: A historical prospective cohort study. Adv Chronic Kidney Dis 13: 183–188, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE: Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant 21: 1257–1262, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Cook JD, Finch CA, Smith NJ: Evaluation of the iron status of a population. Blood 48: 449–455, 1976 [PubMed] [Google Scholar]
- 14.Dallman PR, Yip R, Johnson C: Prevalence and causes of anemia in the United States, 1976 to 1980. Am J Clin Nutr 39: 437–445, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Royston P, Sauerbrei W: Multivariable modeling using cubic regression splines: A principled approach. Stata J 7: 45–70, 2007 [Google Scholar]
- 16.Singer JD, Willett JB: Applied longitudinal data analysis. Modeling change and event occurrence. Oxford, Oxford University Press, Inc., 2003
- 17.Fishbane S, Kowalski EA, Imbriano LJ, Maesaka JK: The evaluation of iron status in hemodialysis patients. J Am Soc Nephrol 7: 2654–2657, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, Gammaro L, Brocco G, Restivo G, Bernich P, Lupo A, Maschio G: The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant 16: 1416–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gunnell J, Yeun JY, Depner TA, Kaysen GA: Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 33: 63–72, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH: Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 19: 141–149, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Harrison PM, Arosio P: The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275: 161–203, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Kwak EL, Larochelle DA, Beaumont C, Torti SV, Torti FM: Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem 270: 15285–15293, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Rogers JT, Bridges KR, Durmowicz GP, Glass J, Auron PE, Munro HN: Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J Biol Chem 265: 14572–14578, 1990 [PubMed] [Google Scholar]
- 24.Gonzalez-Michaca L, Farrugia G, Croatt AJ, Alam J, Nath KA: Heme: A determinant of life and death in renal tubular epithelial cells. Am J Physiol Renal Physiol 286: F370–F377, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Zhou XJ, Laszik Z, Wang XQ, Silva FG, Vaziri ND: Association of renal injury with increased oxygen free radical activity and altered nitric oxide metabolism in chronic experimental hemosiderosis. Lab Invest 80: 1905–1914, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Rao M, Wong C, Kanetsky P, Girndt M, Stenvinkel P, Reilly M, Raj DS: Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int 72: 549–556, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz MI, Carrero JJ, Axelsson J, Lindholm B, Stenvinkel P: Low-grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: Sources and consequences. Clin Nephrol 68: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG: Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 16: 3070–3080, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Liu E, Kopple JD: A low serum iron level is a predictor of poor outcome in hemodialysis patients. Am J Kidney Dis 43: 671–684, 2004 [DOI] [PubMed] [Google Scholar]