Abstract
Background and objectives: Thrombotic microangiopathy (TMA) is a known complication of hematopoietic cell transplantation (HCT). The etiology and diagnosis of TMA in this patient population is often difficult because thrombocytopenia, microangiopathic hemolytic anemia, and kidney injury occur frequently in HCT recipients, and are the result of a variety of insults.
Design, setting, participants & measurements: The authors reviewed renal pathology and clinical data from HCT patients to determine the prevalence of TMA and to identify correlative factors for developing TMA in the kidney. Kidney tissue was evaluated from 314 consecutive autopsies on patients who died after their first HCT (received between 1992 and 1999). Renal pathology was classified into three groups: (1) no renal thrombus (65%), (2) TMA (20%), and (3) isolated thrombosis (15%). Logistic regression models estimated the associations between each histologic category and clinical parameters: donor and recipient gender, patient age, human leukocyte antigen (HLA) matching of the donor and recipient, total body irradiation (TBI), acute graft versus host disease (GVHD), acute kidney injury, medications, and viral infections.
Results: In a multivariate analysis, TMA correlated with acute GVHD grades II to IV, followed by female recipient/male donor, TBI > 1200 cGy, and adenovirus infection. Grades II to IV acute GVHD and female gender were associated with isolated renal thrombus.
Conclusions: TMA in HCT recipients is associated with acute GVHD grades II to IV, recipient/donor mismatch, TBI > 1200 cGy, and adenovirus infection.
Thrombotic microangiopathy (TMA) encompasses a spectrum of clinical diseases characterized by systemic or intrarenal platelet aggregation, thrombocytopenia, and microvascular fragmentation of erythrocytes, resulting in ischemic organ injury. Endothelial damage is thought to represent the inciting factor in TMA syndromes (1). Once the endothelium is damaged, loss of endothelial resistance to thrombus formation, leukocyte adhesion, and increased vascular sheer stress ensues and may augment and perpetuate the injury (2). Although abnormalities in von Willebrand factor and complement pathways have also been associated with inherited and recurrent forms of TMA (2), they have not been found in patients who developed TMA after hematopoietic cell transplant (HCT) (3).
The incidence of TMA syndromes in the setting of HCT ranges between 2% and 21% (4–6). Renal biopsy specimens are difficult to obtain immediately after HCT, and therefore much of the literature on TMA has relied on clinical and laboratory features alone. However, it is difficult to establish the diagnosis of TMA in HCT patients by clinical and laboratory features alone because anemia, thrombocytopenia, and elevations in lactate dehydrogenase (LDH) and creatinine occur frequently because of delayed engraftment, infections, medications, or graft versus host disease (GVHD). Clinical manifestations of TMA range from a fulminant presentation associated with severe acute renal failure and death to a more common, indolent course resulting in the eventual development of chronic kidney disease. In many cases, elevations in serum creatinine may not occur until late in the disease process despite endothelial injury in the kidney, and therefore the diagnosis may be delayed or missed. We hypothesize that renal endothelial injury, consistent with TMA, exists in the absence of a clinical diagnosis of TMA and that this early injury may set the stage for progression to chronic kidney disease. In this study, we examined the renal pathology and clinical data from HCT patients to more accurately identify the prevalence of and risk factors for the development of TMA in the kidney.
Materials and Methods
Patient Selection
Patients who received their first HCT at the Fred Hutchinson Cancer Research Center (FHCRC) during 1992 to 1999, died any time after HCT and were autopsied at FHCRC and had kidney tissue available were included in the study. The study was performed in accordance with a protocol approved by the FHCRC institutional review board.
Histologic Methods and Definitions of TMA
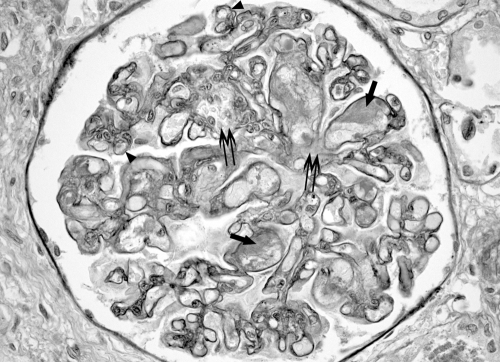
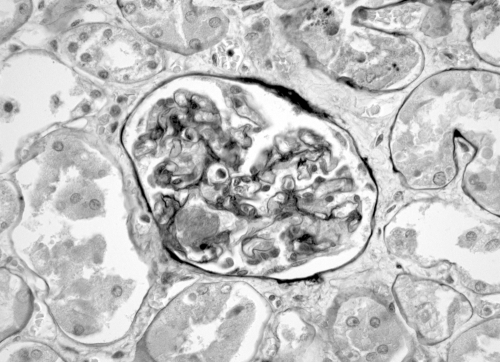
Renal pathology from 322 patients was reviewed by a renal pathologist (S.C.) who was blind to the clinical information of each case. All positive cases and a sample of negative cases were reviewed by a second renal pathologist (C.E.A.). Two sections of renal tissue from each patient, one stained with hematoxylin-eosin and a second stained with periodic-acid Schiff, were reviewed. Renal histologic findings were classified into three groups: (1) no renal thrombosis; (2) typical TMA morphology which includes fibrin thrombi within glomeruli and mesangiolysis, and/or thrombosis involving arterioles or interlobular arteries in conjunction with characteristic intimal swelling in these vessels of the subendothelium and occlusion of capillary loops (Figure 1); and (3) isolated thrombosis, defined as a single glomerular or vascular thrombus without typical TMA morphology (Figure 2).
Figure 1.
Thrombotic microangiopathy involving a glomerulus. There are multiple thrombi in capillary loops, (arrows), segmental mesangiolysis(double arrows) and duplication of basement membranes in some capillary loops (arrowheads). Periodic-acid Schiff stain at 40× magnification.
Figure 2.
Glomerulus showing isolated intracapillary thrombus but with otherwise normal glomerular tuft architecture. Periodic-acid Schiff stain at 40× magnification.
We defined clinical evidence of TMA in two ways to investigate the relationship between clinical parameters in the two weeks before death and histologic findings at death. Definition 1 is hematocrit < 30, platelet count < 100,000, LDH above the upper limits of normal, and a 50% increase in the baseline serum creatinine, in a single day's measurements. Definition 2 is hematocrit < 30, platelet count < 100,000, LDH above the upper limits of normal, and a 50% increase in the serum baseline creatinine, each occurring at least once over the two weeks’ measurements. LDH upper limits of normal are defined as LDH > 425 U/L if less than 10 yr of age, LDH > 350 if age 10 to 15 yr old, and LDH > 250 if 15 yr or older. Medical charts were reviewed before death to determine whether the patients carried a clinical diagnosis or suspicion of TMA. Autopsy reports were reviewed to determine the cause of death.
Statistical Analyses
We defined two outcomes for regression analysis of risk factors for the development of TMA: frequency of typical TMA morphology compared with no renal thrombosis, and frequency of isolated thrombus compared with no renal thrombosis. Logistic regression models were applied to estimate the associations between each of these outcomes and the following pretransplant clinical parameters: donor and recipient gender, patient age (continuous variable), HLA matching of the donor and recipient, TBI exposure, and the post-transplant occurrence of acute GVHD, administration of medications (acyclovir, amphotericin B, cyclosporine, and tacrolimus), viral infection (adenovirus [ADV], herpes simplex, varicella–zoster, and cytomegalovirus [CMV]), and acute kidney injury (AKI). Transplants were classified as match related when the recipient and donor shared a complete haplotype for HLA, and as mismatch related when the donor and recipient did not match on only one HLA antigen, (A, B, or DR). Acute GVHD was graded according to standard clinical criteria (7) and categorized for the models as no acute GVHD or grade I, acute GVHD grades II to IV, or autologous graft. AKI was defined as a tripling of baseline serum creatinine in the first 100 d after transplantation. Maximum total serum bilirubin and serum creatinine levels, bacteremia, fungal infection, intubation, and dialysis in the 14 d before death were also considered as potential predictors of renal thrombosis. A secondary analysis was performed in the subset of 196 patients who received allogeneic transplants and for whom cyclosporine data were available to determine whether cyclosporine dose was associated with the development of TMA. Cumulative cyclosporine dose from the time of transplant to death, expressed as an average dose per day alive divided into quartiles (0 to 99 mg, 100 to 167 mg, 168 to 279 mg, and 280 to 2295 mg), was included as a covariate for this subset analysis. The distributions of cyclosporine total dose and dose per day were compared across renal pathology groups via t test. Only 13 patients received tacrolimus alone for GVHD prophylaxis. Those factors with P ≤ 0.1 in univariate analysis were candidates for the multivariable regression model.
Results
Demographic data and variables identified as potential risk factors for development of TMA or an isolated thrombus are presented in Table 1. The mean age of the cohort was 42.7 yr, with a range of 6 mo to 68 yr. Sixty-four percent of patients received TBI as part of their conditioning regimen before transplant. Sixty-five percent of patients developed acute GVHD grades II to IV after transplant. Forty-one percent of patients developed AKI after transplant, and the mean serum creatinine in the two weeks before death was 1.56 mg/dl. Fifty-nine percent of patients developed a viral infection, 33% a bacterial infection, and 11% a fungal infection after transplant and before death.
Table 1.
Patient and transplant characteristics of the study cohort (N = 314)
| Factor | Number (%) |
|---|---|
| Baseline characteristics | |
| age in years (median & range) | 42.7 (0.6 to 67.8) |
| diagnosis | |
| hematologic malignancies | 160 (51%) |
| myelodysplastic syndrome | 48 (15%) |
| lymphoma | 43 (14%) |
| multiple myeloma | 28 (9%) |
| aplastic anemia | 11 (3.5%) |
| other | 24 (7.5%) |
| patient/donor gender | |
| male/male | 129 (41) |
| male/female | 62 (20) |
| female/female | 75 (24) |
| female/male | 48 (15) |
| Donor type | |
| related-matched | 106 (34) |
| related-mismatched | 44 (14) |
| unrelated | 115 (37) |
| autologous/id twin | 49 (15) |
| TBI conditioning (cGy) | |
| none | 115 (37) |
| ≤ 1200 | 78 (25) |
| > 1200 | 121 (38) |
| Post-transplant features | |
| acute GVHD grade | |
| 0, I | 58 (18) |
| II | 55 (18) |
| III | 85 (27) |
| IV | 61 (19) |
| autologous/syngeneic | 49 (16) |
| missing | 6 (2) |
| incidence of infection | |
| adenovirus | 52 (17) |
| cytomegalovirus | 63 (20) |
| herpes simplex virus | 58 (18) |
| varicella virus | 11 (4) |
| received acyclovir | 246 (81) |
| received amphotericin | 209 (67) |
| Acute kidney injury 2 wk before death | 128 (41) |
| incidence of infection | |
| bacterial | 103 (33) |
| fungal | 35 (11) |
| incidence of dialysis | 82 (26) |
| incidence of intubation | 119 (38) |
| mean total serum bilirubin (mg/dl, median & range) | 4.9 (0.4 to 59.6) |
| mean serum creatinine (mg/dl, median & range) | 1.56 (0.27 to 5.79) |
Patients with negative histology developed AKI at a median of 15 d post-HCT (range 1 to 74), whereas patients with histologic evidence of TMA or an isolated thrombosis developed AKI later, at a median of 32 d (range 0 to 100) and 27 d (range 7 to 95) post-HCT, respectively. Twenty-six percent of all patients required dialysis during the two weeks before death.
Renal Histopathology in the Study Cohort
Two hundred three patients (65%) had no evidence of renal TMA at autopsy; 64 (20%) had evidence of TMA, and most of these had evidence of mesangiolysis; and 47 (15%) had an isolated thrombus noted. Eight patients were excluded from the analysis after review of their renal pathology because the findings could not be classified into one of the three diagnostic categories (six showed aspergillus nephritis, one adenovirus nephritis, and one severe TMA with fibrosis). The study cohort survived for a median number of 43 d after transplantation, with a range of 1 to 720 d. The survival time was similar for the allogeneic and autologous transplant recipients. The median survival time after transplant was shorter in the negative histology group (35 d) compared with the TMA group (59 d) and the isolated thrombosis group (52 d). The main causes of death and numbers of patients were: multiorgan failure (45); infection (70); SOS (30); pulmonary complications (111); GVHD (52); cardiac (19); cerebral hemorrhage (18), and recurrent disease (7). The majority of patients died from combinations of the above complications.
Relation of Histologic Findings to Clinical Variables
The frequency of the histologic findings of TMA and isolated thrombi for each putative risk factor is shown in Table 2, along with the univariate odds ratios for each type of renal thrombotic histology relative to negative thrombotic pathology. On the basis of univariate P values, the candidate factors for the multivariable model of TMA were patient age, patient/donor sex mismatch, donor type, TBI, acute GVHD, incidence of ADV and CMV infection, and AKI. In the multivariable analysis comparing histologic evidence of TMA versus no renal thrombus groups, a female patient–male donor sex match compared with other combinations, TBI > 1200 cGy compared with none, ADV infection, and acute GVHD grades II to IV (compared with grades 0 or I) were each associated with an increased risk of renal TMA at autopsy (Table 3).
Table 2.
Univariate odds ratios with 95% confidence intervals comparing each histologic type to kidneys with no evidence of thrombosis or TMA in the kidney at autopsy
| Factor | Negative (n = 203) N (%) | Isolated renal thrombosis (n = 47)
|
TMA (n = 64)
|
||
|---|---|---|---|---|---|
| N (%) | OR (95% CI) | N (%) | OR (95% CI) | ||
| Baseline characteristics | |||||
| patient/donor gender | |||||
| male/male | 86 (42) | 16 (34) | 1.0 | 27 (42) | 1.0 |
| male/ female | 44 (22) | 8 (17) | 1.0 (0.4 to 2.5) | 10 (16) | 0.7 (0.3 to 1.6) |
| female/female | 51 (25) | 13 (28) | 1.4 (0.6 to 3.1) | 11 (17) | 0.7 (0.3 to 1.5) |
| female/male | 22 (11) | 10 (21) | 2.4 (1.0 to 6.1) | 16 (25) | 2.3 (1.1 to 5.0) |
| donor type | |||||
| related-matched | 63 (31) | 21 (45) | 1.0 | 22 (34) | 1.0 |
| related-mismatched | 26 (13) | 9 (19) | 1.0 (0.4 to 2.6) | 9 (14) | 1.0 (0.4 to 2.4) |
| unrelated | 70 (34) | 14 (30) | 0.6 (0.3 to 1.3) | 31 (48) | 1.3 (0.7 to 2.4) |
| autologous/id twin | 44 (22) | 3 (6) | 0.2 (0.1 to 0.7) | 2 (3) | 0.1 (0.03 to 0.6) |
| TBI conditioning (cGy) | |||||
| none | 84 (41) | 16 (34) | 1.0 | 15 (23) | 1.0 |
| 200 to 1200 | 53 (26) | 9 (19) | 0.9 (0.4 to 2.2) | 16 (25) | 1.7 (0.8 to 3.7) |
| 1240 to 1350 | 54 (27) | 13 (28) | 1.3 (0.6 to 2.8) | 27 (42) | 2.8 (1.4 to 5.7) |
| > 1350 | 12 (6) | 9 (19) | 3.9 (1.4 to 10.9) | 6 (9) | 2.8 (0.9 to 8.6) |
| Median (range) | Median (range) | Median (range) | |||
| agea | 43.1 (0.6 to 67.1) | 46.4 (0.9 to 67.8) | 1.1 (0.9 to 1.4) | 37.3 (1.3 to 67.1) | 0.8 (0.7 to 1.0) |
| Post-transplant features | |||||
| acute GVHD grade | |||||
| 0, I | 48 (24) | 4 (9) | 1.0 | 6 (10) | 1.0 |
| II to IV | 109 (54) | 39 (85) | 4.3 (1.5 to 12.7) | 53 (87) | 3.9 (1.6 to 9.7) |
| autologous/ id twin | 44 (22) | 3 (7) | 0.8 (0.2 to 3.9) | 2 (3) | 0.4 (0.1 to 1.9) |
| ADV infection | |||||
| no | 174 (86) | 39 (83) | 1.0 | 49 (77) | 1.0 |
| yes | 29 (14) | 8 (17) | 1.2 (0.5 to 2.9) | 15 (23) | 1.8 (0.9 to 3.7) |
| CMV infection | |||||
| no | 171 (84) | 34 (72) | 1.0 | 46 (72) | 1.0 |
| yes | 32 (16) | 13 (28) | 2.0 (1.0 to 4.3) | 18 (28) | 2.1 (1.1 to 4.1) |
| HSV infection | |||||
| no | 164 (81) | 37 (79) | 1.0 | 55 (86) | 1.0 |
| yes | 39 (19) | 10 (21) | 1.1 (0.5 to 2.5) | 9 (14) | 0.7 (0.3 to 1.5) |
| varicella virus infection | |||||
| no | 197 (97) | 46 (98) | 1.0 | 60 (94) | 1.0 |
| yes | 6 (3) | 1 (2) | 0.7 (0.1 to 6.1) | 4 (6) | 2.2 (0.6 to 8.0) |
| received acyclovir | |||||
| No | 39 (20) | 10 (22) | 1.0 | 10 (16) | 1.0 |
| Yes | 159 (80) | 36 (78) | 0.9 (0.4 to 1.9) | 51 (84) | 1.3 (0.6 to 2.7) |
| received amphotericin | |||||
| no | 72 (35) | 16 (34) | 1.0 | 17 (27) | 1.0 |
| yes | 131 (65) | 31 (66) | 1.1 (0.5 to 2.1) | 47 (73) | 1.5 (0.8 to 2.8) |
| acute kidney injury | |||||
| no | 122 (60) | 34 (72) | 1.0 | 30 (47) | 1.0 |
| yes | 81 (40) | 13 (28) | 0.6 (0.3 to 1.2) | 34 (53) | 1.7 (1.0 to 3.0) |
| Two weeks before death | |||||
| Bacterial infection | |||||
| no | 133 (66) | 30 (64) | 1.0 | 48 (75) | 1.0 |
| yes | 70 (34) | 17 (36) | 1.1 (0.6 to 2.1) | 16 (25) | 0.6 (0.3 to 1.2) |
| fungal infection | |||||
| no | 176 (87) | 44 (94) | 1.0 | 59 (92) | 1.0 |
| yes | 27 (13) | 3 (6) | 0.4 (0.1 to 1.5) | 5 (8) | 0.6 (0.2 to 1.5) |
| incidence of dialysis | |||||
| no | 152 (75) | 34 (72) | 1.0 | 46 (72) | 1.0 |
| yes | 51 (25) | 13 (28) | 1.1 (0.6 to 2.3) | 18 (28) | 1.2 (0.6 to 2.2) |
| incidence of intubation | |||||
| no | 122 (60) | 30 (64) | 1.0 | 43 (67) | 1.0 |
| yes | 81 (40) | 17 (36) | 0.9 (0.4 to 1.6) | 21 (33) | 0.7 (0.4 to 1.3) |
| Median (range) | Median (range) | Median (range) | |||
| mean bilirubinb | 4.8 (0.4 to 59.6) | 5.4 (0.6 to 43.8) | 1.1 (0.8 to 1.5) | 5.5 (0.5 to 52.1) | 1.1 (0.8 to 1.4) |
| mean serum creatinine | 1.52 (0.40 to 5.79) | 1.56 (0.27 to 4.57) | 0.9 (0.7 to 1.2) | 1.67 (0.55 to 4.74) | 1.1 (0.9 to 1.4) |
Estimate represents the ratio of odds for two patients, one 10 yr older than the other at transplant.
Estimate represents the ratio of odds for two patients, one with mean bilirubin 10 points higher than the other.
Table 3.
Multivariable odds ratios (ORs) of TMA histology with 95% confidence intervals (CIs) compared to no evidence of TMA or renal thrombosis (n = 262)
| Factor | OR | 95% CI | P |
|---|---|---|---|
| Patient/donor gender | |||
| other | 1.0 | — | — |
| female/male | 2.7 | 1.2 to 6.1 | 0.01 |
| TBI conditioning (cGy) | |||
| none | 1.0 | — | — |
| ≤ 1200 | 1.8 | 0.8 to 4.1 | 0.19 |
| > 1200 | 2.2 | 1.0 to 4.7 | 0.04 |
| Acute GVHD grade | |||
| 0, I | 1.0 | — | — |
| II to IV | 3.9 | 1.5 to 9.9 | 0.005 |
| Autologous/id twin | 0.6 | 0.1 to 3.2 | 0.52 |
| Adenovirus infection | |||
| No | 1.0 | — | — |
| Yes | 2.3 | 1.0 to 5.0 | 0.04 |
The candidate predictors for the multivariable model of an isolated renal thrombus were patient gender, donor type, acute GVHD, and incidence of CMV. Acute GVHD grades II to IV, compared with 0 or I (odds ratio [OR] = 4.7, 95% confidence interval [CI,] 1.6 to 14.1), and female gender (OR = 2.4, 95% CI, 1.2 to 4.8) were associated with an increased risk of having an isolated renal thrombus compared with having no renal pathology (Table 4).
Table 4.
Multivariable odds ratios of isolated renal thrombosis with 95% confidence intervals compared to no evidence of TMA (n = 47)
| Factor | OR | 95% CI | P |
|---|---|---|---|
| Patient gender | |||
| male | 1.0 | — | — |
| female | 2.4 | 1.2 to 4.8 | 0.01 |
| Acute GVHD grade | |||
| 0, I | 1.0 | — | – |
| II to IV | 4.7 | 1.6 to 14.1 | 0.006 |
| autologous/id twin | 0.7 | 0.1 to 3.4 | 0.66 |
Subset Analysis: Allograft Recipients
The incidence of cyclosporine use was consistent across renal pathology groups. The median cumulative cyclosporine dose from the time of transplant until the time of death was larger in the TMA group as compared with the negative pathology group, but not significantly so (Table 5). The results of subset analyses of 196 allogeneic transplant recipients were similar to those of the complete cohort (Table 6). The average daily dose of cyclosporine was not associated with the presence of isolated thrombus or TMA, whether or not the model was adjusted for acute GVHD.
Table 5.
Incidence of cyclosporine use and dose by renal pathology in allogeneic transplant recipients (n = 196)
| Pathology finding | n | Any CSA (n, %) | Median cumulative dose (mg) | Median dose per day (mg)a |
|---|---|---|---|---|
| Negative | 144 | 133 (92) | 4706 | 161 |
| TMA | 55 | 53 (96) | 13990 | 176 |
| Isolated | 40 | 38 (95) | 6975 | 174 |
Calculated as the cumulative dose divided by number of days alive post-transplant.
Table 6.
Multivariable odds ratios (ORs) of TMA pathology with 95% confidence intervals (CIs) in allogeneic transplant recipients compared to no evidence of TMA or renal thrombus (n = 196)
| Factor | OR | 95% CI | P |
|---|---|---|---|
| Patient/donor sex | |||
| other | 1.0 | — | — |
| female/male | 2.8 | 1.2 to 6.7 | 0.02 |
| TBI conditioning (cGy) | |||
| none | 1.0 | — | — |
| ≤ 1200 | 1.8 | 0.7 to 4.7 | 0.24 |
| > 1200 | 2.6 | 1.1 to 6.2 | 0.02 |
| CSA dose per day (mg) | |||
| quartile 1 (0 to 99) | 1.0 | — | — |
| quartile 2 (100 to 167) | 1.0 | 0.4 to 2.8 | 0.95 |
| quartile 3 (168 to 279) | 1.4 | 0.5 to 3.7 | 0.47 |
| quartile 4 (280 to 2295) | 0.9 | 0.3 to 2.4 | 0.83 |
| Acute GVHD grade | |||
| 0, I | 1.0 | — | — |
| II to IV | 3.4 | 1.3 to 8.8 | 0.01 |
| Adenovirus infection | |||
| no | 1.0 | — | — |
| yes | 2.8 | 1.2 to 6.8 | 0.02 |
Renal TMA Histology and Clinical Diagnosis
Of the 314 patients in the cohort, 119 (38%) had at least one measurement of each of these parameters in the 14-d period before death (Table 7). Among these 119 patients, the histologic evidence of TMA in the kidney did not correlate with the laboratory evidence of AKI in the two weeks before death. Although the presence of serologic abnormalities is greater in patients with either TMA or isolated thrombosis as opposed to negative pathology, none of these differences was statistically significant. Patients were further classified as having a diagnosis or suspicion of clinical TMA in their charts or no mention of it before death (Table 7). In the positive histology group, a clinical diagnosis or suspicion of TMA correlated with the presence of histologic evidence of TMA in the kidney compared with the negative histology group (35.5% versus 4.5%, Fisher's exact test P < 0.001), but not with the isolated thrombosis group (6.4%). A clinical diagnosis or suspicion of TMA was also associated with laboratory criteria for TMA on the basis of definition 2 (Fisher's exact test P = 0.03).
Table 7.
Frequency of abnormal laboratory values and chart findings suggestive of TMA by renal pathology
| Finding | N | Definition 1 (n, %)a | Definition 2 (n, %)b |
|---|---|---|---|
| Pathology | |||
| negative | 71 | 18 (25) | 39 (55) |
| TMA | 30 | 11 (37) | 22 (73) |
| Isolated | 18 | 6 (33) | 14 (78) |
| Chart | |||
| negative | 99 | 27 (27) | 58 (59) |
| positive | 16 | 8 (50) | 14 (88) |
Definition 1: hematocrit < 30, platelet count < 100,000, LDH above the upper limits of normal and a 50% increase in the baseline serum creatinine, in a single day's measurements.
Definition 2: hematocrit < 30, platelet count < 100,000, LDH above the upper limits of normal, and a 50% increase in the serum baseline creatinine, each occurring at least once over the 2 wk measurements.
Discussion
We found that acute GVHD grades II to IV, patient–donor sex mismatch (female patient and male donor), TBI > 1200 cGY, and ADV infection were associated with an increased risk of TMA in the kidney at autopsy. Our findings are similar to those of studies of TMA based on clinical criteria alone (8–10). The frequency of TMA in this cohort of patients was 20% compared with a frequency of 0.5% to 63.6% reported in the literature, whether defined solely by clinical criteria or by histology as in this cohort (11). Our data suggest, however, that there is little correlation between TMA as diagnosed by clinical criteria and histologic evidence of TMA in the kidney.
Although calcineurin inhibitors such as cyclosporine are frequently implicated as the cause of TMA in patients after transplant, cyclosporine use did not contribute to histologic evidence of TMA in this cohort of patients.
Endothelial injury in the kidney may be secondary to circulating inflammatory cytokines related to GVHD elsewhere in the body or may reflect direct injury to endothelial cells of the kidney by GVHD. Endothelial injury has been described in patients with chronic GVHD, and it is thought that endothelial cells are direct targets of cytotoxic donor T lymphocytes (12). Plasma markers of endothelial injury and coagulation activation are elevated in patients with acute GVHD after HCT, suggesting an association between endothelial injury, acute GVHD, and the subsequent development of TMA (13,14). Newer evidence suggests a role for vascular endothelial growth factor (VEGF) in the development of TMA and GVHD (15,16). Inhibition or loss of function of VEGF leads to development of proteinuria and glomerular endothelial injury consistent with TMA (15), and lower levels of VEGF have been associated with more severe forms of acute GVHD (16). GVHD may lead to endothelial injury in the kidney through a reduction in serum VEGF levels and therefore loss of the protective effects of VEGF on the glomerular filtration barrier. Thus, TMA in the kidney may be a manifestation of renal effects of GVHD. Our finding of patient–donor sex mismatch as a significant risk factor further supports the idea that acute GVHD plays a role in TMA after HCT because these patients are at increased risk for acute GVHD (17,18). The increased risk of GVHD may be related to the selective expression in females of minor histocompatibility antigens on the X-chromosome that are not expressed in males. These minor H antigens may then be recognized by T cells after transplant in females who receive cells from a male donor (17,18).
The significance of a single thrombus is unclear. Its presence may be an early manifestation of TMA or a low-grade TMA. It may be that those patients who develop only a single thrombus have a less severe grade of GVHD. Of course, the finding of a single thrombus may also be due to sampling.
Although elevations in serum creatinine are part of the definition of TMA, we did not find that the histologic changes in the kidney were reflected in serum creatinine measurements in the two weeks before death. This suggests that significant subclinical renal injury occurs early after transplant and that serum creatinine does not accurately measure kidney function in patients with mild renal insufficiency, or in certain other patient populations (e.g., individuals with malnutrition, muscle wasting, cancer, or the elderly) (19,20). Patients undergoing hematopoietic cell transplant may have large fluctuations in their nutritional status, muscle mass, and weight that can influence serum creatinine levels. Only 119 patients (38%) had laboratory data available in the 2 wk before death that could be used to make a clinical diagnosis of TMA, in part because LDH is not part of routine laboratory testing in our patients and is usually drawn only when clinically indicated. However, among patients with LDH measurements, laboratory data did not correlate with a histologic diagnosis of TMA. In the study by Eremina et al. described above, patients also did not have serologic evidence of TMA at the time of biopsy but did have proteinuria (15). In addition, a clinical diagnosis of TMA in the patient's chart correlated with a histologic diagnosis of TMA in approximately one third of the cases. Therefore, a high index of suspicion for the development of TMA is needed in patients after HCT especially those with gender-mismatched donors, TBI > 1200cGy as part of their conditioning regimen, ADV infection, and acute GVHD.
Both TBI and CMV infections are commonly implicated in the development of TMA after transplant (21–25). TBI dose > 1200 cGY was also associated with TMA; however, the effects of TBI were confounded by donor type. In our study, ADV infection rather than CMV infection was associated with an increased risk of TMA. A previous case report has described ADV in a patient mimicking clinical TMA; however, the histologic findings at autopsy in the kidney did not demonstrate TMA (26). In a study of 21 patients from our institution, ADV nephritis was associated with acute kidney injury and diagnosed at autopsy (19 patients) or on biopsy (2 patients) by the presence of virus in the kidney. There was no histologic evidence of TMA in any of these patients (27). We found no evidence that bacterial or fungal infections in the 2 wk before death contributed to the development of TMA after transplant.
In this study, we found no evidence for an association between the average daily dose of cyclosporine and the presence of TMA in the kidney. Martinez et al. similarly found no association between cyclosporine plasma levels and clinical diagnosis of TMA in patients after HCT (28). These findings contradict other studies in which both cyclosporine and tacrolimus have been associated with clinical TMA in the HCT population (24,29–34). Recent reports have also found an association between sirolimus and development of TMA after HCT (35). It may be that our patients died earlier than others and that the duration of therapy of calcineurin inhibitors may be more important for endothelial injury than the cumulative dose.
There are limitations to applying the findings from an autopsy cohort to the general HCT population. Selection biases are inherent as patients who die are obviously sicker than those who survive. Not all patients undergo an autopsy, and even among those who do, the kidney may not always be preserved because autopsies may be limited to certain organs. Most patients who died at home or at an outside institution were not included in this study. Patients in this study died at a median of 43 d after HCT, and risk factors for TMA early after transplant may differ from factors that contribute to the later development of TMA. Finally, we did not evaluate tissue using electron microscopy, which may demonstrate more subtle changes of TMA. Among the strengths of this paper are that the pathologist reviewing the slides was blinded to any clinical information and thus was not biased in her characterizations, and the diagnosis of TMA was made on histologic grounds rather than clinical data, which can often be confounded by other disease processes.
We found that TMA was present in 20% of kidneys at autopsy in patients after HCT and that an additional 15% had evidence of thrombus formation. The risk of TMA is increased four-fold in patients with acute GVHD and is independent of cyclosporine use. We found no evidence that dose of cyclosporine contributes to the development of histologic TMA in the kidney. Therefore, stopping or decreasing the dose of cyclosporine as recommended in many clinical practice guidelines may not be helpful in managing TMA. Rather, continuing treatment of the acute GVHD or directing therapy to mitigate endothelial injury may be of benefit. Although obtaining renal biopsies may not always be practical, they are important for an accurate diagnosis. It is also important to monitor patients with acute GVHD for ongoing and persistent renal damage and urinary abnormalities that can persist after clinical resolution of TMA.
Disclosures
None.
Acknowledgments
We are grateful to Karen Englehart and Charles Mahan for their assistance with the histology. This research was supported by the following: National Institutes of Health Grant K23 DK63038; an American Society of Nephrology/Renal Physicians Association Health Scholars grant; the National Kidney Foundation Young Investigators Grant (S.H.); and National Institutes of Health Grants CA18029 (G.B.M. and D.M.), CA5704, and HL3644 (D.M.).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fogo AB: The role of angiotensin ii and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis 35: 179–188, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ruggenenti P, Noris M, Remuzzi G: Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int 60: 831–846, 2001 [DOI] [PubMed] [Google Scholar]
- 3.van der Plas RM, Schiphorst ME, Huizinga EG, Hene RJ, Verdonck LF, Sixma JJ, Fijnheer R: Von Willebrand factor proteolysis is deficient in classic, but not in bone marrow transplantation-associated, thrombotic thrombocytopenic purpura. Blood 93: 3798–3802, 1999 [PubMed] [Google Scholar]
- 4.Fuge R, Bird JM, Fraser A, Hart D, Hunt L, Cornish JM, Goulden N, Oakhill A, Pamphilon DH, Steward CG, Marks DI: The clinical features, risk factors and outcome of thrombotic thrombocytopenic purpura occurring after bone marrow transplantation. Br J Haematol 113: 58–64, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Paquette RL, Tran L, Landaw EM: Thrombotic microangiopathy following allogeneic bone marrow transplantation is associated with intensive graft-versus-host disease prophylaxis. Bone Marrow Transplant 22: 351–357, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Uderzo C, Fumagalli M, De Lorenzo P, Busca A, Vassallo E, Bonanomi S, Lanino E, Dini G, Varotto S, Messina C, Miniero R, Valsecchi MG, Balduzzi A: Impact of thrombotic thrombocytopenic purpura on leukemic children undergoing bone marrow transplantation. Bone Marrow Transplant 26: 1005–1009, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Thomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE, Lerner KG, Glucksberg H, Buckner CD: Bone-marrow transplantation. N Engl J Med 292: 895–902, 1975 [DOI] [PubMed] [Google Scholar]
- 8.Nakamae H, Yamane T, Hasegawa T, Nakamae M, Terada Y, Hagihara K, Ohta K, Hino M: Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol 81: 525–531, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Daly AS, Hasegawa WS, Lipton JH, Messner HA, Kiss TL: Transplantation-associated thrombotic microangiopathy is associated with transplantation from unrelated donors, acute graft-versus-host disease and venoocclusive disease of the liver. Transfus Apher Sci 27: 3–12, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Holler E, Kolb HJ, Hiller E, Mraz W, Lehmacher W, Gleixner B, Seeber C, Jehn U, Gerhartz HH, Brehm G, Willmanns W: Microangiopathy in patients on cyclosporine prophylaxis who developed acute graft-versus-host disease after HLA-identical bone marrow transplantation. Blood 73: 2018–2024, 1989 [PubMed] [Google Scholar]
- 11.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB: Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: A diagnostic dilemma. Transfusion 44: 294–304, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, Gratwohl A: Endothelial injury mediated by cytotoxic t lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet 359: 2078–2083, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Kanamori H, Maruta A, Sasaki S, Yamazaki E, Ueda S, Katoh K, Tamura T, Otsuka-Aoba M, Taguchi J, Harano H, Ogawa K, Mohri H, Okubo T, Matsuzaki M, Watanabe S, Koharazawa H, Fujita H, Kodama F: Diagnostic value of hemostatic parameters in bone marrow transplant-associated thrombotic microangiopathy. Bone Marrow Transplant 21: 705–709, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Matsuda Y, Hara J, Osugi Y, Tokimasa S, Fujisaki H, Takai K, Ohta H, Kawa-Ha K, Okada S: Serum levels of soluble adhesion molecules in stem cell transplantation-related complications. Bone Marrow Transplant 27: 977–982, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min CK, Kim SY, Lee MJ, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Kim CC, Cho CS: Vascular endothelial growth factor (VEGF) is associated with reduced severity of acute graft-versus-host disease and nonrelapse mortality after allogeneic stem cell transplantation. Bone Marrow Transplant 38: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Nash RA, Pepe MS, Storb R, Longton G, Pettinger M, Anasetti C, Appelbaum FR, Bowden RA, Deeg HJ, Doney K, Martin PJ, Sullivan KM, Sanders J, Witherspoon RP: Acute graft-versus-host disease: Analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood 80: 1838–1845, 1992 [PubMed] [Google Scholar]
- 18.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR: Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood 103: 347–352, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK: Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol 14: 1000–1005, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ross EA, Wilkinson A, Hawkins RA, Danovitch GM: The plasma creatinine concentration is not an accurate reflection of the glomerular filtration rate in stable renal transplant patients receiving cyclosporine. Am J Kidney Dis 10: 113–117, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Waiser J, Budde K, Rudolph B, Ortner MA, Neumayer HH: De novo hemolytic uremic syndrome postrenal transplant after cytomegalovirus infection. Am J Kidney Dis 34: 556–559, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Tarbell NJ, Guinan EC, Niemeyer C, Mauch P, Sallan SE, Weinstein HJ: Late onset of renal dysfunction in survivors of bone marrow transplantation. Int J Radiat Oncol Biol Phys 15: 99–104, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R: Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int 35: 1336–1344, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Cohen EP, Lawton CA, Moulder JE: Bone marrow transplant nephropathy: Radiation nephritis revisited. Nephron 70: 217–222, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Lawton CA, Cohen EP, Murray KJ, Derus SW, Casper JT, Drobyski WR, Horowitz MM, Moulder JE: Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant 20: 1069–1074, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Yabe H, Hattori K, Inoue H, Matsumoto M, Hamanoue S, Hiroi A, Koike T, Kato S, Shimamura K, Yabe M: Fatal adenovirus infection indistinguishable from thrombotic microangiopathy after allogeneic cd34+ peripheral progenitor cell transplantation. Tokai J Exp Clin Med 30: 71–75, 2005 [PubMed] [Google Scholar]
- 27.Bruno B, Zager RA, Boeckh MJ, Gooley TA, Myerson DH, Huang ML, Hackman RC: Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation 77: 1049–1057, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Martinez MT, Bucher C, Stussi G, Heim D, Buser A, Tsakiris DA, Tichelli A, Gratwohl A, Passweg JR: Transplant-associated microangiopathy (tam) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant 36: 993–1000, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Pettitt AR, Clark RE: Thrombotic microangiopathy following bone marrow transplantation. Bone Marrow Transplant 14: 495–504, 1994 [PubMed] [Google Scholar]
- 30.Schriber JR, Herzig GP: Transplantation-associated thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Semin Hematol 34: 126–133, 1997 [PubMed] [Google Scholar]
- 31.Atkinson K, Biggs JC, Hayes J, Ralston M, Dodds AJ, Concannon AJ, Naidoo D: Cyclosporin a associated nephrotoxicity in the first 100 days after allogeneic bone marrow transplantation: Three distinct syndromes. Br J Haematol 54: 59–67, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Zeigler ZR, Rosenfeld CS, Andrews DF, 3rd, Nemunaitis J, Raymond JM, Shadduck RK, Kramer RE, Gryn JF, Rintels PB, Besa EC, George JN: Plasma Von Willebrand factor antigen (vwf:Ag) and thrombomodulin (tm) levels in adult thrombotic thrombocytopenic purpura/hemolytic uremic syndromes (ttp/hus) and bone marrow transplant-associated thrombotic microangiopathy (bmt-tm). Am J Hematol 53: 213–220, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Chappell ME, Keeling DM, Prentice HG, Sweny P: Haemolytic uraemic syndrome after bone marrow transplantation: An adverse effect of total body irradiation? Bone Marrow Transplant 3: 339–347, 1988 [PubMed] [Google Scholar]
- 34.Sarkodee-Adoo C, Sotirescu D, Sensenbrenner L, Rapoport AP, Cottler-Fox M, Tricot G, Ruehle K, Meisenberg B: Thrombotic microangiopathy in blood and marrow transplant patients receiving tacrolimus or cyclosporine a. Transfusion 43: 78–84, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, Ho V, Lee SJ, Soiffer R, Antin JH: Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11: 551–557, 2005 [DOI] [PubMed] [Google Scholar]