Abstract
Background and objectives: In hemodialysis, applicable guidelines recommend regular electrocardiogram (ECG) recordings. However, respective systematic evaluations are absent. Thus, the authors investigated whether routine ECG findings add prognostic information to standard risk assessment in hemodialysis.
Design, setting, participants, & measurements: The relationship between nine common baseline ECG variables and a combined cardiovascular endpoint (CVE; cardiac death, myocardial infarction, stroke), sudden death, stroke, MI, and all-cause death in 1253 patients from the German Diabetes and Dialysis Study was evaluated. All patients were on maintenance hemodialysis, had type 2 diabetes mellitus, and received randomized treatment with atorvastatin or placebo.
Results: During 4 yr of follow-up (March 1998 to March 2004), 469 patients reached the CVE, and 617 died. After adjustment for demographics, comorbidities, and biomarkers in multivariate analysis, patients presenting without sinus rhythm were 89% more likely to die, and the risk of CVE and stroke increased by 75% and 164%, respectively, compared with patients with preserved sinus rhythm. Left ventricular hypertrophy was associated with >2-fold increase in the risk of stroke and a 60% increase in the risk of sudden death.
Conclusions: In hemodialysis patients with type 2 diabetes mellitus, the absence of sinus rhythm is a risk indicator for CVE, stroke, and all-cause death, and left ventricular hypertrophy is associated with stroke and sudden death. Thus, routine ECG recording adds prognostic information to standard risk assessment.
High mortality rates, above 20% per year, show the abysmal prognosis of diabetic hemodialysis patients (1). To ameliorate risk stratification, multiple markers are currently under investigation. In this context, Kidney Disease Outcomes Quality Initiative (K/DOQI) practice guidelines recommend recording of an electrocardiogram (ECG) in every patient at initiation of renal replacement therapy and yearly thereafter (2). Although ECGs are extensively used diagnostic tools, appropriate systematic evaluations regarding the prognostic utility in hemodialysis patients are absent. This is of particular importance, since in this high-risk group ECG interpretation might be compromised by the fact that volume and electrolyte status can influence various ECG variables like the QRS complex, QT interval and P-wave (3,4,5,6).
The German Diabetes and Dialysis Study (4D Study: Die Deutsche Diabetes Dialyse Studie) was designed to test the efficacy and safety of atorvastatin in 1255 patients with type 2 diabetes mellitus on maintenance hemodialysis (7). During this study, baseline ECGs were recorded and analyzed according to prespecified criteria. The study endpoints were precisely documented and centrally adjudicated. Thus, the 4D Study allows the investigation of the prognostic value of easily assessable ECG parameters at baseline for the prediction of cardiovascular events and all-cause death in a large, well characterized population of patients with type 2 diabetes mellitus on maintenance hemodialysis. We hypothesized that increased heart rate, cardiac rhythm abnormalities, QRS-axis deviation, atrioventricular block, signs of myocardial infarction (MI), left ventricular hypertrophy (LVH), complete left and right bundle branch block (LBBB and RBBB), and corrected QT interval are associated with cardiovascular events and all-cause death in patients with type 2 diabetes mellitus on maintenance hemodialysis, supporting the clinical practice of routine ECG recordings.
Materials and Methods
Study Population
Design and methods of the 4D Study have previously been reported in detail (8). Briefly, the 4D Study was a large, randomized, multicenter trial including 1255 patients with type 2 diabetes mellitus and previous duration of maintenance hemodialysis of less than 2 yr. Exclusion criteria included levels of fasting serum LDL cholesterol of <80 mg/dL (2.1 mmol/L) or >190 mg/dL (4.9 mmol/L), triglyceride levels of >1000 mg/dL (11.3 mmol/L); liver function values > 3 times the upper limit of normal or equal to those in patients with symptomatic hepatobiliary cholestatic disease; hematopoietic disease or systemic disease unrelated to end-stage renal disease; vascular intervention, congestive heart failure, or MI within the 3 mo preceding the period of enrolment; unsuccessful kidney transplantation; and hypertension resistant to therapy.
Between March 1998 and October 2002, patients were recruited in 178 dialysis units throughout Germany. After a run-in period of 4 wk, patients were randomly assigned to receive double-blind treatment with either 20 mg of atorvastatin once daily (n = 619) or placebo (n = 636). Follow-up visits were done at 4 wk and then every 6 mo.
Outcome Measures
The primary study endpoint was a combined cardiovascular endpoint (CVE) composed of cardiac death, MI, and stroke, whichever occurred first. All-cause mortality, sudden death, MI, and stroke were defined as secondary outcome measures. Endpoints were prespecified and centrally adjudicated by a specialized committee consisting of 1 cardiologist and 2 nephrologists who were blind to study treatment. All five endpoints were determined to be the outcome measures of the current post hoc analysis. Definitions of these endpoints have previously been reported in detail (7,8).
ECG Methodology
The last standard resting 12-lead ECG recorded before randomization to study treatment was used for this analysis (if there was no ECG available before randomization, the first ECG recorded after randomization was analyzed). Hard-copy ECGs, recorded at either 25 or 50 mm/s paper speeds were collected and processed in a central laboratory. An independent experienced cardiologist who was blind to all clinical information quantified and interpreted the ECGs according to prespecified criteria (Table 1), describing the following characteristics for each recording: (1) cardiac rhythm, (2) heart rate, (3) QRS axis, (4) presence of atrioventricular conduction defects, (5) complete RBBB, (6) complete LBBB, (7) signs of MI, (8) LVH, and (9) corrected QT interval. To validate this analysis, the same reader reanalyzed a random subsample of ECGs. The intraobserver reproducibility was 98% (of 1100 variables, 22 were interpreted differently). Furthermore, a second independent cardiologist interpreted a random subsample of ECGs with an interobserver reproducibility of 96% (of 1100 variables, 41 were differently interpreted) between the first and the second analysis.
Table 1.
Definition of ECG variables
| ECG Variable | Definition |
|---|---|
| Sinus rhythm | Positive P waves in leads I, II, and III, with subsequent QRS complexes. |
| Atrioventricular conduction defects (AV block) | AV block I°: P-Q interval > 0.22 s in the majority of beats in any of leads I, II, III, aVL, aVF; AV block II°-Mobitz: 2:1 or 3:1 block in any lead; AV block-II°-Wenckebach: P-R interval increasing from beat to beat until QRS and T dropped; AV block III°: complete AV block in any lead. |
| QRS axis | In the case of low voltage (QRS amplitude < 0.5mV in leads I, II, and III), artificial pacemaker or complete AV block with ventricular complexes no QRS axis was defined. The following groups were identified: QRS axis −89° to −31°, −30°, 29° to + 29°, + 30°, +31° to +59°, +60°, +61° to +89°, + 90°, +91° to +149°, +150°, +151° to +209°, +210°, +211° to +269 °, +270° (=–90 °). |
| Ventricular conduction defects | Complete left bundle branch block: QRS duration > 0.12 s in a majority of beats in any of leads I, II, III, aVL, or aVF, plus R peak duration > 0.06 s in a majority of beats (of the same QRS pattern) in any of leads I, II, aVL, V5, V6. |
| Complete right bundle branch block: QRS duration > 0.12 s in a majority of beats in any of leads I, II, III, aVL, or aVF, plus R′ > R in V1 or V2; or QRS mainly upright, with R peak duration > 0.06 s in V1 or V2; or S duration > R duration in all beats in lead I or II. | |
| Left ventricular hypertrophy | Sokolow Lyon Index (SV1 or V2 and RV5 or V6 ≥ 3.5 mV). |
| QT interval | QT interval was corrected for the heart rate with the Bazett's QT interval correction formula (QT interval/square root [R-R interval]). The QT interval was measured in the case of absence of sinus rhythm and complete left or right bundle branch block or pacemaker rhythm. |
| Signs of myocardial infarction | Q wave ≥ 0.03 s in at least two adjacent leads except V1 and aVR (adjacent leads are V2-V6; I and aVL, II, III, aVF); abnormal symmetric T wave inversion ≥ 0.1 mV in adjacent leads (adjacent leads are V2-V6; I and aVL, II, III, aVF); abnormal ST depression: each horizontal ST depression ≥ 0.2 mV, 0.08 s after the J point, except in ECGs with bundle branch block, left ventricular hypertrophy, or uncharacteristic repolarization disorder. |
| Baseline heart rate | Ventricular rate, independent of atrial rhythm. |
Statistical Analyses
Baseline data of continuous variables are given as mean ± SD. Absolute and relative frequencies are given for categorical variables. Cumulative incidences and Kaplan-Meier estimates of all outcome measures were calculated. Adjusting for important possible confounders, the Cox proportional hazards regression model was used to calculate the relative risk and corresponding 95% percent confidence intervals (CIs) associated with individual ECG variables and the five endpoints. The following explanatory variables were considered for inclusion into the Cox model: atorvastatin treatment assignment; gender; age; history of smoking; systolic and diastolic BP; body mass index; phosphate; LDL cholesterol; hemoglobin; glycated hemoglobin; albumin; history of coronary artery disease, congestive heart failure, cardiac valve disorder, peripheral vascular disease, and stroke or transitory ischemic attack; and the following ECG variables: cardiac rhythm, heart rate, atrioventricular conduction defects, QRS axis, complete RBBB and LBBB, LVH, signs of MI, and corrected QT interval (regarding the QRS axis, patients were allocated to the following groups: QRS axis −89° to + 29°, +30° to + 89°, +90° to −90°). A forward stepwise selection procedure was used to determine the variables included in the final model. This procedure starts examining the variable with the largest adjusted chi-square statistic, and adds variables to the model if P ≤ 0.25, and retains them if P ≤ 0.15 in the following steps. In the final analysis, a P value ≤ 0.05 was considered to be significant (in an exploratory sense). All P values are reported two-sided. All testing was two-tailed. Analyses were done using SAS version 8.2.
Results
Of 1255 patients, who participated in the 4D Study, 1253 received at least one ECG recording. Of these, 1040 ECGs were recorded before randomization and 213 shortly thereafter. High-quality ECG recordings without missing or misplaced leads or other technical errors were available in 1239 patients. Incomplete high-quality recordings were also analyzed as far as possible (e.g., regarding heart rate and cardiac rhythm). The mean follow-up-period was 3.96 yr (median, 4.0 yr) for patients on atorvastatin and 3.91 yr (median, 4.08 yr) for those on placebo. During follow-up, 617 patients died; of those, 160 died of sudden death. Four hundred sixty-nine patients reached the CVE consisting of cardiac death, nonfatal MI, and stroke, with 103 patients experiencing a stroke and 200 subjects a MI. For baseline characteristics, see Table 2.
Table 2.
Baseline patients’ characteristics
| Characteristic | Overall (N = 1253) | With Sinus Rhythm (n = 1112) | Without Sinus Rhythm (n = 141) | P | Without LVH (n = 1098) | With LVH (n = 155) | P |
|---|---|---|---|---|---|---|---|
| Age, mean yr (SD) | 65.7 (8.3) | 65.3 (8.3) | 69.0 (6.8) | <0.001 | 65.6 (8.4) | 66.5 (7.4) | 0.179 |
| Female, no. (%) | 577 (46) | 510 (45.9) | 67 (47.5) | 0.723 | 522 (47.5) | 55 (35.5) | 0.006 |
| Duration of diabetes, mean yr (SD) | 18.1 (8.8) | 18.2 (8.9) | 17.7 (8.4) | 0.585 | 17.9 (8.9) | 19.9 (8.3) | 0.007 |
| Time on dialysis, mean mo (SD) | 8.3 (6.9) | 8.2 (6.9) | 9.1 (6.6) | 0.128 | 8.4 (7.0) | 7.7 (6.4) | 0.273 |
| Blood pressure, mean (SD), mmHg | |||||||
| systolic | 146 (22) | 146 (22) | 140 (20) | <0.001 | 145 (22) | 152 (23) | <0.001 |
| diastolic | 76 (11) | 76 (11) | 75 (11) | 0.196 | 76 (11) | 77 (12) | 0.122 |
| Smoker (current/former), no. (%) | 506 (40.4) | 449 (40.4) | 57 (40.4) | 0.360 | 430 (39.2) | 76 (49.0) | 0.059 |
| Body mass index, mean kg/m2 (SD) | 27.6 (4.8) | 27.6 (4.8) | 27.6 (4.7) | 0.909 | 28.0 (4.8) | 24.4 (3.4) | <0.001 |
| MI, CABG/ PCI, CHD, no.(%) | 369 (29.4) | 318 (28.6) | 51 (36.2) | 0.077 | 313 (28.5) | 56 (36.1) | 0.060 |
| Congestive heart failure, no. (%) | 444 (35.4) | 372 (33.5) | 72 (51.1) | <0.001 | 380 (34.6) | 64 (41.3) | 0.107 |
| History of cardiac valve disorder, no. (%) | 94 (7.5) | 73 (6.6) | 21 (14.9) | 0.001 | 78 (7.1) | 16 (10.3) | 0.190 |
| Peripheral vascular disease, no. (%) | 560 (44.7) | 494 (44.4) | 66 (46.8) | 0.591 | 486 (44.3) | 74 (47.7) | 0.438 |
| Stroke/transitory ischemic attack, no. (%) | 224 (17.9) | 202 (18.2) | 22 (15.6) | 0.559 | 201 (18.3) | 23 (14.8) | 0.316 |
| Hemoglobin, mean g/dl (SD) | 10.9 (1.3) | 10.9 (1.3) | 11.0 (1.4) | 0.240 | 10.9 (1.3) | 10.7 (1.4) | 0.023 |
| Gycated hemoglobin, mean % (SD) | 6.7 (1.3) | 6.7 (1.3) | 6.9 (1.2) | 0.054 | 6.7 (1.3) | 6.8 (1.3) | 0.396 |
| Albumin, mean g/L (SD) | 3.8 (0.3) | 3.8 (0.3) | 3.9 (0.3) | 0.020 | 3.8 (0.3) | 3.8 (0.3) | 0.507 |
| Phosphate, mean mg/dl (SD) | 6.0 (1.6) | 6.0 (1.6) | 5.9 (1.8) | 0.402 | 6.0 (1.6) | 6.0 (1.6) | 0.612 |
| LDL cholesterol, mean mg/dl (SD) | 126 (30) | 126 (30) | 122 (30) | 0.108 | 126 (30) | 125 (30) | 0.872 |
| Dose of erythropoietin, IU/wk (SD) | 6,030 (3,883) | 5,975 (3,810) | 6,489 (4,433) | 0.214 | 5975 (3878) | 6404 (3904) | 0.210 |
| Arteriovenous fistula, no. (%) | 1168 (93.3) | 1041 (93.7) | 127 (90.1) | 0.109 | 1026 (93.5) | 142 (91.6) | 0.390 |
| Ultrafiltration volume, kg/dialysisa | 2.3 (1.2) | 2.2 (1.2) | 2.3 (1.3) | 0.520 | 2.2 (1.2) | 2.3 (1.1) | 0.540 |
| Duration of dialysis, hr/wk | 12.3 (1.9) | 12.3 (1.9) | 12.3 (2.1) | 0.958 | 12.3 (1.9) | 12.3 (2.1) | 0.993 |
| ECG signs of LVH, no. (%) | - | 145 (13) | 10 (7.1) | 0.042 | - | - | - |
| ECG signs of MI, no. (%) | 176 (14.0) | 141 (12.7) | 35 (24.8) | <0.001 | 155 (14.1) | 21 (13.5) | 0.902 |
The two P values (comparing patients with sinus rhythm to those without, and patients with LVH to those without) were derived by ttest for continuous data and by the Fisher exact test or chi-square test for categorical data. Congestive heart failure is predominantly New York Heart Association II. To convert hemoglobin values to millimoles per liter, multiply by 0.62. To convert values for phosphate to millimoles per liter, multiply by 0.32. To convert values for LDL cholesterol to millimoles per liter, multiply by 0.03. Abbreviations: LVH, left ventricular hypertrophy; MI, myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; CHD, coronary heart disease documented by coronary angiography.
The ultrafiltration volume was calculated on the basis of body weight before and after dialysis at the randomization visit
ECG variables
One thousand one hundred twelve patients (89%) were in sinus rhythm. The most common rhythm disorders were atrial fibrillation (n = 106) and atrial flutter (n = 11), which was documented in 117 out of 141 ECGs without sinus rhythm. Furthermore, pacemaker rhythm (n = 38) and atrial escape rhythm (n = 19) were registered. Atrioventricular conduction defect (AV block) I°-III° was documented in 91 (7%) patients cases (AV block 1°, n = 90 and AV block II°-III°, n = 1). Seven hundred ninety-five ECG scans (63%) showed a QRS axis from −89° to + 29°, 355 (28%) from + 30° to + 89°, and 39 (3%) from + 90° to −90°; in 64 baseline ECGs (5%), the QRS axis could not be determined. Complete RBBB (n = 82) or LBBB (n = 28) was found in 110 ECGs (9%). LVH was diagnosed in 155 patients (12%); signs of MI were present in 176 ECGs (14%). QT interval was measured in 1082 patients. Patients with pre-existing bundle branch block, absence of sinus rhythm, or pacemaker rhythm were excluded from QT-interval analysis.
In univariate analysis, sinus rhythm, LVH, infarction signs, and baseline heart rate were associated with the CVE and all-cause death. In addition, complete LBBB was predictive of mortality and sudden death. ECG signs of MI and baseline heart rate were predictors of MI, and LVH was associated with stroke and sudden death. Baseline heart rate was predictive of sudden death. QT interval, complete RBBB, atrioventricular conduction defects, and QRS axis were not associated with the outcome.
Absolute Risks
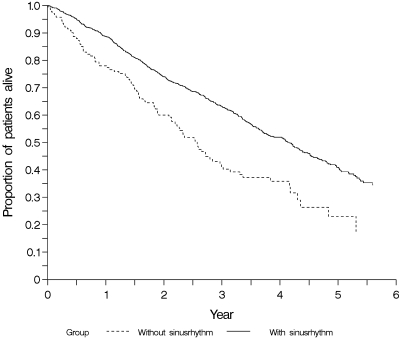
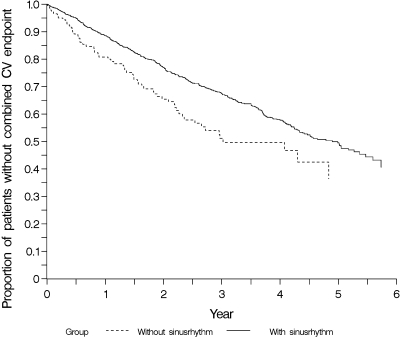
Overall, the risk of mortality was high. After 4 yr of follow-up, the highest proportion of all cause death was seen in patients presenting without sinus rhythm (64%) compared with patients with preserved sinus rhythm (48%) (P < 0.001) (Figure 1). With respect to CVE, similar results were found. Patients without sinus rhythm in the baseline ECG had the highest cumulative incidence of CVE, 50% after 4 yr, compared with 42% in patients with preserved sinus rhythm (P = 0.003) (Figure 2).
Figure 1.
Cumulative incidence estimates for time to all-cause death in subgroups of patients with (n = 1112) and without (n = 141) sinus rhythm in the baseline ECG.
Figure 2.
Cumulative incidence estimates for time to first cardiovascular (CV) event (cardiac death, nonfatal myocardial infarction, or stroke) in patient subgroups with (n = 1112) and without (n = 141) sinus rhythm in the baseline ECG.
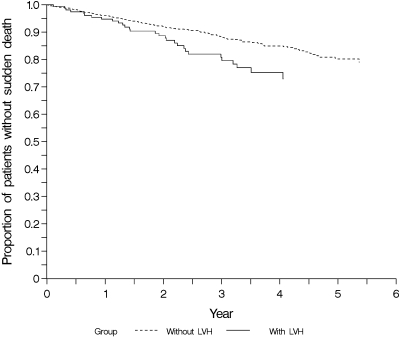
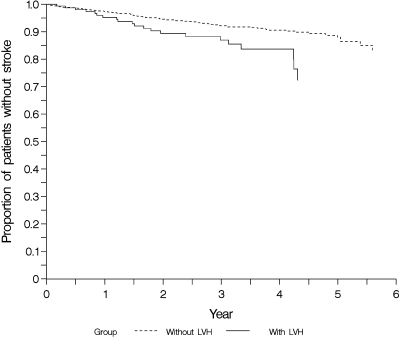
For sudden death, the absolute risk was 25% after 4 yr in patients with ECG signs of LVH and 15% in those without signs of LVH (P = 0.01) (Figure 3). Similar data were found with respect to stroke. The cumulative incidences of stroke after 4 yr were 16% in patients with LVH and 9% in those without LVH (P = 0.02) (Figure 4).
Figure 3.
Cumulative incidence estimates for time to sudden death in subgroups of patients with (n = 155) and without (n = 1098) left ventricular hypertrophy in the baseline ECG.
Figure 4.
Cumulative incidence estimates for time to first stroke in patient subgroups with (n = 155) and without (n = 1098) left ventricular hypertrophy in the baseline ECG.
Relative Risks
The absence of sinus rhythm in the baseline ECG was associated with a marked increase in the risk of all-cause death (HR, 1.89; 95% CI, 1.42 to 2.51; P < 0.001). Consistently, patients presenting without sinus rhythm in the baseline ECG were 75% more likely to experience a CVE (HR 1.75; 95% CI, 1.24 to 2.47; P = 0.001), and more than twice as likely to experience a stroke (HR 2.64; 95% CI, 1.30 to 5.35; P = 0.007). LVH was predictive of sudden death (HR 1.60; 95% CI, 1.05 to 2.44; P = 0.027) and stroke (HR 2.45; 95% CI, 1.49 to 4.05; P < 0.001). There was a trend toward a higher risk of CVE (HR 1.27; 95% CI, 0.97 to 1.65; P = 0.081) and MI (HR 1.44; 95% CI, 0.98 to 2.14; P = 0.066) in patients with signs of MI in their baseline ECG compared with those without. Baseline heart rate, QT interval, complete RBBB and LBBB, atrioventricular conduction defects, and QRS axis were not associated with the outcome in multivariate analyses.
Discussion
The present study investigated the association between 9 routine ECG variables and the outcome in a large prospective patient cohort with a high incidence of prespecified and centrally adjudicated endpoints during 4 yr of follow-up. In patients with type 2 diabetes mellitus on hemodialysis, the absence of sinus rhythm was a risk indicator for all-cause mortality and cardiovascular events. Furthermore, LVH was associated with stroke and sudden death. A number of established ECG risk indicators as infarction signs, LBBB, QT interval, and heart rate were not predictive of the outcome in multivariate analysis.
In patients with normal renal function, the prognostic value of ECG variables is well established. LVH and signs of MI were associated with coronary morbidity, mortality, and stroke in the Framingham Study (9). Strain pattern in hypertensive patients predicted heart failure development and mortality in a subanalysis of the LIFE (The Losartan Intervention for Endpoint Reduction in Hypertension) study (10). An extensive analysis of 12 ECG variables in 38,283 women participating in the Women's Health Initiative found repolarization disorders, signs of MI, and QRS abnormalities to be equally predictive of coronary heart disease morbidity and mortality (11). Repolarization disorders and infarction pattern were also predictors of incident congestive heart failure and all-cause mortality (12). Finally, in atrial fibrillation trials, the preservation of sinus rhythm was associated with a considerable reduction in the risk of death (13,14). Thus, there is a good rationale for baseline ECGs in the above populations. However, information on the prognostic value of the by guidelines recommended routine ECG recording in hemodialysis patients is by now only opinion based (2).
Besides being particularly frequent (11.3% of the cohort; 83% of those had atrial fibrillation or flutter), the absence of sinus rhythm in the baseline ECG was a risk indicator for all-cause death, the CVE, and stroke. So far, only two studies in patients on maintenance hemodialysis treatment reported an increased prevalence of atrial fibrillation and a higher incidence of thrombo-embolic events, hospitalizations, or mortality in patients with atrial fibrillation (15,16). To evaluate the increased prevalence of atrial fibrillation and higher incidence of cardiovascular morbidity and all-cause mortality in hemodialysis, we looked for differences in dialysis specific variables (dialysis shunt, atrial catheter, ultrafiltration volume, duration of dialysis per week) and LVH. With respect to these characteristics, patients with and without sinus rhythm were similar. Significant differences were found regarding age, systolic BP, congestive heart failure, and cardiac valve disorders, suggesting an increased mortality hazard mediated by accompanying risk factors. However, the predictive value of sinus rhythm with respect to all-cause death and CVE persisted even after adjustment for these variables.
LVH diagnosed by ECG criteria was predictive of stroke and sudden death. This is in line with previous data in patients without kidney disease, reporting LVH to be among the risk factors for ischemic stroke and sudden death (17,18). With respect to total mortality and CVE, we found a significant association in the univariate, but not in the multivariate, analyses. This is partly consistent with earlier findings but does not comply with data mainly derived from echocardiographic studies showing a clear association between LVH and mortality in hemodialysis populations (19–23). This might be due to the fact that the sensitivity of hypertrophy markers in the ECG is low (24,25). Thus, ECG and echocardiographic findings cannot be compared directly, and ECG alone underestimates LVH. Furthermore, the QRS amplitude is dependent on various parameters influenced by hemodialysis; for example,. the QRS amplitude increases when body weight, end diastolic volume, potassium, and systolic arterial pressure decrease after hemodialysis (5,6), which might question the validity of LVH assessment by ECG indexes in hemodialysis patients. Finally, multicollinearity might have played a role because LVH is associated with hypertension and anemia; therefore, ECG-based diagnosis of LVH might have dropped out of the multivariate analysis (26).
The remaining variables (signs of MI, baseline heart rate, QRS axis, AV block, complete LBBB or RBBB, and QT interval) showed no statistically significant association with the outcome in multivariate analyses. This was particularly surprising in the case of MI. ECG signs of MI are well established prognostic markers in the general population but there are no data in hemodialysis patients yet. In the 4D Study 14% of patients showed at least one infarction criterion in the baseline ECG. In univariate analysis, this was associated with an increase in CVE, MI, and total mortality. However, this relation did not persist after adjustment for demographics, comorbidity, and biomarkers in the multivariate models. This might be ascribed to the fact that the risk of all-cause death and cardiovascular events associated with ECG signs of MI was not independent of known comorbidities or the low incidence of endpoints. Mortality rates were well below those reported by Herzog et al. (27). In this retrospective analysis, the overall mortality after acute MI among 34,189 patients on long-term dialysis was 90% at 5 yr, with a 26% in-hospital mortality. Of note, in the 4D Study patients with acute cardiovascular events within the last 3 mo before study entry were not allowed to be randomized to study treatment, thus some patients (18% of the study cohort) in the 4D Study were selected survivors of MI.
There are some limitations to be mentioned. First, the ECG reading was not performed on a computerized basis but by an experienced cardiologist, reflecting usual procedures and evaluating clinically relevant variables. Thus, this analysis reflects daily routine practice. Second, even if the large cohort of 1253 patients was analyzed, this was a selected population of German hemodialysis patients with type 2 diabetes mellitus randomized into the 4D Study. Therefore, the relationship between ECG variables and risk may not be generalizable to other populations. Other potential limitations may be that data about anticoagulation use were not available and that LVH was diagnosed using the Sokolow index only, without considering other methods, for example, the Cornell index.
In conclusion, the absence of sinus rhythm was an important risk indicator for all-cause death and cardiovascular events in patients with type 2 diabetes mellitus on hemodialysis. LVH was predictive of stroke and sudden death. Thus, routine ECG recording adds prognostic information to standard risk assessment. However, only these two out of a set of nine routinely assessed ECG parameters were predictive of outcome in multivariate analysis.
Disclosures
None.
Acknowledgments
We thank all investigators and study nurses who participated in the 4D Study (a complete list is available at www.uni-wuerzburg.de/nephrologie); without their collaboration this article would not have been written. Special thanks go to the event committee: J. Mann (chair), J. Bommer, P. Schanzenbächer, P. Schollmeyer, and M. Schartl.
This work was presented as an abstract at the American Society of Nephrology Renal Week 2008.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: 112-119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: 1-153, 2005 [PubMed] [Google Scholar]
- 3.Covic A, Diaconita M, Gusbeth-Tatomir P, Covic M, Botezan A, Ungureanu G, Goldsmith DJ: Hemodialysis increases QT(c) interval but not QT(c) dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplant 17: 2170-2177, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Madias JE: P waves in patients with changing edematous states: Implications on interpreting repeat P wave measurements in patients developing anasarca or undergoing hemodialysis. Pacing Clin Electrophysiol 27: 749-756, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Fuenmayor AJ, Vasquez CJ, Fuenmayor AM, Winterdaal DM, Rodriguez D: Hemodialysis changes the QRS amplitude in the electrocardiogram. Int J Cardiol 41: 141-145, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Ojanen S, Koobi T, Korhonen P, Mustonen J, Pasternack A: QRS amplitude and volume changes during hemodialysis. Am J Nephrol 19: 423-427, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238-248, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Wanner C, Krane V, Marz W, Olschewski M, Asmus HG, Kramer W, Kuhn KW, Kutemeyer H, Mann JF, Ruf G, Ritz E, Deutsche Diabetes-Dialyse-Studie (4D) Study Group: Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): Demographic and baseline characteristics. Kidney Blood Press Res 27: 259-266, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, Abbott RD: A prognostic comparison of asymptomatic left ventricular hypertrophy and unrecognized MI: The Framingham Study. Am Heart J 111: 391-397, 1986 [DOI] [PubMed] [Google Scholar]
- 10.Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Dahlof B; LIFE Study Investigators: Electrocardiographic strain pattern and prediction of new-onset congestive heart failure in hypertensive patients: The Losartan Intervention for End point Reduction in Hypertension (LIFE) study. Circulation 113: 67-73, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A: Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: The Women's Health Initiative. Circulation 113: 473-480, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Rautaharju PM, Kooperberg C, Larson JC, LaCroix A: Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women: The Women's Health Initiative. Circulation 113: 481-489, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD, Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators: A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 347: 1825-1833, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C: Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: A Danish investigation of arrhythmia and mortality on dofetilide (DIAMOND) substudy. Circulation 104: 292-296, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Vazquez E, Sanchez-Perales C, Lozano C, Lozano C, Garcia-Cortes MJ, Borrego F, Guzman M, Perez P, Pagola C, Borrego MJ, Perez V: Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. Am J Cardiol 92: 868-871, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, Valsecchi MG: Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis 51: 255-262, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bots ML, Nikitin Y, Salonen JT, Elwood PC, Malyutina S, Freire de Concalves A, Sivenius J, Di Carlo A, Lagiou P, Tuomilehto J, Koudstaal PJ, Grobbee DE: Left ventricular hypertrophy and risk of fatal and non-fatal stroke. EUROSTROKE: A collaborative study among research centers in Europe. J Epidemiol Community Health 56: S8–S13, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritz E, Wanner C: The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 3: 920-929, 2008 [DOI] [PubMed] [Google Scholar]
- 19.De Lima JJ, Sesso R, Abensur H, Lopes HF, Giorgi MC, Krieger EM, Pileggi F: Predictors of mortality in long-term haemodialysis patients with a low prevalence of comorbid conditions. Nephrol Dial Transplant 10: 1708-1713, 1995 [PubMed] [Google Scholar]
- 20.Koch M, Thomas B, Tschope W, Ritz E: Survival and predictors of death in dialysed diabetic patients. Diabetologia 36: 1113-1117, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Foley RN, Culleton BF, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Cardiac disease in diabetic end-stage renal disease. Diabetologia 40: 1307-1312, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024-2031, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Nakamura S, Sasaki O, Nakahama H, Inenaga T, Kimura G: Left ventricular hypertrophy is a risk factor independent of hypertension in survival of hemodialyzed patients. Ren Fail 24: 175-186, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Alfakih K, Walters K, Jones T, Ridgway J, Hall AS, Sivananthan M: New gender-specific partition values for ECG criteria of left ventricular hypertrophy: recalibration against cardiac MRI. Hypertension 44: 175-179, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP: Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 81: 815-820, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Rigatto C, Foley R, Jeffery J, Negrijn C, Tribula C, Parfrey P: Electrocardiographic left ventricular hypertrophy in renal transplant recipients: Prognostic value and impact of blood pressure and anemia. J Am Soc Nephrol 14: 462-468, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Herzog CA, Ma JZ, Collins AJ: Poor long-term survival after acute MI among patients on long-term dialysis. N Engl J Med 339: 799-805, 1998 [DOI] [PubMed] [Google Scholar]