Abstract
Background and objectives: The prevalence of chronic kidney disease (CKD) has increased over the past two decades. The sensitivity of serum creatinine (sCr) to identify CKD is low. As a result, many healthcare centers report estimated GFR (eGFR) with routine blood work. The aim of this study was to determine the cost-effectiveness of automatic eGFR reporting compared with reporting sCr alone.
Design, setting, participants, & measurements: A Markov model was designed to evaluate the cost-effectiveness of reporting eGFR compared with reporting sCr alone in a hypothetical cohort of 60-yr-old individuals undergoing annual blood chemistry testing over 18 yr. Paths and path probabilities were identical between the two arms, except for the sensitivity and specificity of eGFR and sCr to detect CKD.
Results: eGFR reporting was dominant with a cost/effectiveness ratio of $16,751/quality-adjusted life year (QALY) versus $16,779/QALY for sCr reporting. Monte Carlo microsimulations in a hypothetical cohort of 10,000 patients demonstrated that over 18 yr, an average of 13 fewer deaths, 29 fewer ESRD events, and 11,348 more false positive CKD (FP-CKD) cases occurred with eGFR reporting. A sensitivity analysis revealed that decreasing the FP-CKD quality of life by > 2% rendered sCr reporting more cost-effective than eGFR reporting. If FP-CKD reduced quality of life by 5%, the incremental cost-effectiveness ratio for sCr reporting versus eGFR reporting would be $4367/QALY.
Conclusion: A decision analysis suggests that reporting eGFR may be beneficial, but this limited benefit was reversed with virtually any reduction in quality of life caused by incorrect diagnosis of CKD.
The prevalence of all stages of chronic kidney disease (CKD) in the United States has been estimated to be 13.1%; the great majority of these patients are unaware of their disease (1,2). The elevated morbidity and mortality associated with CKD (3,4), including cardiovascular disease and progression to end-stage renal disease (ESRD), have spurred physicians to develop methods of detecting and treating CKD before ESRD occurs.
Although examining serum creatinine alone is an insensitive method of detecting CKD (5,6), equations have been developed that use serum creatinine in addition to clinical and demographic data to estimate the GFR. The most extensively studied of these equations is the Modification of Diet in Renal Disease (MDRD) equation. Although a more sensitive measure than serum creatinine alone for CKD detection, MDRD underestimates measured GFR (mGFR) in healthy patients (7–11). False positive diagnosis with CKD can engender costs from additional clinical evaluations and, potentially, reduce quality of life.
Interest in identifying patients with unrecognized CKD has led many healthcare center laboratories to report eGFR automatically when a serum creatinine test is requested. As of November 2007, Connecticut, Louisiana, Michigan, New Jersey, Pennsylvania, and Tennessee have mandated eGFR reporting (12,13). Despite this important change in practice, few studies have investigated the impact of routine reporting of eGFR (14).
The aim of this study was to assess whether routine reporting of eGFR is cost-effective compared with reporting serum creatinine alone. We hypothesized that reporting eGFR with serum creatinine would increase detection of true CKD, but also lead to the false identification of healthy individuals as CKD patients, thereby limiting the cost-effectiveness of the strategy of universal reporting of eGFR. We also hypothesized that the cost-effectiveness of routine reporting of eGFR would be strongly influenced by any decrement in health-related quality of life resulting from a false positive diagnosis of CKD.
Materials and Methods
Model Design
A state-transition Markov model with two arms was constructed (15); one arm assessed the cost and effect of serum creatinine reporting, and the other assessed the cost and effect of routine eGFR reporting. All probabilities, costs, and rewards within a state were identical between the two arms, except for the sensitivity and specificity of serum creatinine and eGFR reporting with respect to identifying cases of CKD. The base case patient was 60 yr of age and was followed for 18 cycles, 1 yr in length. Sixty years was chosen because of the increasing prevalence of CKD after age 60 (1). Eighteen cycles were performed to capture the effects of eGFR reporting until the average age at the end of life in the United States, based on life expectancy at birth: 78 yr (16). Patients had either a serum creatinine or creatinine plus eGFR measurement every cycle.
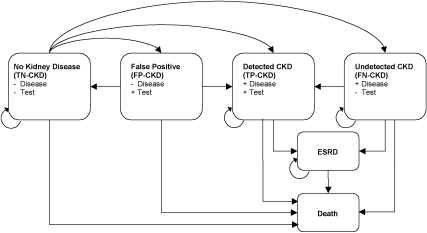
Six health states were included in the model: (1) no kidney disease or early stages of kidney disease, which included all patients with GFR ≥60 ml/min/1.73 m2 in the “true negative” state (TN-CKD); (2) CKD in the “true positive” state (TP-CKD); (3) CKD in the “false negative” state (FN-CKD); (4) no kidney disease or early stages of disease in the “false positive” state (FP-CKD), among patients with a GFR ≥60 ml/min/1.73 m2 and an incorrect diagnosis; (5) ESRD, which included all patients with eGFR < 15 ml/min per 1.73 m2 and did not distinguish among transplant recipients, hemodialysis patients, and peritoneal dialysis patients; and (6) death. Figure 1 shows these health states and possible transitions between states.
Figure 1.
State transition model showing the possible transitions of patients between health states. An individual patient could be in only 1 of the 6 possible states during a single cycle. TN-CKD, true negative state for patient without chronic kidney disease (GFR ≥ 60 ml/min/1.73 m2); FP-CKD, false positive state for patient without chronic kidney disease; TP-CKD, true positive state for patient with chronic kidney disease; FN-CKD, false negative state for patient with chronic kidney disease; CKD, chronic kidney disease stages 3 to 4 (GFR 15 to 59 ml/min/1.73 m2); ESRD, end stage renal disease (GFR < 15 ml/min/1.73 m2).
CKD, both true positive and false negative, included only National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) CKD stages 3 to 4, in which GFR is 15 to 59 ml/min/1.73 m2 (17). The model was designed in this way because the MDRD equation has been validated in stages 3 to 5 of CKD and because nephrology evaluation is usually obtained in these later stages of disease. We combined stages 3 and 4 because some important studies of CKD outcomes did not distinguish between these stages (18–21).
Patients were distributed initially between TN-CKD, TP-CKD, FN-CKD, and FP-CKD on the basis of CKD prevalence and test characteristics (see Transition Probabilities) and were then able to move through the model as follows (see Figure 1):
(1) Patients in the TN-CKD state could not move directly to ESRD. We assumed that patients with such rapid progression would be a minimal fraction of the total ESRD population and would be detected regardless of eGFR reporting practices. (2) TP-CKD and FN-CKD patients could not return to the TN-CKD or FP-CKD states. Thus, acute kidney injury was not considered in our model because we assumed a nephrologist would follow the great majority of these patients regardless of eGFR reporting practices. (3) Patients could not return to TN-CKD, TP-CKD, FN-CKD, or FP-CKD after entering ESRD. (4) TP-CKD patients could not transition to FN-CKD. (5) Death was the only absorbing state.
On the basis of the sensitivity and specificity characteristics of eGFR and serum creatinine, some patients in each cycle moved from TN-CKD to FP-CKD. We assumed that the majority of actual patients falsely diagnosed with CKD would have further evaluation of their renal function with an outpatient evaluation, after which they would be correctly recognized as having no kidney disease. All FP-CKD patients incurred the one-time costs of diagnostic workup and returned to the TN-CKD state after one cycle. In summary, the model treats eGFR < 60 ml/min/m2 or an elevated serum creatinine as possible evidence of CKD; these patients are then assumed to undergo additional confirmatory evaluation by a nephrologist.
Transition Probabilities
Movement between states was dependent on incidence and prevalence of CKD, sensitivity and specificity of serum creatinine or eGFR to detect CKD, the probability of progressing from CKD to ESRD with and without optimal renal management, mortality without CKD, mortality for CKD patients with and without optimal renal management, and mortality with ESRD. Optimal renal management in this study included BP control and the use of angiotensin-converting enzyme inhibitors (18,20-22). Values and data sources are listed in Table 1.
Table 1.
Prevalence, incidence, mortality, progression to ESRD, effect of therapy, and test characteristics
| Probabilities | Age (yr) | Base Case (%/yr) | Range | Source |
|---|---|---|---|---|
| Annual incidence of CKD | 50-64 | 0.71 | Estimated from Coresh (1) | |
| 65+ | 2.29 | |||
| Annual mortality in the United States | 60 | 0.97 | Arias (23) | |
| 70 | 2.36 | |||
| Annual mortality of patients with ESRD | 62 | 14.9 | USRDS ADR (24) | |
| 67 | 18.9 | |||
| 74.5 | 26.0 | |||
| Prevalence of CKD at age 60 | 0.149 | 0.075 to 0.224 | Coresh (1) | |
| Annual mortality of patients with CKD | 0.050 | 0.0076 to 0.17 | Foley, Go, Gerstein, Jafar, Sarnak (3, 18, 19, 20, 21) | |
| Annual probability of progressing from CKD to ESRD | 0.076 | 0.023 to 0.160 | Foley, Jafar, Sarnak (19–21) |
| Effect of therapy on mortality and progression | |||
|---|---|---|---|
| Effect of therapy on annual mortality of CKD patients (CKD mortality coefficient) | 1 | 0.76 to 1.00 | Gerstein, Sarnak, Jafar (18, 20, 21) |
| Effect of therapy on annual progression from CKD to ESRD (CKD progression coefficient) | 0.728 | 0.637 to 0.819 | Jafar, Sarnak (20, 21) |
| Test characteristics of eGFR and serum creatinine | |||
|---|---|---|---|
| sensitivity of eGFR in detecting CKD | 0.924 | 0.919 to 0.928 | Rigalleau, Froissart (25, 26) |
| specificity of eGFR in detecting CKD | 0.835 | 0.784 to 0.885 | Rigalleau, Froissart (25, 26) |
| sensitivity of serum creatinine in detecting CKD | 0.559 | 0.385 to 0.90 | Newman, Xia, Oddoze (27, 28, 29) |
| specificity of serum creatinine in detecting CKD | 0.950 | 0.90 to 0.975 | Newman, Xia, Oddoze (27, 28, 29) |
eGFR was derived using the abbreviated MDRD equation. ESRD, end stage renal disease (glomerular filtration rate < 15 ml/min/1.73 m2); CKD, chronic kidney disease, stages 3 to 4 (glomerular filtration rate 15 to 59 ml/min/1.73 m2); USRDS ADR, United States Renal Data Service, Annual Data Report.
Prevalence and Incidence
Age-specific prevalence of CKD (stages 3 to 4) was estimated from National Health and Nutrition Examination Survey (NHANES) (1). Discrete values for these prevalence estimates presented in the published report only in graphical form were obtained from the authors (1). (Personal communication: Dr. Josef Coresh). We estimated age-specific incidence from NHANES data based on differences in prevalence across age groups.
Mortality, Morbidity and Effects of Therapy
Age-specific mortality in U.S. patients without CKD was estimated from United States Life Tables (23). We estimated annual mortality in CKD patients from published studies (3,18–21). Stage-specific mortality was published in Go et al. (3), so for CKD patients in our study, a weighted average was calculated to represent mortality in stages 3 to 4
The effect of therapy on mortality was represented by a factor (CKD mortality coefficient) by which annual CKD mortality was multiplied (< 1 = protective, 1 = no effect, > 1 = harmful). We found conflicting data regarding the effect of appropriate medical treatment on mortality in CKD patients (18,20,21). Given these data, we used 1 as the CKD mortality coefficient, meaning therapy had no effect on mortality, but we examined lower values in a sensitivity analysis.
The effect of therapy on CKD progression to ESRD was represented by a CKD progression coefficient by which annual probability of progressing from CKD to ESRD was multiplied (20,21). Age-specific mortality in ESRD was estimated from United States Renal Data System (USRDS) Annual Data Report (ADR) data (24).
Sensitivity and Specificity of Serum Creatinine Versus eGFR
We searched for published studies that estimated the sensitivity and specificity of eGFR and/or serum creatinine in the general population with reference to mGFR (such as evaluated by iothalamate clearance) and that defined CKD as a GFR < 60 ml/min/1.73 m2. Currently available studies, however, used a variety of eGFR, serum creatinine, and mGFR thresholds to define the testing characteristics of eGFR and serum creatinine. In addition, some of the studies were not performed exclusively in the general population.
On the basis of studies that examined the four-variable MDRD equation, we selected a mean eGFR sensitivity of 0.924 and specificity of 0.835 as base-case values for our model (25,26). These values were used in conjunction with other variables to transition patients into the four health states of TN-CKD, FN-CKD, TP-CKD, and FP-CKD.
We selected a mean sensitivity of 0.559 and specificity of 0.950 of serum creatinine for our base-case model using data from studies that compared serum creatinine alone to mGFR (27–29). These studies used cut-offs for abnormal serum creatinine that ranged from 1.06 to 1.36 mg/dl and mGFR thresholds to define CKD that ranged from 60 to 72 ml/min and ml/min/1.73 m2.
Utilities
Values for health utilities are presented in Table 2. A maximum utility of 1 was allocated to patients in the state of TN-CKD, and a minimum utility of 0 was reserved for death; CKD was assigned an intermediate value based on a study of CKD patients (30). We assigned identical QALYs to the states of FN-CKD and TP-CKD. The health state of FP-CKD was given a base case utility of 1. For ESRD, we used a mean QALY value based on 62 quality-of-life estimates in different ESRD populations (31). Health utilities were discounted at an annual rate of 3%.
Table 2.
Utilities by health state in quality-adjusted life years (QALY)
| Health state | Utilities (QALY)
|
Source | ||
|---|---|---|---|---|
| Base Case | Range
|
|||
| Lower | Upper | |||
| TN-CKD | 1 | 1 | 1 | |
| CKD (FN-CKD and TP-CKD) | 0.79 | 0.71 | 0.87 | TTO, HUI (30) |
| ESRD | 0.64 | 0.14 | 0.9 | All values from Tengs (31) |
| FP-CKD | 1 | 0.95 | 1 | Estimate |
TN-CKD, no chronic kidney disease stages 3 to 4 (glomerular filtration rate ≥ 60 ml/min/1.73 m2); CKD, chronic kidney disease stages 3 to 4 (glomerular filtration rate 15 to 59 ml/min/1.73 m2); FN-CKD, undetected chronic kidney disease stages 3 to 4; TP-CKD, detected chronic kidney disease stages 3 to 4; ESRD, end stage renal disease (glomerular filtration rate < 15 ml/min/1.73 m2); FP-CKD, false-positive chronic kidney disease due to false positive test result; TTO, time trade-off; HUI-3, Health Utilities Index.
Costs
Medical costs are presented in Table 3. Annual medical costs were estimated for the health states of TN-CKD, FN-CKD, TP-CKD, and ESRD using the USRDS ADR (24) and data from a case-control study by Smith et al. (32).
Table 3.
Total annual medical costs by health state and for a one-time nephrology evaluation
| Description | Cost ($)
|
Source | ||
|---|---|---|---|---|
| Base Case | Range
|
|||
| Lower | Upper | |||
| Single nephrology evaluation for CKDa | 266 | 133 | 399 | Based on CMS laboratory fee schedule (33) |
| Annual medical costs for patients with CKD (FN-CKD or TP-CKD), ages 65+ | 20,784 | 15,588 | 25,980 | USRDS ADR, 2007 (24) |
| Annual medical costs for patients without CKD stages 3-4 (TN-CKD), ages 65+ | 11,760 | 8,820 | 14,700 | Smith, USRDS ADR, 2007 (32, 24) |
| Annual medical costs for patients with ESRD, ages 65+ | 68,808 | 51,606 | 86,010 | USRDS ADR, 2007 (24) |
| One-time death cost | 37,611 | 28,208 | 47,014 | Hoover (34) |
Evaluation consists of new patient visit (CPT code 99203, outpatient visit of new patient with presenting problem of moderate severity), renal metabolic panel (CPT 80069), complete blood count (CPT 85025), urinalysis (CPT 81001), limited retroperitoneal ultrasound with image documentation (CPT 76775), urine protein (CPT 84156), urine creatinine (CPT 82570), and repeat patient visit (CPT 99213). CKD, chronic kidney disease, stages 3 to 4 (glomerular filtration rate 15 to 59 ml/min/1.73 m2); FN-CKD, false negative state for patient with chronic kidney disease, stages 3 to 4; TP-CKD, true positive state for patient with chronic kidney disease, stages 3 to 4; USRDS ADR, United States Renal Data System, Annual Data Report; TN-CKD, true negative state for patient without chronic kidney disease (glomerular filtration rate ≥ 60 ml/min/1.73 m2); ESRD, end stage renal disease (glomerular filtration rate < 15 ml/min/1.73 m2); CMS; Center for Medicare and Medicaid Services.
There are no USRDS ADR cost data for a healthy, control population (which corresponds to TN-CKD in our model). Annual health costs for TN-CKD patients were, therefore, estimated using a ratio derived from an article by Smith et al. that examined a population of 27,998 patients with and without CKD enrolled in the Kaiser Permanente health plan (32). The CKD patients in this study were a mixture of those with CKD that was recognized (TP-CKD) and unrecognized (FN-CKD) by their physicians (personal communication with Dr. Smith). We multiplied the ratio of medical costs for healthy controls to those for CKD patients in stages 3 to 4 from Smith et al. by the costs documented in the ADR for CKD patients as follows:
Annual health costs in TN-CKD=(Annual health costs of controls from Smith/Annual health costs of CKD patients stage 3 to 4 from Smith) × (Annual health costs in CKD patients from ADR)
No data were found that distinguished between health costs in recognized versus unrecognized CKD; therefore, the states of TP-CKD and FN-CKD were assigned identical costs.
Annual health costs for ESRD were estimated for patients over age 65 on the basis of costs and point prevalence counts of ESRD documented in the ADR by age category (24).
All individuals who transitioned to the states of TP-CKD or FP-CKD were assigned the one-time cost of an outpatient nephrology evaluation. We conferred with a group of academic nephrologists at our institution to define a set of tests that would likely be performed for newly diagnosed CKD. This consisted of a new patient visit, a repeat patient visit, a renal metabolic panel, complete blood count, urinalysis, urine protein, urine creatinine, and a limited retroperitoneal ultrasound (33). The state of FP-CKD was assigned annual health costs equaling those of TN-CKD plus the additional cost of the one-time evaluation.
A one-time cost was assigned when patients transitioned to death representing the average incremental costs of care during the final year of life (34). All costs were discounted at an annual rate of 3% and adjusted to 2005 dollars.
Statistical Analyses
A Markov model was created using Pro Suite 2008 (TreeAge; Williamstown, MA). The initial distribution of the population was based on the prevalence of CKD stages 3 to 4 (1). Thereafter, the incidence of CKD determined transitions from TN-CKD/FP-CKD to CKD. When multiple studies offered different values for estimates in the model, we performed sensitivity analyses over the range of published values (Tables 1 to 3). CKD prevalence at age 60 was varied by 50%; estimated incidence of CKD was varied from the estimated incidence at age 50 to age 65+. Testing costs were varied by 50% above and below the base case value. All other costs were varied by 25%.
Ten Monte Carlo microsimulations of 10,000 hypothetical patients were performed using the base-case values to estimate the absolute number of transitions between health states. In addition, a Monte Carlo probabilistic sensitivity analysis (second order uncertainty analysis) using 1000 trials was performed. This probabilistic sensitivity analysis incorporated normal distributions for all variables except QALY of FP-CKD, CKD mortality coefficient, QALY adjustment for ESRD, and specificity of serum creatinine, for which custom distributions based on normal distribution, but that did not extend to values greater than 1, were used.
Results
As shown by base-case values over an 18-yr period, the eGFR strategy was dominant (more effective and less costly). The total cost per patient was $185,933 for the eGFR strategy, with an effect of 11.100 QALYs/patient and a cost-effectiveness ratio of $16,751 per QALY. The strategy of serum creatinine alone was associated with a slightly higher total cost of $186,088 and a lower effect of 11.090 QALYs/patient, yielding a cost-effectiveness ratio of $16,779 per QALY (Table 4).
Table 4.
Cost, effect, cost/effect and incremental cost-effectiveness ratios per person generated after 18 one-year cyclesa
| Base CareValues and Variables | Sensitivity Analysis Boundary | Reporting Strategy | Cost ($) | Effect (QALY) | Cost/ Effect ($/QALY) | Descriptive Outcome | ICER ($/QALY)b | See Figure |
|---|---|---|---|---|---|---|---|---|
| Base case values | SCr | 186,088 | 11.090 | 16,779 | eGFR more effective and less costly (dominant) | NA | ||
| eGFR | 185,933 | 11.100 | 16,751 | |||||
| QALY of FP-CKD | Lower | SCr | 186,088 | 11.068 | 16,813 | SCr more cost- effective | 4,376 | 3a |
| eGFR | 185,933 | 11.032 | 16,853 | |||||
| Upper | SCr | 186,088 | 11.090 | 16,779 | eGFR more effective and less costly (dominant) | NA | ||
| eGFR | 185,933 | 11.100 | 16,751 | |||||
| Annual probability of progressing from CKD to ESRD | Lower | SCr | 177,752 | 11.276 | 15,764 | SCr more cost- effective | 20,289 | 3b |
| eGFR | 177,824 | 11.279 | 15,765 | |||||
| Upper | SCr | 194,479 | 10.891 | 17,857 | eGFR more effective and less costly (dominant) | NA | ||
| eGFR | 194,142 | 10.905 | 17,802 | |||||
| Sensitivity of SCr | Lower | SCr | 186,422 | 11.082 | 16,822 | eGFR more effective and less costly (dominant) | NA | 3c |
| eGFR | 185,933 | 11.100 | 16,751 | |||||
| Upper | SCr | 185,725 | 11.099 | 16,733 | SCr more cost-effective | 433,324 | ||
| eGFR | 185,933 | 11.100 | 16,751 |
QALY, quality adjusted life year; ICER, incremental cost-effectiveness ratio; SCr, serum creatinine; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease, stages 3 to 4 (glomerular filtration rate 15 to 59 ml/min/1.73 m2); FP-CKD, false-positive state for patient without chronic kidney disease, stages 3 to 4; ESRD, end stage renal disease (glomerular filtration rate < 15 ml/min/1.73 m2).
Results calculated using base case values and the upper and lower boundary values used in one-way sensitivity analyses.
ICER results as generated by TreeAge, and differ slightly from ICER values calculated from table due to differing numbers of significant digits.
Examination of effect alone over the 18-yr period revealed that 105 patients would be required to have an eGFR annually to achieve a net increase of one QALY. The eGFR strategy was also dominant when absolute years of life (instead of QALYs) were used as the outcome, with a mean effect of 11.596 yr for eGFR reporting versus 11.586 yr for reporting serum creatinine alone.
The average of 10 Monte Carlo microsimulations of 10,000 patients over 18 yr revealed that 13 fewer deaths and 29 fewer transitions to ESRD occurred with eGFR reporting. Notably, eGFR reporting generated 11,348 more FP-CKD cases compared with serum creatinine alone and resulted in 2072 fewer patient-years spent with undetected CKD (FN-CKD) (Table 5).
Table 5.
Mean results of 10 Monte Carlo microsimulations
| Patient Outcomes | Estimated Glomerular Filtration Rate (eGFR) Reporting | Serum Creatinine Reporting | Mean Difference Using eGFR Reporting |
|---|---|---|---|
| Deaths | 4,627 | 4,640 | −13 |
| Transitions from CKD (FN-CKD or TP-CKD) to ESRD | 1,259 | 1,288 | −29 |
| Number of FP-CKD categorizations | 17,015 | 5,667 | 11,348 |
| Patient-years spent in FN-CKD | 253 | 2,325 | −2,072 |
| Patient-years spent in TP-CKD | 22,196 | 19,842 | 2,354 |
Each microsimulation was composed of 10,000 trials of 18 one-year cycles. Serum creatinine and eGFR measurements occurred once per cycle. CKD, chronic kidney disease, stages 3 to 4 (glomerular filtration rate 15 to 59 ml/min/1.73 m2); FN-CKD, false negative state for patient with chronic kidney disease, stages 3 to 4; TP-CKD, true positive state for patient with chronic kidney disease, stages 3 to 4; ESRD, end stage renal disease (glomerular filtration rate < 15 ml/min/1.73 m2); FP-CKD, false-positive state for patient with chronic kidney disease, stages 3 to 4.
Sensitivity Analyses
One-way sensitivity analyses identified three variables that had an important impact on the model output. When effectiveness alone was the outcome, the only variable that rendered the eGFR strategy inferior to serum creatinine was the QALY assigned to FP-CKD. For the sensitivity analyses of the other two variables, eGFR was consistently more effective, but was less cost-effective within the ranges examined (Figures 2 and 3).
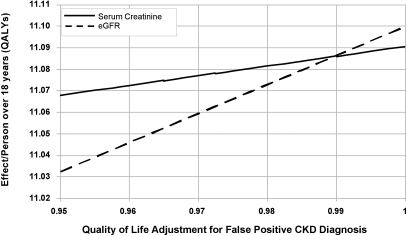
Figure 2.
One-way sensitivity analysis of quality of life assigned to the state of false positive chronic kidney disease (FP-CKD). Estimated GFR and serum creatinine reporting strategies are compared with effectiveness (QALYs) as the measured outcome.
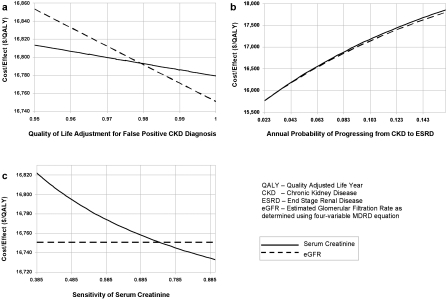
Figure 3.
One-way sensitivity analyses of three variables. (a) Quality of life adjustment for patients with false positive diagnosis of CKD. (b) Annual probability of progressing from CKD to ESRD. (c) Sensitivity of serum creatinine to identify CKD. Estimated GFR and serum creatinine reporting strategies are compared with cost-effectiveness (dollars/quality adjusted life year) as the measured outcome. Base case results: eGFR $16,751/QALY, serum creatinine $16,779/QALY; eGFR dominates. CKD, chronic kidney disease stages 3 to 4 (GFR 15 to 59 ml/min/1.73 m2); ESRD, end stage renal disease (GFR < 15 ml/min/1.73 m2).
Assuming that the quality of life was diminished when a patient had a false positive test, we decreased the QALY for FP-CKD from 1 to 0.95. Below a QALY of 0.98, serum creatinine became the more cost-effective strategy. Using a quality of life for FP-CKD of 0.95, eGFR reporting yielded 0.035 fewer QALYs/person over 18 yr than serum creatinine reporting (Figure 2). Using this 5% reduction in the QALYs assigned to FP-CKD, the eGFR strategy was also less cost-effective than the serum creatinine strategy at $16,853 per QALY versus $16,813 per QALY, respectively, yielding an incremental cost-effectiveness ratio (ICER) of $4367 per QALY gained with serum creatinine reporting (Figure 3a).
One-way sensitivity analyses demonstrated that the cost-effectiveness of serum creatinine reporting became superior to eGFR reporting over plausible ranges of two variables: 1) annual probability of progressing from CKD to ESRD, and 2) sensitivity of serum creatinine to detect CKD (Figures 3b and 3c). At the lower bound of annual probability of progressing from CKD to ESRD, reporting serum creatinine was found to be more cost-effective than reporting eGFR with an ICER of $20,289 per QALY gained by eGFR reporting. At the upper bound of sensitivity of serum creatinine, reporting serum creatinine was more cost-effective than reporting eGFR with an ICER of $433,324 per QALY gained by eGFR reporting (Table 4).
Sensitivity analysis also showed that if the cost of patient work-up for a positive test was $652/person or more, reporting serum creatinine became the more cost-effective strategy. This cost was, however, outside of our predefined plausible range.
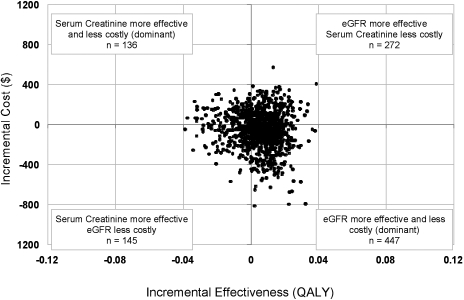
The distribution of cost-effectiveness ratios from a Monte Carlo probabilistic sensitivity analysis of 1000 trials revealed no clearly preferable strategy (Figure 4). eGFR reporting generated a mean cost-effectiveness of 17,796 $/QALY (±1267 $/QALY). Serum creatinine reporting generated mean cost-effectiveness of 17,809 $/QALY (±1273 $/QALY).
Figure 4.
Monte Carlo probabilistic analysis, results of 1000 trials plotting incremental cost versus incremental effect. eGFR reporting is compared with the baseline strategy of serum creatinine reporting. QALY, quality-adjusted life year
Discussion
The development of the GFR estimating equations and advocacy for early CKD diagnosis have led to a rapid shift in practice toward the automatic reporting of eGFR with serum creatinine. Although our analysis demonstrated that reporting eGFR is dominant compared with reporting serum creatinine alone under base-case assumptions, the difference in cost-effectiveness between the two strategies was modest and highly sensitive to any decrement in quality of life related to false positive diagnosis of CKD. In addition, sensitivity analyses revealed two other variables that render reporting serum creatinine the more cost-effective approach. These findings suggest that the policy of routine reporting of eGFR should be regarded with caution, particularly until the effect on quality of life and costs to FP-CKD patients are better understood.
eGFR reporting was the most effective and cost-effective strategy when analyzed using the separate outcomes of absolute life years, QALYs, and cost/QALY. The superiority of the eGFR reporting strategy results from the fact that eGFR identified more cases of CKD, and treatment of CKD led to fewer cases of ESRD. The limited magnitude of the effect seen with eGFR reporting is likely a result of the limited effects of therapy on CKD progression and mortality.
The value assigned to the false-positive diagnosis of CKD had a strong effect on the model outcomes. In one-way sensitivity analyses, eGFR reporting became less effective than serum creatinine when the QALY for FP-CKD was decreased by greater than 1%. Our Monte Carlo microsimulations also demonstrated the tradeoff between increased sensitivity and decreased specificity with eGFR reporting. In a hypothetical population of 10,000 individuals followed over 18 yr, there were, on average, 13 fewer deaths and 29 fewer cases of ESRD, but 11,348 more false positive diagnoses of CKD with eGFR reporting compared with serum creatinine alone. Furthermore, our Monte Carlo probabilistic sensitivity analysis, which considers jointly the uncertainties in our decision model, failed to indicate a clearly preferable strategy.
The range of possible harmful outcomes from false positive diagnosis of CKD includes health-related, economic, and psychologic consequences. Possible negative health consequences include unnecessary renal biopsies and medications such as angiotensin-converting enzyme inhibitors with their associated costs and potential adverse effects, which we did not include in our analysis. The economic burden of a false positive diagnosis with CKD also merits consideration. In this study, the financial burden of a positive test for CKD was incurred whether or not a patient had true disease and was, therefore, a greater source of increased cost in the eGFR reporting strategy as a result of the high number of FP-CKD patients. This diagnostic evaluation was limited to two visits with a nephrologist and limited tests that were estimated to cost $266. However, the cost of the clinical evaluation of a positive test for CKD was based on opinion and could be an underestimate (35). In addition, we did not include a one-time cost of software installation to allow eGFR reporting, because many institutions have already made this investment (12). Lastly, patients with a false positive diagnosis could suffer adverse outcomes such as anxiety, employment discrimination, and denial of health or life insurance (36–38).
Other factors besides the quality of life of FP-CKD patients proved influential in our model. Serum creatinine reporting was more cost-effective than eGFR reporting within the boundaries of two variables: the annual probability of progressing from CKD to ESRD, and the sensitivity of serum creatinine in identifying patients with CKD (Figure 3, b and c). Varying the annual probability of progressing from CKD to ESRD did not result in a large difference in cost-effectiveness between the two strategies, and both strategies became less cost-effective as the probability of progression to ESRD increased (Figure 3b).
As the sensitivity of serum creatinine was increased to 0.75 and higher, however, this strategy became substantially more cost-effective than eGFR reporting (Figure 3c). The influence of this variable on cost-effectiveness is highlighted because the sensitivity of serum creatinine for diagnosis of CKD in clinical practice is uncertain. In contrast to eGFR, which defines CKD using widely published numerical guidelines, (17) the value at which a serum creatinine is considered abnormal varies between physicians. For instance, a study by Mendelssohn showed that family physicians in Ontario generally did not refer patients with serum creatinine levels in the range of 1.4 to 1.7 mg/dl to a nephrologist (39), whereas the studies that we cited defined abnormal serum creatinine as those values higher than 1.06 to 1.36 mg/dl (27,28,29).
Our study has several limitations. Although published data were used to specify our model, precise estimates for some important variables were not available. For instance, limited data exist regarding health costs in CKD patients. The annual health costs of CKD were estimated using one retrospective study that did not distinguish between patients diagnosed with CKD and those who were undiagnosed (32). Thus, both TP-CKD and FN-CKD groups were assumed to have similar costs of care. The undiagnosed group may, in reality, have more unintended hospital admissions than the diagnosed group. However, the diagnosed group likely has more outpatient visits and medication prescriptions. Therefore, it is unclear whether one CKD group would have greater costs than the other, but a difference in costs could influence the outcomes of our model.
Our model examines the effects of eGFR reporting among the elderly, although the true prevalence of CKD in this population is not known. This uncertainty arises, in part, because declines in renal function among the elderly may represent “normal” senescence rather than a pathologic disease process. As a result, there has been substantial debate about the appropriate epidemiologic approach to measuring CKD in an elderly population (40,41). For instance, the estimate of CKD prevalence that we used for our hypothetical population of 60-yr-old individuals was taken from a study that relied on the MDRD equation to measure CKD in the general population (1). The MDRD equation, however, may lack precision in older individuals (9,42,43). We addressed this uncertainty about the prevalence of CKD by varying the estimate by ± 50% in our sensitivity analysis around the base-case value.
An additional limitation is that the quality of life of CKD patients was assumed to be the same in our model whether or not CKD was diagnosed. As discussed above with respect to patients falsely diagnosed with CKD, a “true positive” diagnosis of CKD could similarly lead to anxiety, employment discrimination, and denial of insurance. However, in patients with true disease, identification might also allow for treatments that improve quality of life, such as correction of anemia.
Conclusions
Automatic reporting of eGFR was the dominant strategy providing more QALYs with fewer costs, but this benefit was modest and was reversed with virtually any reduction in quality of life caused by the incorrect diagnosis of CKD. eGFR reporting also resulted in a far higher number of patients falsely diagnosed with CKD. Despite the widespread enthusiasm and increasing adoption of routine eGFR reporting, our study shows that clinicians and policy-makers should carefully examine the consequences of this practice. Additional studies are needed to quantify the impact of false diagnosis of CKD on quality of life, the probability of progressing from CKD to ESRD, and the sensitivity with which serum creatinine and eGFR reporting alone identifies patients with CKD. Until these data become available, the superiority of eGFR reporting compared with serum creatinine alone remains uncertain.
Conflict of interest and financial support.
Disclosures
Dr. den Hartog received support from the Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania. Dr. Reese received support from National Institutes of Health Grant K23-DK078688-01. Dr. Feldman received support from NIH K24-DK002651.
The study was exempt from the Institutional Review Board's approval because human subjects and personal data were not used in the study.
Acknowledgments
We thank Dr. Joseph Coresh for providing unpublished data relating to prevalence of CKD by age group (1). Preliminary data were presented at the American Society of Nephrology Renal Week, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, Collins AJ, Kusek JW, Levey AS, Greene T: Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 73: 1310–1315, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Duncan L, Heathcote J, Djurdjev O, Levin A: Screening for renal disease using serum creatinine: Who are we missing? Nephrol Dial Transplant 16: 1042–1046, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Swedko PJ, Clark HD, Paramsothy K, Akbari A: Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 163: 356–360, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Vervoort G, Willems HL, Wetzels JF: Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: Validity of a new (MDRD) prediction equation. Nephrol Dial Transplant 17: 1909–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS: Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43: 112–119, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Knight EL, Hogan ML, Singh AK: A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 14: 2573–2580, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Kidney disease mortality–Michigan, 1989-2005. MMWR Morb Mortal Wkly Rep 56: 225–227, 2007 [PubMed] [Google Scholar]
- 13.McDonough DP: New Jersey's experience: Mandatory estimated glomerular filtration rate reporting. Clin J Am Soc Nephrol 2: 1355–1359, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Glassock RJ, Winearls, C: Screening for CKD with eGFR: Doubts and dangers. Clin J Am Soc Nephrol 3: 1563–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnenberg FA, Beck JR: Markov models in medical decision making: a practical guide. Med Decis Making 13: 322–338, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Minino AM, Heron MP, Murphy SL, Kochanek KD: Deaths: final data for 2004. Natl Vital Stat Rep 55: 1–119, 2007 [PubMed] [Google Scholar]
- 17.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266, 2002 [PubMed] [Google Scholar]
- 18.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS: The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med 142: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study G: The Effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Arias E: United States life tables, 2002. Natl Vital Stat Rep 53: 1–38, 2004 [PubMed] [Google Scholar]
- 24.U.S. Renal Data System: USRDS 2007 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007
- 25.Rigalleau V, Lasseur C, Perlemoine C, Barthe N, Raffaitin C, Liu C, Chauveau P, Baillet-Blanco L, Beauvieux MC, Combe C, Gin H: Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or modification of Diet in Renal Disease study equation? Diabetes Care 28: 838–843, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP: Serum cystatin C measured by automated immunoassay: A more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47: 312–318, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Xia LH, Bing XG, An XT: Serum cystatin C assay for the detection of early renal impairment in diabetic patients. J Clin Lab Anal 18: 31–35, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oddoze C, Morange S, Portugal H, Berland Y, Dussol B: Cystatin C is not more sensitive than creatinine for detecting early renal impairment in patients with diabetes. Am J Kidney Dis 38: 310–316, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM: Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68: 2801–2808, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Tengs TO, Wallace A: One thousand health-related quality-of-life estimates. Med Care 38: 583–637, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB: Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol 15: 1300–1306, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Center for Medicare and Medicaid Services: Clinical Laboratory Fee Schedule and Physician Fee Schedule, Baltimore, Center for Medicare and Medicaid Services, 2007
- 34.Hoover DR, Crystal S, Kumar R, Sambamoorthi U, Cantor JC: Medical expenditures during the last year of life: Findings from the 1992-1996 Medicare current beneficiary survey. Health Serv Res 37: 1625–1642, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR: Screening for proteinuria in US adults: A cost-effectiveness analysis. JAMA 290: 3101–3114, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Brewer NT, Salz T, Lillie SE: Systematic review: The long-term effects of false-positive mammograms. Ann Intern Med 146: 502–510, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Golin CO, Johnson AM, Fick G, Gabow PA: Insurance for autosomal dominant polycystic kidney disease patients prior to end-stage renal disease. Am J Kidney Dis 27: 220–223, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Clase CM: Glomerular filtration rate: Screening cannot be recommended on the basis of current knowledge. BMJ 333: 1030–1031, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendelssohn DC, Kua BT, Singer PA: Referral for dialysis in Ontario. Arch Intern Med 155: 2473–2478, 1995 [PubMed] [Google Scholar]
- 40.Fliser D, Zeier M, Nowack R, Ritz E: Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 3: 1371–1377, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Fliser D, Franek E, Ritz E: Renal function in the elderly–Is the dogma of an inexorable decline of renal function correct? Nephrol Dial Transplant 12: 1553–1555, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Glassock RJ, Winearls C: An epidemic of chronic kidney disease: Fact or fiction? Nephrol Dial Transplant 23: 1117–1121, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Lamb EJ, Webb MC, Simpson DE, Coakley AJ, Newman DJ, O'Riordan SE: Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: is the modification of diet in renal disease formula an improvement? J Am Geriatr Soc 51: 1012–1017, 2003 [DOI] [PubMed] [Google Scholar]