Abstract
Background and objectives: Chronic kidney disease (CKD) has recently assumed epidemic proportion, becoming a troubling emerging cause of morbidity, especially if it progresses to terminal stage (ESRD). The authors aimed to evaluate whether neutrophil gelatinase-associated lipocalin (NGAL), a novel specific biomarker of acute kidney injury, could predict the progression of CKD.
Design, setting, participants, & measurements: Serum and urinary NGAL levels, together with a series of putative progression factors, were evaluated in a cohort of 96 patients (mean age: 57 ± 16 years) affected by nonterminal CKD (eGFR ≥15 ml/min/1.73 m2) of various etiology. Progression of CKD, assessed as doubling of baseline serum creatinine and/or onset of ESRD, was evaluated during follow-up.
Results: At baseline, both serum and urinary NGAL were inversely, independently, and closely related to eGFR. After a median follow-up of 18.5 mo (range 1.01 to 20), 31 patients (32%) reached the composite endpoint. At baseline, these patients were significantly older and showed increased serum creatinine, calcium-phosphate product, C-reactive protein, fibrinogen, daily proteinuria, and NGAL levels, whereas eGFR values were significantly lower. Univariate followed by multivariate Cox proportional hazard regression analysis showed that urinary NGAL and sNGAL predicted CKD progression independently of other potential confounders, including eGFR and age.
Conclusion: In patients with CKD, NGAL closely reflects the entity of renal impairment and represents a strong and independent risk marker for progression of CKD.
Whatever the primary disease process, the rate of decline of kidney function is recognized as strictly influenced by several secondary components. However, although hypertension, proteinuria, hyperlidemia, and inflammation represent some important modifiable risk factors, by themselves, these elements are not sufficient to properly explain renal outcomes in patients affected by chronic kidney disease (CKD) (1,2).
Recent observations have pointed out the crucial role of the renal tubule in the genesis and progression of CKD; independently of the primary disease and the eventual presence of superimposed damaging conditions, the pathogenic mechanisms causing progressive renal destruction converge on a common tubulo-interstitial pathway characterized by tubular atrophy and hypoxia, peritubular capillary injury, and interstitial fibrosis, ultimately explaining the irreversible evolution to terminal uremia (3). In accordance with this point of view, it is now widely accepted that in some CKD-associated diseases, such as diabetic nephropathy, the rate of deterioration in renal function, and the overall renal long-term outcome, are more accurately associated with the degree of renal tubulo-interstitial impairment than with the severity of glomerular lesions.
Indeed, several tubular proteins have been reported to be strictly involved in the experimental pathogenesis of tubular damage and its progression to terminal fibrosis, leading to uremia (4).
No less important, as described by several authors, many of these factors, such as the cellular carrier liver-type fatty acid binding protein (L-FABP), endothelin-1, β-2 microglobulin and N-acetyl-β-glucosaminidase (NAG) can acquire an important clinical impact if considered as predictors of severity and progression of specific CKD-related conditions (5–7).
In a recent study (8), we pointed out that subjects with membranous nephropathy and impaired renal function showed exaggeratedly increased baseline levels of neutrophil gelatinase-associated lipocalin (NGAL), a small 25-kD protein massively released from renal tubular cells after various injuring stimuli. Moreover, subjects with higher baseline NGAL showed a considerably increased risk of worsening residual renal function within 1 yr compared with those with lower baseline NGAL values. This attributed to NGAL an interesting predictive value, although confined to a small and pathologically homogeneous population of patients. Starting from these assumptions, the main aim of the present prospective study was, on the contrary, to examine the eventual predictive value of serum and urinary NGAL measurement for the progression of CKD in a wider cohort of patients with nonadvanced chronic kidney disease of various etiology.
Materials and Methods
Patients and Baseline Data
We examined 96 white European patients with various degrees of renal impairment referred to the CKD outpatient clinic of the Department of Internal Medicine of Messina University Hospital from January to March 2006. The study was approved by the local Ethic Committee, and all patients gave written informed consent.
Inclusion criteria were presence of CKD of stages 2 to 4 according to the National Kidney Foundation's classification and a stable renal function, defined as the absence of any transitory or permanent doubling in serum creatinine levels for at least 5 mo before starting the study.
To minimize potential confounding factors, patients with serum creatinine above 6 mg/dl and/or estimated glomerular filtration rate (GFR) <15 ml/min (National Kidney Foundation stage 5); malignancy; liver, thyroid, or infectious diseases; severe proteinuria (>3.5 g/d), inflammatory states; alterations in leukocyte count or formula; and treatment with steroids or immunosuppressors, were excluded from the study.
CKD was the consequence of biopsy-confirmed glomerulonephritis in 25 patients (26%), diabetic nephropathy in 19 (20%), autosomal polycystic kidney disease in 25 (26%), other types of kidney disease in 21 (22%), and unknown in the remaining six.
Patients history was carefully recorded by interview and confirmed by checking patients record, also recording drug prescription. Clinical examination, including assessment of BMI and blood glucose, was performed. BP was measured three times, and the average value was considered for data analysis.
Laboratory Measurements
Blood samples were taken in the morning before any food intake, and the second urine minction of the day was also collected. Common biochemical parameters, including urea, creatinine, uric acid, serum lipids and electrolytes, albumin, hemoglobin, proteinuria, fibrinogen, and C-reactive protein (CRP) were measured at baseline in all patients, according to standard methods in the routine clinical laboratory. eGFR was assessed using the Modification of Diet in Renal Disease formula, equation 7, derived by Levey et al. (9). Further blood samples were immediately placed into chilled vacutainer tubes containing potassium ethylenediamine tetracetate, and the plasma was promptly separated in a refrigerated centrifuge. The samples were then stored at −80°C until assayed. Ten milliliters of fresh urine was mixed with 1 ml of 10 mM Tris buffer, pH 8.6, with 0.05% Tween 20 and 0.01% NaN3 containing protease inhibitors (10 mM benzamidine, 10 mM aminocaproic acid, 20 mM ethylenediamine tetracetate, and aprotinin). This mixture was centrifuged at 3000 rpm for 8 min and stored at −80°C until assayed.
NGAL was measured in the blood and urine using a commercial available ELISA kit (Antibody Shop, Gentofte, Denmark), according to the manufacturer's instructions. All specimens were diluted often to obtain concentration for the optimal density according to the ELISA kit instruction. Coefficients of variation (and 95% confidence intervals [CIs]) for the serum and urine NGAL assays were 3.0% (1.2 to 4.0) and 2.1% (1.3 to 4.0), respectively, for intra-assay variation, and 8.2% (2.2 to 11.2) and 9.1% (6.8 to 18.1) for interassay variation. The enzymatic reactions were quantified in an automatic microplate photometer. All measurements were made in a triplicate and blinded manner. NGAL levels were expressed as nanograms per milliliter; NGAL was also measured in a small group of 14 healthy subjects with normal serum creatinine, well-matched with CKD patients for age, gender, and BP (see Table 1).
Table 1.
Baseline demographic, somatometric, clinical and laboratory data of the study population
| Parameter | Control Subjects | All Patients | Progressors | Nonprogressors | P |
|---|---|---|---|---|---|
| n:14 | n:96 | n:31 (32%) | n:65 (68%) | ||
| Gender, M/F | 7/7 | 48/48 | 15/16 | 33/32 | 0.54 |
| Age, yrs | 58 ± 13 | 57 ± 16 | 61 ± 16 | 56 ± 17 | 0.03 |
| BMI, kg/m2 | 26.9 ± 4.7 | 27.1 ± 5 | 26.2 ± 7.1 | 28.3 ± 6.5 | 0.45 |
| BP, mmHg | |||||
| systolic | 128 ± 16 | 130 ± 18 | 130 ± 7 | 130 ± 11 | 0.85 |
| diastolic | 75 ± 10 | 77 ± 9 | 78 ± 9 | 76 ± 8 | 0.32 |
| Diabetics, n, % | — | 21 (22%) | 8 (25%) | 13 (20%) | 0.10 |
| eGFR, ml/min/1.73 m2 | 111.6 ± 18.7 | 41.8 ± 19.1 | 34.3 ± 11.4 | 44.4 ± 13.6 | 0.02 |
| Serum creatinine, mg/dl | 0.9 ± 0.3 | 2.83 ± 1.37 | 3.19 ± 1.48 | 2.66 ± 1.28 | 0.03 |
| Albumin, g/dl | 4.02 ± 0.83 | 3.99 ± 0.64 | 4.11 ± 0.60 | 3.94 ± 0.65 | 0.56 |
| Calcium, mg/dl | 9.39 ± 1.3 | 9.04 ± 0.90 | 9.03 ± 1.09 | 9.04 ± 0.63 | 0.79 |
| Phosphate, mg/dl | 3.91 ± 0.92 | 4.26 ± 1.17 | 4.78 ± 1.26 | 4.15 ± 1.12 | 0.03 |
| Ca × P product, mg2/dl2 | 32.7 ± 3.15 | 38.9 ± 10.4 | 40.2 ± 11.6 | 37.4 ± 9.6 | 0.04 |
| Hemoglobin, g/dl | 14.9 ± 1.6 | 12.1 ± 1.8 | 12.0 ± 1.7 | 12.3 ± 1.8 | 0.77 |
| Cholesterol, mg/dl | 201.3 ± 32.5 | 187.1 ± 43.4 | 182.5 ± 30.1 | 189.6 ± 41.3 | 0.81 |
| Triglycerides, mg/dl | 97.5 ± 26.7 | 156.3 ± 71.6 | 151.8 ± 74.3 | 159.4 ± 71.1 | 0.11 |
| C-reactive protein, mg/L | 0.35 (0.09-0.54) | 7.2 (1.5-42.4) | 9.2 (1.8-45.1) | 6.6 (1.5-31.6) | 0.01 |
| Fibrinogen, mg/dl | 255.7 ± 58.5 | 339.5 ± 98.8 | 343.2 ± 95.2 | 321.1 ± 100.0 | 0.04 |
| Uric acid, mg/dl | 5.04 ± 0.68 | 6.74 ± 1.88 | 6.59 ± 1.92 | 6.82 ± 1.87 | 0.47 |
| Proteinuria, mg/24 h/1.73 m2 | 70.7 (10.3-91.8) | 450.6 (30.4-1150.0) | 520.8 (50.1-1230.2) | 393.1 (30.4-985.4) | 0.009 |
| Serum NGAL, ng/ml | 35.4 (18.9-46.5) | 515.4 (58.9-1405.5) | 906.8 (265.8-1460.0) | 420.3 (62.0-1118.5) | 0.002 |
| Urinary NGAL, ng/ml | 6.6 (2.1-9.6) | 195.6 (4.1-801.6) | 352.4 (6.8-823.1) | 125.6 (4.1-560.3) | 0.005 |
Prospective Follow-Up and Renal Outcome
After the baseline assessments, patients were followed prospectively until the end of the observation period or the primary study endpoint was reached. This latter was defined by the combined outcome of doubling of baseline serum creatinine, an accepted surrogate index of GFR slope (10), and/or the onset of end-stage renal disease (ESRD). Patients were personally contacted in case they missed any appointment and at the study end date, to avoid eventual loss during follow-up.
Statistical Analyses
Statistical analyses were performed with NCSS for Windows (version 4.0), the MedCalc (version 8.0) software, and the GraphPad Prism (version 4.0) package. Data were presented as mean ± SD, median (range), or percentage frequency, as appropriate. Differences between groups were established by unpaired t test for normally distributed values and by Kruskal-Wallis analysis followed by Dunn's test for nonparametric values. Dichotomized values were compared using the χ2 test. Pearson or Spearman correlation coefficients were used as appropriate to test correlations between eGFR and other variables. Before correlations were tested, all non-normally distributed values were log-transformed to better approximate normal distributions. Variables incorporated into the Modification of Diet in Renal Disease formula (9) were excluded from analysis. Receiver operating characteristics (ROC) analysis was used to calculate the area under the curve (AUC) for NGAL and eGFR and to find the best NGAL cut-off values for identifying the progression to renal endpoint. Kaplan-Meier curves were generated to assess renal survival in subjects with serum and urinary NGAL values above and below the optimal ROC-derived cut-off levels. Adjusted risk estimates for progression endpoint were calculated using univariate followed by multivariate Cox proportional hazard regression analysis. Exploratory graphical analysis and test of specific violations indicated no departure from the assumption of proportional hazards. All results were considered significant if P was <0.05.
Results
Patients Baseline Characteristics
The main baseline characteristics of the study cohort are summarized in Table 1. Mean age of patients was 57 ± 16 years, and exactly one half of them (n = 48) were male. Twenty-one patients (22%) had diabetes. Mean serum creatinine was 2.83 ± 1.37 mg/dl, with a mean estimated GFR of 41.8 ± 19.1 ml/min/1.73 m2 (range 16.2 to 80.5). Serum NGAL levels were significantly higher compared with those measured in healthy controls (515.4 [58.9–1405.5] versus 35.4 [18.9 to 46.5] ng/ml, P < 0.0001), as were uNGAL levels (195.6 [4.1 to 801.6] versus 6.6 [2.1 to 9.6] ng/ml, P < 0.0001).
Correlates of Estimated GFR
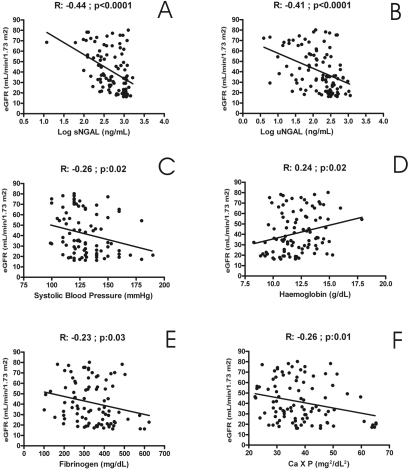
On univariate analysis, the estimated GFR was found to be directly correlated to hemoglobin (R = 0.24, P = 0.02), whereas inverse significant correlations were evidenced with fibrinogen (R = −0.23, P = 0.03), calcium-phosphate product (R = −0.26, P = 0.01), systolic blood pressure (R = −0.26, P = 0.02), and very closely with uNGAL (R = −0.41, P < 0.0001) and sNGAL (R = −0.44, P < 0.0001). Figure 1 provides a graphical resume of these findings. On the contrary, any significant correlation was described with other parameters, such as age, gender, BMI, cholesterol, triglycerides, CRP, or proteinuria (R ranging from 0.06 to 0.18, P > 0.06). Using eGFR as dependent variable in a multiple regression model including all previously reported univariate correlates, only the associations with serum NGAL (sNGAL) (β = −0.31, P = 0.005), urinary NGAL (uNGAL) (β = −0.27, P = 0.01) and fibrinogen (β = −0.22, P = 0.003) remained significant. Of note, this model explained about 34% of the total variance of eGFR. Table 2 provides a resume of these reports.
Figure 1.
Univariate baseline statistical correlations (Pearson coefficient) of estimated GFR (MDRD formula). Significant correlations was evidenced with serum neutrophil gelatinase-associated lipocalin sNGAL (A), urinary NGAL (uNGAL) (B), systolic blood pressure (C), hemoglobin (D), fibrinogen (E), and calcium-phosphate product (F). sNGAL and uNGAL were found to be the best eGFR correlates among all these variables.
Table 2.
Univariate and multiple regression analysis of estimated GFR at baseline
| Variable | Partial R | β | P |
|---|---|---|---|
| Log sNGAL | −0.44 (P < 0.0001) | −0.31 | 0.005 |
| Log uNGAL | −0.41 (P < 0.0001) | −0.27 | 0.01 |
| Fibrinogen | −0.23 (P = 0.03) | −0.22 | 0.03 |
| Calcium phosphate product | −0.26 (P = 0.01) | −0.06 | 0.52 |
| Haemoglobin | 0.24 (P = 0.02) | 0.02 | 0.83 |
| Systolic blood pressure | −0.26 (P = 0.02) | −0.05 | 0.60 |
Multiple R = 0.58, R2 = 34%; P < 0.0001. β is the standardized coefficient of correlation. sNGAL, serum NGAL; uNGAL, urinary NGAL.
Progression Endpoint During the Follow-Up Period
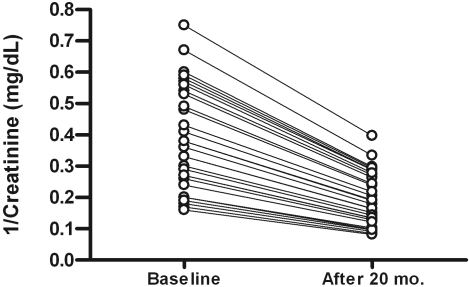
During the observational period (median follow-up of 18.5 mo; range 1.01 to 20), 31 patients (32%) reached the composite renal endpoint. In particular, 24 patients had doubled baseline creatinine levels, whereas seven patients experienced a severe worsening in renal function, which soon required dialytic treatment (Figure 2); these latter patients did not differ from other progressors with regard to main baseline clinical and laboratory parameters.
Figure 2.
Linear decline of 1/creatinine (mg/dl) in patients who have experienced a progression in CKD during the follow-up period.
None of the progressors experienced a regression of serum creatinine to baseline values during the observational period. This excluded the possibility that the appearance of acute kidney injury (AKI) was mistakenly interpreted as a CKD progression.
The remaining 65 patients (68%) who did not experience a progression in CKD completed the whole observational period (20 mo). At baseline, progressor subjects were significantly older and showed increased serum creatinine and serum phosphate levels, as well as calcium-phosphate product, C-reactive protein, fibrinogen and daily proteinuria. As expected, they also showed lower eGFR values. In contrast, no difference was noticed for gender, surrogate nutritional parameters (serum albumin and BMI), or serum lipids (triglycerides and cholesterol) and BP. Table 1 displays main data and statistical differences between patients with or without CKD progression during follow-up.
NGAL and Progression of CKD
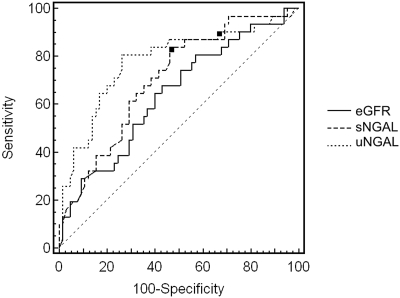
Progressor subjects presented significantly increased sNGAL and uNGAL values at baseline compared with nonprogressors. ROC analysis showed an AUC for eGFR, sNGAL, and uNGAL of 0.64 (95% CI, 0.53 to 0.73), 0.70 (95% CI, 0.60 to 0.79), and 0.78 (95% CI, 0.68 to 0.85) respectively. Both sNGAL and uNGAL areas were statistically different with respect to that of eGFR (P = 0.03). On the contrary, the difference between the two NGAL areas was nonsignificant (P = 0.25). For sNGAL the best cut-off level was found to be 435 ng/ml (sensitivity 83.9%, specificity 53.8%), whereas for uNGAL it was 231 ng/ml (sensitivity 80.6%, specificity 73.8%). Figure 3 shows reports from the ROC analysis.
Figure 3.
Receiver operating characteristics curves of eGFR, serum neutrophil gelatinase-associated lipocalin (sNGAL), and urinary NGAL (uNGAL) considering progression of CKD as status variable. The area under the curve for eGFR, sNGAL, and uNGAL was 0.64 (95% CI, 0.53 to 0.73), 0.70 (95% CI, 0.60 to 0.79), and 0.78 (95% CI, 0.68 to 0.85) respectively. Both sNGAL and uNGAL areas were significantly different than that of eGFR (P = 0.03). On the contrary, the difference between the two NGAL areas was nonsignificant (P = 0.25). Black squares represent the best cut-off values to predict the progression of CKD. For sNGAL this value was found to be 435 ng/ml, with a sensitivity of 83.9 (95% CI, 66.3 to 94.5) and a specificity of 53.8 (95% CI, 41.0 to 66.3), whereas for uNGAL it was 231 ng/ml with a sensitivity of 80.6 (95% CI, 62.5 to 92.5) and a specificity of 73.8 (95% CI, 61.5 to 84.0).
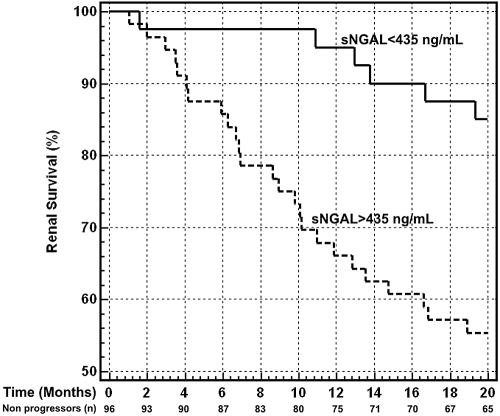
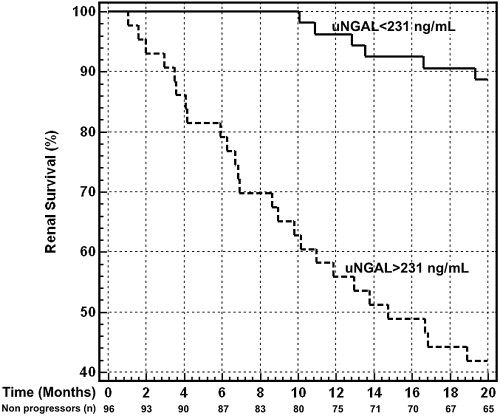
Kaplan-Meier survival curves in patients with sNGAL and uNGAL levels above and below the optimal cut-off are presented in Figure 4 and Figure 5. Subjects with sNGAL values above 435 ng/ml experienced a significantly faster evolution to endpoint (P = 0.002), with a mean follow-up time to progression of 14.9 mo (95% CI, 13.1 to 17.6) compared with 18.9 mo (95% CI, 17.8 to 19.6) for sNGAL below the cut-off. Similar but stronger reports were evidenced if subjects were categorized according to uNGAL values. Subjects with uNGAL values above 231 ng/ml showed a significantly faster progression to endpoint (P < 0.0001), with a mean follow-up time of 13.2 mo (95% CI, 11.9 to 15.9) compared with 19.2 mo (95% CI, 17.9 to 19.8) for uNGAL below the cut-off.
Figure 4.
Kaplan-Meier survival curves of renal end-point in patients with serum neutrophil gelatinase-associated lipocalin (sNGAL) levels above and below the optimal receiver operating characteristics cut-off level of 435 ng/ml. Patients with sNGAL >435 ng/ml showed a significantly faster progression to endpoint (P = 0.002, log-rank test), with a hazard ratio of 3.37 (95% CI, 1.86 to 7.62). The number of nonprogressor subjects still in the study at specific time points is reported.
Figure 5.
Kaplan-Meier survival curves of renal end-point in patients with urinary neutrophil gelatinase-associated lipocalin (uNGAL) levels above and below the optimal ROC cut-off level of 231 ng/ml. Patients with uNGAL >231 ng/ml showed a significantly faster progression to endpoint (P < 0.0001, log-rank test), with a hazard ratio of 7.45 (95% CI, 3.58 to 15.53). The number of nonprogressor subjects still in the study at specific time points is reported.
Using the median of sNGAL (195 ng/ml) and uNGAL (515 ng/ml) instead of best ROC-derived cut-off value, a similar trend in both survival curves was observed (data not shown).
Univariate/Multiple Cox Regression Analysis and Progression of CKD
To identify putative risk factors associated with progression of CKD, we performed a Cox regression analysis out, inserting in the model all variables that were different at baseline in patients who reached the end-point during the whole follow-up period (age, calcium-phosphate product, fibrinogen, CRP and proteinuria). Univariate analysis showed that only eGFR (HR 0.78; 95% CI, 0.63 to 0.96; P = 0.02), sNGAL (HR 1.02; 95% CI, 1.01 to 1.03; P = 0.0003), and uNGAL (HR 1.04; 95% CI, 1.02 to 1.05; P < 0.0001) were significantly associated with endpoint, whereas calcium-phosphate product, fibrinogen, CRP, proteinuria, and even age failed to reach statistical significance. Table 3 summarizes the unadjusted hazard ratios for the study outcome associated with various parameters taken into account.
Table 3.
Univariate Cox proportional hazards regression model for progression of CKD
| Variable | Units of Increase | HR | 95% CI | χ2 | P |
|---|---|---|---|---|---|
| Age | 1 yr | 1.01 | 0.99 to 1.03 | 1.23 | 0.12 |
| eGFR | 10 ml/min/1.73 m2 | 0.78 | 0.63 to 0.96 | 2.27 | 0.02 |
| Ca X P | 1 mg2/dl2 | 1.02 | 0.99 to 1.06 | 1.54 | 0.12 |
| Fibrinogen | 10 mg/dl | 1.04 | 0.99 to 1.05 | 0.27 | 0.78 |
| CRP | 1 mg/L | 0.99 | 0.95 to 1.03 | 0.16 | 0.86 |
| uNGAL | 10 ng/ml | 1.04 | 1.02 to 1.05 | 5.20 | <0.0001 |
| sNGAL | 10 ng/ml | 1.02 | 1.01 to 1.03 | 3.61 | 0.0003 |
| Proteinuria | 100 mg/24 h/1.73 m2 | 1.03 | 0.99 to 1.04 | 0.85 | 0.39 |
HR, hazard ratio; CI, confidence interval; Ca X P, calcium phosphate product; CRP, C-reactive protein; sNGAL, serum NGAL; uNGAL, urinary NGAL.
A multiple Cox regression was constructed, simultaneously inserting into the model all of the variables found to be significantly associated with endpoint at univariate analysis (sNGAL, uNGAL, and eGFR). In addition, age was also inserted in this model as it commonly represents one of the most important risk factors for CKD progression, although in this population it was not found to be statistically associated with renal endpoint.
Results from this analysis indicated that both uNGAL and sNGAL predicted higher risk of CKD progression independently of eGFR and age. In detail, the increase of 10 ng/ml of uNGAL was associated with a 3% increased risk of progression (HR 1.03; 95% CI, 1.02 to 1.04; P = 0.0005), whereas the increase of 10 ng/ml of sNGAL increased this risk by 2% (HR 1.02; 95% CI, 1.01 to 1.03; P = 0.04).
Results remained substantially unchanged if CKD progression was defined as doubling of creatinine only and the seven patients who started dialytic treatment were censored. Table 4 summarizes data from multivariate Cox analysis.
Table 4.
Multivariate Cox proportional hazards regression model for progression of CKD
| Variable | Units of Increase | HR | 95% CI | χ2 | P |
|---|---|---|---|---|---|
| Age | 1 yr | 1.00 | 0.98–1.02 | 0.71 | 0.47 |
| eGFR | 10 ml/min/1.73 m2 | 0.97 | 0.94–0.99 | 1.07 | 0.05 |
| uNGAL | 10 ng/ml | 1.03 | 1.02–1.04 | 3.47 | 0.0005 |
| sNGAL | 10 ng/ml | 1.02 | 1.01–1.03 | 1.82 | 0.04 |
HR, hazard ratio; CI, confidence interval; sNGAL, serum NGAL; uNGAL, urinary NGAL.
Discussion
Findings from the present study clearly indicate that NGAL represents a novel risk marker of CKD progression. If predictive value of baseline eGFR confirms the general suggestion that an already impaired renal function is an important factor for the subsequent progression of renal damage, remarkably, both urinary and sNGAL showed a most impressive predictive power in such a contest even after adjustment for eGFR. This suggests that NGAL would not be a simple surrogate index of baseline eGFR, but a marker on its own, predicting CKD progression beyond the information provided by GFR estimation.
In recent years, CKD has become a severe public health problem. The 2007 Annual Data Report of the U.S. Renal Data System estimated a dramatically increasing overall prevalence of CKD (11), especially for earlier stages (12). Furthermore, CKD ranks today as an independent risk condition for cardiovascular disease; this risk increases in parallel with the worsening of renal function and is maximum in the terminal stage (ESRD) (13). For these reasons, the identification of new environmental, genetic, and biologic factors involved in CKD progression represents an intriguing but difficult challenge, becoming imperative as it is widely realized that traditionally considered risk factors alone, such as hypertension or proteinuria, are no longer sufficient to fully explain and predict different evolutions of this disease.
Recently, several other modifiable and nonmodifiable factors were investigated as putative risk conditions of CKD progression. For example, independent studies correlated male gender to a significantly faster progression to ESRD (14), whereas this association was debated by other authors because it seemed to be strongly confounded by other factors (15). Higher serum phosphate and calcium-phosphate product, unlike serum calcium, were associated with an increased independent risk of progressive CKD (16), as well as several circulating factors such as fibroblast growth factor 23, apolipoprotein-A4, asymmetric dimethylarginine, and B-type natriuretic peptide (17–20).
In recent years, however, different studies have underlined the crucial role played by the renal tubule in the genesis of progressive acute and chronic kidney disease and its evolution to terminal stage (3).
The search for early, specific substances able to reveal the onset of acute kidney injury has uncovered NGAL as one of the most promising biomarkers in the future of clinical nephrology (21).
This small 25-kD protein, belonging to the “lipocalins” superfamily, is massively released in blood and urine from injured tubular cells after various conditions potentially detrimental to the kidney in experimental and human clinical models. No less important, NGAL release from renal tubule occurs soon after damage, notably preceding the rise in serum creatinine and thus allowing the initiation of preventive therapeutic measures in a timely manner.
On the basis of these unique properties, recent works have validated the reliability of NGAL as a specific, sensible, and early predictor of AKI after cardiac surgery, contrast administration, septic shock, and even renal transplantation (22–26).
In our study, NGAL was measured in a cohort of patients affected by nonadvanced CKD with stable renal function. Interestingly, apart from the already cited predictive value, a strict, independent, and inverse correlation with estimated GFR was described for both sNGAL and uNGAL, suggesting that under these particular conditions this protein may also represent a surrogate index of residual renal function, similar to what has previously been described elsewhere (27–29).
Recently, Mori and Nakao (30) proposed an interesting theory which might explain the relationship between NGAL and GFR, suggesting that the increase in NGAL is not just the passive consequence of a reduced renal clearance. This hypothesis, called the “forest fire theory, ” assumes that the increase in NGAL in chronic kidney disease (“forest fire”) is the consequence of a sustained production by “inflamed” but vital tubular cells, whereas the rise in serum creatinine and the contraction of GFR are the mere passive result of a general loss of functional cells or nephrons. From this point of view, NGAL would represent a real-time indicator of how much active kidney damage exists within the overall condition of chronic renal impairment.
Conclusions
We have clearly demonstrated that in subjects affected by nonterminal CKD, NGAL represents a novel, independent renal predictor of CKD progression that also provides a good reflection of the severity of renal disease. However, the present study has some limitations that should be mentioned.
First, it was a single-center study, and the cohort of patients was relatively small. These limitations did not allow us, for example, to evaluate the influence of different primary diseases on CKD progression and the eventual relationship with corresponding NGAL levels. Confirmation in wider cohorts is indispensable to attribute general validity to our reports. Second, although the enrolled patients showed miscellaneous primary diseases causing CKD, the resulting percentage distribution of renal pathology was not representative of a typical CKD population.
Finally, the duration of follow-up time was chosen to be relatively brief on purpose. Notwithstanding, the primary endpoint was reached by one third of the participants, and the statistical model was powerful enough to establish independent relationships between NGAL and progression of CKD. Further in-depth examinations should be undertaken to verify whether these findings can be confirmed in a longer observational period and to determine whether therapeutic measures targeting NGAL balance would be helpful in delaying the progression of CKD.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, de Jong P, de Zeeuw D, Remuzzi G, Kamper AL, Levey AS: Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol 18: 1959–1965, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Eddy AA, Neilson EG. Chronic kidney disease progression: J Am Soc Nephrol 17: 2964–2966, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Phillips AO: The role of renal proximal tubular cells in diabetic nephropathy. Curr Diab Rep 3: 491–496, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bruzzi I, Remuzzi G, Benigni A: Endothelin: A mediator of renal disease progression. J Nephrol 10: 179–183, 1997 [PubMed] [Google Scholar]
- 6.Hofstra JM, Deegens JK, Willems HL, Wetzels JF: Beta-2-microglobulin is superior to N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic membranous nephropathy. Nephrol Dial Transplant 23: 2546–2551, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Kimura K: Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: A multicenter trial. J Lab Clin Med 145: 125–133, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bolignano D, Coppolino G, Lacquaniti A, Nicocia G, Buemi M: Pathological and prognostic value of urinary neutrophil gelatinase-associated lipocalin (NGAL) in macroproteinuric patients with worsening renal function. Kidney Blood Press Res 31: 274–279, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Rossing P: Doubling of serum creatinine: Is it sensitive and relevant? Nephrol Dial Transplant 13: 244–246, 1998 [DOI] [PubMed] [Google Scholar]
- 11.United States Renal Data System: Annual Report of the US Renal Data System 2007. National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2007
- 12.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Zoccali C: The burden of cardiovascular disease in patients with chronic kidney disease and in end-stage renal disease. Contrib Nephrol 161: 63–67, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329. 2000 [DOI] [PubMed] [Google Scholar]
- 15.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, Marcantoni C, Becker G, Shahinfar S, De Jong PE, De Zeeuw D, Kamper AL, Strangaard S, Levey AS: The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP: Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1: 825–831, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F; MMKD Study Group, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P: Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Boes E, Fliser D, Ritz E, König P, Lhotta K, Mann JF, Müller GA, Neyer U, Riegel W, Riegler P, Kronenberg F: Apolipoprotein A-IV predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease Study. J Am Soc Nephrol 17: 528–536, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C: Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: A competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, Ritz E: Asymmetric dimethylarginine and progression of chronic kidney disease: The Mild to Moderate Kidney Disease Study. J Am Soc Nephrol 16: 2456–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bolignano D, Donato V, Coppolino G, Campo C, Buemi A, Lacquaniti A, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis 52: 595–609, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P: NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 22: 2089–2095, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q: Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 108: c176–c181, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR: Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 36: 1297–1303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P: Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6: 1639–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant 23: 414–416, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M: Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol 27: 373–378, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Bolignano D, Lacquaniti A, Coppolino G, Campo S, Arena A, Buemi M: Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res 31: 255–258, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Mori K, Nakao K: Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int 71: 967–970, 2007 [DOI] [PubMed] [Google Scholar]