Abstract
Background and objectives: The existence and prevalence of cerebral salt wasting (CSW) or the preferred term, renal salt wasting (RSW), and its differentiation from syndrome of inappropriate antidiuretic hormone (SIADH) have been controversial. This controversy stems from overlapping clinical and laboratory findings and an inability to assess the volume status of these patients. The authors report another case of RSW without clinical cerebral disease and contrast it to SIADH.
Design, setting, participants, & measurements: Three patients with hyponatremia, hypouricemia, increased fractional excretion (FE) of urate, urine sodium >20 mmol/L, and concentrated urines were infused with isotonic saline after collection of baseline data.
Results: One patient with RSW had pneumonia without cerebral disease and showed increased plasma aldosterone and FEphosphate, and two patients with SIADH had increased blood volume, low plasma renin and aldosterone, and normal FEphosphate. The patient with RSW responded to isotonic saline by excretion of dilute urines, prompt correction of hyponatremia, and normal water loading test after volume repletion. Hypouricemia and increased FEurate persisted after correction of hyponatremia. Two patients with SIADH failed to dilute their urines and remained hyponatremic during 48 and 110 h of saline infusion.
Conclusions: The authors demonstrate appropriate stimulation of ADH in RSW. Differences in plasma renin and aldosterone levels and FEphosphate can differentiate RSW from SIADH, as will persistent hypouricemia and increased FEurate after correction of hyponatremia in RSW. FEphosphate was the only contrasting variable at baseline. The authors suggest an approach to treat the hyponatremic patient meeting criteria for SIADH and RSW and changing CSW to the more appropriate term, RSW.
The differentiation of SIADH from cerebral salt wasting syndrome (CSW) or the preferred term, renal salt wasting (RSW), represents one of the diagnostic conundrums that includes the fundamental question of the existence and prevalence of RSW (1–4). This conundrum exists because of multiple overlapping clinical associations and laboratory abnormalities that characterize both syndromes and the dearth of specific parameters that distinguish one syndrome from the other (1–4). The increasing acceptance of RSW as a clinical entity among internists and the realization that RSW might be more common than previously acknowledged collectively intensify the need to differentiate one syndrome from the other (1,3,4–6). The divergent therapeutic goals make this differentiation even more important: to fluid-restrict in SIADH or provide salt and water supplementation in RSW.
In general, neurosurgeons believe RSW to be more common than SIADH, whereas internists believe RSW to be rare. RSW is best defined as extracellular volume (ECV) depletion caused by a tubular defect in sodium transport with or without hyponatremia or urinary sodium (UNa) > 20 mmol/L. Unfortunately, the clinical assessment of ECV, the only differentiating variable on first encounter, is fraught with errors unless invasive procedures, such as determination of blood volume by radioisotope dilution methodology, pulmonary wedge, or central venous pressures are used (1–4). The determination of ECV by tenuous clinical criteria in the original description of RSW may have contributed to the belief that this was a case of SIADH and not RSW (7). On the basis of the definition of RSW and the difficulty of clinically assessing ECV, the best method of estimating the prevalence of RSW is to determine ECV by invasive methods. Only three reports involving more than a single patient meet these criteria, and in these studies, 67% to 100% of hyponatremic neurosurgical patients were found to be hypovolemic with high UNa, suggesting that RSW is not rare but is more common than SIADH in neurosurgical patients (8–10). Moreover, UNa, plasma renin, aldosterone, and atrial/brain natriuretic peptide; serum and urinary urate excretion rates; determination of hemoglobin, hematocrit, serum total protein, and albumin; and clinical assessment of ECV are inconsistent, inaccurate, or not available on first encounter.
To bring clarity to a perplexing clinical controversy, we unequivocally described RSW in a patient without clinical cerebral disease. We demonstrated the value of determining serum urate levels in hyponatremia and fortified our contention that the persistence of hypouricemia and elevated fractional excretion (FE) of urate after correction of hyponatremia differentiate RSW from SIADH, since the hypouricemia and elevated FEurate in SIADH normalize after correction of the hyponatremia (1,5,11–18). Differences in urate metabolism, however, are evident only after correction of the hyponatremia and would not contribute to differentiating SIADH from RSW on first encounter.
We describe three patients with hyponatremia with coexistent hypouricemia and increased FEurate, one with RSW without cerebral disease and the other two as SIADH with cerebral/neurologic disease. Both groups responded differently to saline infusions and in the case of RSW, there was persistence of hypouricemia and increased FEurate after correction of hyponatremia
Materials and Methods
This study was approved by the Institutional Review Board in accordance with the Declaration of Helsinki. Every patient signed an informed consent. Baseline studies included serum sodium, potassium, chloride, carbon dioxide, blood urea nitrogen (BUN), creatinine, urate, phosphate, calcium, glucose and osmolality, plasma renin and aldosterone, routine urinalysis and urine osmolality (Uosm), creatinine, sodium, potassium, urate, and phosphate while on unrestricted food intake. The morning serum cortisol, TSH, and T4 were normal in all three patients. Blood volume was obtained in two subjects at baseline, using 51Cr-labeled red blood cells and 125I serum albumin by standard dilution techniques. All three patients received isotonic saline at 50 to 75 ml/h while on unrestricted food and fluid intake. Serum electrolytes, BUN, creatinine, urate, and phosphate were determined concomitantly with urine osmolality, sodium, potassium, creatinine, urate, and phosphate at regular intervals during the study.
Results
Patient 1-RSW, An 80-yr-old male, was admitted with a 3-day history of sore throat, cough productive of white phlegm, malaise, fever of 102°F and pneumonia on chest x-ray. On physical examination he had a pulse of 100 bpm, temperature 98.9°F, and BP 124/76 mmHg. He had rales in the right lower lung fields and was cognitively intact, with a normal neurologic examination. The serum creatinine was 1.0 mg/dl, BUN 14 mg/dl, serum sodium 125 mmol/L, potassium 4.3 mmol/L, urate 2.5 mg/dl, phosphate 3.5 mg/dl, osmolality 263 mosm/kg, UNa 39 mmol/L, and Uosm 386 mosm/kg (Table 1). The patient initially received a rapid intravenous infusion of 500 ml isotonic saline followed by a constant infusion at 75 ml/h for 4.5 h, in addition to 250 ml of saline containing doxycycline before being switched to oral Ofloxacin. He received a total of 1340 ml of saline as serum sodium increased from 125 to 132 mmol/L and the Uosm decreased from 386 to 284 mosm/kg. On the basis of pneumonia, hyponatremia, UNa of 39 mmol/L, and Uosm of 386 mosm/kg, the diagnosis of SIADH was made by an internist and the patient was fluid restricted to <750 ml/d for the next 22 h, at the end of which time there was no evidence of postural hypotension. At the end of the period of fluid restriction, the Uosm increased from 284 to 570 mosm/kg and UNa to 60 mmol/L. At this time, the supine plasma renin was 1.63 ng/ml/h (normal, 0.15 to 2.33 ng/ml/h), aldosterone 26.2 ng/ml (normal, 1 to 16 ng/ml), FENa 0.30%, FEurate 37.9% (normal 5 to 10%), and FEphosphate 23.1% (normal <20%).
Table 1.
Summary of baseline data and after correction of hyponatremia in patient 1 with RSW and baseline data in patients 2 and 3 with SIADH
| Serum
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Na, mmol/L | K, mmol/L | Cl, mmol/L | CO2, mmol/L | BUN, mg/d | Creatinine, mg/d | Uric Acid, mg/d | Phosphate, mg/d | |
| Patient 1 | 125 | 4.8 | 89 | 22 | 14 | 1 | 2.5 | 3.4 |
| Correction | 138 | 1.5 | ||||||
| Patient 2 | 126 | 3.7 | 97 | 25 | 11 | 0.7 | 1.6 | 2.7 |
| Patient 3 | 131 | 3.8 | 97 | 28 | 14 | 0.6 | 1.8 | 3.5 |
| Plasma | ||
|---|---|---|
| Renin, ng/ml/h | Aldosterone, ng/dl | |
| Patient 1 | 1.63 | 26.2 |
| Patient 2 | <0.15 | <1.6 |
| Patient 3 | 0.3 | 4.3 |
| Urine
|
|||||||
|---|---|---|---|---|---|---|---|
| Osmolality, mosm/kg | Na, mmol/L | K, mmol/L | FENa, % | FEK, % | FEUA, % | FEPi, % | |
| Patient 1 | 386 | 39 | 39 | 0.31 | 8 | 37.9 | 23.1 |
| Correction | 24.6 | ||||||
| Patient 2 | 534 | 161 | 28 | 1.85 | 10.3 | 21.1 | 7.4 |
| Patient 3 | 303 | 99 | 30 | 1.48 | 15.8 | 16.7 | 10.5 |
Note persistence of hypouricemia and increased FEurate after correction of hyponatremia and elevated plasma renin and aldosterone levels and increased FEphosphate in patient 1 with RSW as compared to low plasma renin and aldosterone levels and normal FEphosphate in patients 2 and 3 with SIADH.
Saline Infusion
Isotonic saline was infused at 50 ml/h for 36 h. The creatinine clearance was 94 ml/min, and 24 h urate excretion was 664 mg. His medications included Ofloxacin 400 mg orally twice daily and Colace 100 mg.
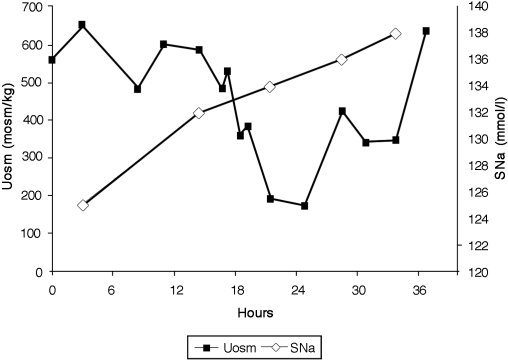
As noted in Figure 1, Uosm gradually decreased to a nadir of 178 mosm/kg at 25 h and rose overnight to 640 mosm/kg by midmorning the following day. The serum sodium increased from 132 to 138 mmol/L, urate decreased from 2.5 to 1.5 mg/dl, and FEurate remained elevated at 24.6% when his serum sodium was 138 mmol/L. His weight increased from 164 lb to 168 lbs.
Figure 1.
Graph demonstrating serum sodium concentrations in mmol/L and urine osmolalities in mosm/kg during infusion of 0.9% saline at 50 ml/h over 36 h. Note dilution of urine at 24 h of the study and prompt correction of hyponatremia 36 h after initiation of saline therapy.
Water Loading Test
After correction of hyponatremia, a water loading test was performed. The patient received 20 ml/kg BW or 1500 ml of water by mouth within 30 min. His baseline Uosm and Posm were 570 and 272 mosm/kg, respectively. The Uosm reached a nadir of 131 mosm/kg at 2.5 h, and he excreted 1240 ml or 82.7% of the ingested water after 4 h (normal >80%, Table 2). The normal water loading test confirms normal ADH response after volume repletion.
Table 2.
Water loading test in patient 1 after ECV repletion
| Time, min | Urine Volume, ml | Uosm, mosm/kg water | Posm, mosm/kg water | Cosm, mosm/kg water | CH2O, ml/min | TCH2O, ml/min |
|---|---|---|---|---|---|---|
| 0 | 570 | 272 | ||||
| 45 | 75 | 323 | 1.98 | — | 0.31 | |
| 90 | 350 | 150 | 4.29 | 3.49 | ||
| 150 | 450 | 131 | 3.61 | 3.89 | ||
| 210 | 145 | 179 | 1.59 | 0.83 | ||
| 240 | 75 | 192 | 1.78 | 0.72 |
The patient ingested 20 ml/kg water, 1500 ml, in 30 min. He excreted 1240 ml of urine in 4 h or 82.7% of the 1500 ml water ingested at time 0, normal >80%. Note: the lowest Uosm of 131 mosm/kg at 150 min is higher than would be expected in a normal subject and is consistent with a free-water clearing defect that is characteristic of RSW.
Patient 2-SIADH, a 76-yr-old male with hypertension, coronary artery disease, peripheral vascular disease, and resection of a glioblastoma followed by radiation therapy 7 mo before admission, was readmitted with aphasia and headache. He had a recurrence of the glioblastoma, which was surgically resected. His baseline serum sodium was 122 mmol/L, potassium 3.8 mmol/L, CO2 25 mmol/L, BUN 11 mg/dl, creatinine 0.7 mg/dl, urate 1.6 mg/dl, phosphate 2.7 mg/dl, osmolality 251 mosm/kg, plasma renin <0.15 ng/ml/h, aldosterone <1.6 ng/ml, UNa 161 mmol/L, UK 28 mmol/L, Uosm 534 mosm/kg, FEurate 21.1%, and FEphosphate 10.4%. The red cell volume was 24 ml/kg (normal 22 to 30 ml/kg), plasma volume was 55.3 ml/kg (normal 30 to 45 ml/kg), and blood volume was 79.3 ml/kg (normal 52 to 75 ml/kg), consistent with SIADH.
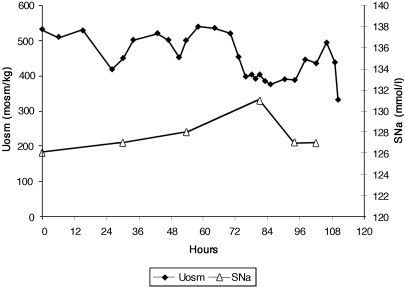
Saline was infused at a rate of 75 ml/h for 110 h. Uosm remained hypertonic and FEurate was not determined because the hyponatremia never corrected (Figure 2).
Figure 2.
Graph demonstrating serum sodium concentrations in mmol/L and urine osmolalities in mosm/kg during infusion of 0.9% saline at 75 ml/h over 110 h. Note failure of urine osmolality to dilute during the course of the study and failure of serum sodium to correct during the entire study.
Patient 3-SIADH, a 72-yr-old female, presented with a 20-yr history of hypertension and 3-yr history of weakness and hyponatremia after a “bad flu.” She had autonomic dysfunction with a BP of 168/90 mm/Hg lying, and 150/90 and 130/90 mmHg standing for 1 and 2 min, respectively, with no change in pulse rates. Pertinent laboratory data included a serum sodium of 134 mmol/L, osmolality 265 mosm/kg, BUN 6 mg/dl, creatinine 0.6 mg/dl, urate 1.7 mg/dl and phosphate 3.8 mg/dl. The Uosm was 303 mosm/kg, UNa 99 mmol/L, FENa 1.48%, FEurate 16.7%, FEphosphate 10.8%, and FEK 15.8%. The supine plasma renin was 0.29 ng/ml/h and aldosterone 4.3 ng/dl (Table 1). Red cell volume was 23 ml/kg (normal, 22 to 30 ml/kg), plasma volume was 54 ml/kg (normal, 30 to 45 ml/kg), and blood volume was 77 ml/kg (normal, 52 to 75 ml/kg), consistent with SIADH. Isotonic saline was infused at a rate of 75 ml/h for 48 h. Serum sodium never exceeded 135 mmol/L and did not correct while the patients was in the hospital.
Discussion
The present report illustrates several important differentiating parameters that not only prove the existence of RSW but also emphasize differences between RSW and SIADH with regard to plasma renin and aldosterone levels, FEphosphate, and response to saline infusion (1,6,19,20). The normal renal, thyroid, and adrenal function; elevated plasma aldosterone; increased FEphosphate; persistent hypouricemia; increased FEurate after correction of hyponatremia; generation of dilute urines with prompt correction of hyponatremia in response to saline; and normal water loading test after volume repletion collectively support the diagnosis of RSW in patient 1.
Patient 1 has RSW, which is pathophysiologically different from SIADH. In SIADH, ADH levels are autonomously increased with respect to serum osmolality and ECV, hence the term “inappropriate secretion of ADH.” The elevated ADH levels increase water reabsorption, concentrating the urine and preventing excretion of ingested water. This inappropriate water retention leads to increasing hyponatremia. There is an initial salt-wasting phase when sodium excretion exceeds sodium intake, which transitions into a steady-state phase when sodium and water intake match sodium and water excretion, or vasopressin escape that is in part due to a decrease in aquaporin 2 and urea transport UT-A3 (21,22). UNa can be <20 mmol/L if sodium intake is low (23). Uosm is always increased above plasma osmolality, although 2 L of isotonic saline infused at 16 ml/min has been shown to dilute the urine to 151 mosm/kg by unknown mechanisms (24). As seen in patients 2 and 3, the inappropriate water retention increased ECV and decreased plasma renin and aldosterone (20). Fluid restriction of 750 to 1000 ml per day is the recommended treatment for SIADH, although Schwartz et al. increased serum sodium from 109 to 135 mmol/L over an 8-day balance study, with daily fluid and sodium intake averaging 2648 ml and 314.5 mmol, respectively (24). Thus, serum sodium can increase at high fluid intake as long as sodium intake is high and the intake sodium concentration exceeds the excreted sodium concentration (24).
In contrast to SIADH, RSW evolves by physiologic mechanisms that normally occur with ECV depletion, which in the case of RSW results from a defect in renal sodium transport. At the onset of RSW, sodium output exceeds sodium intake to reduce ECV, which sets off a series of compensatory changes that include increased plasma ADH, renin, and aldosterone; decreased glomerular filtration rates; and activation of hemodynamic and neural adjustments that create a new steady-state condition for sodium and water balance at a lower ECV. ECV depletion leads to a reduction in effective arterial volume as pressure in the arterial system and contraction of the venous capacitance vessels lead to prerenal azotemia, a condition characterized by increased solute reabsorption, including urea, uric acid, and phosphorus, when renal function is intact (25). In RSW, however, the reduction in ECV is a consequence of defective sodium transport that is associated with defects in urate; urea; and, in this and some other cases, phosphate transport. Some of the characteristic findings of prerenal azotemia are therefore not applicable in RSW. Renin production in RSW can be variable, depending on salt intake despite ECV depletion, because the major defect in salt transport resides in the proximal tubule (15,26). Thus, the salt supply to the juxtaglomerular apparatus will reflect salt intake as compared with a decreased supply in a volume-depleted patient with normal kidneys (27). Alternatively, UNa can be <20 mmol/L if sodium intake is low (6). This was evident in our hip fracture hyponatremic patient without cerebral disease (6). She had a UNa of 6 mmol/L that was attributed to anorexia and reduced sodium intake while fluid restricted, resulting in an erroneous diagnosis of SIADH. The coexisting hypouricemia and increased FEurate, however, were inconsistent with a volume-depleted patient with normal kidneys, that is, prerenal azotemia, but consistent with SIADH and RSW. The diagnosis of RSW was based on a 7% reduction in blood volume; increased plasma renin, aldosterone and FEphosphate; undetectable levels of ADH resulting from elimination of the volume stimulus for ADH secretion by saline and inhibition by the coexisting plasma hypo-osmolality; generation of free water excretion; and prompt correction of hyponatremia (6).
The extent of the ECV depletion in RSW depends on the severity of the defect in tubular sodium transport that induces mild to severe ECV depletion, which sets off the compensatory changes noted above. Thus, fluid restriction in patients with severe RSW to treat an erroneous diagnosis of SIADH has been reported to lead to an array of clinical consequences of ECV depletion that include weakness, loss of appetite, severe postural hypotension, disturbances in gait and mentation, slurred speech, somnolence, syncope, and even aggravation of their underlying disease (6,12,28,29). These consequences of fluid restriction in severe RSW respond to saline infusions that eliminate the volume stimulus for ADH secretion and allow the coexistent hypo-osmolality to inhibit ADH secretion, induce free water excretion, and correct the hyponatremia. This sequence of physiologic events was clearly demonstrated in patient 1 and our hip fracture patient (Figure 1) (6).
Treatment of hyponatremic patients, who meet the criteria for SIADH and RSW is not well defined, largely because of overlapping clinical and laboratory findings and our inability to differentiate SIADH from RSW. There is uniform agreement that symptomatic hyponatremia should be treated with hypertonic saline, but treatment of the asymptomatic patient poses a therapeutic dilemma, to restrict water for SIADH or to administer isotonic saline in RSW. Depending on the severity of the defect in renal sodium transport, the patient with RSW can deteriorate clinically if fluid restricted for an erroneous diagnosis of SIADH. We are encountering with greater frequency clinical deterioration of patients with RSW who are fluid restricted for an erroneous diagnosis of SIADH with or without cerebral disease (6,12,29). This outcome may be more common in patients with intracranial diseases such as those with subarachnoid hemorrhage in whom fluid restriction increased morbidity and mortality, because RSW is more common than SIADH in these patients (28). Of the blood volume studies that have been reported in a group of hyponatremic neurosurgical patients, the UNa reported in two of three studies ranged from 23 to 203 mmol/L, and blood volume was decreased in 67% to100%, suggesting that RSW was more common than SIADH (8–10). Moreover, 10 patients with AIDS had hyponatremia, hypouricemia, elevated FEurate, elevated plasma renin and aldosterone levels, UNa >40 mmol/L, central venous pressures of 0 cmH2O, and postural hypotension that responded to saline (30). On the basis of these reports, the recent proposal to infuse hypertonic saline to hyponatremic neurosurgical patients has merit as a therapeutic first option (4). We should include hyponatremic patients with coexistent hypouricemia and FEurate >10% without cerebral disease to this list. Subsequent decisions to water restrict or administer isotonic saline depend on plasma renin and aldosterone levels and whether FEurate returns to normal or remains persistently elevated after correction of the hyponatremia. However, if the baseline FEphosphate is >20%, we advocate isotonic saline as the first therapeutic option, because increased FEphosphate is a feature of RSW and not SIADH. Regardless of the type of saline administered, serum sodium concentration should not increase by more than 10 mmol/L/d in chronic hyponatremia to minimize the development of osmotic demyelination syndrome. Fluid intake should also be monitored to avoid congestive heart failure. In the rare patient with SIADH or RSW, the desalinization syndrome can be observed when the administration of isotonic saline exacerbates the hyponatremia (31). This occurs when the total UNa and UK exceed 150 mmol/L, in which case hypertonic saline and water restriction should be instituted.
The present report does not resolve the conundrum of differentiating RSW from SIADH on first encounter, because the response to saline infusion, determination of plasma renin or aldosterone, or persistence of hypouricemia and elevated FEurate after correction of the hyponatremia are not available at this time. It does, however, strengthen our contention that RSW not only exists but occurs in the absence of clinical cerebral disease (1,6,11,12). It is ironic that patient 1 had RSW without cerebral disease and patient 2 had SIADH with cerebral disease, a diagnosis that would customarily be reversed when applying standard clinical practices. We have encountered several patients with RSW without clinical cerebral disease, including patient 1 in this report, hip fracture, bronchogenic carcinoma with normal CT scan of brain, uncomplicated Hodgkin's disease and metastatic pancreatic carcinoma (6,12). These cases led us to advocate replacing the outmoded term, CSW, with RSW (1,6,12).
The mechanisms by which hypouricemia and elevated FEurate develop in SIADH and RSW appear to be different and not well defined. In SIADH, the hypouricemia and increased FEurate are present only during hyponatremia and normalize after correction of the hyponatremia (16–18). The hypervolemia in SIADH is an unlikely cause as intense volume expansion with saline failed to attain the high FEurates seen in SIADH and RSW (32–34). This has been reviewed in our in-depth review of renal urate transport (11). The V1 receptor activity of ADH is untenable because ADH levels are still elevated when FEurate normalizes with the correction of hyponatremia (16,17,18,35) and induction of SIADH by dDAVP increased FEurate without V1 activity (36).
It would appear that multiple transport abnormalities involving the proximal tubule in RSW are reminiscent of the Fanconi syndrome. We have not investigated amino acid excretion rates, and we have never observed glucosuria in any of our patients. None of our patients appear to have acid base abnormalities, but amino acid and glucose transport or acid base disturbances have not been systematically investigated. Abnormalities in sodium and urate transport appear to be more common than that noted for phosphate transport, and azotemia is clearly not a feature of RSW. The BUN-to-creatinine ratios of 20.0 in our previous case and 14.0 in patient 1 with RSW and 15.7 and 23.3, respectively, in patients 2 and 3 with SIADH provide evidence against the proposal that an increase in the BUN-to-creatinine ratio distinguishes RSW from SIADH (3). The absence of a disproportionate rise in BUN over the rise in creatinine in RSW has been addressed by others (1,6,37). Volume depletion in the presence of normal kidney function would lead to prerenal azotemia with the disproportionate rise in BUN and urate, and decreased FEurate as part of the increase in proximal solute and water reabsorption (38). The increase in FEurate and hypouricemia in RSW and SIADH indicates an underlying proximal tubular transport defect that also affects urea reabsorption through an inability to generate a favorable concentration gradient for urea to be reabsorbed passively, contributing to the absence of azotemia (12). Determination of FEurate can, therefore, be a useful tool to assess proximal tubule function in various disease states, being low in prerenal azotemia with normal kidneys (38) and high in those with proximal tubule dysfunction such as RSW, or transiently high in SIADH. The administration of plasma from our patients with neurosurgical and Alzheimer's diseases who had hypouricemia, increased FEurate, and normal serum sodium as seen in RSW resulted in a 30% increase in FElithium and a significant increase in FENa (14,15,26). Because lithium is transported mainly in the proximal tubule on a one-to-one basis with sodium in the absence of osmotic diuretics (39,40), these studies suggest that a plasma natriuretic factor reduces proximal and possibly distal tubular sodium transport in RSW. It is also probable that the natriuretic factor inhibits the tubule transporters of urate, phosphate, and urea in addition to sodium.
In summary, we present data that distinguish SIADH from RSW by several distinctive differences. As noted in Table 1, both groups presented with hyponatremia, hypouricemia, increased FEurate, and concentrated urines with high UNa. Differences, however, can be noted by the increased aldosterone levels and persistence of hypouricemia and increased FEurate after correction of the hyponatremia in RSW as compared with SIADH. These differences, however, are not observable on first encounter with the patient, as we are confronted with the ever-present enigma of assessing the state of low ECV in RSW and high to high normal ECV in SIADH. One clue might be the presence of FEphosphate of >20% that, when present on first encounter, would lead one to favor the diagnosis of RSW and institute saline supplementation immediately (Table 1).
In the absence of an elevated FEphosphate, it might be prudent to use hypertonic saline as suggested, (4) and to await results of the renin and aldosterone determinations or persistence of an elevated FEurate after correction of hyponatremia to decide whether to water restrict or administer isotonic saline. As in any case of chronic hyponatremia, it is prudent to increase serum sodium by <10 mmol/L/24 h to prevent osmotic demyelination syndrome. Lastly, the absence of clinical cerebral disease should not eliminate RSW from consideration. We are encountering more patients with RSW without clinical cerebral disease, who are erroneously being treated for SIADH with harmful consequences. It is time to consider CSW an outmoded term that is confining, misleading, and inappropriate.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
S.B.'s current affiliation is Kaiser Permanente WLA Medical Center, Los Angeles, California.
References
- 1.Maesaka JK, Gupta S, Fishbane S: Cerebral salt wasting syndrome: Does it exist? Nephron 82: 100–109, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Oh MS, Carroll HS: Cerebral salt-wasting syndrome, we need better proof of its existence. Nephron 82: 110–114, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Palmer BF: Hyponatremia in a neurosurgical patient: Syndrome of inappropriate antidiuretic hormone secretion versus cerebral salt wasting. Nephrol Dial Transplant 15: 262–268, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Sterns RH, Silver SM: Cerebral salt wasting versus SIADH: What difference? J AM Soc Nephrol 19: 194–196, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Maesaka JK: An expanded view of SIADH, hyponatremia, and hypouricemia. Editorial. Clin Neph 46: 79–83, 1996 [PubMed] [Google Scholar]
- 6.Maesaka JK, Miyawaki N, Palaia T, Fishbane S, Durham J: Renal salt wasting without cerebral disease: Value of determining urate in hyponatremia. Kidney Int 71: 822–826, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Peters JP, Welt LG, Sims EA, Orloff J, Needham J: A salt-wasting syndrome associated with cerebral disease. Trans Assoc Am Physicians 63: 57–64, 1950 [PubMed] [Google Scholar]
- 8.Nelson PB, Seif SM, Maroon JC, Robinson AG: Hyponatremia in intracranial disease: Perhaps not the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J Neurosurg 55: 938–941, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J: Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol 18: 211–216, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Sivakumar V, Rajshekhar V, Chandy MJ: Management of neurosurgical patient with hyponatremia and natriuresis. Neurosurgery 43: 269–274, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Maesaka JK, Fishbane S: The regulation of renal urate excretion. A critical review. Am J Kid Dis 32: 917–933, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Maesaka JK, Batuman V, Yudd M, Salem M, Sved AF, Venkatesan J: Hyponatremia and hypouricemia: Differentiation from the syndrome of inappropriate secretion of antidiuretic hormone. Clin Nephrol 33: 174–178, 1990 [PubMed] [Google Scholar]
- 13.Maesaka JK, Cusano AJ, Thies HL, Siegal FP, Dreisbach AW: Hypouricemia in acquired immunodeficiency syndrome. Am J Kidney Dis 15: 252–257, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Maesaka JK, Venkatesan J, Piccione JM, Decker R, Dreisbach AW, Thies HL, Wetherington JD: Abnormal renal urate transport in patients with intracranial disease. Am J Kidney Dis 19: 10–15, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Maesaka JK, Wolf-Klein GP, Piccione JM, Ma C: Hypouricemia abnormal renal tubular urate transport and plasma natriuretic factors in patients with Alzheimer's disease. J Am Geriatr Soc 41: 501–506, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Beck LH: Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med 301: 528–530, 1979 [DOI] [PubMed] [Google Scholar]
- 17.Decaux G, Prospert F, Soupart A, Musch W: Evidence that chronicity of hyponatremia contributes to the high urate clearance observed in the syndrome of inappropriate secretion of antidiuretic hormone. Am J Kidney Dis 36: 745–751, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Dorhout Mees EJ, Blom van Assendelft P, Nieuwenhuis MG: Elevation of urate clearance caused by inappropriate antidiuretic hormone secretion. Acta Med 189: 69–72, 1971 [DOI] [PubMed] [Google Scholar]
- 19.Vogel JH: Aldosterone in the cerebral salt wasting. Circulation 127: 44–50, 1963 [DOI] [PubMed] [Google Scholar]
- 20.Fichman MP, Micheldakis AP, Horton R: Regulation of aldosterone in the syndrome of inappropriate antidiuretic hormone secretion (SIADH). J Clin Endocrinol Metab 39: 136–144, 1974 [DOI] [PubMed] [Google Scholar]
- 21.Jaenike JR, Waterhouse C: The renal response to sustained administration of vasopressin and water in man. J Clin Endocrinol Metab 21: 231–242, 1961 [DOI] [PubMed] [Google Scholar]
- 22.Hoorn EJ, Hoffert JD, Knepper MA: Combined proteomics and pathways analysis of collecting duct reveals a protein regulatory network activated in vasopressin escape. J Am Soc Nephrol 16: 2852–2863, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decaux G, Musch W: Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol ePress, April 23, 2008 [DOI] [PubMed]
- 24.Schwartz WB, Bennett W, Curelop S: A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med 23: 529–542, 1957 [DOI] [PubMed] [Google Scholar]
- 25.Abuelo JG: Normotensive ischemic acute renal failure. N Engl J Med 357: 797–805, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Maesaka JK, Venkatesan J, Piccione JM, Decker AW, Dreisbach A, Wetherington JD: Plasma natriuretic factor(s) in patients with intracranial disease, renal salt wasting and hyperuricosuria. Life Sci 52: 1875–1882, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Harris RC, Zhang MZ, Cheng HF: Cyclooxygenase-2 and the renin-angiotensin system. Acta Physiol Scand 181: 543–547, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Wijdicks EF, Vermeulen M, Hijdra A, van Gijn J: Hyponatremia and cerebral infarction in patients with ruptured intracranial aneurysm: Is fluid restriction harmful. Ann Neurol 17: 137–140, 1985 [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez OM, Lin HY: Refractory Hyponatremia. Kidney Int 71: 79–82, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Cusano AJ, Thies HL, Siegal FP, Dreisbach AW, Maesaka JK: Hyponatremia in patients with acquired immunodeficiency syndrome. J AIDS 3: 949–953, 1990 [PubMed] [Google Scholar]
- 31.Steele A, Gowrishankar M, Abrahamson S, Mazer CD, Feldman RD, Halperin ML: Postoperative hyponatremia despite near-isotonic saline infusion: A phenomenon of desalination. Ann Intern Med 126: 20–25, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Diamond H, Maisel A: Influence of volume expansion, serum sodium, and fractional excretion of sodium on urate excretion. Pflügers Arch 356: 47–57, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Cannon PJ, Svahn DS, Demartini FE: The influence of hypertonic saline infusions upon the fractional reabsorption of urate and other ions in normal and hypertensive man. Circulation 41: 97–108, 1970 [DOI] [PubMed] [Google Scholar]
- 34.Steele TH: Evidence for altered renal urate reabsorption during changes in volume of extracellular fluid. J Lab Clin Med 74: 288–299, 1969 [PubMed] [Google Scholar]
- 35.Decaux G, Namias B, Gulbis B: Soupart A: Evidence in hyponatremia related to inappropriate secretion of ADH that V1 receptor stimulation contributes to the increase in renal urate clearance. J Am Soc Nephrol 7: 805–810, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Boer WH, Koomans HA, Dorhout Mees EJ: Lithium clearance during the paradoxical natriuresis of hypotonic expansion in man. Kidney Int 32: 376–381, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Tanneau RS, Moal M-C, Rouhart F, Cueymes J-M, Bourbigot B: Hypouricemia with high urate clearance in hyponatremia: Is it always a clue for increased effective volemia?. Letter. Clin Nephrol 44: 128, 1995 [PubMed] [Google Scholar]
- 38.Steele TH, Oppenheimer S: Factors affecting urate excretion following diuretic administration in man. Am J Med 47: 564–574, 1969 [DOI] [PubMed] [Google Scholar]
- 39.Koumans HA, Boer WH, Dorhout Mees EJ: Evaluation of lithium clearance as a marker of proximal tubule sodium handling. Kidney Int 36: 2–12, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Leyssac PP, Holstein-Rathlou N-H, Skott P, Alfrey AC: A micropuncture study of proximal tubular transport of lithium during osmotic diuresis. Am Jour Physiol 258: F1090–F1095, 1990 [DOI] [PubMed] [Google Scholar]