Abstract
The ABCG2 transporter breast cancer resistance protein (BCRP) has been identified in several physiological sites. It has been suggested to play an important role in disposition of many drugs and environmental toxins. We investigated the effects of several antipsychotic drugs, including risperidone, 9-hydroxy-risperidone (paliperidone), olanzapine, quetiapine, clozapine, haloperidol and chlorpromazine, and a positive control inhibitor Ko143 on functions of BCRP in MCF7 and BCRP over-expressing MCF7/MX100 cell lines using a BCRP prototypical substrate mitoxantrone. Our findings indicated that the tested antipsychotics rank order of potency of inhibition of BCRP according to concentrations required to reach 50% of maximum inhibition (IC50) was as follows: Ko143 (0.07 μM) > risperidone (38.1 μM) > clozapine (42.0 μM) > paliperidone (51 μM) > chlorpromazine (52.2 μM) > quetiapine (66.1 μM) > olanzapine = haloperidol (>100.0 μM). We further tested the effects of various concentrations of risperidone on the BCRP-mediated transport of oestrone-3-sulfate in a colon carcinoma cell line, Caco-2, a widely used model to study drug absorption. Our findings show that risperidone at concentrations ranging from 1 to 100 μM significantly inhibited intracellular accumulation of oestrone-3-sulfate in Caco-2 cell monolayers. The present results suggest that a potential source of pharmacokinetic interactions exists between BCRP substrates and several antipsychotics.
The breast cancer resistance protein (BCRP, ABCG2) belongs to the energy-dependent adenosine triphosphate-binding cassette (ABC) transporter family. Among the ABC transporter proteins, BCRP is unique for its half-transporter property, which possesses six putative transmembrane domains, four potential glycosylation sites and one ATP-binding domain. Recent studies suggested that BCRP may function as a homodimer or tetramer with unknown partner(s) bridged by disulfide bonds [1,2].
The BCRP is expressed at very high levels in the apical surface of the placental syncytiotrophoblast, and to a lesser extent in liver canaliculi, colon and small intestinal mucosal surfaces, cardiac muscle, pancreas, adrenal cortex, thyroid, parathyroid, ovary, vein and capillary endothelia. BCRP has been confirmed to exert a great impact on drug absorption, distribution and excretion. The identified substrates of BCRP include irinotican [3], methotrexate [4], mitoxantrone [5], topotecan [6,7], sulfated oestrogens [8], SN-38 (a major active metabolite of irinotecan) [9], and porphyrins and porphyrin-like compounds [10]. Many studies have demonstrated that BCRP shares many similarities with another multidrug resistance transporter P-glycoprotein in terms of in vivo protective function, tissue distribution, and substrate and inhibitor specificity. It has been further demonstrated that these two transporters work in concert in limiting brain and placenta penetration of many dual substrates of these two transporters. Inhibition of the transporter activity by P-glycoprotein and/or BCRP inhibitors can significantly increase brain accumulation of substrates of the two transporters (e.g. topotecan and imatinib) [7,11].
The important role of BCRP in protecting the body from harmful substances has been further emphasized by the findings of its involvement in the transport of dietary carcinogens, such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and conjugated metabolites of benzo[a]pyrene [10–13]. In addition, a number of studies have consistently confirmed that modulation of BCRP function by BCRP modulators, such as fumitremorgin C, GF120918, pantoprazole and omeprazole, can result in significant drug–drug interactions or associated toxicities [3,7,14,15].
Antipsychotics, including risperidone, paliperidone (or 9-OH-RSP), olanzapine, quetiapine, clozapine, haloperidol and chlorpromazine, are the primary medications used for treatment of various psychotic and bipolar disorders. Despite the recognized importance of BCRP in drug disposition, the potential risks for its interaction with anti-psychotics have not been studied. We have previous studied the interaction potentials of the antipsychotics and P-glycoprotein transport activity [16,17]. In the present study, we assessed the inhibitory potential of risperidone, paliperidone, olanzapine, quetiapine, clozapine, haloperidol and chlorpromazine on BCRP-mediated transcellular transport of mitoxantrone and oestrone-3-sulfate using similar approaches with application of the MCF7, MCF7/MX100 and/or Caco-2 cell lines.
Materials and Methods
Materials
Risperidone, paliperidone and methyl-risperidone were obtained from Janssen Pharmaceutica (Titusville, NJ, USA). Olanzapine and quetiapine were gifts from Eli Lilly & Company (Indianapolis, IN, USA) and AstraZeneca Pharmaceuticals (Wilmington, DE, USA), respectively. Haloperidol, chlorpromazine hydrochloride, clozapine, mitoxantrone and oestrone-3-sulfate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ko143 was kindly provided by Dr. Alfred Shinkel (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Foetal bovine serum, trypsin and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Hyclone Co. (Logan, UT, USA). Modified improved minimum essential medium was obtained from Invitrogen (Carlsbad, CA, USA). Non-essential amino acid solution was obtained from Stemcell Technologies Inc. (Vancouver, British Columbia, Canada). Dulbecco’s phosphate-buffered saline, penicillin and streptomycin were purchased from Mediatech Inc. (Herndon, VA, USA). All other reagents were of the purest grade available.
Cell culture conditions
MCF7 and MCF7/MX100 cells
The wild-type breast carcinoma cell line MCF7 and the BCRP over-expressing drug resistance cell line MCF7/MX100 were kindly provided by Dr. Rob W. Robey from the National Cancer Institute (Bethesda, MD, USA). The MCF7 cells were cultured with improved minimum essential medium containing 4500 mg/l glucose, 4 mM L-glutamine and sodium pyruvate supplemented with 10% foetal bovine serum, 1% non-essential amino acids, 100 U/ml penicillin and 100 μg/ml streptomycin at 37° in an atmosphere of 5% CO2 and 95% relative humidity. The MCF7/MX100 cells were cultured under identical conditions with MCF7 except that the media containing 100 nM mitoxantrone to maintain BCRP expression [18]. Cells were supplemented with fresh media every 2–3 days. All cultured cells used in the experiment were between passages 5–25.
Caco-2 cells
A human colon carcinoma cell line, Caco-2 cell line (passage 17) was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Caco-2 cells were cultured under standard cell culture conditions with DMEM containing 4500 mg/l glucose, 4 mM L-glutamine and sodium pyruvate supplemented with 10% foetal bovine serum, 1% non-essential amino acids, 100 U/ml penicillin and 100 μg/ml streptomycin at 37° in an atmosphere of 5% CO2 and 95% relative humidity [19,20]. Cells were supplemented with fresh media every 2–3 days until reaching confluence. All cultured cells used in the experiment were between passages 20–40.
Flow cytometric assay of intracellular mitoxantrone accumulation
MCF7 and MCF7/MX100 cells were grown to 80–90% confluence and harvested with 0.25% trypsin/2.21 mM ethylenediaminetetraacetic acid in Hank’s buffered salt solution for drug accumulation experiment. The cells were suspended in DMEM containing 5 μM mitoxantrone and filtrated through 70 μm nylon mesh screen (Spectrum Laboratory Corp., New Brunswick, NJ, USA). The cell numbers were approximately 106 in each reaction in a final incubation volume of 0.5 ml. After adding various concentrations (0–100 μM) of the tested antipsychotic drugs or a positive control inhibitor Ko143 (0–10 μM), cells were incubated in incubation media with serum-free DMEM without antibiotic at 37° in an atmosphere of 5% CO2 and 95% relative humidity for 90 min. to equilibrate mitoxantrone accumulation into the cells (the time chosen for incubation was based on an examination of the linearity of the rate of mitoxantrone accumulation into the cells). The final concentration of the dimethyl sulfoxide (DMSO) for each reaction containing the tested inhibitors or their respective controls was 1% (v/v). No effect of the vehicle on mitoxantrone accumulation was observed at this concentration. All incubations were conducted in triplicate.
Intracellular fluorescence of mitoxantrone was analysed with a FACScan flow cytometer (Becton Dickinson Immune Cytometry Systems, Mountain View, CA, USA) equipped with an argon laser using a previous established method with minor modification [17]. In brief, intracellular fluorescence of 10,000 events was measured logarithmically through a 635-nm band-pass filter at an excitation wavelength of 488 nm [21]. Cell debris was eliminated by gating on forward versus side scatter. The blank histogram, cells in medium containing vehicle (1% DMSO), yielded negligible cellular autofluorescence. The MCF7 and MCF7/MX100 cells in medium containing mitoxantrone alone or Ko143 (1 μM), or various concentrations of the tested antipsychotics, generated the negative control, positive control and inhibitor histograms, respectively. The median fluorescence (ΔF) of the tested compounds was used to calculate the percentage of inhibitory effect (E%) of BCRP-mediated mitoxantrone intracellular accumulation using the following equation:
where ΔFi is the median fluorescence of tested inhibitor at concentration i, ΔF0 is the median fluorescence of tested inhibitor at concentration 0; ΔFmax is the median fluorescence with maximal inhibition of BCRP activity by 1 μM Ko143. The concentration of inhibitor sufficient to cause 50% of maximal increment of intracellular mitoxantrone fluorescence (IC50) values was estimated by fitting the mean of triplicate data to the sigmoidal dose–response equation using GraphPad 4.0 software (Intuitive Software for Science, San Diego, CA, USA).
Oestrone-3-sulfate uptake experiment in Caco-2 cells
Caco-2 cells were plated at a density of 5 × 104 cell/ml in 24-well cell culture plates (Costar Corp., Suwanee, GA, USA). Cells were supplemented with fresh media every 3–4 days until reaching confluence (visually inspected through microscope). All cultured Caco-2 cells used in the experiment were between passages 20–40. All experiments were performed in four replicates.
Ninety minutes before initiation of the experiment, the medium in the culture plates was replaced with serum-free DMEM. Oestrone-3-sulfate (100 μM) was added to the incubation media with various concentrations of risperidone (1–100 μM) and control vesicles containing 1% DMSO. After incubation for 30 min. (within this incubation time, the rate of intracellular accumulation of oestrone-3-sulfate versus time is linear), the culture medium was removed. Cells were then washed twice with ice-cold phosphate-buffered saline, followed by extraction twice with 1 ml methanol. The methanol solution was evaporated under slow nitrogen flow at 45°. The residues were reconstituted with 100 μl mobile phase (30% 0.1 M ammonium acetate: 70% methanol), and was assayed by high performance liquid chromatography for oestrone-3-sulfate [22]. The remaining cells were solubilized by 1 ml 0.5 M NaOH. The protein concentration in each cultured well was assayed [23] using Bio-Rad dye reagent (Richmond, CA, USA) with bovine serum albumin as the standard.
Statistical analysis
Data are presented as mean ± S.D. of three to four independent determinations. Effects of Ko143 on mitoxantrone accumulation in MCF7 and MCF7/MX100 cells were analysed with one-way ANOVA, using Turkey’s test for post hoc comparisons. Effects of putative inhibitors on intracellular mitoxantrone and oestrone-3-sulfate accumulation were analysed using unpaired t-test (two-tailed). P-values of <0.05 were considered statistically significant.
Results
Antipsychotic drugs inhibit cellular uptake of mitoxantrone in MCF7 and MCF7/MX100 cells
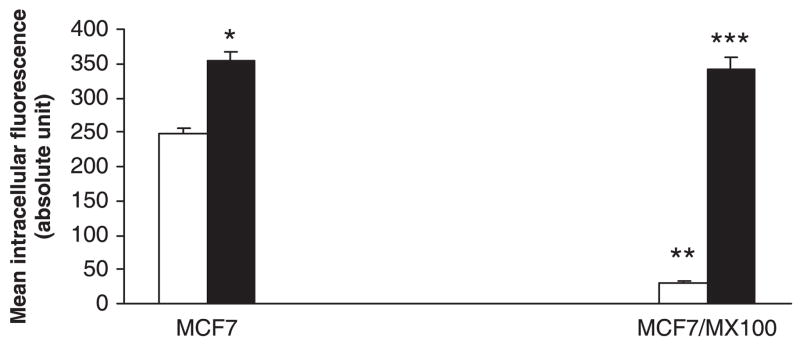
After incubation at 37° for 90 min., the intracellular fluorescence of mitoxantrone in the wild-type MCF7 cells was 8.1-fold higher than that in the BCRP over-expression cell line MCF7/MX100 (P < 0.001), indicating that BCRP was over-expressed in the MCF7/MX100 cells but not in the MCF7 cells (fig. 1). Treatment of the MCF7/MX100 cells with a positive control inhibitor of BCRP, Ko143 (0.01–1 μM) [7], significantly increased the intracellular fluorescence of mitoxantrone in the MCF7/MX100 cells (P < 0.001). While in the wild-type MCF7 cells, Ko143 increased the intracellular fluorescence of mitoxantrone to a significant (P < 0.05) but for a lesser degree compared to those in the MCF7/MX100 cells (fig. 1). There was no statistically significant difference between MCF7/MX100 cells and MCF7 cells regarding their intracellular fluorescence of mitoxantrone in the presence of Ko143 (1 μM) (P = 0.34).
Fig. 1.
Effect of Ko143 on intracellular accumulation of mitoxantrone in MCF7 and MCF7/MX100 cells. Bars represent mean ± S.D. of three determinations. **P < 0.001, compared to MCF7 cells. *P < 0.05 and ***P < 0.001, compared to those without 1 μM Ko143. Empty bars represent vesicle controls, solid bars represent treatment with Ko143.
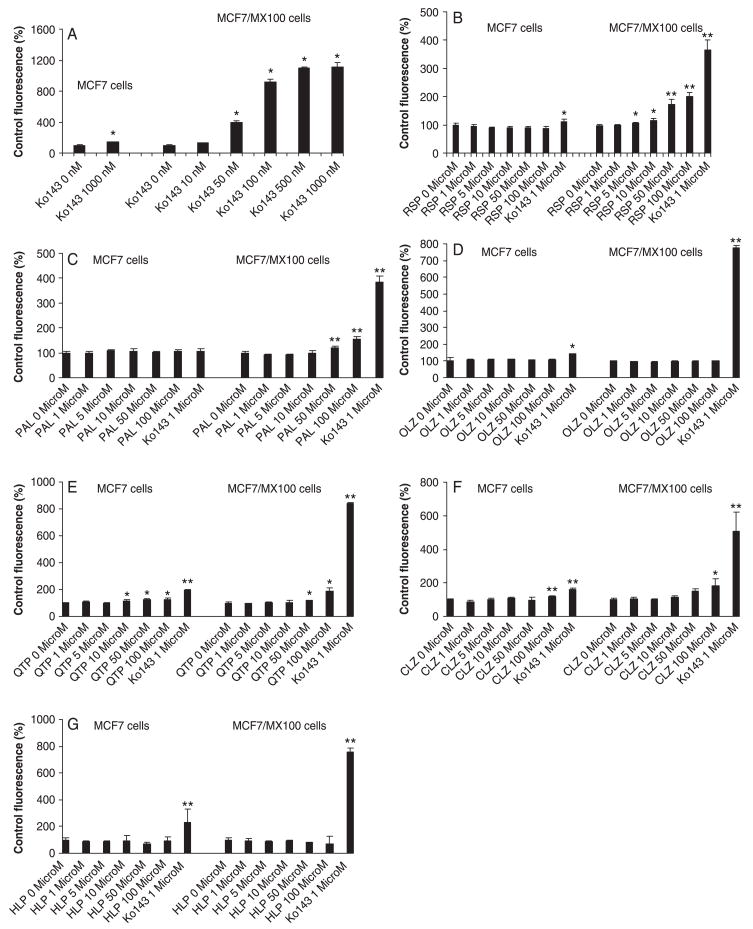
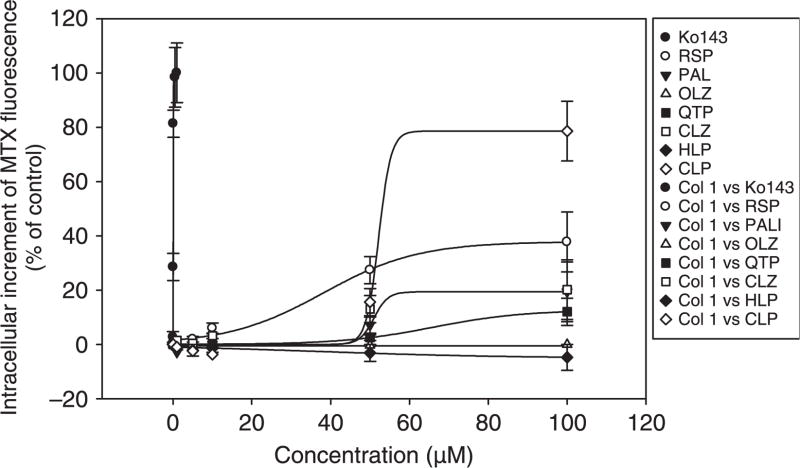
At concentrations ranging from 1 to 100 μM, all the tested antipsychotic drugs except olanzapine and haloperidol showed various degrees of inhibitory effects on the cellular uptake of mitoxantrone in MCF7/MX100 cells (fig. 2A–H). Based on examination of the concentration-dependent inhibitory effects of the putative inhibitors on the intracellular accumulation of mitoxantrone in the MCF7/MX100 cells, the IC50 values were estimated (fig. 3; table 1). According to these IC50 values, the rank order of the inhibitory potency of the tested inhibitors for BCRP was: Ko143 (0.07 μM) > risperidone (38.1 μM) > clozapine (42.0 μM) > paliperidone (51 μM) > chlorpromazine (52.2 μM) > quetiapine (66.1 μM) > olanzapine = haloperidol (>100.0 μM) (table 1). However, when taking into consideration of the plasma concentration and tissue accumulation of the drugs based on typical values reported in the biomedical literature [24–33], the tested antipsychotics especially chlorpromazine, paliperidone and risperidone may possess the potential to inhibit BCRP activity (table 1).
Fig. 2.
Effects of Ko143 (A), risperidone (B), paliperidone (C), olanzapine (D), quetiapine (E), clozapine (F), haloperidol (G) and chlorpromazine (H) on intracellular accumulation of mitoxantrone in MCF7 and MCF7/MX100 cells. Bars represent mean ± S.D. of three determinations. *P < 0.01, **P < 0.001.
Fig. 3.
Concentration-dependent effect of Ko143, of risperidone, paliperidone, olanzapine, quetiapine, clozapine, haloperidol and chlorpromazine on intracellular accumulation of mitoxantrone in MCF7/MX100 cells. Data represent mean ± S.D. of three determinations.
Table 1.
Estimated IC50 values of the antipsychotics on BCRP-mediated mitoxantrone transport in the MCF7/MX100 cells, and the mean maximal plasma concentration (Cmax) and tissue accumulation of the antipsychotics.
| IC50 (μM) | Cmax (μM) | Cmax/IC50 | Liver:plasma partition ratio | Cmax * liver:plasma partition ratio/IC50 | |
|---|---|---|---|---|---|
| Ko143 | 0.07 | – | – | – | – |
| Risperidone | 38.1 | 0.41 | 0.01 | 22.35 | 0.2 |
| Paliperidone | 51.0 | 0.41 | 0.008 | 67.55 | 0.5 |
| Olanzapine | >100 | 0.32 | <0.003 | 32.76 | <0.09 |
| Clozapine | 42.0 | 2.52 | 0.06 | 1.8–3.57 | 0.1–0.2 |
| Quetiapine | 66.1 | 0.82 | 0.012 | 3.5–8.78 | 0.04–0.10 |
| Chlorpromazine | 52.2 | 0.33 | 0.006 | 97.79 | 0.6 |
| Haloperidol | >100 | 0.054 | 0.001 | 13.4–53.710 | 0.01–0.05 |
The superscript numbers for Cmax refer to the following references:
Aravagiri et al. 1998;
Hiemke et al. 2004;
Suzuki et al. 2001; and
Yeung et al. 1993. The mean liver to plasma partition ratios were calculated from mean tissue to plasma area under concentration time curve ratios which were obtained from reference 16. The superscript numbers for liver:plasma partition ratio refer to the following original references:
Aravagiri et al. 2002;
Aravagiri et al. 1999;
Manjunath and Venkateswarlu 2005;
Hopenwasser et al. 2004;
Sgaragli et al. 1995;
Miyazaki et al. 1986.
Risperidone inhibited cellular uptake of oestrone-3-sulfate into Caco-2 cells
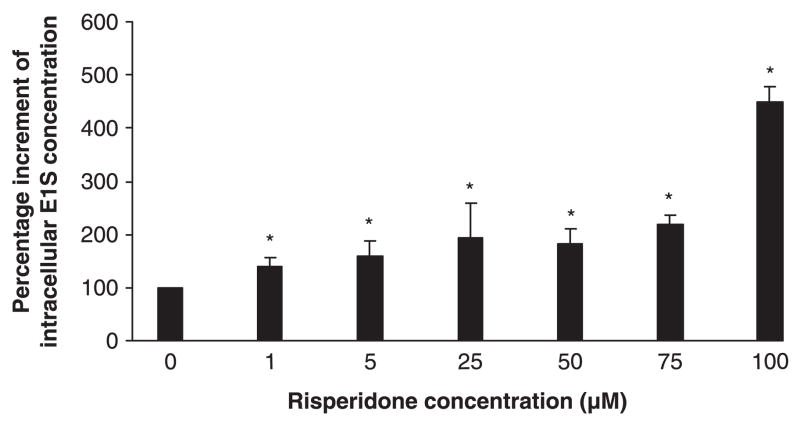
Caco-2 cell line is a widely used model to study drug absorption. Oestrone-3-sulfate has been reported to be a putative substrate of BCRP, but not P-glycoprotein or multi-drug resistance association protein [3,8]. In the present study, at all tested concentrations (1–100 μM), risperidone showed a significant and dose-dependent effect on BCRP-mediated intracellular accumulation of oestrone-3-sulfate in the Caco-2 cells (P < 0.05; fig. 4).
Fig. 4.
Concentration-dependent effect of risperidone on intra-cellular accumulation of oestrone-3-sulfate in Caco-2 cells. Risperi-done at all tested concentrations (1–100 μM) showed a statistically significant effect (P < 0.01) on BCRP-mediated transcellular transport of oestrone-3-sulfate. All data represent mean of triplicate determinations. *P < 0.01.
Discussion
The present findings provide evidence that several commonly used antipsychotic drugs exhibited various degrees of inhibitory effects on BCRP activity. Among these antipsychotics, risperidone, clozapine, paliperidone, chlorpromazine and quetiapine clearly exhibited moderate to mild inhibitory effects on BCRP activity and its transport efficiency. Olanzapine and haloperidol exhibited no apparent inhibitory effect on BCRP activity (table 1; fig. 2B,D,G). The widely used anti-psychotics risperidone was one of the most potent inhibitors of BCRP among the tested antipsychotics. It inhibitory effects were consistently demonstrated in two cell lines over-expressing BCRP (MCF7/MX100 and Caco-2 cells) with application of two prototypic substrates of BCRP (mitoxantrone and oestrone-3-sulfate) [13,34–36].
In general, the plasma levels of the antipsychotics in patients are relatively low compared to the current estimated IC50 values for BCRP (table 1). However, the tested antipsychotics have extensive tissue accumulation, with the tissue to plasma ratios ranging from 1.8-fold to 97.7-fold [16]. Based on this fact, the present findings suggest the existence of potential pharmacokinetic drug–drug interactions between BCRP substrates and several antipsychotics, especially risperidone, paliperidone and chlorpromazine.
The present findings may have potential application for safety treatment of the antipsychotics. BCRP has long been recognized to confer multidrug resistance in cancer and inflammation chemotherapies. Like another multidrug resistance transporter P-glycoprotein (ABCB1), modulation of BCRP may influence drugs with a variety of structural features and therapeutic purposes in terms of absorption, disposition, and ultimately, therapeutic outcome. Inhibition of BCRP’s barrier function in the intestine by BCRP modulators, such as pantoprazole, omeprazole and gefitinib, has been associated with significantly increased oral bioavailability and elevated plasma levels of known substrates methotrexate, topotecan and imatinib [14,15,37]. There are also supporting data for an important role of BCRP in drug excretion into bile [10,11], in transporting drugs across placenta [10], in transporting drugs into human milk [38] and in limiting drug penetration across the blood brain barrier [39].
We have previously studied the antipsychotics relative to their inhibitory potential on P-glycoprotein activity. Our past studies clearly indicated that several antipsychotics, particularly risperidone and olanzapine, are potent inhibitors of P-glycoprotein [16]. These findings in combination with the present findings verified that several antipsychotics, including chlorpromazine, risperidone, quetiapine, paliperidone and clozapine, are dual inhibitors of both P-glycoprotein and BCRP. However, olanzapine (3.9 μM) and haloperidol (9.1 μM) are selective inhibitors of P-glycoprotein with no apparent inhibitory effect on BCRP with concentrations up to 100 μM. The selectively inhibitory effects of olanzapine and haloperidol for P-glycoprotein but not BCRP suggest potential applications of these two drugs to be utilized as in vivo/vitro tools in studying substrate specificities of P-glycoprotein and BCRP.
In conclusion, the present study assessed the inhibitory effects of several antipsychotics on BCRP activity. The findings suggest that several antipsychotics at clinical circumstances may inhibit BCRP activity and cause drug–drug interactions with BCRP substrates.
Acknowledgments
This work was supported by National Institutes of Health grant MH071811-01A1. None of the authors has conflicting interests which interfere with the integrity of the content of the article.
References
- 1.Kage K, Tsukahara S, Sugiyama T, Asada S, Ishikawa E, Tsuruo T, et al. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int J Cancer. 2002;97:626–30. doi: 10.1002/ijc.10100. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279:19781–9. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 3.Xia CQ, Yang JJ, Gan LS. Breast cancer resistance protein in pharmacokinetics and drug-drug interactions. Expert Opin Drug Metab Toxicol. 2005;1:595–611. doi: 10.1517/17425255.1.4.595. [DOI] [PubMed] [Google Scholar]
- 4.Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64:5804–11. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]
- 5.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 6.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, et al. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–63. [PubMed] [Google Scholar]
- 7.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, et al. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–25. [PubMed] [Google Scholar]
- 8.Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64:610–8. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- 9.Bessho Y, Oguri T, Achiwa H, Muramatsu H, Maeda H, Niimi T, et al. Role of ABCG2 as a biomarker for predicting resistance to CPT-11/SN-38 in lung cancer. Cancer Sci. 2006;97:192–8. doi: 10.1111/j.1349-7006.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–6. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 11.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–9. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 12.van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, et al. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63:6447–52. [PubMed] [Google Scholar]
- 13.Ebert B, Seidel A, Lampen A. Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis. 2005;26:1754–63. doi: 10.1093/carcin/bgi139. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–9. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 15.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Wang JS, Zhu HJ, Markowitz JS, Donovan JL, Devane CL. Evaluation of antipsychotic drugs as inhibitors of multidrug resistance transporter P-glycoprotein. Psychopharmacology (Berl) 2006;187:415–23. doi: 10.1007/s00213-006-0437-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang JS, DeVane CL, Gibson BB, Donovan JL, Markowitz JS, Zhu HJ. Population pharmacokinetic analysis of drug-drug interactions among risperidone, bupropion, and sertraline in CF1 mice. Psychopharmacology (Berl) 2006;183:490–9. doi: 10.1007/s00213-005-0209-y. [DOI] [PubMed] [Google Scholar]
- 18.Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Res. 2002;62:5035–40. [PubMed] [Google Scholar]
- 19.Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, et al. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther. 2006;317:850–7. doi: 10.1124/jpet.105.098541. [DOI] [PubMed] [Google Scholar]
- 20.Zhu HJ, Wang JS, Markowitz JS, Donovan JL, Gibson BB, Devane CL. Risperidone and paliperidone inhibit P-glycoprotein activity in vitro. Neuropsychopharmacology. 2007;32:757–64. doi: 10.1038/sj.npp.1301181. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Escarp M, Martinez-Munoz V, Sales-Pardo I, Barquinero J, Domingo JC, Marin P, et al. Flow cytometry-based approach to ABCG2 function suggests that the transporter differentially handles the influx and efflux of drugs. Cytometry A. 2004;62:129–38. doi: 10.1002/cyto.a.20072. [DOI] [PubMed] [Google Scholar]
- 22.Ohsaki M, Matsumoto T, Sakura N, Ueda K. Enzymatic diagnosis of steroid sulfatase deficiency by high performance liquid chromatography. Clin Chim Acta. 1993;215:165–71. doi: 10.1016/0009-8981(93)90123-l. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Aravagiri M, Marder SR, Wirshing D, Wirshing WC. Plasma concentrations of risperidone and its 9-hydroxy metabolite and their relationship to dose in schizophrenic patients: simultaneous determination by a high performance liquid chromatography with electrochemical detection. Pharmacopsychiatry. 1998;31:102–9. doi: 10.1055/s-2007-979308. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki H, Matsunaga Y, Nambu K, Oh-e Y, Yoshida K, Hashimoto M. Disposition and metabolism of [14C]-haloperidol in rats. Arzneimittelforschung. 1986;36:443–52. [PubMed] [Google Scholar]
- 26.Yeung PK, Hubbard JW, Korchinski ED, Midha KK. Pharmacokinetics of chlorpromazine and key metabolites. Eur J Clin Pharmacol. 1993;45:563–9. doi: 10.1007/BF00315316. [DOI] [PubMed] [Google Scholar]
- 27.Sgaragli GP, Valoti M, Palmi M, Frosini M, Giovannini MG, Bianchi L, et al. Rat tissue concentrations of chlorimipramine, chlorpromazine and their N-demethylated metabolites after a single oral dose of the parent compounds. J Pharm Pharmacol. 1995;47:782–90. doi: 10.1111/j.2042-7158.1995.tb06741.x. [DOI] [PubMed] [Google Scholar]
- 28.Aravagiri M, Teper Y, Marder SR. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos. 1999;20:369–77. doi: 10.1002/1099-081x(199911)20:8<369::aid-bdd200>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Aravagiri M, Marder SR. Brain, plasma and tissue pharmacokinetics of risperidone and 9-hydroxyrisperidone after separate oral administration to rats. Psychopharmacology (Berl) 2002;159:424–31. doi: 10.1007/s00213-001-0933-x. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Someya T, Shimoda K, Hirokane G, Morita S, Yokono A, et al. Importance of the cytochrome P450 2D6 genotype for the drug metabolic interaction between chlorpromazine and haloperidol. Ther Drug Monit. 2001;23:363–8. doi: 10.1097/00007691-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Hiemke C, Dragicevic A, Grunder G, Hatter S, Sachse J, Vernaleken I, et al. Therapeutic monitoring of new antipsychotic drugs. Ther Drug Monit. 2004;26:156–60. doi: 10.1097/00007691-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Hopenwasser J, Mozayani A, Danielson TJ, Harbin J, Narula HS, Posey DH, et al. Postmortem distribution of the novel anti-psychotic drug quetiapine. J Anal Toxicol. 2004;28:264–7. doi: 10.1093/jat/28.4.264. [DOI] [PubMed] [Google Scholar]
- 33.Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107:215–28. doi: 10.1016/j.jconrel.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, et al. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 35.Xiao Y, Davidson R, Smith A, Pereira D, Zhao S, Soglia J, et al. A 96-well efflux assay to identify ABCG2 substrates using a stably transfected MDCK II cell line. Mol Pharm. 2006;3:45–54. doi: 10.1021/mp050088t. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A, Dai Y, Vethanayagam RR, Hebert MF, Thummel KE, Unadkat JD, Ross DD, et al. Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol. 2006;58:374–83. doi: 10.1007/s00280-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 37.Reid T, Yuen A, Catolico M, Carlson RW. Impact of omeprazole on the plasma clearance of methotrexate. Cancer Chemother Pharmacol. 1993;33:82–4. doi: 10.1007/BF00686028. [DOI] [PubMed] [Google Scholar]
- 38.Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–9. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 39.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64:3296–301. doi: 10.1158/0008-5472.can-03-2033. [DOI] [PubMed] [Google Scholar]