Abstract
Background
Drug transport proteins may be instrumental in controlling the concentration of fentanyl at µ-receptors in the brain and may provide potential therapeutic targets for controlling an individual response to opioid administration. P-glycoprotein (P-gp) efflux transporter and organic anion transport protein inward transporters (OATP, human; Oatp, rat) have been implicated in fentanyl and verapamil (only P-gp) transport across the blood-brain barrier. We hypothesized that transport proteins P-gp and Oatp mediate opioid uptake in a drug and organ-specific manner, making them excellent potential targets for therapeutic intervention.
Methods
Opioid (fentanyl or loperamide) was administered by IV infusion to Sprague Dawley rats alone or in combination with competitive substrates of P-gp (verapamil) or Oatp (pravastatin, naloxone). Plasma, lung and brain were collected over 10 minutes and at 60 minutes after opioid infusion and opioid concentration determined using liquid chromatography/mass spectrometry (LC/LC-MS/MS). Continuous electroencephalogram was used to determine the in vivo response to fentanyl and loperamide in the presence and absence of verapamil.
Results
Loperamide brain:plasma (PB) and lung:plasma (PL) partitioning was increased 2 and 5 fold respectively in the presence of verapamil. Verapamil administration was lethal unless the loperamide dose was reduced by half (0.95 mg/kg to 0.475 mg/kg). Fentanyl brain:plasma and lung:plasma were reduced 4 and 6 fold, respectively, by pravastatin and naloxone, whereas verapamil had much less effect. Electroencephalogram results indicated that verapamil reduced the fentanyl-induced central nervous system (CNS) effect and increased the loperamide CNS effect.
Conclusion
Protein transporters appear to be organ and drug-specific in vivo, affecting first-pass pulmonary uptake and CNS response to opioid administration. Further, data suggest that transport protein inhibition may prove useful for normalizing an individual response to opioids.
Introduction
Fentanyl, a widely used, potent, synthetic opioid analgesic, has a high degree of intra- and interindividual potency variability (1). Opioids such as fentanyl and loperamide cross cell membranes via specific transport proteins including P-glycoprotein transporters (P-gp), which actively efflux drug from endothelial cells to the circulation, as well as by passive diffusion (2). Evidence of discreet control of opioid uptake by transport proteins, specific for opioid and/or organ, could provide valuable information for designing targeted therapeutics to mediate opioid effect and account for significant interindividual differences in opioid dose response.
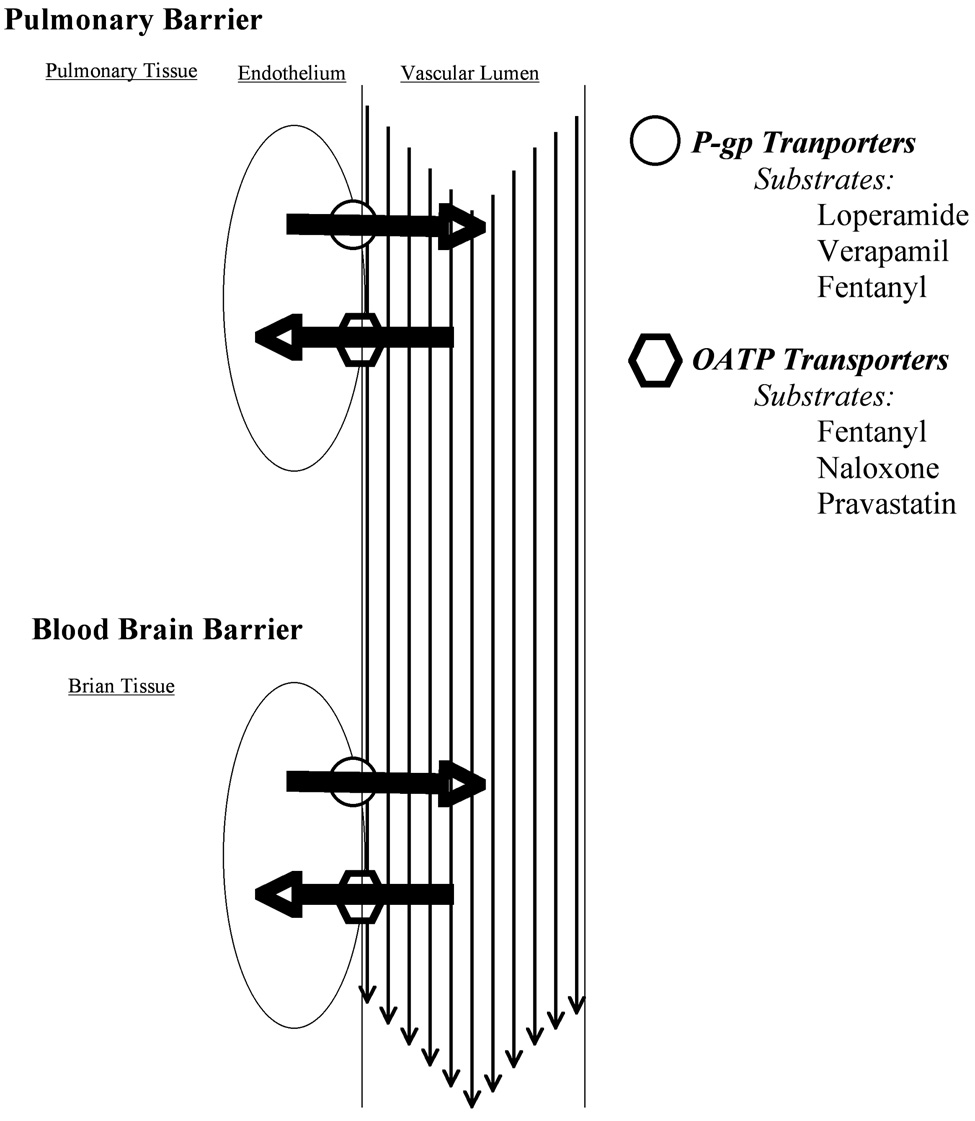
We previously demonstrated that verapamil, a competitive substrate for P-gp, reduces endothelial concentration of fentanyl by a saturable energy-dependent transport process in brain and lung endothelial cell cultures, while suggesting that verapamil may also inhibit an inward protein transporter (3–5). Organic anion transporters (referred to as OATP in humans, Oatp in rats) are active protein transporters that have been identified on the luminal and abluminal sides of the blood-brain barrier (BBB) (6). Opioid transport across the BBB via Oatp has been reported for Tyr-D-Penicillamine-Gly-Phe-D-Penicillamine (DPDE) and deltorphin II (7). Thus, the fentanyl central nervous system intrinsic effect may be mediated by active Oatp transport in vivo (Figure 1).
Figure 1.
Protein transporters P-glycoprotein and organic anion transport protein (oatp) in the vascular endothelium of the lung (first-pass uptake) and brain (blood-brain barrier) are depicted with large dark arrows indicating the direction of substrate transport by each transport and lighter arrows in the vessel lumen indicating the direction of blood flow.
In contrast, transport of loperamide, a potent opioid that is widely used for treatment of diarrhea through activation of opioid receptors in the intestinal tract (8,9), appears to be mediated at the BBB exclusively by the outward P-gp transporter, thus limiting its central effect (2). Thus, alteration of opioid transport by P-gp inhibition would likely have opposite effects on the pharmacokinetics of fentanyl and loperamide. We studied the transport of fentanyl and loperamide to the brain and lungs of rats and hypothesized differential effects of transporter inhibition by verapamil on opioid pharmacokinetics.
Specifically, because in vitro studies suggest that fentanyl transport is primarily mediated by outward transporters whereas loperamide transport is exclusively mediated by outward transporters, we hypothesized that verapamil, a substrate for both inward and outward protein transporters, would reduce fentanyl uptake by brain and lung endothelium yet would increase loperamide uptake in these tissues. Consequently, verapamil would reduce fentanyl’s central effects and increase loperamide’s central effect, as measured by electroencephalogram (EEG) delta wave response. Further, inhibition of inward, energy-dependent Oatp by nalaxone and pravastatin would decrease brain and lung uptake of fentanyl.
Methods
Pharmacokinetic and Pharmacodynamic Study Protocol
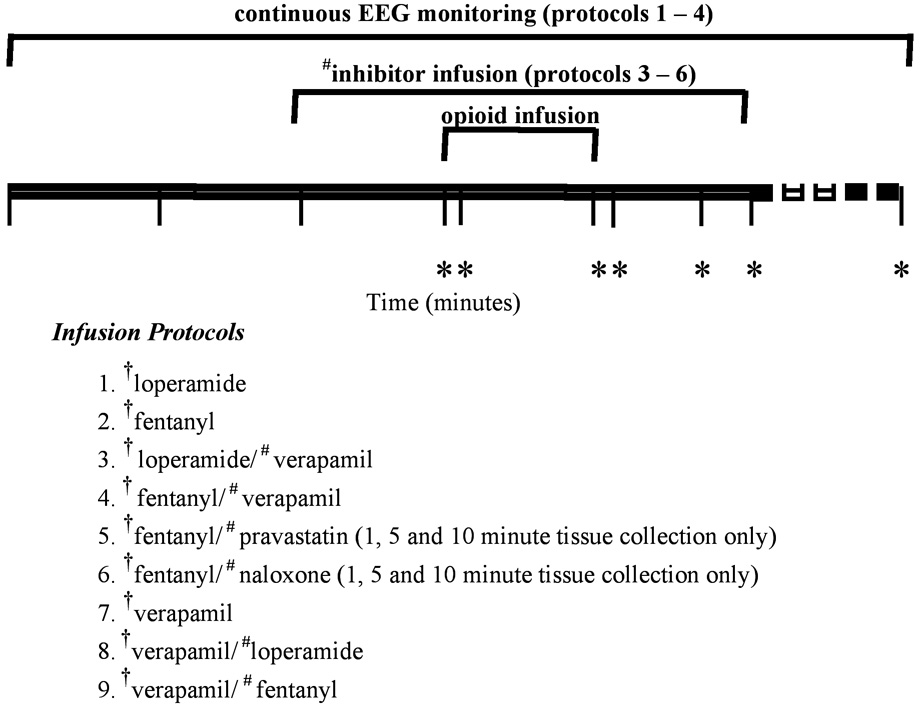
Pharmacokinetics of opioids in rats were determined by collecting plasma, lung and brain at 0, 1, 5, 6, 8, 10 and 60 minutes after initiation of a 5 minute infusion of an opioid in the presence and absence of competitive substrate (Figure 2). Pharmacodynamics in rats were assessed by measuring delta wave activity by EEG for each opioid in the presence and absence of a competitive substrate, with each animal acting as its own control (Figure 2).
Figure 2.
Study design indicating the time frame of drug administration, tissue collections and protocols (n=4 animals per time point per protocol). *blood and tissue collection time points, # competetive substrates: verapamil, pravastatin and naloxone, † indicates which was drug measured by liquid chromatography/mass spectrometry (LC/LC-MS/MS) in brain, lung and plasma at each time point
Animals
Experiments were performed with approval from the University of Colorado Health Sciences Center and Colorado State University Animal Care and Use Committee. Male Sprague-Dawley rats with indwelling cannulae (jugular venous catheter for drug infusion and carotid artery catheter for blood collection) were purchased (300–350 g, Harlan, Madison, WI). Animals were kept on a 12-hour light/dark cycle and fed standard laboratory chow. For the pharmacokinetic study, tissue was collected from animals (n = 4) at each of 7 time points for 5 protocols and at each of 3 time points for 2 protocols (total n = 164). For the pharmacodynamic studies, each animal (n = 8) was studied with an opioid alone and 5 days later by the same opioid in the presence of a competitive substrate (and vice versa). Four animals were used for each of 2 opioids (fentanyl, loperamide).
Infusions
Intravenous infusions of fentanyl citrate (5 min, 5.25 µg/kg/min) or loperamide hydrochloride (5 min, 0.95 mg/kg/min loperamide alone and 0.475mg/kg/min when administered with verapamil) were administered (Abbott Laboratories, North Chicago, IL). Doses for fentanyl and verapamil were based on doses used in previous studies (10,11). The loperamide dose increased rat brain delta wave activity. However, loperamide infusion (0.95 mg/kg/ml) in the presence of verapamil resulted in death, therefore the loperamide dose was reduced to 0.475 mg/min/kg when administered in the presence of verapamil (Sigma Aldrich, St. Louis, MO). On separate occasions, target-controlled infusions (TCI) of verapamil (target plasma concentration of 1 mg/ml), a constant infusion of pravastatin sodium (1 mg/kg/mm: Bristol-Myers Squibb, Princeton, NJ) or naloxone hydrochloride (0.1mg/kg/min: Abbott Laboratories, North Chicago, IL) were administered for 15 minutes, beginning 5 minutes prior to the opioid infusion and continuing for 10 minutes after the start of opioid infusion (Table 1). TCI for verapamil was used in an attempt to produce consistent competitive substrate concentration for the pharmacodynamic studies.
Table 1.
The effect of verapamil on lung and brain partitioning of loperamide and fentanyl.
| PL | PB | |
|---|---|---|
| loperamide | 1.09 | 0.006 |
| loperamide + verapamil |
5.74* | 0.01* |
| fentanyl | 0.367# | 0.142 |
| fentanyl + verapamil |
0.367# | 0.082* |
Significantly different from treatment with the same opioid in the absence of verapamil.
AIC increased with individual coefficients for lung partitioning therefore the PL is reported for the combined model.
PL = lung: plasma partition coefficient, PB = brain: plasma partition coefficient
TCI was accomplished using a Harvard 22 syringe pump (Harvard Apparatus, Holliston, Massachusetts), controlled via a serial connection to a Pentium-based computer running the TCI software STANPUMP (written by Steven L. Shafer, MD) and using a 3-compartment pharmacokinetic model for verapamil. The pharmacokinetic model was derived from a one-compartment rat model for verapamil and a similar 3-compartmental model for thiopental in the rat from which the total volume of distribution of verapamil (1-compartment model) was divided into central and peripheral compartments in proportion to the thiopental model. Central and peripheral compartments had identical inter-compartmental clearances set to equal the thiopental model (10,12).
Tissue Collection
Blood (5 mL) was collected over 3 to 5 seconds from the carotid catheter prior to decapitation and drawn into tubes containing citrate as an anticoagulant. The time 0 blood draw was collected just prior to starting the opioid infusion and the 5 min blood draw was begun exactly when the infusion was stopped. Plasma was separated following a standard centrifugation protocol (400×g, 10 min, 4°C). Brain was collected from the skull of decapitated animals and flash-frozen in liquid nitrogen. Lungs were collected after thoracotomy and were immediately frozen in liquid nitrogen.
Opioid Analytic Methods
Rat brain and lung (10 mg) were homogenized with 2 ml KH2PO4 buffer pH=7.4 (1.0 M) using a teflon-glass manual homogenizer and stored at −80°C until analysis using specific and highly sensitive high-performance liquid chromatography (HPLC)/ mass spectrometry (LC/LC-MS/MS) assays for fentanyl, loperamide and/or verapamil developed in our laboratory. Samples were extracted using online extraction and were quantified using an API4000 tandem quadrupole mass spectrometer as detector (Applied Biosystems, Foster City, CA). Two linked HPLC systems (LC/LC), one for extraction (I) and one for analytical separation (II), comprising the following components: HPLC I: Agilent G1312A binary pump, G1322A degasser and Leap Technologies HTS PAL autosampler (CTC Analytics, Zwingen, Switzerland) equipped with a cooled stack temperature controller, a 6-port LC injection valve and a fast wash station; HPLC II: Agilent G1312A binary pump, a G1316A column thermostat and an API4000 mass spectrometer. The 2 HPLC systems were connected via a motorized 6-port column-switching valve (Rheodyne, Cotati, CA; for connection details, Reference 22). The mass spectrometer, HPLC components, LEAP autosampler were controlled and all data were processed by Applied Biosystems Analyst software version 1.3 (Applied Biosystems, Foster City, CA).
On the day of analysis, plasma and homogenized brain and lung were thawed on ice and prepared by adding plasma, brain or lung (200 µL) to internal standard solution (400 µL, 0.06 M ZnSO4 solution containing water and methanol, 30:70, v/v) and internal standard (10 µL, alfentanil for opioids and (±)-methoxy verapamil hydrochloride for verapamil), resulting in a final concentration of 50 ng/mL. After vortexing (2 min), the samples were centrifuged (13000 g × 5 min) to remove precipitated proteins. The resulting supernatant (100 µL) was injected into the HPLC system (series 1100, Agilent Technologies, Santa Clara, CA).
For on-line sample clean up, supernatants were loaded onto the extraction column (4.6 × 12.5 mm, 5 µm particle size, Eclipse XDB-C8, Agilent) and washed with a high flow (5 mL/min) of 80% 0.1% formic acid/ 20% methanol for 1 min. The switching valve was then activated and the analytes were back-flushed onto the C8 analytical column (4.6 × 150 mm, 5 µm particle size, Eclipse XDB-C8, Agilent). A linear gradient was used: methanol increased from 55% to 100% in 4 min and was kept at 100% for 1 min (flow rate 1.0 mL/min, column temperature 40°C). Analytes eluted from the HPLC column were introduced into the turbo-ion spray source. Zero-grade air for nebulizing and turbo gases was provided by a Zero Air Generator (Analytical Gas Systems). Ultra-high-purity nitrogen (99.999%) was used as collision activated dissociation gas (CAD), and the curtain gas provided by a nitrogen generation system (Agilent, Palo Alto, CA). Ionization was achieved in the positive Multiple Reaction Monitory (MRM) mode. MRM sensitivities for each analyte were simultaneously optimized by direct infusion of each compound (0.1 µg/mL, in 80% methanol/20%, 0.1% formic acid).
For the quantification of all opioids, the MRM parameters were: source temperature = 480°C and ion spray voltage = 5000V, and gases set at 20 for nebulizer gas, 15 for turbo gas, 15 for curtain gas and 8 for CAD gas (all arbitrary units). The declustering potential was 50V. The dwell time for each transition was 200 ms. Data were collected and the major product ion transitions were monitored: fentanyl m/z 337.5 Π 188.4, loperamide m/z 477.5 Π 266.1, internal standard alfentanil m/z 417.4 Π 197.3, verapamil m/z= 455.6 Π 165.6 and m/z= 485.6 Π 333.5 for the internal standard (±)-methoxy verapamil.
Assay Validation
The assay was completely validated following current Food and Drug Administration guidelines (CDER (2001)) United States Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research and Center for Veterinary Medicine, Guidance for the Industry, Bioanalytical Method Validation, May 2001 (www.fda.gov/cder, accessed 5-24-2005). Key method performance parameters were: linear range from 0.033 to 16.67 ng/mL for rat plasma, lung and brain. The regression coefficients (r) were always better than 0.999. The lower limits of quantitation (LLOQ) on the column was 3.3 pg. Intra-day accuracies were between 81.8% and 119.7%, and intra-day precisions were between 2.3% and 20.4% at the lower limit of quantitation (LLOQ). At the upper limit of quantitation (ULOQ), intra-day accuracies and precisions were between 99.4%–100.3% and 0.9%–3.9% in plasma and tissues, respectively. Inter-day accuracies were between 80.5%–119.5% at LLOQ, 99%–100.8% at ULOQ, and inter-day precisions were between 3.6%–18.0% at LLOQ, and 2.1%–3.8% at ULOQ. There were no matrix interferences, carryover or ion suppression.
Surgery to Install the EEG Transmitter
Prior to surgery, ketamine hydrochloride (75 mg/kg) and xylazine hydrochloride (15 mg/kg) were administered intraperitoneally. Anesthetized animals were immobilized in a stereotaxic apparatus (Stoelting Co, Wood Dale, IL). A straight midline skin incision was made on the scalp to expose the skull. Using a dremel tool (1/8 inch bit), 2 holes located 2 mm on either side of midline and 2 mm anterior to the bregma suture were then drilled in the skull. Using blunt forceps, the skin dorsal to the neck was dissected to form a subcutaneous pouch into which the transmitter was placed (EEG transmitters (CTA-F40), receivers, and telemetry system purchased from Data Sciences International, Arden Hills, MN, USA). Once the bone was totally dry, the screws, soldered to the end of the transmitter leads, were placed into the holes and adhered with a small amount of dental acrylic cement.
EEG Procedures
One day after surgery, EEG signals were recorded. The rats and their respective transmitters and receivers were entered into the telemetry system. Signals were recorded for 15 minutes to establish a baseline EEG before the start of opioid infusion. Signals were continuously recorded for 60 minutes after the start of drug infusion (Table 1). EEG signals were captured, low and high filters set at 0.5 and 100 Hz, respectively, and immediately recorded with model A1000 spectral EEG monitor (Aspect Medical Systems, Norwood, MA) coupled to a data logger with EEG data handling software (Data Sciences International, Arden Hills, MN) for offline analysis. The delta wave was then extracted from the raw data within the range of 0.5 – 4.5 Hz, using Brain Vision Analyzer Software (Brain Product GmbH, München, Germany) and a running 20 sec average of the power in the delta range was recorded as the baseline (no drug) and drug effects.
Statistics
Pharmacokinetics
Drugs, for which pharmacokinetics were determined, are referred to as pk drugs (fentanyl, loperamide and verapamil). Conversely, drugs used for their properties of transport protein inhibition (fentanyl, loperamide, verapamil, pravastatin and naloxone) are referred to as competitive substrates, specific to each protocol. For pharmacokinetics analysis, a naive pooled-data technique supported by SAAM II software (SAAM Institute, Seattle, WA) was used. To test model parsimony, the Akaike information criterion (AIC) was used (13). To determine if a covariate (elimination clearance and volumes of the central and peripheral compartments, and tissue partitioning) for an experiment using a competitive substrate for the transport protein produced a statistically significant change in the pharmacokinetic parameter, the AIC with the added covariate in the model was compared with the AIC of the simpler model without the covariate. The comparison was considered significant if the AIC was lower than that of the simpler model (13).
Data Analysis
Data from the pk drug infused alone or with a competitive substrate were lumped into separate models with linked parameters, such as elimination clearance and the volumes of central and peripheral compartments. Plasma kinetics were modeled either with a 1 or 2-compartment open model. In the 2-compartment open model, intercompartmental clearance (ClI) was assumed to be 0.1 ml/kg/min for fentanyl and 0.01 ml/kg/min for loperamide, because the distribution volume of loperamide was approximately 10 fold less than that of fentanyl. Furthermore, within a 3-fold range around these selected inter-compartmental clearances the model predicted values did not change substantially and the coefficient of variation around the paramter was large. The remaining parameters, volume of distribution (V1 and V2) and elimination clearance (ClE) were then fit to the plasma fentanyl or plasma loperamide concentration-time data. Data fit with this model had a lower AIC than either a 1-compartment model or a 2-compartment model in which ClI was an adjustable parameter.
Conversely, the 1-compartment open model best-fit data from experiments in which verapamil was the pk drug and opioids considered the competitive substrate. In the 1-compartment open model, V1 and ClE were fit to the plasma verapamil or plasma fentanyl concentration-time data. Data fit with this model had a lower AIC than a 2-compartment open model. To test the hypothesis that the competitive substrate affects the plasma pharmacokinetics of the pk drug, all data were first fit to a unified pharmacokinetic model for the pk drug; the null hypothesis was assumed and data from pk drug alone and pk drug plus competitive substrate were grouped together. Each of the adjustable parameters (ClE, V1, V2) was then fit so that each parameter could have a covariate modifier for the condition in which the competitive substrate was administered. Accordingly, covariate parameters are such that data from experiments with pk drug alone and pk drug with the competitive substrate are considered as 2 sets of data rather than 1. To test if the pharmacokinetic parameters from the 2 experiments (pk drug alone or in the presence of competitive substrate) were statistically significantly different, the AIC with the added covariate in the model was compared with the AIC of the simpler model (without the covariate); the model with the lowest AIC was selected (5). A 1-compartment open model was used for the data from experiments in which fentanyl was the pk drug and pravastatin and naloxone were the competitive substrates, because only 3 time points were determined and less data points were available in these studies without pharmacodynamics.
Tissue Partitioning
Lung and brain concentration-time data were modeled by adding partition coefficients, PB and PL, to describe the ratio between plasma pk drug concentration and those in brain and lung, respectively. To test the hypothesis that the competitive substrate changes tissue: plasma partitioning for the brain and lung, all data were first fit to a unified pharmacokinetic model for the pk drug and then each of the tissue:plasma partition coefficients (PB, PL) were fit so that each coefficient could have a covariate parameter and AIC evaluated as described above for statistical significance. The pravastatin and naloxone experiments were used to test the role of Oatp in lieu of verapamil having little effect on fentanyl tissue partitioning and only 1, 5 and 10-minute time points were determined. Thus, a 1-compartment model was used to analyze these data.
Pharmacokinetic/Pharmacodynamic Modeling
The fentanyl and loperamide pharmacokinetic models in the absence and presence of verapamil were used to construct the pharmacokinetic/pharmacodynamic models in the manner of Sheiner et al. (14) in which a plasma effect-site equilibration rate constant describes the time lag between the increase and decrease of plasma drug concentrations and the observed effect. Thus, estimates of effect-site concentrations that would be proportional to blood concentrations at steady-state are obtained. A sigmoid Emax pharmacodynamic model was used to describe the relationship between effect-site drug concentration and EEG effect:
in which EC is the EEG effect at drug effect-site concentration C, E0 is the no-drug effect, Emax is the intrinsic activity maximum effect, EC50 is the drug concentration at 50% maximum effect, and γ is the Hill coefficient, which was fixed at 2 (15). The parameters of this model were analyzed across treatments with covariates for the presence of verapamil and AIC was evaluated as described above to test for statistical significance.
Results
Loperamide Pharmacokinetics
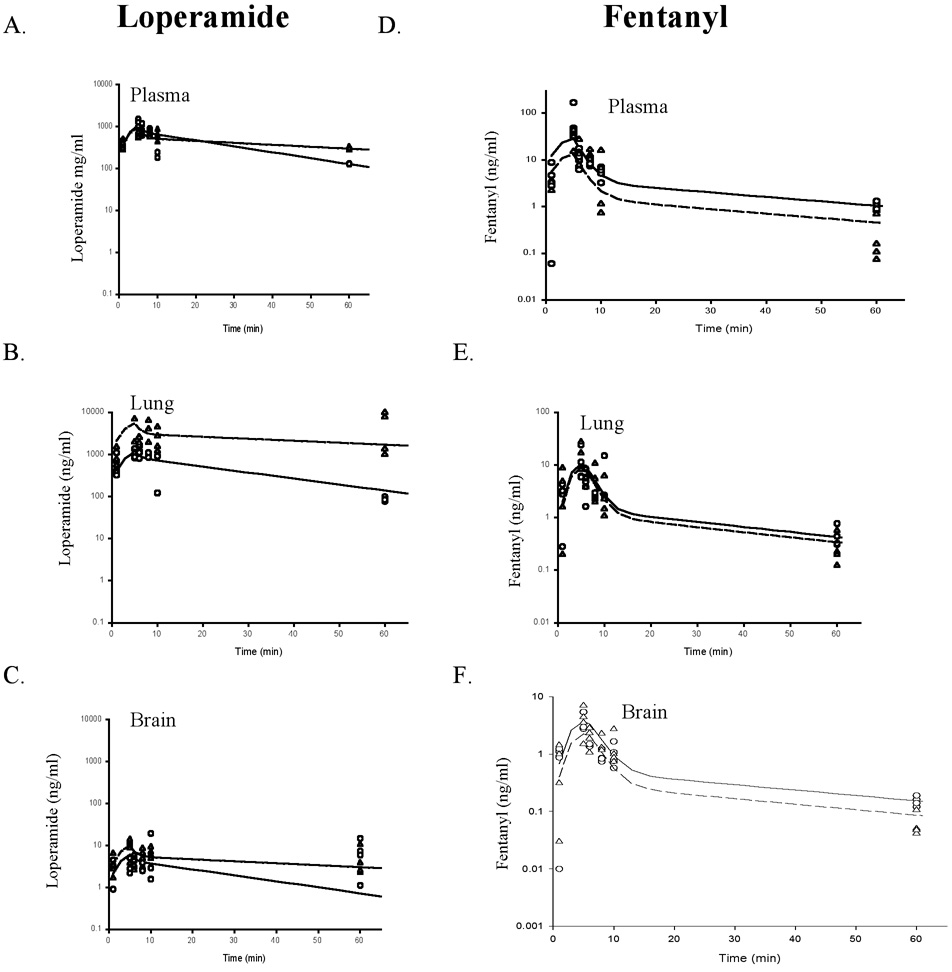
Verapamil had a far greater effect on the pharmacokinetics of loperamide than on fentanyl. The dose of loperamide (0.95 mg/kg) was the lowest dose that elicited an EEG response. When verapamil was added to the loperamide (0.95 mg/kg), all animals died. The loperamide dose was then cut in half (0.475 mg/kg) when used in combination with verapamil. Modeling determined that while verapamil increased loperamide PB and PL in comparison to the full dose of loperamide alone, the change in PL was larger than that in PB, 5.3-fold versus 2-fold, respectively (Table 1, Figure 3).
Figure 3.
Loperamide (A–C) and fentanyl (D–F) concentration in Sprague-Dawley rats’ arterial plasma (A, D), lung (B, E), and brain (C, F) in the absence and presence of verapamil (n = 4 per time point per protocol). The symbols represent measured drug concentrations, whereas the lines represent drug concentrations predicted by the model: Solid line – modeled opioid only, dashed line – modeled opioid with verapamil, circles – measured opioid only, upright triangles – measured opioid with verapamil.
Fentanyl Pharmacokinetics
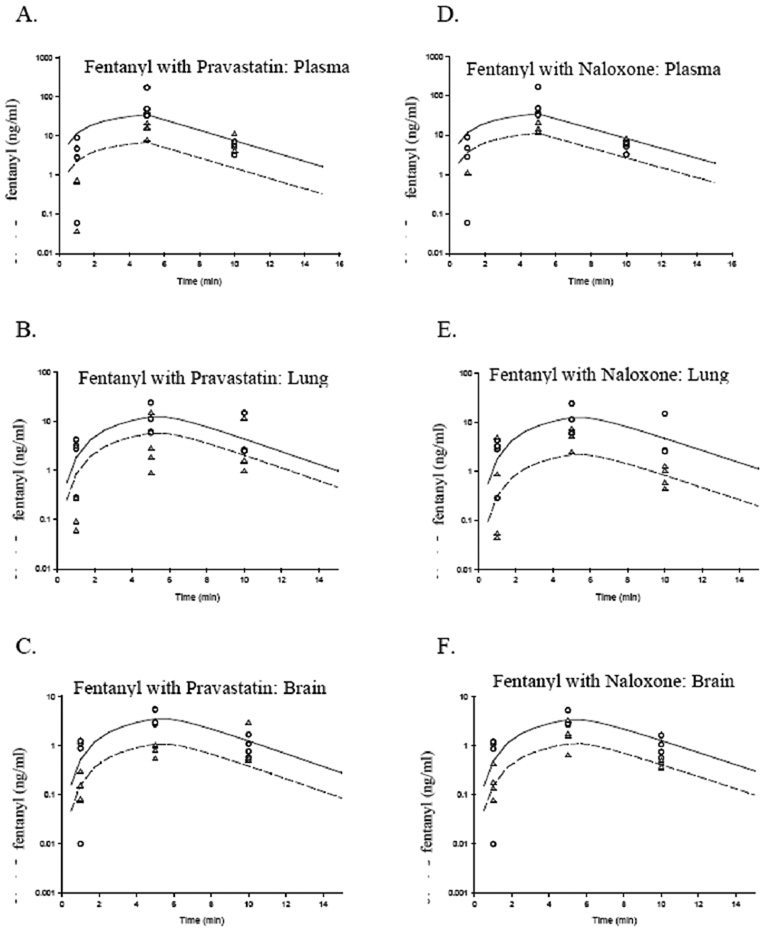
Modeling to determine brain and lung partitioning showed that verapamil decreased fentanyl PB, but had no effect on PL (Table 1, Figure 3). Further studies were performed to determine if Oatp had a more primary role in fentanyl pharmacokinetics in lieu of the fact that the competitive substrate for P-gp had such a mild effect on fentanyl pharmacokinetics. Oatp competitive substrates, pravastatin and naloxone, had an extremely large effect on fentanyl PB and PL, reducing partitioning by 4.2 and 5.9 fold, respectively (Table 2, Figure 4).
Table 2.
The effect of pravastatin and naloxone on lung and brain partitioning of fentanyl.
Significantly different from treatment with only fentanyl.
PL = lung: plasma partition coefficient, PB = brain: plasma partition coefficient
Figure 4.
Fentanyl concentration in Sprague-Dawley rats’ arterial plasma (A, D), lung (B, E), and brain (C, F) in the absence and presence of pravastatin (A–C) and naloxone (D–F) (n = 4 per time point per protocol). The symbols represent measured drug concentrations, whereas the lines represent drug concentrations predicted by the model: Solid line – modeled fentanyl only, dashed line – modeled fentanyl with verapamil, circles – measured fentanyl only, upright triangles – measured fentanyl with verapamil
Verapamil Pharmacokinetics
To determine if the opioids could have, in turn, altered the partitioning of verapamil for lung and brain, the pharmacokinetics of verapamil in the presence of fentanyl and loperamide were determined. Modeling to determine tissue partitioning indicated that fentanyl slightly reduced verapamil PB, yet slightly increased PL, whereas loperamide increased PB and PL of verapamil (Table 3).
Table 3.
The effect of loperamide and fentanyl on lung and brain partitioning of verapamil.
Significantly different from treatment with only verapamil.
Pharmacodynamics
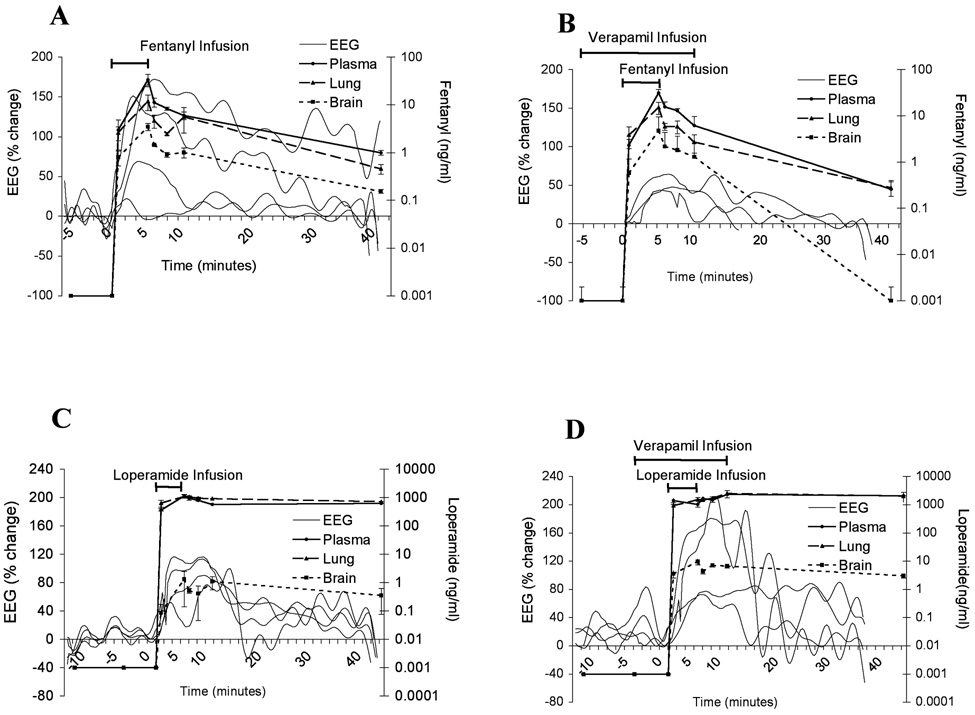
To determine if verapamil altered the central nervous system effect of fentanyl and loperamide, the delta wave of the EEG response was determined with administration of opioid alone and opioid with competitive substrate. A rising delta wave power indicates a greater opioid effect. Verapamil had opposite effects on the opioid-induced EEG for fentanyl and loperamide. Verapamil reduced delta wave activity in response to fentanyl but enhanced delta wave activity in response to loperamide (Figure 5).
Figure 5.
Fentanyl (A,B) and loperamide (C,D) concentrations in arterial plasma and the delta wave electroencephalogram (EEG) response in the absence (A,C) and presence of loperamide (B,D) in Sprague-Dawley rats (n = 4 per time point in pk studies, each animal (n = 4) was studied with pharmacokinetics (pk) drug and its competitive inhibitor except for n=3 for loperamide alone for EEG studies). Data presented as mean ± S.E.M.
Pharmacokinetic/pharmacodynamic modeling
Pharmacokinetic/pharmacodynamic modeling indicated that verapamil increased fentanyl EC50 by 4.7 fold and reduced ke0 by .45 fold, respectively, but the EC50 and ke0 of loperamide were decreased by 0.3 and 0.4 fold, respectively (Table 4).
Table 4.
Fentanyl and loperamide concentrations at 50% maximum electroencephalogram effect.
| EC50 (ng/ml) |
ke0 | Tmax (min) |
|
|---|---|---|---|
| loperamide | 141 | 1.54 | 1.1 |
| loperamide + verapamil |
42 | 0.62 | 1.8 |
| fentanyl | 7 | 0.44 | 1.2 |
| fentanyl + verapamil |
33 | 0.20 | 1.3 |
EC50 is the drug concentration at 50% maximum effect
Tmax is the time to maximal effect
ke0 is the equilibration rate effect
Discussion
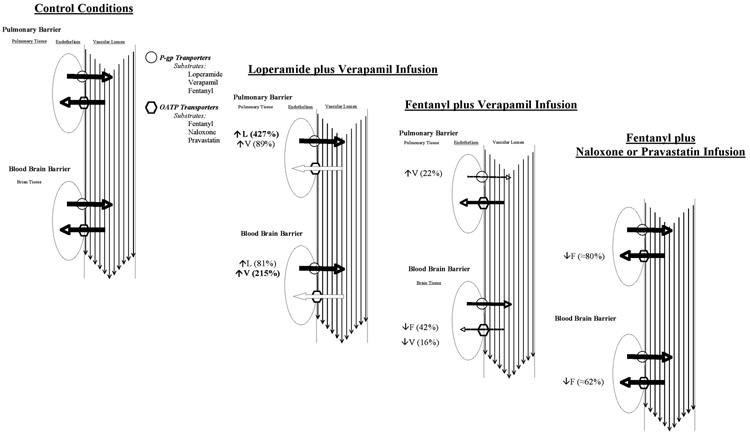
The data support the hypothesis that specific transport proteins are responsible for differential tissue partitioning of opioids and variable EEG effects in a drug and organ-specific manner (Figure 6). Verapamil, a competitive substrate of P-gp, caused death in all loperamide-treated animals unless the loperamide dose was reduced by half (from 0.95 to 0.475 mg/kg). Lung and brain partitioning of loperamide were increased 2 and 5-fold, respectively, and the EC50 was substantially reduced in the presence of verapamil. Competitive substrates of Oatp reduced fentanyl brain:plasma partitioning 4-fold, whereas verapamil only reduced fentanyl:brain partitioning by approximately 0.57 fold as reflected by the increased EC50. Interestingly, Oatp competitive substrates (pravastatin and naloxone) had a much greater effect on tissue partitioning of fentanyl than P-gp substrate (verapamil). The small effect of verapamil on fentanyl may be a consequence of the fact that they are substrates of both outward and inward transporters, most likely P-gp and Oatp, respectively. Thus, our data indicate that fentanyl tissue partitioning is primarily mediated by Oatp inward transporters, whereas verapamil tissue partitioning is primarily mediated by P-gp outward transporters (Figure 6).
Figure 6.
The effect of competitive substrates for protein transporters on opioid partitioning to lung and brain tissue and the hypothesized effect on transport proteins P-glycoprotein (P-gp) and organic anion peptide transporters (OATP) in determining pharmacokinetics of and by opioids. Values adjacent to transporter most hypothesized to be most affected. Solid line indicates no change in transporter activity, dashed line indicates reduced transporter activity, thinner dashed line indicates greater reduction in transporter activity
Study Limitations
The drugs used to inhibit P-gp and/or Oatp transport have other actions, such as cardiovascular effects, cholesterol lowering actions and µ-receptor effects. Verapamil treatment reduced the rat’s cardiac output by 1/3 in all protocols, yet it produced opposite effects in the 2 study drugs (determined in our laboratory by Doppler flow echocardiography, data not reported here). Our data also indicate that verapamil not only inhibits efflux transport via P-gp, but blocks drug influx via an unidentified inward transporter in vivo. Pravastatin and naloxone were used to inhibit Oatp. Pravastatin inhibits OATP1B1 and OATP1B3 and naloxone inhibits transport of deltorphins at the BBB (7,16). Because pravastatin also inhibits multidrug-resistant associated protein 2 (17) and it is unclear which Oatp is inhibited by naloxone, we cannot conclude exactly which Oatp is instrumental in fentanyl uptake. However, it is likely that an Oatp is involved, as both pravastatin and naloxone inhibited influx. Naloxone binds Oatp but is also a µ-receptor antagonist, precluding the ability to study its effect on fentanyl pharmacodynamics.
Loperamide Pharmacokinetics
Loperamide, a µ-receptor agonist, was thought to not cross the BBB, however, with a sufficient IV dose we showed that central effects occurred (18). In the current study, verapamil had such a strong effect on brain partitioning of loperamide that the animals could not survive the insult and the loperamide dose had to be cut in half. Verapamil increased loperamide PB and loperamide increased verapamil PB by 1.8- and 3-fold, respectively, providing evidence to support that both drugs are P-gp substrates. Also, first –pass pulmonary uptake of loperamide was mediated by P-gp inhibition. Verapamil treatment caused a 5.3-fold increase in loperamide PL and, in turn, loperamide increased verapamil PL by 1.9-fold. These data are supported by previous research, which indicated that P-gp mediates loperamide partitioning in brain (2,19).
Fentanyl Pharmacokinetics
Although fentanyl and verapamil have been identified as substrates of P-gp efflux transporters, our previous in vitro and current in vivo data suggest that P-gp-mediated partitioning is overshadowed by an unidentified inward transporter (5). Furthermore, because fentanyl and verapamil mutually excluded partitioning of each other into the brain, results also suggest that fentanyl and verapamil are substrates of the same inward transporter in the brain.
Recently, organic anion transporter polypeptides (designated Oatp in rat, OATP in human) were found in endothelial cells lining capillaries in the brains of rats and humans (20,21). Besides sharing drug substrates (including opioids (7,22)) with P-gp efflux transporters, these transporters were also reported to serve as inward transporters for deltorphin in the brain (7). Because fentanyl and verapamil are known substrates of P-gp efflux transporters and data from the present study indicated that these drugs might also be substrates of an unidentified inward transporter in the brain, the effect of Oatp competitive substrates (pravastatin and naloxone) on fentanyl pharmacokinetics were assessed. Oatp competitive substrates pravastatin and naloxone reduced fentanyl PB and PL. The results suggest that Oatp may play a significant role in first pass pulmonary uptake and brain partitioning of fentanyl. It is important to note that, while verapamil reduced fentanyl PB by 0.57 fold, pravastatin and naloxone caused even greater reduction in fentanyl PB (0.24 and 0.31 fold, respectively). These data are consistent with the idea that verapamil blocks both fentanyl efflux (via P-gp) and influx (possibly via Oatp), whereas pravastatin and naloxone only block influx (via Oatp) (Figure 6). By reducing transport in both directions, verapamil would likely have less effect on PB than pravastatin and naloxone.In vitro data from Waters et al. (23) support our data suggesting that verapamil may be a competitive substrate for an active infflux transporter as well as the P-gp efflux transporter. They report that the KEQ (ratio of cell-associated fentanyl concentration to supernatant fentanyl concentration plotted as a function of fentanyl supernatant concentration) becomes lower as verapamil concentration increases. However, verapamil reduced fentanyl KEQ in a concentration-dependent manner, having its greatest effect when the fentanyl concentration was low. The authors hypothesized that the concentrations at which verapamil was effective in vitro would not be sufficient to alter fentanyl lung partitioning, which is supported by the current in vivo data. In fact, when fentanyl was administered as a competitive substrate for verapamil partitioning, lung partitioning was increased by 1.15 fold, likely via fentanyl binding P-gp transporters.
Pharmacokinetic/Pharmacodynamic Model
Our results indicate that in the presence of verapamil the distribution of loperamide to the brain increased, resulting in smaller EC50 (Table 4) and suggesting that more loperamide was present at the effect-site at any given steady-state blood concentration. However, verapamil decreased the distribution of fentanyl to the brain, and hence EC50 was greater (Table 4), suggesting that less fentanyl was at the effect-site at any given steady-state blood concentration.
According to the effect-site model introduced by Sheiner, et al. (14), the effect-site equilibration rate constant ke0 is assumed to be the same for entry and egress, so that concentration values could be related back to those measured in the blood. Because verapamil changed the pharmacokinetic model parameters of fentanyl and loperamide and ke0 is a model-dependent parameter, we cannot interpret the changes ke0.
Because of loperamide’s handling by P-gp at the BBB, P-gp inhibition by verapamil allowed more loperamide to enter the brain resulting in increased Emax and decreased EC50. The EC50 is smaller because more loperamide is at the effect-site at any given steady-state blood concentration, probably not because of changed neural effects such as opioid receptor binding.
In conclusion, our results indicate that primary control of loperamide and fentanyl tissue partitioning is mediated by different protein transporters, likely a form of P-gp-mediated efflux for loperamide and Oatp-mediated influx for fentanyl. These data suggest that transport proteins variably regulate fentanyl and loperamide pharmacokinetics and pharmacodynamics and may provide potential targets for therapeutics designed to modulate inter- and intra-individual responses to opioids.
Acknowledgments
Financial Support:
Supported by grant No. R01- GM47502.09 from the National Institutes of Health and in part by the Nema Foundation, Malaysia.
Footnotes
Disclaimers:
Senior authors MC Tissot van Patot and TK Henthorn contributed equally to this work.
Conflict of Interest:
None
Implications Statement
These findings suggest that transport proteins variably regulate fentanyl and loperamide pharmacokinetics and pharmacodynamics and may provide potential targets for therapeutics designed to modulate individual responses to opioids. The interindividual variability of fentanyl response may be related to currently unappreciated transporter interactions with other drugs, such as statins or variable transporter expression, thus affecting pharmacodynamics.
References
- 1.Hanks GW, Reid C. Contribution to variability in response to opioids. Supportive Care in Cancer. 2005;13:145–152. doi: 10.1007/s00520-004-0730-2. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol. 2004;67:269–276. doi: 10.1016/j.bcp.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Waters CM, Avram MJ, Krejcie TC, Henthorn TK. Uptake of fentanyl in pulmonary endothelium. J Pharmacol Exp Ther. 1999;288:157–163. [PubMed] [Google Scholar]
- 4.Henthorn TK, Krejcie TC, Avram MJ, Jensen TR, Waters CM. Transporter-mediated pulmonary endothelial uptake of fentanyl. Int J Clin Pharmacol Ther. 1998;36:74–75. [PubMed] [Google Scholar]
- 5.Henthorn TK, Liu Y, Mahapatro M, Ng KY. Active transport of fentanyl by the blood-brain barrier. J Pharmacol Exp Ther. 1999;289:1084–1089. [PubMed] [Google Scholar]
- 6.Hagenbuch B, Gao B, Meier PJ. Transport of xenobiotics across the blood-brain barrier. News Physiol Sci. 2002;(17):231–234. doi: 10.1152/nips.01402.2002. [DOI] [PubMed] [Google Scholar]
- 7.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- 8.Heel RC, Brogden RN, Speight TM, Avery GS. Loperamide: a review of its pharmacological properties and therapeutic efficacy in diarrhoea. Drugs. 1978;15:33–52. doi: 10.2165/00003495-197815010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Heykants J, Michiels M, Knaeps A, Brugmans J. Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 5: the pharmacokinetics of loperamide in rats and man. Arzneimittelforschung. 1974;24:1649–1653. [PubMed] [Google Scholar]
- 10.Bhatti MM, Foster RT. Pharmacokinetics of the enantiomers of verapamil after intravenous and oral administration of racemic verapamil in a rat model. Biopharm Drug Dispos. 1997;18:387–396. doi: 10.1002/(sici)1099-081x(199707)18:5<387::aid-bdd26>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Cox EH, Kerbusch T, Van der Graaf PH, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effect of synthetic opioids in the rat: correlation with the interaction at the mu-opioid receptor. J Pharmacol Exp Ther. 1998;284:1095–1103. [PubMed] [Google Scholar]
- 12.Gustafsson LL, Ebling WF, Osaki E, Harapat S, Stanski DR, Shafer SL. Plasma concentration clamping in the rat using a computer-controlled infusion pump. Pharm Res. 1992;9:800–807. doi: 10.1023/a:1015863824277. [DOI] [PubMed] [Google Scholar]
- 13.Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22:431–445. doi: 10.1007/BF02353864. [DOI] [PubMed] [Google Scholar]
- 14.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 15.Ebling WF, Lee EN, Stanski DR. Understanding pharmacokinetics and pharmacodynamics through computer stimulation: I. The comparative clinical profiles of fentanyl and alfentanil. Anesthesiology. 1990;72:650–658. doi: 10.1097/00000542-199004000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Seithel A, Eberl S, Singer K, Auge D, Heinkele G, Wolf NB, Dorje F, Fromm MF, Konig J. The influence of macrolide antibiotics on the uptake of organic anions and drugs mediated by OATP1B1 and OATP1B3. Drug Metab Dispos. 2007;35:779–786. doi: 10.1124/dmd.106.014407. [DOI] [PubMed] [Google Scholar]
- 17.Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- 18.Nozaki-Taguchi N, Yaksh TL. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist--loperamide. Anesthesiology. 1999;90:225–234. doi: 10.1097/00000542-199901000-00029. [DOI] [PubMed] [Google Scholar]
- 19.Sadeque AJ, Wandel C, He H, Shah S, Wood AJ. Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther. 2000;68:231–237. doi: 10.1067/mcp.2000.109156. [DOI] [PubMed] [Google Scholar]
- 20.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 21.Gao B, Stieger B, Noe B, Fritschy JM, Meier PJ. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem. 1999;47:1255–1264. doi: 10.1177/002215549904701005. [DOI] [PubMed] [Google Scholar]
- 22.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 23.Waters CM, Krejcie TC, Avram MJ. Facilitated uptake of fentanyl, but not alfentanil, by human pulmonary endothelial cells. Anesthesiology. 2000;93:825–831. doi: 10.1097/00000542-200009000-00033. [DOI] [PubMed] [Google Scholar]