Abstract
Recirculation of blood lymphocytes through the liver occurs under normal conditions as part of the process of immune surveillance. In response to injury or infection recruitment from blood increases and the nature and distribution of the infiltrate will determine the type and outcome of the resulting hepatitis. Recruitment from blood occurs via the hepatic sinusoids and is controlled by interactions between circulating lymphocytes and the highly specialised sinusoidal endothelial cells. This is a low flow vascular bed and the molecular basis of recruitment differs from other tissues. In this review we outline the molecular basis of lymphocyte recruitment to the liver and the effect on it of the local tissue microenvironment and how dysregulation of these processes can lead to uncontrolled inflammation and liver damage.
Introduction
Lymphocyte Homing and Migration
The immune system has developed to respond to foreign antigen in a rapid and specific manner. A vital part of this process involves lymphocyte recirculation and homing. These complex systems of lymphocyte trafficking are designed to facilitate both continual immune surveillance and the ability to recognize foreign antigen wherever it enters the body (Butcher & Picker 1996). As a result, lymphocytes are continually recirculating between specific sites and they need to migrate into tissues where antigen is found since they are only activated by direct contact with pathogen derived antigen (von Andrian & Mackay 2000). A T-cell which has not encountered antigen previously, termed a naïve T-cell, is programmed to migrate to secondary lymphoid organs (peripheral lymph nodes, Peyers patches, tonsils, spleen) where it interacts with dendritic cells which present antigen that they have taken up in the periphery (Banchereau & Steinman 1998). If the T-cell recognizes the antigen presented by the dendritic cell it becomes an activated effector T-cell, and gains the ability to migrate to the site of inflammation as well other areas of the secondary lymphoid tissue to interact with B-cells. Once the antigen is cleared most T-effector cells die but memory T-cells remain to either patrol the peripheral tissue or remain in lymphoid organs (Sallusto et al. 1999) in case of subsequent reoccurrence of the same antigen challenge.
Throughout the processes described above lymphocytes need to have the ability to cross vascular barriers from blood into tissue, from tissue into the lymphatics and then back into the blood. The migration from blood into tissue takes place in post capillary venules (except in the spleen, lung and liver). In all cases, the key interaction is between the lymphocyte and the endothelial cell lining the vessel (Springer 1995). Lymphocytes interact with endothelium under conditions of blood flow by initially tethering and rolling on the vessel wall before undergoing arrest and transendothelial migration into tissue. This is known as the multi-step adhesion cascade and distinct families of adhesion molecules and chemotactic cytokines control which lymphocyte subsets are recruited at a particular site (Springer 1995). Table 1 summarises the major adhesion molecules which play a role in the leucocyte adhesion cascade. In the initial step known as rolling, lymphocytes are captured from the blood by selectins (Tedder et al. 1995) or members of the immunoglobulin superfamily (Alon et al. 1995;Berlin et al. 1995;Lalor et al. 1997) which induce rolling on the vessel wall allowing G-protein coupled receptors (GPCRs) on the lymphocyte surface to be activated by specific chemokines sequestered in the endothelial glycocalyx (Adams & Lloyd 1997;Campbell et al. 1996). Activation of these receptors triggers an intracellular signaling cascade which clusters integrins on the lymphocyte membrane at sites of contact and alters integrin confirmation to a ‘high affinity’ state allowing efficient binding to immunoglobulin family receptors on the endothelium resulting in arrest and firm adhesion to the vessel wall (Campbell et al. 1998;Tanaka et al. 1993). The final step is transmigration through the endothelial layer. This is a complex and poorly understood process which involves multiple adhesion molecules and chemokines (Imhof & urrand-Lions 2004). Over the last decade further insights have improved our understanding of each step of the adhesion cascade (Ley et al. 2007).
Table 1.
Summary of the major molecular determinants of the leucocyte adhesion cascade
| Molecule | Expression | Role in Adhesion Cascade |
|---|---|---|
| L selectin | Leucocytes | Rolling |
| P selectin | Endothelial cells and Platelets | Rolling |
| E selectin | Endothelial cells | Rolling |
| Selectin Ligands | ||
| Sialyl Lewisx | Various cells regulated by fucosyl transferase VII | Ligand for all selectins which is presented on the following molecules : |
| PSGL-1 | Leucocytes | Main ligand for P-selectin but binds all selectins |
| PNAd | High endothelial venules | Ligand for L-selectin |
| CLA | Skin homing leucocytes | Ligand for E-selectin |
| β2 Integrins | Firm adhesion and transmigration | |
| αL β2 | Leucocytes | |
| αM β2 | Myeloid cells | |
| α4 Integrins | Rolling and Firm Adhesion | |
| α4 β1 | Leucocytes except neutrophils | |
| α4 β7 | Gut homing leucocytes | |
| Immunoglobulin Superfamily | ||
| ICAM-1 | Increased expression on endothelial cells with cytokines | Ligand for β2 Integrins: firm adhesion and transmigration |
| ICAM-2 | Constitutive on endothelium | Ligand for β2 integrins: firm adhesion and transmigration |
| VCAM-1 | Induced expression on endothelial cells with cytokines | Ligand for α4 Integrins: rolling and firm adhesion |
| MAdCAM-1 | High endothelial venules on gut associated lymphoid tissue | Ligand for L-selectin and rolling and α4 β7: firm adhesion |
| PECAM-1 | Endothelial cells and leucocytes | Transmigration |
| JAM A,B,C | Endothelial cells | Transmigration |
PSGL-1, P-selectin glycoprotein ligand 1; PNAd, Peripheral-node addressin; CLA, Cutaneous lymphocyte antigen; ICAM-1, Intercellular adhesion molecule 1; ICAM-2, Intercellular adhesion molecule 2; VCAM-1, Vascular cell adhesion molecule 1; MAdCAM-1, Mucosal vascular addressin cell adhesion molecule 1; PECAM-1, Platelet-endothelial cell adhesion molecule 1; JAM, Junctional adhesion molecule.
Rolling
Capture and rolling adhesion is classically mediated by the selectins (L-selectin, P-selectin and E-selectin), which bind to glycosylated ligands (McEver & Cummings 1997). L-selectin is expressed by most leucocytes, whereas E- and P-selectin are predominantly expressed on endothelial cells. The selectin-ligand interaction involves a ‘catch-bond’ phenomenon in which each bond is strengthened with increasing shear stress (Marshall et al. 2003). In addition a transport phenomenon between selectin ligands and selectins allows the rolling motion of the cell to generate new bonds before old ones are broken (Yago et al. 2007). In some circumstances capture can be mediated by integrins, particularly α4 integrins and their endothelial ligands VCAM-1 and MAdCAM-1 (Berlin et al. 1995;Sigal et al. 2000). Selectins and integrins may also cooperate to mediate ‘slow rolling’. Here initial rolling triggered by engagement of selectins leads to integrin activation and triggering of slow rolling as evidenced for the β2 integrins, LFA-1 (lymphocyte function-associated antigen 1, αLβ2) and MAC-1 (macrophage receptor 1, αmβ2) (Dunne et al. 2002).
Activation and Arrest
The arrest of lymphocytes is triggered by chemokines and other chemoattractants that activate integrins to bind immunoglobulin superfamily members such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells (Campbell et al. 1996;Campbell et al. 1998). During inflammation, endothelial cells are activated by cytokines to express adhesion molecules and synthesize chemokines that are presented on their luminal surface via binding to glycosaminoglycans (GAGs) in the endothelial glycocalyx (Johnson et al. 2005). The binding of chemokines to GPCRs on the lymphocyte surface leads to integrin activation via ‘inside-out’ signaling. This induces the integrin to undergo a conformational change from a bent low affinity state to an extended conformation which opens up the ligand binding pocket (Arnaout et al. 2005). The pathway that leads to this activation has not been completely described but there appear to be three stages involving phospholipase C (PLC), activation of small GTPases and finally transitional integrin conformational changes through association with actin-binding proteins such as Talin-1 (Ley et al. 2007).
Transendothelial Cell Migration
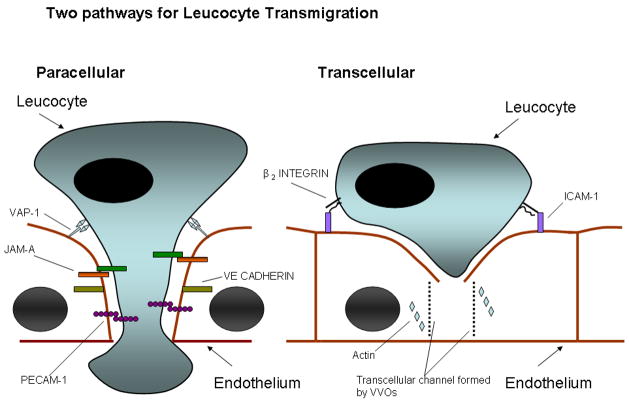
The initial step in migration is crawling on the vessel wall which is integrin-and ICAM-1 dependent (Phillipson et al. 2006;Schenkel et al. 2004). Initiation of transmigration may involve several mechanisms including chemoattractants acting under conditions of shear stress and interactions between integrins and endothelial cell ligands (ICAM-1 and VCAM-1) which stimulate endothelial cells to promote migration. Adherent leucocytes can induce the formation of ‘docking sites’ or ‘transmigratory cups’ which are rich in ICAM-1 and VCAM-1 (Barreiro et al. 2002;Carman & Springer 2004). Following this there are two possible routes for migration, paracellular and transcellular (Figure 1). In the paracellular route, ligation of adhesion molecules leads to weakening of intra-endothelial contacts. This has been shown for ICAM-1 ligation which leads to release of intracellular calcium and activation of p38 mitogen activated protein kinase and Ras homologue (RHO) GTPase which results in opening up of endothelial junctions (Huang et al. 1993;Millan & Ridley 2005). In addition endothelial junctional molecules which bind leucocytes such as platelet/endothelial cell adhesion molecule 1 (PECAM 1) and junctional adhesion molecule A (JAM A) are mobilized to junctions and thereby create a haptotactic gradient which guides leucocytes to the junctional zone (Muller 2003). The molecules directly implicated in migration include immunoglobulin superfamily members- PECAM-1,ICAM-1,ICAM-2,JAM-A,B and C and endothelial cell–selective adhesion molecule (ESAM), as well as the non-immunoglobulin molecule CD99 (Ley et al. 2007). Other molecules implicated include poliovirus receptor (PVR, CD155), and several ectoenzymes, VAP-1 (Vascular adhesion protein-1) and CD157 and leucocyte specific protein 1 (LSP1) (Liu et al. 2005;Reymond et al. 2004;Salmi & Jalkanen 2005). Thus several interactions must occur in sequence for transmigration to take place as demonstrated by the fact that blockade of CD99 and PECAM-1 exerts an additive effect on leucocyte transmigration (Lou et al. 2007;Schenkel et al. 2002).
Figure 1.
Schematic representation of two pathways that may be taken by leucocytes during transmigration across endothelial layers. The paracellular route involves loosening of endothelial tight junctions (maintained by VE-cadherin) and migration of the leucocyte through the junction by binding to adhesion molecules ( PECAM-1 and JAM-A). In the context of the liver we believe that VAP-1 also plays a significant role in this process. The transcellular route involves interaction with ICAM-1 on the endothelial surface which leads to a channel forming through the endothelial cell by the formation of VVOs (vesiculo-vacuolar organelles). This channel is supported by cytoskeletal proteins such as actin.
The transcellular route has been demonstrated in the central nervous system and at sites of inflammation as well as in in vitro models (Engelhardt & Wolburg 2004;Millan et al. 2006). It appears to involve a minority of leucocytes (5–20% of cells binding cytokine treated HUVEC). The pathway involves translocation of apical ICAM-1 to caveolae and F–actin rich regions and the transport of caveolin-1 to the basal membrane resulting in a channel through which the leucocyte can migrate (Cinamon et al. 2004;Millan et al. 2006).
The lymphocyte adhesion cascade is the generally accepted pathway by which lymphocytes interact with endothelium but it is clear that there are specific molecular determinants which regulate lymphocyte homing to specific tissues. Such tissue-specificity is demonstrated by the molecular regulation of lymphocyte homing to skin and the intestine which have been described in recent years (Butcher & Picker 1996;Robert & Kupper 1999). It is tempting to speculate that tissue-specific signals will also be involved in the liver; certainly recruitment to the liver involves unusual combinations of adhesion receptors although a truly tissue-specific liver homing receptor remains elusive (Lalor et al. 2002b).
Lymphocyte Recruitment to the Liver
The human liver contains a significant number of resident lymphocytes, estimated at 1010 (Racanelli & Rehermann 2006). This lymphocyte population is comprised of conventional lymphocytes (B cells, CD4+ and CD8+ T cells) and unconventional lymphocytes (NK and NKT cells). During the development of inflammatory liver diseases, lymphocyte recruitment increases markedly and the intrahepatic localization of these infiltrating lymphocytes will determine the nature and severity of disease. For example a parenchymal infiltrate leads to a lobular hepatitis as seen in viral hepatitis, whereas portal infiltrates around bile ducts are seen in biliary diseases such as primary biliary cirrhosis and primary sclerosing cholangitis (Lalor et al. 2002b).
The hepatic vasculature
The liver’s vascular supply has a major effect on hepatic immune regulation. The liver has a unique dual blood supply. Arterial blood from the systemic circulation via the hepatic arteries comprises only 25% of the blood supply, the rest coming from portal venous blood which drains the gut and splanchnic organs. The hepatic artery and portal vein both drain into the hepatic sinusoids and blood then flows from the portal area into the hepatic venules at the centre of the hepatic lobule. These venules connect to form the hepatic veins that drain into the inferior vena cava. A response to infection and injury is characterized by lymphocytes binding to and migrating across endothelial cells that line the hepatic microvasculature. But the endothelial cells lining the hepatic microvasculature are not uniform in structure (Aird 2007). The portal venules and hepatic arterioles are lined by endothelium which is similar to vascular endothelium but there is still heterogeneity within these vessels. The portal venules are lined by spindle shaped endothelium which is non-fenestrated but covered with short microvilli. At the transition from portal venule to the hepatic sinusoid, the endothelium is smooth and large and contains many actin fibres. This allows the endothelium to act as part of a sphincter which controls blood flow into the sinusoids. Endothelial heterogeneity is most striking in the hepatic sinusoids which form a unique capillary bed lined by discontinuous endothelium containing fenestrae; open pores 100 to 200nm in diameter which make up 6–8% of the endothelial surface, and lacking a classical basement membrane (Braet & Wisse 2002). This unique architecture is in part a consequence of the low flow sinusoidal environment, estimated to be 400–450 m/sec compared with 500–1000 m/sec in other capillary beds. This allows the sinusoidal endothelium to function as a sieve and the fenestrae to act as dynamic filters for fluids, solutes and particles (Braet & Wisse 2002).
A unique environment for lymphocyte recruitment
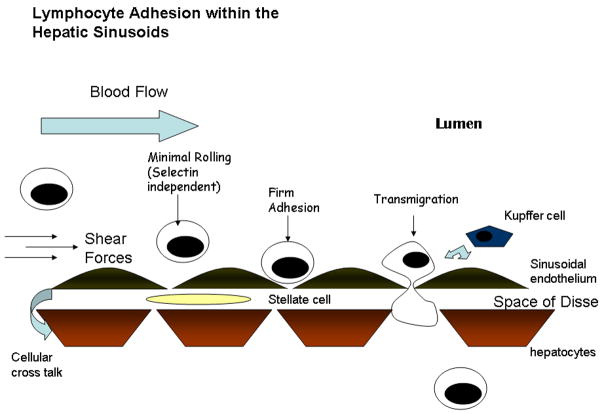
There is now a significant body of evidence that lymphocyte interactions within the hepatic microvasculature do not conform to the classical pathway of leucocyte adhesion described earlier (Lee & Kubes 2008) (Figure 2). Intravital experiments show that although leucocytes are capable of adhesion and migration across different regions of the microvasculature, the majority adhere to the hepatic sinusoids (Wong et al. 1997). This is unlike leucocyte extravasation in most other tissues, which usually takes place in the post-capillary venules. Hepatic sinusoids have several functions which set them apart from other endothelial beds. They are the primary site for filtration in the liver and also display highly efficient scavenging function allowing the liver to remove soluble waste molecules and colloids from the circulation (Smedsrod 2004). This function is mediated by scavenging receptors such as the mannose receptor and stabilin-2. Thirdly they have an important role in antigen presentation being able to process and present antigens to both CD4 and CD8 T cells and to tolerize immune responses to CD8 cells (Knolle & Limmer 2001). In keeping with this unique pattern of receptor expression, hepatic sinusoidal endothelial cells (HSEC) display differences in adhesion molecule expression compared with other endothelial cells. A fundamental example is the lack of expression of P-selectin in vitro or in vivo and the markedly reduced expression of E-selectin (Adams et al. 1996). Furthermore, there is no upregulation of these receptors on HSEC in diseases such as PBC (Steinhoff et al. 1993). In contrast portal vascular endothelium does upregulate selectins in inflammation, highlighting the heterogeneity within the hepatic microvasculature (Adams et al. 1994).
Figure 2.
Schematic representation of adhesion cascade within the hepatic sinusoids- The low shear environment allows selectin independent recruitment with a brief rolling step leading to firm adhesion. The final step is transmigration through the specialised endothelial layer. There are many factors which influence this process by modulating the expression, secretion and presentation of adhesion molecules and chemokines. It is becoming clear that the microenvironment plays a significant role and the cellular cross-talk between kupffer cells, hepatocytes, stellate cells and the endothelium allows paracrine interactions to alter the molecules expressed and presented.
Endothelial adhesion molecules involved in lymphocyte recruitment to the liver
Intravital experiments confirmed that lymphocyte recruitment within the hepatic sinusoids was selectin independent (Wong et al. 1997). In view of these findings, lymphocyte recruitment was initially thought to be due to ‘physical trapping’ but subsequent studies have demonstrated that lymphocytes do interact with sinusoidal endothelium via adhesion receptors. ICAM-1 is constitutively expressed on post-capillary venules and only minimally on true capillaries, in keeping with the fact that leucocyte adhesion occurs at the post-capillary venules (Iigo et al. 1997) but in the liver the density of ICAM-1 in the hepatic sinusoids is comparable to the central venules (Iigo et al. 1997). VCAM-1 is not expressed in normal liver tissue but it is markedly upregulated on sinusoidal endothelium in inflammatory liver diseases (Volpes et al. 1992). Multiple strands of evidence implicate both ICAM-1 and VCAM-1 in lymphocyte adhesion to hepatic sinusoids. Thus ICAM-1 deficient mice demonstrate reduced leucocyte adhesion to hepatic sinusoids (Wong et al. 1997) and VCAM-1 has been shown to mediate lymphocyte capture and adhesion via α4 integrins. In vitro studies with human HSEC show reduced lymphocyte adhesion to hepatic sinusoids when VCAM-1 and ICAM-1 are blocked (Edwards et al. 2005;Lalor et al. 2002a).
Non-classical endothelial adhesion molecules in the hepatic sinusoids
ICAM-1 and VCAM-1 play a role in adhesion of leucocytes to endothelium, but the liver sinusoids also express another adhesion receptor known as VAP-1 which appears to play a more specific role for lymphocyte recruitment to the liver. VAP-1 is a 170kDa homodimeric glycoprotein that has monoamine oxidase activity. It is expressed constitutively on hepatic sinusoids but is absent from other non-inflamed vessels in extralymphoid organs (Lalor et al. 2002a). In vitro, VAP-1 mediates lymphocyte adhesion to human hepatic sinusoidal endothelium (Lalor et al. 2002a) and in vivo experiments on mice using intravital microscopy have suggested that VAP-1 regulates the adhesion of certain T-cell subsets (Bonder et al. 2005). VAP-1 has also been implicated in the recruitment of lymphocytes to rodent liver transplants (Martelius et al. 2004). The precise role of VAP-1 is unclear. It is heavily sialydated suggesting it might mediate rolling adhesion and treatment of liver endothelium with sialidase reduces lymphocyte adhesion. However our detailed observations using HSEC monolayers in vitro under flow suggest liver endothelial cells support little classical rolling and instead VAP-1 may mediate initial tethering interactions. VAP-1 also supports transmigration (see below). In addition, the enzyme activity of VAP-1 may modulate the function of other adhesion molecules particularly ICAM-1 and VCAM-1 because provision of substrate to VAP-1 on hepatic endothelial cell monolayers results in the NFκ-B-dependent upregulation of VCAM-1 and ICAM-1 which results in enhanced lymphocyte adhesion from flow (Lalor et al. 2007a).
Another adhesion molecule which is widely expressed but appears to play a particular role in the liver is CD44 which has been shown to mediate sequestration of neutrophils in hepatic sinusoid during sepsis (McDonald et al. 2008). Unlike in other vascular beds the adhesion of neutrophils within hepatic sinusoids is independent of selectins. There are numerous molecules that may potentially mediate selectin-independent tethering in the liver sinusoids including α4 integrins, VAP-1 and CD44 all of which have been reported to support leucocyte–endothelial interactions under flow. A recent study examining neutrophil adhesion on hepatic sinusoids using intravital microscopy suggests that the CD44 ligand hyaluronan is increased on sinusoids during sepsis where it can support CD44 mediated adhesion. Moreover CD44 alone was necessary and sufficient for reversible neutrophil adhesion within liver sinusoids in vivo (McDonald et al 2008). This occurred as a consequence of an increased deposition of serum-derived HA-associated protein on sinusoidal endothelium which enhanced the CD44-dependent adhesion of leucocytes (McDonald et al. 2008). CD44 has been shown to support lymphocyte trafficking in other situations but whether it can also support lymphocyte adhesion to hepatic sinusoids is yet to be defined (DeGrendele et al. 1997).
Recent evidence implicates two other receptors in lymphocyte adhesion to hepatic endothelium, namely the mannose receptor and common lymphatic endothelial and vascular endothelial receptor-1 (CLEVER-1) (Irjala et al. 2001;Irjala et al. 2003;Salmi et al. 2004). Both these molecules are part of the scavenger receptor family. The mannose receptor has been shown to a play a role in endocytic clearance of certain glycoproteins and phagocytosis of microrganisms by macrophages (Taylor et al. 1990). In addition it appears to modulate the levels of endogenous proteins which bear high mannose oligosaccharides such as lysosomal enzymes and tissue plasminogen activator. The extracellular region of the mannose receptor allows recognition of a diverse range of glycoconjugate ligands (McGreal et al. 2005;Taylor et al. 1990;Taylor et al. 2005). The receptor was firstly shown to be expressed on afferent and efferent lymphatic systems but not on high endothelial venules (HEVs). A role in lymphocyte homing was then suggested by blockade of the mannose receptor which led to a significant decrease in lymphocyte binding in a static in-vitro assay. The mannose receptor is also a counter–receptor for L-selectin, which has already been shown to play an important role in lymphocyte trafficking within HEVs (Irjala et al. 2001;Irjala et al. 2003).
CLEVER-1 (also known as FEEL-1 and Stabilin-1) is a large glycoprotein which is made up of multiple EGF-like repeats, tandem fasciculin-like domains, a proteoglycan link protein-like sequence and 2 RGD motifs (Salmi et al. 2004). Like the mannose receptor it has scavenger receptor properties and has been shown to internalize modified LDLs, whole bacteria and advanced glycation end products (Politz et al. 2002;Tamura et al. 2003). Recent studies have shown that CLEVER-1 is expressed constitutively by lymphatic endothelium and during inflammation by vascular endothelium. This was followed by the demonstration that CLEVER-1 mediates lymphocyte adhesion and migration through cultured lymphatic endothelium (Salmi et al. 2004). Both mannose receptor and CLEVER-1 have been demonstrated to be expressed on hepatic sinusoidal endothelium and another related molecule stabilin-2 or FEEL-2 has been shown to mediate lymphocyte adhesion to sinsuoidal endothelial cells, thus adding to the molecules potentially involved at this site (Jung et al. 2007).
In some inflammatory conditions hepatic endothelium can be induced to express the endothelial adhesion molecule MAdCAM-1 which is normally confined to mucosal endothelium in the bowel (Briskin et al. 1997). It plays a critical role in the compartmentalization of lymphocyte trafficking by recruiting gut-specific mucosal lymphocytes. However in immune-mediated liver diseases that complicate inflammatory bowel disease MAdCAM-1 can be induced in the liver by as yet unknown factors, with the result that mucosal T cells are recruited to the liver where they drive immune mediated damage. MAdCAM-1 is absent from normal liver endothelium and not induced in most other inflammatory diseases (Hillan et al. 1999).
Chemokines involved in lymphocyte recruitment to the liver
As discussed above, chemokines play a critical role in several steps of the adhesion cascade involving triggering of integrin-mediated adhesion and migration responses. Chemokines are crucial for lymphocyte trafficking to the liver and particular chemokines secreted by liver cells contribute to the compartmentalization of lymphocyte recruitment within the liver (Figure 3). The CCR5 ligands CCL3–5 are strongly expressed in vascular endothelium within portal tracts where they mediate lymphocyte recruitment in a range of inflammatory diseases including graft versus host disease, immune mediated liver disease and graft rejection (Goddard et al. 2001;Murai et al. 1999). Lymphocytes are also found within normal portal tracts and basal levels of some of these chemokines may support trafficking of lymphocytes through the non-inflamed liver. One of the critical events in the progression of injury in hepatitis is infiltration of the parenchyma, either as a component of interface hepatitis and extension of portal inflammation or as direct lobular infiltration in lobular hepatitis. Lymphocyte recruitment into the parenchyma via inflamed hepatic sinusoids is a feature of progressive liver injury in chronic viral infection and autoimmune disease and is associated with strong expression of the CXCR3 ligands CXCL9–11 on sinusoidal endothelium (Harvey et al. 2003;Shields et al. 1999). These chemokines are not only secreted by endothelium but also by cholangiocytes, hepatocytes and stellate cells in the inflamed liver. Whatever their source they can be presented on sinusoidal endothelium. For example the process of transcytosis allows chemokines produced by stellate cells or underlying hepatocytes in the local microenvironment to be transported from the basolateral to the luminal surface of the endothelium, or chemokines secreted by ‘upstream’ cholangiocytes can be captured by retention in the proteoglycan-rich endothelial glycocalyx (Curbishley et al. 2005). Further evidence that CXCR3 ligands are important for lymphocyte infiltration into the liver come from several studies showing that lymphocytes isolated from inflamed human liver parenchyma express high levels of CXCR3 and migrate to CXCR3 ligands in vitro (Harvey et al. 2003;Narumi et al. 1997;Shields et al. 1999). In vitro human experiments show that activation of CXCR3 on lymphocytes by its ligands promotes lymphocyte adhesion and transmigration across HSEC (Curbishley et al. 2005). In vivo experiments suggest that CXCR3 and its ligands play a significant role in recruitment of virus specific CD8+ T cells to the murine liver (Hokeness et al. 2007).
Figure 3.
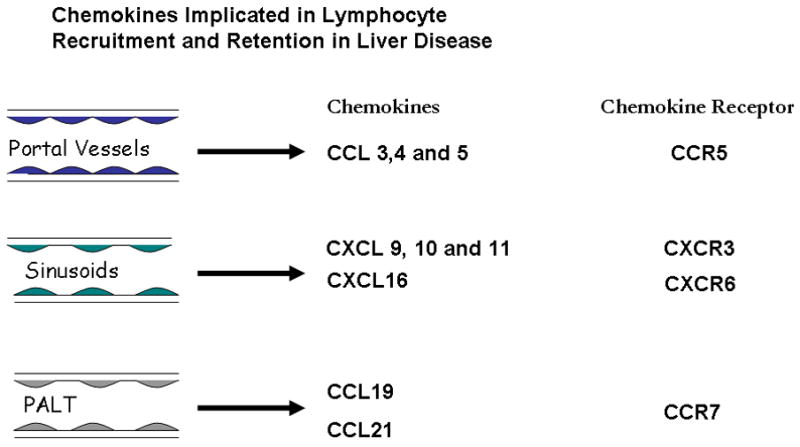
Summary of chemokines involved in the pathogenesis of liver disease. Lymphocytes can be recruited across the portal endothelium and this appears to be mediated by the chemokines CCL3–5, which are ligands for CCR5. Recruitment across sinusoidal endothelium is mediated by the chemokines CXCL9–11 as well as CXCL16. In some forms of chronic hepatic inflammation there is neovessel formation within the portal tracts which has characteristics of lymphoid tissue. This has been termed portal associated lymphoid tissue (PALT) and has been shown to be associated with the chemokines CCL19 and CCL21 which are ligands for CCR7.
A significant proportion of liver-infiltrating lymphocytes in inflamed liver tissue also express the chemokine receptor CXCR6. These cells include CD4, CD8 and NKT cells. The CXCR6 ligand is a transmembrane chemokine CXCL16 expressed by cholangiocytes and hepatocytes in inflamed liver and sinusoidal endothelium in normal liver (Heydtmann et al. 2005). CXCR6 has been shown to mediate trafficking of NKT cells in murine sinusoids (Geissmann et al. 2005) and the increased expression of CXCL16 on inflamed epithelial cells led us to suggest that it may act to localize and retain effector lymphocytes at epithelial cells such as infected hepatocytes in viral hepatitis (Heydtmann et al. 2005). Another chemokine that is highly expressed on inflamed endothelium is CCL28. This chemokine was originally isolated from the gut but is widely expressed at mucosal surfaces throughout the body. We detected increased expression of CCL28 on inflamed endothelium, cholangiocytes and hepatocytes in a variety of liver diseases and found increased numbers of T cells expressing the CCL28 receptor, CCR10 in the inflamed human liver (Eksteen et al. 2006). However to our surprise a high proportion of these cells were phenotypically and functionally regulatory cells; they were CD4+ and CD25high, expressed FoxP3 and suppressed naïve T cell activation in vitro. When compared with Treg in blood the CCR10+, liver-derived Treg expressed high levels of CXCR3 and low levels of CCR7 suggesting they have a tissue infiltrating phenotype and leading us to propose that they also use CXCR3 to enter the liver but then localized in inflamed bile ducts using CCR10.
Further evidence for the important role of chemokines in liver inflammation is the suggestion that aberrant homing of mucosal T-cells to the liver underlies the pathogenesis of primary sclerosing cholangitis (PSC) (Adams & Eksteen 2006). This is supported by the finding of CCL25, which is usually present in the gut, on hepatic endothelium in PSC and its role in promoting the recruitment of lymphocytes bearing its receptor, CCR9 (Eksteen et al. 2004).
In addition to a role in recruitment to the liver, chemokines are also important for exit of effector cells via lymphatics out of tissues (Bromley et al. 2005;Debes et al. 2005). We observed that a subset of liver infiltrating lymphocytes express the lymph node homing receptor CCR7. The expression of CCR7 and L-selectin on central memory cells enables them to traffic to lymph nodes via HEV (Campbell et al. 2001a;Campbell et al. 2001b), however the liver-infiltrating CCR7+ T cells we described expressed low levels of L-selectin, which distinguishes them from classical central memory cells and precludes them from entering lymph nodes via HEV. We suggest that CCR7 is involved in trafficking to and from the liver, a possibility that is supported by the presence of the CCR7 ligands CCL19 and CCL21 on CD31+/CD34+ neovessels and portal lymphatic vessels in inflamed liver tissue. The presence of CCL19 on the abluminal aspect of lymphatic vessels and CCL21 on sinusoidal endothelium is consistent with a role in promoting exit from tissue into the lymphatics (Grant et al. 2002) and suggests that CCR7 ligands provide a route for CCR7+ lymphocytes to exit the liver to regional lymph nodes (Heydtmann et al. 2006).
Transendothelial migration across the hepatic sinusoids
Lymphocyte transmigration across sinusoidal endothelium again involves different receptors compared with those implicated in transmigration through vascular endothelium. The tight junctions of vascular endothelium express PECAM/CD31 which has been shown to mediate leucocyte transmigration (Ley et al. 2007). Liver sinusoidal cells lack tight junctions and express lower levels of CD31 than vascular endothelium and blocking CD31 in mice does not inhibit neutrophil extravasation in the liver (Chosay et al. 1998). Additional experiments using both knockout mice and blocking antibodies suggested that junctional adhesion molecule A (JAM-A) mediated neutrophil transmigration but did not play a role in T-cell migration (Khandoga et al. 2005). The precise molecular basis of lymphocyte transmigration across sinusoidal endothelium is not known but in vitro studies using flow-based transmigration assays have suggested a role for distinct combinations of receptors. Thus ICAM-1 and LFA-1 are implicated as they are in other vascular beds, but in addition there is an important role for VAP-1 which not only mediates adhesion, but also drives transmigration of adherent lymphocytes under flow (Lalor et al. 2002a). This molecule is constitutively expressed in the liver and is not regulated by the same proinflammatory signals as ICAM-1 and VCAM-1. It is not yet clear how VAP-1 mediates adhesion and transmigration and the nature of its leukocyte ligand is not clear. Lalor et al., have also shown that the enzymatic activity of VAP-1 can mediate NFκ-B-dependent activation of human HSEC leading to expression of adhesion molecules and chemokines (Lalor et al. 2007a). These factors as well as the finding that circulating levels of VAP-1 are significantly increased in chronic inflammatory liver diseases makes this molecule an attractive therapeutic target for such conditions (Lalor et al. 2007b). Figure 4 summarises the molecular determinants of lymphocyte recruitment on the hepatic sinusoids.
Figure 4.
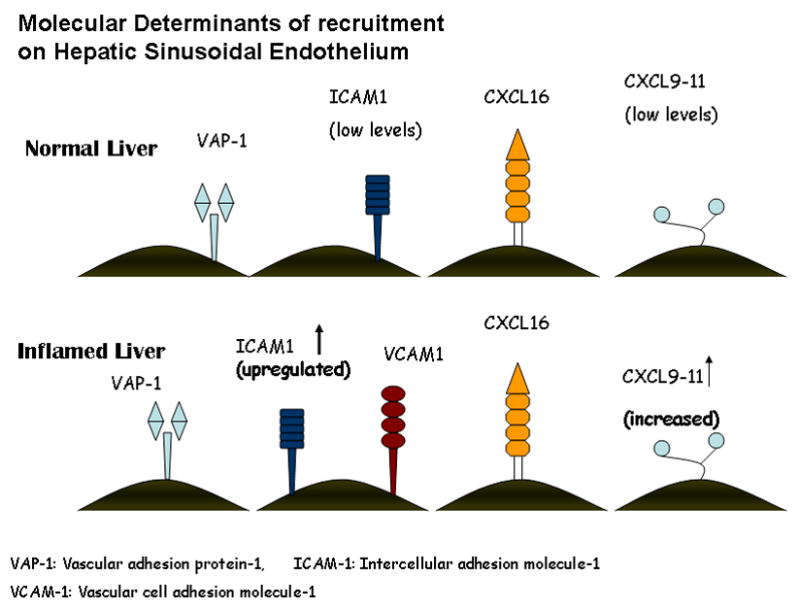
Molecular determinants of lymphocyte recruitment to the sinusoids. VAP-1 is expressed in both the normal and inflamed liver and appears to play a role in the brief rolling step and transmigration. ICAM-1 mediates firm adhesion and is present in the normal liver but there is increased expression in the inflamed liver where there is also a role for VCAM-1 in firm adhesion. ICAM-1 binds to the integrin αLβ2 and VCAM-1 binds to the integrin α4β1. The interferon inducible chemokines CXCL9–11 and the chemokine CXCL16 appear to play an important role in the recruitment to the hepatic sinusoids in the inflamed liver. CXCL9–11 are ligands for the chemokine receptor CXCR3 and CXCL16 binds to CXCR6. CXCR3 and CXCR6 have both been found on liver infiltrating lymphocytes.
The role of other cells in the hepatic microenvironment
Traditionally, studies of lymphocyte trafficking have focused on lymphocyte endothelial interactions and in terms of the liver we have concentrated on the hepatic sinusoidal endothelium. But these cells exist in a complex multi-cellular microenvironment where other cells types and signals will influence behaviour of the endothelium via paracrine interactions. This is particularly relevant in the liver where cells such as Kupffer cells, stellate cells and hepatocytes are in close proximity to sinusoidal endothelium. These cells are capable of secreting cytokines and other inflammatory mediators that may influence interactions between lymphocytes and hepatic endothelium.
Kupffer cells, the resident liver macrophages, patrol the hepatic sinusoids ready to phagocytose foreign particles. Activation of Kupffer cells leads to the release of cytokines and other factors (oxygen radicals, Platelet activating factor) which have the ability to promote leucocyte adhesion (Granger 1997). Kupffer cells may also have a physical role in leucocyte adhesion by promoting the trapping of leucocytes within the sinusoids (Jaeschke & Smith 1997). Furthermore interactions between kupffer cells and other liver cell types profoundly effects their behavior and secretion of cytokines (Alabraba et al. 2007). Kupffer cells may also regulate recruitment directly by providing adhesive ligands as has been shown for dendritic cells (Matsuno et al. 2002;Uwatoku et al. 2001).
Stellate cells are fibroblasts situated in the space of Disse and are the central mediators of matrix formation during liver injury and fibrosis (Friedman 2008). It is likely that they also play a direct role in the inflammatory process (Safadi et al. 2004). They are known to secrete chemokines and have been shown to express cell adhesion molecules important for lymphocyte adhesion such as ICAM-1 and VCAM-1 (Knittel et al. 1999;Smith et al. 1997). Our own unpublished data show that stellate cells and activated liver myofibroblasts can support lymphocyte adhesion and promote migration suggesting to us that they may be critical regulators of post-endothelial migration and positioning of cells within the liver parenchyma.
Hepatocytes can also have direct effects on sinusoidal endothelium to promote adhesion. In vitro studies demonstrated that co-culture of human HSEC with hepatocytes led to increased expression of adhesion receptors and this was associated with levels of lymphocyte adhesion equivalent to those seen following stimulation by inflammatory cytokines (Edwards et al. 2005). These effects were not only due to secretion of soluble mediators because the maximum effects were seen when cells were co-cultured in physical contact. Thus secretion of growth factors and cytokines and contact-dependent conditioning will both be involved in determining how sinusoidal endothelial cells respond to paracrine interactions within the sinusoids. Further co-culture experiments will help to clarify the influence various resident cells have on HSEC and their role in lymphocyte recruitment.
The route taken by trafficking lymphocytes
We have discussed the major determinants of lymphocyte homing to the liver. Clarifying this process is not only crucial in understanding the pathogenesis of many liver diseases but also the immune regulation within the normal liver. The precise anatomical route of lymphocytes entering and trafficking through the liver is poorly understood but detailed in vivo studies recently published shed important light on this. It has been clear for many years that lymphocytes are continually migrating from blood into tissue and then into the lymphatic system. The recirculation of lymphocytes was proven by demonstrating the depletion of lymphocytes in experimental animals by the continuous removal of lymph from a thoracic duct fistula (Gesner & Gowans 1962). One of the best described routes is within the lymph node where naïve lymphocytes continuously home to T cell areas via high endothelial vessels allowing them to interact with dendritic cells which have entered the lymph node via the afferent lymph vessels. If the lymphocytes are activated they are imprinted with receptors that allow them to migrate via blood to peripheral tissues whereas lymphocytes which do not recognize antigen leave the lymph node via the efferent lymph vessels into the thoracic duct (von Andrian & Mempel 2003).
The liver, with its large population of resident lymphocytes, is also patrolled by continual trafficking of lymphocytes from blood which then return from the liver into hepatic lymph nodes via lymphatics in large numbers (Xu et al. 2008). This is in keeping with the large antigen load that the liver receives from the gut and the periphery. In terms of where exactly lymphocytes enter from blood into liver tissue, the exact route is still not clear. Recent studies in a rat model provide further insight into the route taken by lymphocytes within the liver (Xu et al. 2008). Using a model where the hepatic lymph nodes were either removed or ligated the authors showed that the transit time within the liver was approximately 3–4 hours, but lymphocytes appeared within the portal area within 0.5 hours. Their studies suggest that most lymphocytes enter via the sinusoids in the periportal regions with the subsequent route taken determined by signals received from the hepatic environment. Lymphocytes may then either exit the liver via lymphatic vessels located in the hepatic portal area to hepatic lymph nodes or be retained within the liver to carry out immune surveillance in the non-inflamed liver or as effector cells in the context of inflammatory disease. Once the lymphocytes enter the liver, the signals which mediate their retention are unclear. As discussed above interactions with epithelial cells or stromal cells may be important, but an additional structure develops during some forms of chronic hepatic inflammation that may act as a portal for ongoing recruitment and retention. Neovessels are formed in the portal tracts which are similar to high endothelial venules seen in lymphoid tissue (Garcia-Monzon et al. 1995) and in both animals and humans they can become organized into ‘portal tract associated lymphoid tissue’ (PALT) (Grant et al. 2002;Yoneyama et al. 2001). These tertiary lymphoid follicles within the portal tract include discreet T and B cell areas and contain dendritic cells and stromal cells. The development of this tissue provides an ideal environment for recruitment and retention of lymphocytes within the liver, and appears to be partly explained by the presence of CCL19 and CCL21 in such lymphoid follicles in chronic inflammatory liver disease. In secondary lymphoid tissues CCL19 and CCL21 lead to the recruitment and organization of naïve T-cells and dendritic cells and they have been implicated in the formation of tertiary lymphoid tissues in chronic inflammation as part of the process of lymphoneogenesis. Such structures provide potential targets for anti-inflammatory treatment, particularly anti-TNF therapy and diseases such as PBC and PSC that are associated with PALT should in theory respond to such biological therapy (Grant et al. 2002).
Conclusion
Our understanding of lymphocyte homing and trafficking has evolved hugely in the last few years, and insights into organ-specific recruitment pathways provide insights into disease pathogenesis. Significant advances have been made in our comprehension of hepatic lymphocyte recruitment, how this differs from recruitment to other organs and how the processes are modulated in inflammatory liver disease. Further work is required to identify the molecular basis of recruitment via the hepatic microvasculature and how the hepatic microenvironment influences and drives disease processes. The determinants of lymphocyte recruitment to the liver may well represent therapeutic targets for inflammatory liver diseases, which may provide a means of preventing the pathological progression to fibrosis and cirrhosis, without disturbing physiological lymphocyte recruitment.
Acknowledgments
Grant support
Shishir Shetty is supported by a BRET-CORE fellowship.
The work is supported by grants from the European Commission (QLG1-CT-1999-00295); the Medical Research Council G0300101 and the National Institutes of Health 5RO1AA014257. We are grateful to our colleagues at the Queen Elizabeth Hospital, Birmingham UK for help with sample collection.
List of Abbreviations
- GPCRs
G-Protein coupled receptors
- VCAM-1
Vascular cell adhesion molecule
- MAdCAM-1
Mucosal vascular addressin cell adhesion molecule-1
- LFA-1
Lymphocyte function-associated antigen-1
- MAC-1
Macrophage receptor-1
- ICAM-1
Intercellular adhesion molecule-1
- GAGs
Glycosoaminoglycans
- PLC
Phospholipase C
- RHO
Ras homologue
- PECAM-1
Platelet/endothelial cell adhesion molecule-1
- JAM
Junctional adhesion molecule
- ESAM
Endothelial cell-selective adhesion molecule
- PVR
Poliovirus receptor
- VAP-1
Vascular Adhesion Protein-1
- LSP1
Leucocyte specific protein
- HUVEC
Human umbilical vein endothelial cell
- NK cell
Natural Killer cell
- NK-T cell
Natural Killer-T cell
- HSEC
Hepatic sinusoidal endothelial cell
- CLEVER-1
Common lymphatic endothelial and vascular endothelial receptor-1
- HEVs
High endothelial venules
- PSC
Primary sclerosing cholangitis
- PALT
Portal tract associated lymphoid tissue
Footnotes
Liver Research Group, 5th Floor, Institute of Biomedical Research, Wolfson Drive, The Medical School, University of Birmingham, Birmingham B15 2TT, UK
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DH, Burra P, Hubscher SG, Elias E, Newman W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology. 1994;19(3):588–594. doi: 10.1002/hep.1840190308. [DOI] [PubMed] [Google Scholar]
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6(3):244–251. doi: 10.1038/nri1784. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hubscher SG, Fisher NC, Williams A, Robinson M. Expression of E-selectin and E-selectin ligands in human liver inflammation. Hepatology. 1996;24(3):533–538. doi: 10.1002/hep.510240311. [DOI] [PubMed] [Google Scholar]
- Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349(9050):490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- Alabraba EB, Lai V, Boon L, Wigmore SJ, Adams DH, Afford SC. Coculture of human liver macrophages and cholangiocytes leads to CD40-dependent apoptosis and cytokine secretion. Hepatology. 2007 doi: 10.1002/hep.22011. [DOI] [PubMed] [Google Scholar]
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128(6):1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout MA, Mahalingam B, Xiong JP. INTEGRIN STRUCTURE, ALLOSTERY, AND BIDIRECTIONAL SIGNALING. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;%19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol JID - 0375356. 2002;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80(3):413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Bonder CS, Norman MU, Swain MG, Zbytnuik LD, Yamanouchi J, Santamaria P, Ajuebor M, Salmi M, Jalkanen S, Kubes P. Rules of recruitment for Th1 and th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity. 2005;23(2):153–163. doi: 10.1016/j.immuni.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1(1):1–17. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR, Newman W, Ringler DJ. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6(9):895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279(5349):381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Butcher EC, Wu L. CCR7 expression and memory T cell diversity in humans. The Journal of Immunology. 2001a;166(2):877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134(1):255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. The Journal of Immunology. 2001b;166(11):6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167(2):377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosay JG, Fisher MA, Farhood A, Ready KA, Dunn CJ, Jaeschke H. Role of PECAM-1 (CD31) in neutrophil transmigration in murine models of liver and peritoneal inflammation. Am J Physiol. 1998;274(4 Pt 1):G776–G782. doi: 10.1152/ajpgi.1998.274.4.G776. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173(12):7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR3 Activation Promotes Lymphocyte Transendothelial Migration across Human Hepatic Endothelium under Fluid Flow. Am J Pathol. 2005;167(3):887–899. doi: 10.1016/S0002-9440(10)62060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6(9):889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278(5338):672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99(1):336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- Edwards S, Lalor PF, Nash GB, Rainger GE, Adams DH. Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology. 2005;41(3):451–459. doi: 10.1002/hep.20585. [DOI] [PubMed] [Google Scholar]
- Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hubscher SG, Briskin M, Salmon M, Adams DH. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200(11):1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, Adams DH. Epithelial Inflammation Is Associated with CCL28 Production and the Recruitment of Regulatory T Cells Expressing CCR10. J Immunol. 2006;177(1):593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34(11):2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Monzon C, Sanchez-Madrid F, Garcia-Buey L, Garcia-Arroyo A, Garcia-Sanchez A, Moreno-Otero R. Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in portal tracts. Gastroenterology. 1995;108(1):231–241. doi: 10.1016/0016-5085(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3(4):e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesner BM, Gowans JL. The output of lymphocytes from the thoracic duct of unanaesthetized mice. Br J Exp Pathol. 1962;43:424–30. 424–430. [PMC free article] [PubMed] [Google Scholar]
- Goddard S, Williams A, Morland C, Qin S, Gladue R, Hubscher SG, Adams DH. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72(12):1957–1967. doi: 10.1097/00007890-200112270-00016. [DOI] [PubMed] [Google Scholar]
- Granger DN. Cell adhesion and migration. II. Leukocyte-endothelial cell adhesion in the digestive system. Am J Physiol. 1997;273(5 Pt 1):G982–G986. doi: 10.1152/ajpgi.1997.273.5.G982. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Goddard S, Ahmed-Choudhury J, Reynolds G, Jackson DG, Briskin M, Wu L, Hubscher SG, Adams DH. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160(4):1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74(3):360–369. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, Salmon M, Adams DH. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006;177(1):729–738. doi: 10.4049/jimmunol.177.1.729. [DOI] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174(2):1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Hillan KJ, Hagler KE, MacSween RN, Ryan AM, Renz ME, Chiu HH, Ferrier RK, Bird GL, Dhillon AP, Ferrell LD, Fong S. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver. 1999;19(6):509–518. doi: 10.1111/j.1478-3231.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP. CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection. J Virol. 2007;81(3):1241–1250. doi: 10.1128/JVI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AJ, Manning JE, Bandak TM, Ratau MC, Hanser KR, Silverstein SC. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J Cell Biol. 1993;120(6):1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigo Y, Suematsu M, Higashida T, Oheda JI, Matsumoto K, Wakabayashi Y, Ishimura Y, Miyasaka M, Takashi T. Constitutive expression of icam-1 in rat microvascular systems analyzed by laser confocal microscopy. American Journal Of Physiology-Heart And Circulatory Physiology. 1997;42:H138–H147. doi: 10.1152/ajpheart.1997.273.1.H138. [DOI] [PubMed] [Google Scholar]
- Imhof BA, urrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4(6):432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, Alanen K, Grenman R, Salmi M, Jalkanen S. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol. 2003;33(3):815–824. doi: 10.1002/eji.200323859. [DOI] [PubMed] [Google Scholar]
- Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, Jalkanen S. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194(8):1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61(6):647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- Johnson Z, Proudfoot AE, Handel TM. Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005;16(6):625–636. doi: 10.1016/j.cytogfr.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Jung MY, Park SY, Kim IS. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with alphaMbeta2 integrin. J Leukoc Biol. 2007;82(5):1156–1165. doi: 10.1189/jlb.0107052. [DOI] [PubMed] [Google Scholar]
- Khandoga A, Kessler JS, Meissner H, Hanschen M, Corada M, Motoike T, Enders G, Dejana E, Krombach F. Junctional adhesion molecule-A deficiency increases hepatic ischemia-reperfusion injury despite reduction of neutrophil transendothelial migration. Blood. 2005;106(2):725–733. doi: 10.1182/blood-2004-11-4416. [DOI] [PubMed] [Google Scholar]
- Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, Ramadori G. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999;154(1):153–167. doi: 10.1016/s0002-9440(10)65262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22(8):432–437. doi: 10.1016/s1471-4906(01)01957-3. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Clements JM, Pigott R, Humphries MJ, Spragg JH, Nash GB. Association between receptor density, cellular activation, and transformation of adhesive behavior of flowing lymphocytes binding to VCAM-1. Eur J Immunol. 1997;27(6):1422–1426. doi: 10.1002/eji.1830270619. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002a;169(2):983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002b;80(1):52–64. doi: 10.1046/j.1440-1711.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Sun PJ, Weston CJ, Martin-Santos A, Wakelam MJ, Adams DH. Activation of vascular adhesion protein-1 on liver endothelium results in an NF-kappaB-dependent increase in lymphocyte adhesion. Hepatology. 2007a;45(2):465–474. doi: 10.1002/hep.21497. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Tuncer C, Weston C, Martin-Santos A, Smith DJ, Adams DH. Vascular adhesion protein-1 as a potential therapeutic target in liver disease. Ann N Y Acad Sci. 2007b;1110:485–496. doi: 10.1196/annals.1423.051. [DOI] [PubMed] [Google Scholar]
- Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. Journal of Hepatology. 2008;48(3):504–512. doi: 10.1016/j.jhep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Liu L, Cara DC, Kaur J, Raharjo E, Mullaly SC, Jongstra-Bilen J, Jongstra J, Kubes P. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J Exp Med. 2005;201(3):409–418. doi: 10.1084/jem.20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178(2):1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423(6936):190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- Martelius T, Salaspuro V, Salmi M, Krogerus L, HOCKERSTEDT K, Jalkanen S, Lautenschlager I. Blockade of vascular adhesion protein-1 inhibits lymphocyte infiltration in rat liver allograft rejection. Am J Pathol. 2004;165(6):1993–2001. doi: 10.1016/S0002-9440(10)63250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Nomiyama H, Yoneyama H, Uwatoku R. Kupffer cell-mediated recruitment of dendritic cells to the liver crucial for a host defense. Dev Immunol. 2002;9(3):143–149. doi: 10.1080/1044667031000137610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, McAvoy EF, Lam F, Gill V, de la MC, Savani RC, Kubes P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205(4):915–927. doi: 10.1084/jem.20071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(11 Suppl):S97–103. [PubMed] [Google Scholar]
- McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Current Opinions in Immunology. 2005;17(1):18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8(2):113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- Millan J, Ridley AJ. Rho GTPases and leucocyte-induced endothelial remodeling. Biochem J. 2005;385(Pt 2):329–337. doi: 10.1042/BJ20041584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24(6):327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104(1):49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. The Journal of Immunology. 1997;158(11):5536–5544. [PubMed] [Google Scholar]
- Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203(12):2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, Longati P, Velten FW, Johansson S, Goerdt S. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J. 2002;362(Pt 1):155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med. 2004;199(10):1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N Engl J Med. 1999;341(24):1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127(3):870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [see comments] Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5(10):760–771. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- Salmi M, Koskinen K, Henttinen T, Elima K, Jalkanen S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood. 2004;104(13):3849–3857. doi: 10.1182/blood-2004-01-0222. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3(2):143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5(4):393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163(11):6236–6243. [PubMed] [Google Scholar]
- Sigal A, Bleijs DA, Grabovsky V, van Vliet SJ, Dwir O, Figdor CG, van Kooyk Y, Alon R. The LFA-1 integrin supports rolling adhesions on ICAM-1 under physiological shear flow in a permissive cellular environment. J Immunol. 2000;165(1):442–452. doi: 10.4049/jimmunol.165.1.442. [DOI] [PubMed] [Google Scholar]
- Smedsrod B. Clearance function of scavenger endothelial cells. Comp Hepatol. 2004;3(Suppl 1):S22, S22. doi: 10.1186/1476-5926-2-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. 827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- Steinhoff G, Behrend M, Schrader B, Duijvestijn AM, Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia - lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2 and LFA-3. American Journal of Pathology. 1993;142:481–488. [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Adachi H, Osuga J, Ohashi K, Yahagi N, Sekiya M, Okazaki H, Tomita S, Iizuka Y, Shimano H, Nagai R, Kimura S, Tsujimoto M, Ishibashi S. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J Biol Chem. 2003;278(15):12613–12617. doi: 10.1074/jbc.M210211200. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361(6407):79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Conary JT, Lennartz MR, Stahl PD, Drickamer K. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J Biol Chem. 1990;265(21):12156–12162. [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB Journal. 1995;9(10):866–873. [PubMed] [Google Scholar]
- Uwatoku R, Suematsu M, Ezaki T, Saiki T, Tsuiji M, Irimura T, Kawada N, Suganuma T, Naito M, Ando M, Matsuno K. Kupffer cell-mediated recruitment of rat dendritic cells to the liver: roles of N-acetylgalactosamine-specific sugar receptors. Gastroenterology. 2001;121(6):1460–1472. doi: 10.1053/gast.2001.29594. [DOI] [PubMed] [Google Scholar]
- Volpes R, van den Oord JJ, Desmet VJ. Vacular adhesion molecules in acute and chronic liver disease. Hepatology. 1992;15:269–275. doi: 10.1002/hep.1840150216. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. Journal of Clinical Investigation. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XD, Ueta H, Zhou S, Shi C, Koga D, Ushiki T, Matsuno K. Trafficking of recirculating lymphocytes in the rat liver: rapid transmigration into the portal area and then to the hepatic lymph. Liver Int. 2008;28(3):319–330. doi: 10.1111/j.1478-3231.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through L-selectin at threshold shear. Biophys J. 2007;92(1):330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193(1):35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]