Abstract
Granulomatous experimental autoimmune thyroiditis (G-EAT) is induced by mouse thyroglobulin (MTG)-sensitized splenocytes activated in vitro with MTG and IL-12. Thyroid lesions reach maximal severity 20 days after cell transfer, and usually resolve or progress to fibrosis by day 60 depending on the extent of thyroid damage at day 20. Our previous studies indicated that neutralization of TNF-α or FasL had no effect on G-EAT induction, but neutralization of TNF-α promoted, while neutralization of FasL inhibited G-EAT resolution. TNF-related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily. This study was undertaken to define the role of endogenous TRAIL in G-EAT development and/or resolution. Neutralization of endogenous TRAIL had little effect on G-EAT induction, but significantly inhibited G-EAT resolution and increased thyroid fibrosis. This correlated with higher expression of pro-inflammatory cytokines and preferential expression of the pro-apoptotic molecule TRAIL, and anti-apoptotic molecules FLIP and Bcl-xL on inflammatory cells in thyroids of anti-TRAIL-treated recipients. Furthermore, CD4+ T cells outnumbered CD8+ T cells in thyroids of anti- TRAIL-treated recipients. The results suggest that endogenous TRAIL is not required for G-EAT development in recipients, but is critical for G-EAT resolution. Endogenous TRAIL might promote resolution, at least in part, through modulation of the balance between pro- and anti-inflammatory cytokines, and the expression pattern of pro- and anti-apoptotic molecules of thyroid epithelial cells (TEC) and inflammatory cells.
Introduction
Mouse thyroglobulin (MTG)-sensitized splenocytes activated in vitro with MTG and IL-12 induce a severe granulomatous form of experimental autoimmune thyroiditis (G-EAT) after transfer to syngeneic recipients [1]. G-EAT lesions are characterized by infiltration of inflammatory cells and destruction of thyroid follicles [2–4]. Thyroid lesions reach maximal severity 20 days after cell transfer, and inflammation resolves or progresses to fibrosis by day 60 depending on the extent of thyroid damage at day 20 [1– 6]. CD4+ T cells are the primary effector cells for G-EAT [3] and CD8+ T cells are important for G-EAT resolution [5].
Members of the TNF superfamily such as TNF-α and Fas ligand (FasL) play important roles in development of autoimmune diseases [7]. Neutralization of TNF-α promotes G-EAT resolution and decreases fibrosis, suggesting that TNF-α is anti-inflammatory in G-EAT [8]. In contrast, neutralization of FasL inhibits G-EAT resolution and G-EAT resolution is delayed in FasL-deficient gld (generalized lymphoproliferative disorder) mice, suggesting that FasL is anti-inflammatory in G-EAT [9]. TNF-related apoptosis-inducing ligand (TRAIL) is a type II membrane protein belonging to the TNF superfamily [10]. TRAIL and its receptor, death receptor 5 (DR5), are constitutively expressed in various tissues [11,12] and upregulated upon cell activation [13]. TRAIL induces apoptosis by interacting with DR5 and this signal is propagated through caspase-8 and caspase-3 [10,11]. The anti-apoptotic molecule FLIP (Fas-associated death domain-like IL (interleukin)-1β-converting enzyme inhibitory protein) inhibits death receptor-mediated apoptosis by blocking activation of caspase-8 [11,14,15]. FLIP is an important inhibitor of the initial upstream steps of death receptor-mediated apoptosis (11,14). Bcl-xL, an important anti-apoptotic molecule belonging to the Bcl-2 family, inhibits apoptosis by regulating mitochondrial membrane potential and cytochrome c release [16]. Although TRAIL does not induce apoptosis of most normal cells [17], TRAIL has been shown to inhibit collagen induced arthritis and experimental autoimmune encephalomyelitis [18,19]. A recent study showed that exogenous recombinant TRAIL inhibits development of EAT in naïve mice [20], but it is not clear whether endogenous TRAIL is required for development and/or resolution of G-EAT. Here we define the role of endogenous TRAIL in autoimmune thyroiditis and ask whether endogenous TRAIL is required for development and/or resolution of G-EAT.
Materials and Methods
Mice
CBA/J mice were generated in our breeding colony at the University of Missouri. CBA/J FLIP transgenic mice used for thyroid epithelial cells (TEC) culture were described previously [14,15]. All mice were bred and maintained in accordance with University of Missouri institutional guidelines for animal care.
G-EAT induction
CBA/J donors were injected i.v. twice at 10-day intervals with 150 µg MTG and 15 µg LPS (Sigma) [1]. Seven days later, donor splenocytes were activated in vitro with 25 µg/ml MTG and 5 ng/ml IL-12 (Peprotech) [3]. Cells were harvested after 72 h and 3 × 107 cells were transferred i.v. to 500-Rad irradiated syngeneic recipients. Recipient thyroids were evaluated 20 (peak of disease), or 60 days after cell transfer [3].
Neutralization of TRAIL
The hybridoma producing neutralizing rat anti-mouse TRAIL mAb (N2B2) was described previously [21]. TRAIL mAb was purified from culture supernatant using protein G-Sepharose. Recipients were given 0.3 mg TRAIL mAb or normal rat IgG (Jackson ImmunoResearch) i. p. the day after cell transfer and every 5–7 days until termination of the experiment.
Evaluation of G-EAT histopathology and fibrosis and Immunohistochemistry
(see Supplementary materials)
RT-PCR
Individual thyroid lobes were homogenized in TRIzol (Invitrogen). RNA was extracted, reverse transcribed and cDNA was amplified as previously described in detail [14]. Most primers used in this study have been described previously [14,15,22]. The primers for TRAIL are: sense: 5’-TCACCAACGAGATGAAGCAGC-3’, antisense: 5’- CTCACCTTGTCCTTTGAGACC-3’, and for DR5 are: sense: 5’-GTACCAAATGCTG CTCAAGTGG-3’, antisense: 5’-GTAAACCTCCCGGATTTCACTG-3’.
Confocal laser scanning double-immunofluorescence microscopy
Apoptosis was detected using an in situ cell death kit (Roche) [15] (For details see Supplementary materials).
Isolation of infiltrating inflammatory cells from thyroids
Isolation of thyroid-infiltrating inflammatory cells was performed using phycoerythrin (PE) labeled anti-CD45 mAb (eBioscience) and a positive selection EasySep PE selection kit (Stem Cell Technologies) [14]. TRIzol (Invitrogen) was used to extract RNA from the isolated inflammatory cells and the unbound fraction (mainly TEC) for RT-PCR analysis. Keratin primers were used to assess the extent of contamination of inflammatory cell fractions by TEC and CD4 and CD8 primers were used to assess inflammatory cells. Primers for CD4 and CD8 were described previously [14]. Primer sequences for keratin are: sense: 5’- CATGCAAGAGCTCAGAAAGGTC-3’, antisense: 5’-CCTCTTGGTACTTCTGAGGC AAC-3’.
Culture of primary TEC
Mouse primary TEC cultures were generated using conditions previously described by ourselves [22,23] and others [24] (For details see Supplementary materials).
Cytokine and TRAIL treatment of cultured TEC
60–70% confluent TEC were cultured for 4 d with 50 IU/ml IL-1β (eBioscience) and 50 IU/ml TNF-α (eBioscience) in the presence or absence of recombinant mouse IL-10 (0–20 ng/ml, Peprotech). Cells were then treated overnight with 0.8 µg/ml recombinant mouse TRAIL (eBioscience) [22,23,25,26].
Determination of apoptosis of cultured TEC
Apoptosis of cultured TEC was determined by TUNEL assay using an Apoptag kit (Chemicon) [30,32] (For details see Supplementary materials).
Statistical analysis
All experiments were repeated three times. Statistical analysis was performed using an unpaired two-tailed Student’s t test. A value of p < 0.05 was considered significant and is designated by the asterisk or diamond in the figure legends.
Results
Effect of TRAIL neutralization on G-EAT induction and resolution
To determine if neutralization of endogenous TRAIL influences G-EAT induction or resolution, G-EAT was induced by adoptive transfer of MTG-sensitized splenocytes activated with MTG and IL-12 in vitro. Recipients were given rat IgG or anti-TRAIL as described in Methods. Thyroid lesions in all recipients reached maximal severity 20 days after cell transfer, and G-EAT severity scores were similar (4.6 ± 0.2 vs. 4.1 ± 0.2, p > 0.05; Fig 1A), suggesting that TRAIL neutralization has little effect on G-EAT development. By day 60, thyroid lesions of most IgG-treated recipients had begun to resolve, while thyroids of anti-TRAIL-treated recipients had ongoing inflammation and fibrosis. Differences in G-EAT severity scores in rat IgG- and anti-TRAIL-treated recipients were highly significant (2.8± 0.5 vs. 4.8 ± 0.1, p < 0.01, Fig. 1B). There was extensive fibrosis and atrophy in thyroids of anti-TRAIL-treated recipients at day 60, but fibrosis was minimal in thyroids of IgG-treated controls (data not shown). These results suggest that endogenous TRAIL plays a role in promoting G-EAT resolution and inhibiting thyroid fibrosis.
Figure 1. Neutralization of TRAIL inhibits G-EAT resolution.
G-EAT severity scores of individual mice 20 and 60 days after cell transfer are shown using the histopathology criteria described in Methods. Recipient mice were given rat isotype IgG (control) or anti-TRAIL as described in Methods. A: Thyroid lesions in both IgG-treated and anti-TRAIL-treated recipients reached maximal severity 20 days after cell transfer, and the G-EAT severity scores are similar (average severity score: 4.6 ± 0.2 vs. 4.1 ± 0.2, p > 0.05). B: By day 60, differences in G-EAT severity scores in IgG-treated and anti-TRAIL-treated recipients were highly significant (average severity score: 2.8± 0.5 vs. 4.8 ± 0.1, p < 0.01). Results are representative of three separate experiments. A significant difference between the two groups is indicated by the asterisk (p < 0.05).
Effect of TRAIL neutralization on pro- and anti-inflammatory cytokine expression
The balance between pro- and anti-inflammatory cytokines produced by thyroid-infiltrating inflammatory cells could directly contribute to G-EAT outcome by regulating expression of pro- or anti-apoptotic molecules [6,15,27]. RT-PCR was used to assess the effect of TRAIL neutralization on mRNA expression of pro- and anti-inflammatory cytokines at day 20. Expression of cytokine mRNA was undetectable in thyroids of naïve (normal) mice (Fig. 2). Expression of the pro-inflammatory cytokines IFN-γ, TNF-α and IL-17 was higher (Fig. 2, A–C) and expression of anti-inflammatory cytokines IL-10 and IL-13 (Fig. 2, D and E) was lower in anti-TRAIL-treated compared to rat IgG-treated recipients. This was also supported by protein expression of pro- and anti-inflammatory cytokines in thyroids by IHC (data not shown). This suggests that neutralization of TRAIL can alter the balance between pro- and anti-inflammatory cytokines in thyroids. TGF-β was increased in thyroids of anti-TRAIL-treated compared to rat IgG-treated recipients both at day 20 and day 60 (data not shown). This is consistent with the increased fibrosis in thyroids of anti-TRAIL-treated recipients, suggesting that the profibrotic function of TGF-β rather than its anti-inflammatory function is predominant in G-EAT as shown previously [37].
Figure 2. Neutralization of TRAIL increases pro-inflammatory and decreases anti-inflammatory cytokine mRNA expression.
mRNA was isolated from single thyroid lobes of individual mice 20 days after cell transfer and amplified as described in Methods. All thyroids had comparable 4-5+ severity scores. mRNA expression of pro-inflammatory cytokines IFN-γ, TNF-α and IL-17 (A–C), and anti-inflammatory cytokines IL-10 and IL-13 (D and E) is shown. Results are expressed as the mean ratio of cytokine densitometric U/HPRT ± SEM (×100) of 5–6 mice/group, and are representative of three independent experiments. A significant difference between anti-TRAIL-treated mice and IgG-treated mice is indicated by the asterisk (p < 0.05).
Effect of TRAIL neutralization on expression of pro- and anti-apoptotic molecules on TEC vs. thyroid-infiltrating inflammatory cells
The balance and distribution pattern of pro- and anti-apoptotic molecules on TEC vs. infiltrating inflammatory cells might be one mechanism that determines whether an autoimmune response resolves or progresses to fibrosis [6,15,22]. We first examined whether TRAIL neutralization influenced expression of mRNA for pro-apoptotic molecules such as TRAIL and DR5 and anti-apoptotic molecules such as FLIP and Bcl-xL. There was no significant difference between the two groups in expression of mRNA for these pro- and anti-apoptotic molecules in thyroids (data not shown), suggesting that TRAIL neutralization has little effect on expression of mRNA for pro- and anti-apoptotic molecules in thyroids.
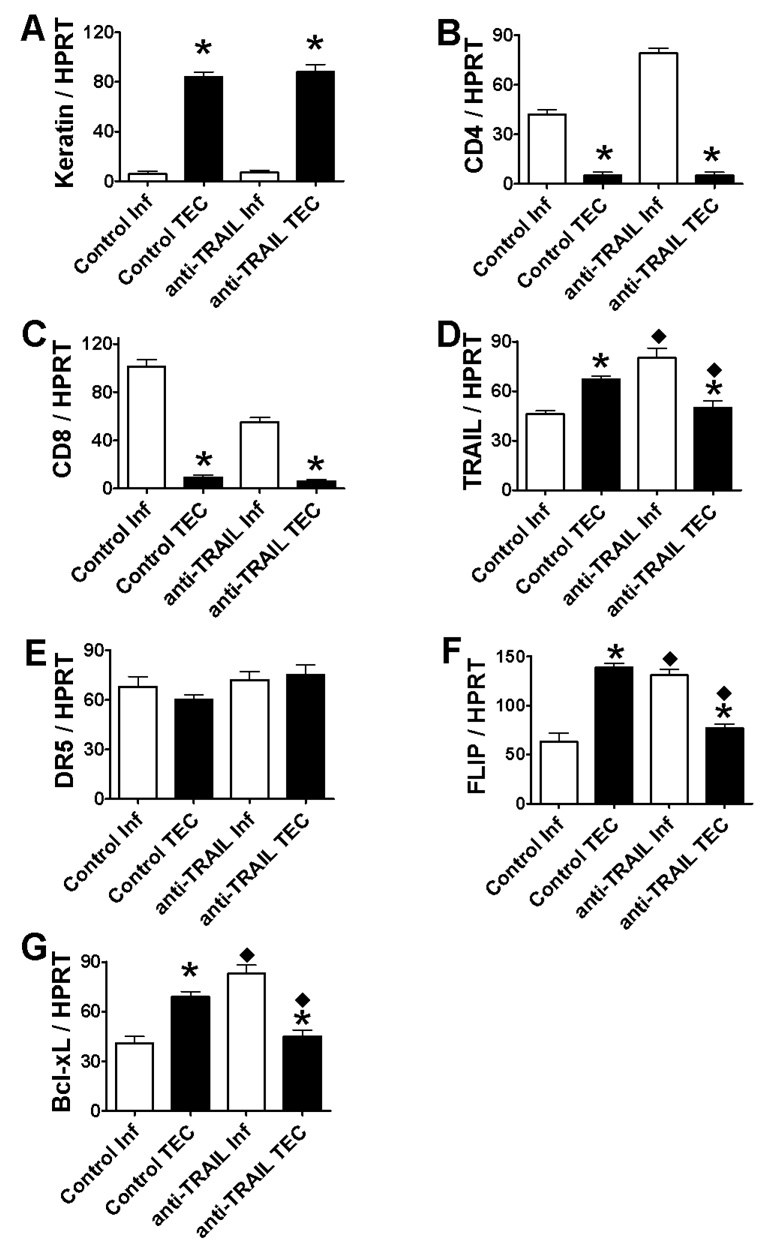
To determine if TRAIL neutralization influences the site of expression of pro- and anti-apoptotic molecules (TEC vs. thyroid-infiltrating inflammatory cells), pro- and anti-apoptotic molecule expression on TEC vs. thyroid-infiltrating inflammatory cells was examined by RT-PCR ( Fig. 3) and IHC (Fig. 4, A–H). Thyroid-infiltrating inflammatory cells and TEC were isolated from thyroids of IgG-treated and anti-TRAIL-treated recipients with 4–5+ G-EAT severity scores at day 20 as described in Methods. Cells positively selected by CD45+ magnetic beads (mainly inflammatory cells) expressed minimal cytokeratin message (Fig. 3A) but highly expressed CD4 and CD8 message (Fig. 3, B and C), suggesting minimal contamination of inflammatory cells by TEC. In contrast, cells not selected by CD45+ magnetic beads (mainly TEC) highly expressed cytokeratin message (Fig. 3A), but little CD4 or CD8 message (Fig. 3, B and C). TEC and inflammatory cells in thyroids of IgG-treated or anti-TRAIL-treated recipients all expressed similar DR5 message (Fig. 3E), but TRAIL, FLIP and Bcl-xL were more highly expressed on TEC in thyroids of IgG-treated recipients (Fig. 3, D, F and G) and on inflammatory cells in thyroids of anti-TRAIL-treated recipients (Fig. 3, D, F and G). TRAIL, FLIP and Bcl-xL were also more highly expressed in inflammatory cells and lower in TEC when the same cell types (inflammatory cells or TEC) were compared.
Figure 3. mRNA expression of pro- and anti-apoptotic molecules on TEC vs. thyroid-infiltrating inflammatory cells in thyroids.
Thyroid-infiltrating inflammatory cells were isolated from thyroids of 5–6 IgG-treated or anti-TRAIL-treated recipients with 4–5+ G-EAT severity scores at day 20 as described in Methods. The fraction that was depleted of inflammatory cells was obtained and designated TEC fraction (see Methods). RNA was extracted and cDNA was prepared and amplified with specific primers as described in Methods. Expression of keratin, CD4 and CD8 mRNA (A–C), or pro-apoptotic molecules TRAIL and DR5 mRNA (D and E), and anti-apoptotic molecules FLIP and Bcl-xL (F and G) is shown. Results are expressed as the mean ratio of densitometric U/HPRT ± SEM (×100), and are representative of two independent experiments. A significant difference between inflammatory cells and TEC isolated from IgG-treated (control) or anti-TRAIL-treated mice is indicated by the asterisk (p < 0.05). TRAIL, FLIP and Bcl-xL were also expressed higher in inflammatory cells and lower in TEC when the same cell types (inflammatory cells or TEC) were compared between the two groups (control vs. anti-TRAIL). A significant difference is indicated by the diamond (p < 0.05).
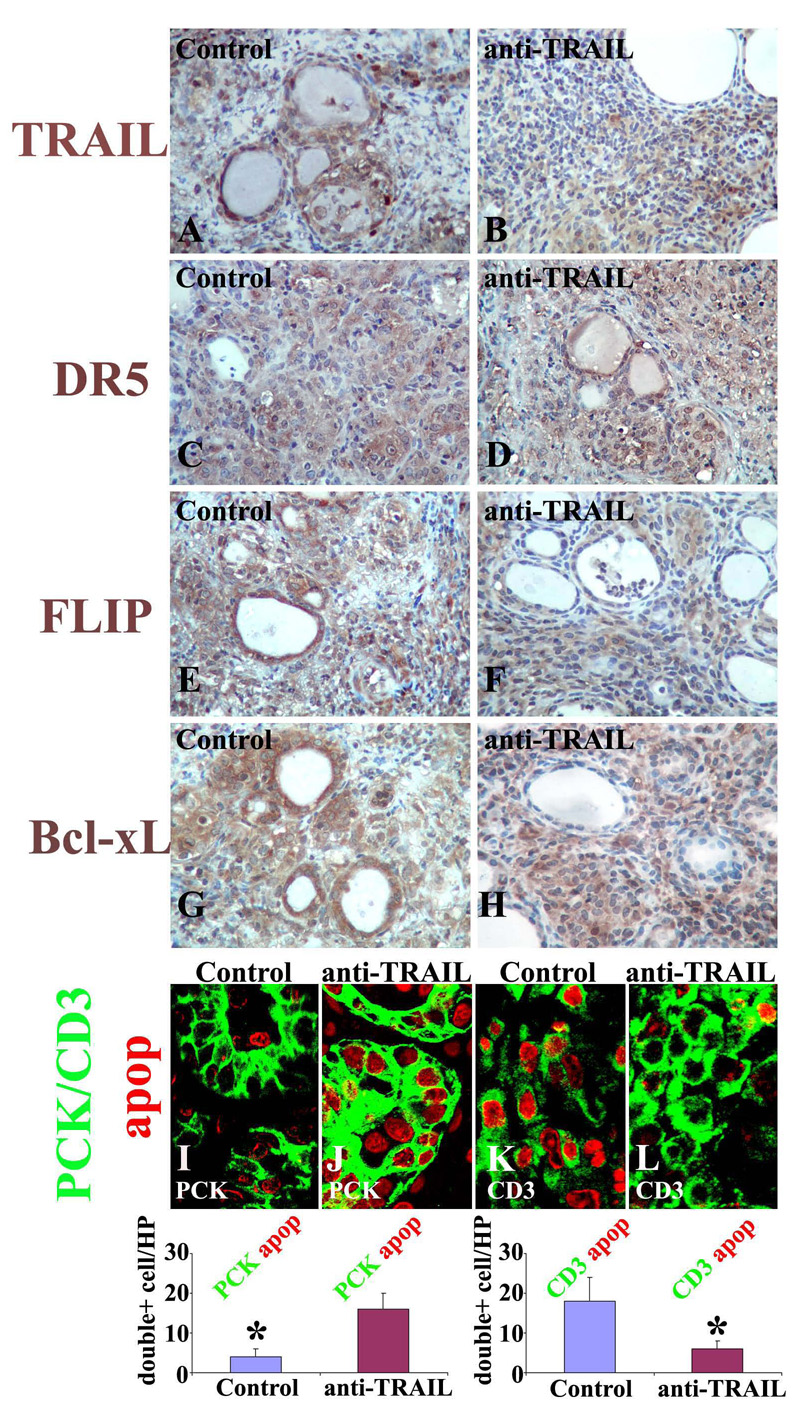
Figure 4. Neutralization of TRAIL alters the expression pattern of pro- and anti-apoptotic molecules on TEC vs. thyroid-infiltrating inflammatory cells.
IHC of TRAIL (A and B), DR5 (C and D), FLIP (E and F) and Bcl-xL (G and H) on paraffin sections of thyroids of IgG-treated recipients (left column) and anti-TRAIL-treated recipients (right column) and confocal dual-color immunofluorescence microscopy on frozen sections of thyroids of IgG-treated and anti-TRAIL-treated recipients (I–L) 20 days after cell transfer. TEC were identified by pan-cytokeratin (PCK, green, I and J), T cells were identified by CD3 (green, K and L) and apoptosis was detected as red nuclear staining. Double positive cells (red circled by green in I–L) in 5–6 randomly selected high power fields of three individual mice/group (magnification: ×800) were manually counted and summarized (M and N). Shown are representative areas on slides of thyroids of at least 3 individual mice/group with comparable 4–5+ G-EAT severity scores. A significant difference of apoptotic PCK+ cells (M) or CD3+ cells (N) in thyroids of IgG-treated vs. anti-TRAIL-treated recipients is indicated by the asterisk (p < 0.05). Original magnification: A–H ×400, I–L <800.
Pro- and anti-apoptotic protein expression by TEC vs. infiltrating inflammatory was also examined by IHC (Fig. 4, A–H). Consistent with the results in Fig. 3, TRAIL was mainly expressed by TEC in thyroids of IgG-treated recipients (Fig. 4A) and mainly by inflammatory cells in anti-TRAIL-treated recipients (Fig. 4B). DR5 did not show a differential expression pattern on TEC or inflammatory cells in G-EAT thyroids (Fig. 4, C and D). Fas also did not show a differential expression pattern, whereas FasL, like TRAIL, was mainly expressed by TEC in thyroids of IgG-treated recipients and mainly by inflammatory cells in anti-TRAIL-treated recipients (data not shown). The anti-apoptotic molecules FLIP and Bcl-xL were mainly expressed on TEC in thyroids of IgG-treated recipients (Fig. 4, E and G), and mainly on infiltrating inflammatory cells in thyroids of anti-TRAIL-treated recipients (Fig. 4, F and H).
A fluorescence apoptosis kit was used to determine the distribution pattern of apoptotic cells in the two groups (Fig. 4, I–L). TEC were identified by pan-cytokeratin (PCK, green) and T cells were identified by CD3 (green). Apoptosis was detected as red nuclear staining. Many apoptotic cells were detected in both groups at day 20. Only a few TEC were apoptotic in thyroids of IgG-treated recipients, while many TEC were apoptotic in thyroids of anti-TRAIL-treated recipients (red circled by green, Fig. 4, I vs. J). In contrast, many CD3+ cells were apoptotic in thyroids of IgG-treated recipients, whereas only a few CD3+ cells were apoptotic in thyroids of anti-TRAIL-treated recipients (Fig. 4, K vs. L). Double positive cells (red circled by green in Fig. 4, I–L) were manually counted and summarized (Fig. 4, M and N). These results suggest that TRAIL neutralization alters the expression pattern of pro- and anti-apoptotic molecules on TEC vs. thyroid-infiltrating inflammatory cells, resulting in increased apoptosis of TEC and decreased apoptosis of inflammatory cells.
Sensitization of primary TEC to TRAIL-induced apoptosis in vitro is locked by transgenic overexpression of FLIP
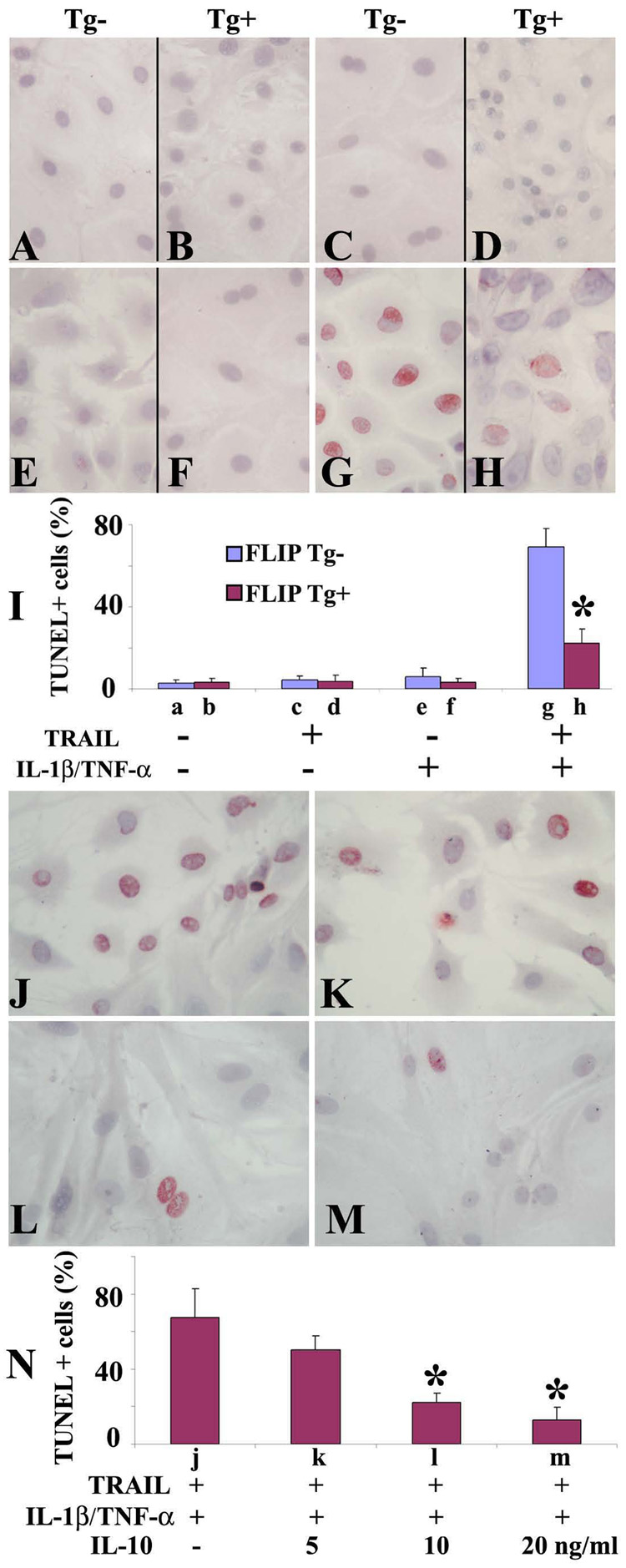
As shown in Fig. 3 and Fig. 4, anti-apoptotic FLIP and pro-apoptotic TRAIL were mainly expressed on TEC and only a few TEC were apoptotic in thyroids of IgG-treated recipients that would resolve by day 60. This suggests that coexpression of FLIP with TRAIL on TEC in thyroids of IgG-treated recipients might protect TEC from TRAIL-induced apoptosis. Cultured human TEC are resistant to TRAIL-induced apoptosis, but can be sensitized in vitro by IL-1β and TNF-α to undergo TRAIL-induced apoptosis [25]. To determine if murine TEC can be sensitized by IL-1β and TNF-α to undergo TRAIL-induced apoptosis and if transgenic overexpression of FLIP can protect TEC from apoptosis in vitro, 60–70% confluent TEC from FLIP Tg+ and Tg- naïve mice were treated with or without TRAIL in the presence or absence of IL-1β and TNF-α, and apoptosis was detected by TUNEL staining (Fig. 5, A–I). No TUNEL+ cells (red) were detected in TEC of FLIP Tg- or Tg+ mice cultured in the absence of TRAIL and cytokines (Fig. 5, A, B and I), or in the presence of cytokines (IL-1β and TNF-α) or TRAIL alone (Fig. 5, C–F and I), even using 3-fold higher concentrations of cytokines or TRAIL (data not shown). However, when TEC pretreated with cytokines (IL-1β and TNF-α) were stimulated by TRAIL, many TUNEL+ cells were detected in TEC of FLIP Tg- mice (Fig. 5, G and I), whereas only a few TUNEL+ cells were detected in TEC of FLIP Tg+ mice (Fig. 5, H and I). TUNEL+ cells were manually counted and the results are summarized in Fig. 5I (bars a–h correspond to Fig. 5, A–H). These results show that TEC are resistant to TRAIL-induced apoptosis, but can be sensitized by IL-1β and TNF-α to undergo apoptosis in vitro. Apoptosis is inhibited by transgenic overexpression of FLIP on TEC. These in vitro results are consistent with the idea that coexpression of FLIP and TRAIL on TEC might protect TEC from TRAIL-induced apoptosis, contributing to the earlier resolution of G-EAT in controls.
Figure 5. Transgenic overexpression of FLIP and addition of IL-10 protect TEC from TRAIL-induced apoptosis in vitro.
60–70% confluent cultured TEC from Tg- and Tg+ mice were treated with or without TRAIL(0.8 µg/ml) in the presence or absence of IL-1β (50 IU/ml) and TNF-α (50 IU/ml). Apoptosis was detected by TUNEL staining (A–H). TUNEL+ cells (red) in 5–6 randomly selected high power fields of three wells/group were manually counted (magnification: ×400) and the results are summarized (I). 60–70% confluent cultured TEC were pretreated with IL-1β (50 IU/ml) and TNF-α (50 IU/ml) for 4 days in the presence (5–20 ng/ml) or absence of IL-10 and stimulated with TRAIL (0.8 µg/ml) for 20h. Apoptosis was detected by TUNEL staining. TUNEL+ cells (J–M, red) in 5–6 randomly selected high power fields of three wells/group were manually counted and summarized (N). Bars a–m correspond to A–M. The percentage of TUNEL+ cells decreased in a dose-dependent manner in the presence of IL-10 (J–N). Shown are representative areas on slides. A significant difference in the percentage of TUNEL+ cells of TEC (A–H, Tg- vs. Tg+; J–M, presence of IL-10 vs. absence of IL-10) is indicated by the asterisk (p < 0.05). Original magnification: ×400.
IL-10 protects TEC from TRAIL-induced apoptosis in vitro
Our previous study showed that IL–10 promotes G-EAT resolution [22] and protects cultured TEC from Fas-mediated apoptosis [22]. IL-10 was higher (Fig. 3D) in thyroids of IgG-treated recipients with less apoptosis of TEC (Fig. 4, I vs. K) compared to thyroids of anti-TRAIL-treated recipients with predominant apoptosis of TEC at day 20, suggesting that IL-10 might protect TEC from TRAIL-induced apoptosis. To directly address this possibility, 60–70% confluent TEC were pretreated with cytokines (IL-1β and TNF-α) for 4 days in the presence or absence of various concentrations of IL-10 (0–20 ng/ml) and stimulated with TRAIL for 20 h. TUNEL+ cells (red, Fig. 5, J–M) were manually counted and results are summarized in Fig. 5N. In the absence of IL-10, TEC cultured in the presence of cytokines and TRAIL underwent extensive apoptosis (68 ± 15%), while the percentage of TUNEL+ cells decreased in a dose-dependent manner in the presence of IL-10 (Fig. 5, J–N). Minimal apoptosis was observed when TEC were cultured with TRAIL in the absence of cytokines or with cytokines in the absence of TRAIL (data not shown), suggesting that IL-10 protects TEC from TRAIL-induced apoptosis. These in vitro results are consistent with the idea that increased anti-inflammatory cytokines such as IL-10 contributes to the earlier resolution of G-EAT in controls.
Discussion
The G-EAT model is an excellent model for studying mechanisms that determine if an autoimmune inflammation will resolve or progress to fibrosis [1–6]. The objective of this study was to define the role of endogenous TRAIL in G-EAT. Neutralization of endogenous TRAIL had little effect on development of adoptively transferred G-EAT, but significantly inhibited G-EAT resolution and resulted in increased fibrosis. These results suggest that TRAIL might be a member of an inhibitor protein subfamily that promotes resolution of autoimmune diseases by downregulating autoimmune inflammation during the recovery stage. Several levels of control could be involved in the delayed resolution of G-EAT when endogenous TRAIL is neutralized. TRAIL neutralization increases pro-inflammatory and decreases anti-inflammatory cytokines, and alters the expression pattern of pro- and anti-apoptotic molecules by TEC vs. inflammatory cells, and increases apoptosis of TEC.
As a member of the TNF superfamily, TRAIL induces apoptosis of tumor cells, but the roles of TRAIL in autoimmune disease are not fully understood [7,18]. Apoptosis plays an important role in autoimmune diseases [6,11,28], and death receptor pathways other than Fas and TNFR-1 have only recently become recognized as potentially important in disease pathogenesis [14,15,18–20,29]. Specifically, inhibition of TRAIL has been implicated in exacerbation of collagen induced arthritis and experimental autoimmune encephalomyelitis in mice [18,19]. Although exogenous recombinant TRAIL inhibits induction of EAT when given to naïve mice [20], our results suggest that endogenous TRAIL does not inhibit G-EAT development, since TRAIL neutralization in recipients had little effect on disease severity at day 20. This is consistent with our previous findings that anti-TNF-α or anti-FasL also had little influence on G-EAT development [8,9]. Using IFN-γ−/−, IL-12−/−, and IL-4−/− mice, we found that these individual cytokines were also not required for severe G-EAT development [1,6,30], suggesting that the combined effects of several pro- and anti-inflammatory cytokines or pro- and anti-apoptotic molecules direct or determine the outcome and development of G-EAT.
Although endogenous TRAIL is not required for G-EAT development, endogenous TRAIL contributes to G-EAT resolution since resolution was inhibited when TRAIL was neutralized. The precise mechanism whereby TRAIL contributes to G-EAT resolution is still unclear. Cytokines play a critical role in the regulation of autoimmune diseases. TRAIL neutralization might inhibit G-EAT resolution due to its ability to modulate the balance between pro- and anti-inflammatory cytokines. The pro-inflammatory cytokines IFN-γ, TNF-α and IL-17 are important for development and/or progression of most autoimmune diseases [6,7,15,31,32]. Pro-inflammatory cytokines can promote destruction of thyroid follicles by promoting TEC apoptosis [14,15,22,33,34], and increased expression of pro-inflammatory cytokines in recipient thyroids might contribute to the increased TEC apoptosis and follicle destruction in anti-TRAIL-treated recipients. In this regard, neutralization of TNF-α or deficiency of IFN-γ promotes G-EAT resolution in this model [6,8], whereas anti-inflammatory cytokines such as IL-10 can function as immunoregulatory molecules to inhibit pro-inflammatory cytokines [30,34–36]. IL-10 is higher in FLIP Tg+ recipient thyroids whose lesions resolve by day 60 [14,15], and IL-10 deficiency or IL-10 neutralization inhibits G-EAT resolution in this model [22]. Furthermore, IL-10 protects TEC from TRAIL-mediated apoptosis in vitro (Fig. 5). Thus, the balance between pro- and anti-inflammatory cytokines might contribute to the distinct outcome of G-EAT in controls and anti-TRAIL-treated recipients. One anti-inflammatory cytokine increased in thyroids of anti-TRAIL-treated recipients is TGF-β, suggesting its profibrotic function is predominant in G-EAT. This is consistent with increased fibrosis in thyroids of anti-TRAIL-treated recipients, and neutralization of TGF-β decreases fibrosis and promotes resolution in this model [37].
The ability of TRAIL neutralization to inhibit G-EAT resolution might correlate with its ability to modulate the balance between pro- and anti-apoptotic molecules. Our previous studies demonstrated a role for apoptosis in G-EAT resolution [6,9,14,15,22]. Since anti-TRAIL only functionally blocks TRAIL, the TRAIL distribution pattern in thyroids could be detected using polyclonal anti-TRAIL. TRAIL, FasL and anti-apoptotic molecules were mainly expressed on TEC in controls, and mainly on infiltrating inflammatory cells in anti-TRAIL-treated recipients. Pro-apoptotic molecules expressed on TEC can induce apoptosis of both TEC and inflammatory cells. Thus the coexpression of pro- and anti- apoptotic molecules on TEC can protect TEC from apoptosis during G-EAT development, resulting in decreased apoptosis of TEC and increased apoptosis of inflammatory cells in controls (Fig. 3 and Fig. 4). In contrast, the coexpression of pro- and anti- apoptotic molecules on inflammatory cells protects inflammatory cells from apoptosis, resulting in decreased apoptosis of inflammatory cells and increased apoptosis of TEC in anti-TRAIL-treated recipients. However, further investigation is needed to clarify the precise pathway leading to increased pro-inflammtory cytokine production and predominant coexpression of pro- and anti-apoptotic molecules by TRAIL neutralization.
In summary, our data established that TRAIL has little effect on G-EAT development, but promotes its resolution. It is important to further investigate the precise mechanisms whereby endogenous TRAIL promotes G-EAT resolution. Such studies may be helpful in designing TRAIL-based drugs for treatment of human autoimmune diseases.
Acknowledgments
This work was supported by National Institutes of Health Grant DK35527 and the Arthritis Foundation Eastern Missouri Chapter. We thank Drs. Robert Ortmann and Vincent DeMarco for helpful discussions, and Daniel Schnurr and Alicia Duren for excellent technical assistance.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Braley-Mullen H, Johnson M, Sharp GC, Kyriakos M. Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol. 1985;93:132–143. doi: 10.1016/0008-8749(85)90394-6. [DOI] [PubMed] [Google Scholar]
- 2.Braley-Mullen H, Sharp GC, Bickel JT, Kyriakos M. Induction of severe granulomatous experimental autoimmune thyroiditis in mice by effector cells activated in the presence of anti-interleukin 2 receptor antibody. J Exp Med. 1991;173:899–912. doi: 10.1084/jem.173.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braley-Mullen H, Sharp GC, Tang H, Chen K, Kyriakos M, Bickel JT. Interleukin-12 promotes activation of effector cells that induce a severe destructive granulomatous form of murine experimental autoimmune thyroiditis. Am J Pathol. 1998;152:1347–1358. [PMC free article] [PubMed] [Google Scholar]
- 4.Braley-Mullen H, Sharp GC. Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis. Int Rev Immunol. 2000;19:535–555. doi: 10.3109/08830180009088511. [DOI] [PubMed] [Google Scholar]
- 5.Braley-Mullen H, McMurray RW, Sharp GC, Kyriakos M. Regulation of the induction and resolution of granulomatous experimental autoimmune thyroiditis in mice by CD8+ T cells. Cell Immunol. 1994;153:492–504. doi: 10.1006/cimm.1994.1045. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Mechanisms of spontaneous resolution versus fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol. 2003;171:6236–6243. doi: 10.4049/jimmunol.171.11.6236. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Decreasing TNF± results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leuk Biol. 2007;81:306–314. doi: 10.1189/jlb.0606402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Chen K, Sharp GC, Braley-Mullen H. Fas ligand is required for resolution of granulomatous experimental autoimmune thyroiditis. J Immunol. 2004;173:7615–7621. doi: 10.4049/jimmunol.173.12.7615. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, et al. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–2647. [PubMed] [Google Scholar]
- 11.Wang SH, Mezosi E, Wolf JM, Cao Z, Utsugi S, Gauger PG, et al. IFNγ sensitization to TRAIL-induced apoptosis in human thyroid carcinoma cells by upregulating Bak expression. Oncogene. 2004;23:928–935. doi: 10.1038/sj.onc.1207213. [DOI] [PubMed] [Google Scholar]
- 12.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 13.Mariani SM, Krammer PH. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur J Immunol. 1998;28:1492–1498. doi: 10.1002/(SICI)1521-4141(199805)28:05<1492::AID-IMMU1492>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, DeMarco VG, Sharp GC, Braley-Mullen H. Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice. Endocrinology. 2007;148:5734–5745. doi: 10.1210/en.2007-0939. [DOI] [PubMed] [Google Scholar]
- 15.Fang Y, Wei Y, DeMarco V, Chen K, Sharp GC, Braley-Mullen H. Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice. Am J Pathol. 2007;171:875–887. doi: 10.2353/ajpath.2007.060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 18.Song K, Chen Y, Goke R, Wilmen A, Seidel C, Goke A, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–1104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilliard B, Wilmen A, Seidel C, Liu TS, Goke R, Chen Y. Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis. J Immunol. 2001;166:1314–1319. doi: 10.4049/jimmunol.166.2.1314. [DOI] [PubMed] [Google Scholar]
- 20.Wang SH, Cao Z, Wolf JM, Antwerp MV, Baker JR., Jr Death ligand tumor necrosis factor-related apoptosis-inducing ligand inhibits experimental autoimmune thyroiditis. Endocrinology. 2005;146:4721–4726. doi: 10.1210/en.2005-0627. [DOI] [PubMed] [Google Scholar]
- 21.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, et al. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol. 1999;163:1906–1913. [PubMed] [Google Scholar]
- 22.Fang Y, Sharp GC, Braley-Mullen H. Interleukin-10 promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol. 2008;172:1591–1602. doi: 10.2353/ajpath.2008.071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Braley-Mullen H. Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1β converting enzyme inhibitory protein are protected from Fas-mediated apoptosis. Endocrinology. 2008;149:3321–3329. doi: 10.1210/en.2008-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HS, Carayanniotis G. Induction of goitrous hypothyroidism by dietary iodide in SJL mice. Endocrinology. 2007;148:2747–2752. doi: 10.1210/en.2007-0082. [DOI] [PubMed] [Google Scholar]
- 25.Bretz JD, Mezosi E, Giordano TJ, Gauger PG, Thompson NW, Baker JR., Jr Inflammatory cytokine regulation of TRAIL-mediated apoptosis in thyroid epithelial cells. Cell Death Differ. 2002;9:274–286. doi: 10.1038/sj.cdd.4400965. [DOI] [PubMed] [Google Scholar]
- 26.Bharhani MS, Borojevic R, Basak S, Ho E, Zhou P, Croitoru K. IL-10 protects mouse intestinal epithelial cells from Fas-induced apoptosis via modulating Fas expression and altering caspase-8 and FLIP expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:820–829. doi: 10.1152/ajpgi.00438.2005. [DOI] [PubMed] [Google Scholar]
- 27.Fang Y, Yu S, Braley-Mullen H. Contrasting roles of IFN-γ in murine models of autoimmune thyroid diseases. Thyroid. 2007;17:989–994. doi: 10.1089/thy.2007.0261. [DOI] [PubMed] [Google Scholar]
- 28.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Sharp GC, Peterson KE, Braley-Mullen H. Induction of granulomatous experimental autoimmune thyroiditis in IL-4 gene-disrupted mice. J Immunol. 1998;160:155–162. [PubMed] [Google Scholar]
- 31.Chen D. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Ann Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 33.Stassi G, Di Liberto D, Todaro M, Zeuner A, Ricci-Vitiani L, Stoppacciaro A, et al. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nat Immunol. 2000;1:483–488. doi: 10.1038/82725. [DOI] [PubMed] [Google Scholar]
- 34.Bretz JD, Arscott PL, Myc A, Baker JR., Jr Inflammatory cytokine regulation of Fas-mediated apoptosis in thyroid follicular cells. J Bio Chem. 1999;274:25433–25438. doi: 10.1074/jbc.274.36.25433. [DOI] [PubMed] [Google Scholar]
- 35.Gangi E, Vasu C, Cheatem D, Prabhakar B. IL-10 producing CD4+CD25+ regulatory T cells play a critical role in granulocyte macrophage colony stimulatory factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 36.Serhan C, Savill J. Resolution of inflammation: the beginning programs the end. Nature Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Inhibition of TGFβ1 by anti- TGFβ1 antibody or lisinopril reduced thyroid fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol. 2002;169:6530–6538. doi: 10.4049/jimmunol.169.11.6530. [DOI] [PubMed] [Google Scholar]