Abstract
Background
We sought to identify criteria for the intraoperative assessment of sentinel lymph node (SLN) involvement in women with early breast cancer. We also sought to determine whether the SLN nomogram developed by the Memorial Sloan-Kettering Cancer Center (MSKCC) to predict nonsentinel lymph node (NSLN) involvement when the SLN is positive would accurately predict NSLN involvement in our patient population.
Methods
We performed 405 SLN biopsies in 397 women between January 1998 and June 2005. We determined factors associated with SLN metastases using univariate and multivariate logistic regression. Ninety women who had 1 positive SLN or more and underwent axillary lymph node dissection (ALND) had complete data for analysis. We applied the MSKCC nomogram retrospectively to this subset of women, and we calculated the probability of NSLN involvement and compared it with the observed rate.
Results
Multifocality and the presence of lymphovascular invasion were predictive of SLN involvement. Ductal carcinoma in situ was negatively associated with SLN involvement. Intraoperative evaluation identified 57 (63%) of the 90 women with involved SLN, of which 26 (29%) had involved NSLN. Application of the MSKCC nomogram to our data set produced an area under the receiver operator characteristic curve of 0.71. The nomogram tended to overestimate the probability of NSLN involvement in our population.
Conclusion
Lymphovascular invasion and multifocality were associated with SLN involvement. Women with small low-grade tumours may not require routine intraoperative evaluation of SLNs. The MSKCC nomogram appears to be most useful as a decision aid in selecting those women with an involved SLN in whom ALND may be omitted.
Abstract
Contexte
Nous voulions établir les critères justifiant l'évaluation periopératoire de l'atteinte du ganglion sentinelle (GS) chez les femmes atteintes d'un cancer du sein peu avancé. Nous voulions aussi déterminer si le nomogramme mis au point par le Memorial Sloan-Kettering Cancer Center (MSKCC) pour prédire l'envahissement des ganglions non sentinelles (GNS) lorsque le GS est positif allait permettre de prédire avec justesse l'envahissement des GNS chez notre population de patientes.
Méthodes
Nous avons effectué 405 biopsies du GS chez 397 femmes entre janvier 1998 et juin 2005. Nous avons relevé les facteurs associés aux métastases du ganglion sentinelle par analyse de régression logistique univariée et multivariée. Pour l'analyse, nous disposions de données complètes sur 90 femmes qui présentaient un GS positif ou plus et avaient subi un évidement ganglionnaire axillaire. Nous avons appliqué le nomogramme du MSKCC de manière rétrospective à cette série de patientes, nous avons calculé la probabilité d'envahissement du GNS et nous l'avons comparée au taux observé.
Résultats
La multifocalité et la présence d'un envahissement lymphovasculaire ont été des éléments prédictifs de l'atteinte du GS. Le carcinome intracanalaire non infiltrant était négativement associé à l'envahissement du GS. L'évaluation periopératoire a révélé la présence d'une atteinte du GS chez 57 de ces 90 femmes (63 %), dont 26 (29 %) présentaient un envahissement des GNS. L'application du nomogramme du MSKCC à notre ensemble de données a produit une aire sous la courbe des caractéristiques receveur/opérateur de 0,71. Le nomogramme a eu tendance à surestimer la probabilité d'envahissement du GNS dans notre population.
Conclusion
L'envahissement lymphovasculaire et la multifocalité ont été associés à l'atteinte du GS. Les femmes dont les tumeurs sont petites et de bas grade pourraient ne pas nécessiter d'emblée une évaluation peropératoire du GS. Le nomogramme du MSKCC semble le plus utile pour aider à choisir les patientes présentant un envahissement du GS chez qui on peut omettre l'évidement ganglionnaire axillaire.
The morbidity of surgical treatment for many women with early stage breast cancer has been reduced with the adoption of breast-conserving surgery1 and sentinel lymph node biopsy (SLNB).2 Integration of SLNB into the surgical management of breast cancer in Canada has been limited by a number of factors, including lack of available resources in some areas and ongoing controversies surrounding the indications for its use and interpretation of the results.3
Sentinel lymph node biopsy has an inherent false-negative rate of 5%–10%4,5 that cannot be entirely eliminated, even with multiple sectioning of the sentinel lymph node (SLN).6 Although the false-negative rate of SLNB is higher than that of axillary lymph node dissection (ALND),7 the clinical significance of this difference is diminished by the frequent use of adjuvant systemic therapy in node-negative disease. Given the selection of lower-risk patients for SLNB, the rate of axillary recurrence following a negative SLNB is very low,8–11 even when surveillance imaging is added.12 This rate has been reported to be less than that in the population of women undergoing ALND.7 Thus concerns about axillary recurrence when the SLN is negative appear to be unfounded.
Many institutions conduct intraoperative assessments of SLNs to select patients for ALND. Such assessments are resource-intensive, often result in prolongation of the surgical procedure and infrequently result in a change in intraoperative management, since most SLNs are free of metastases. Even when SLNs are involved, the sensitivity of intraoperative assessment is only 60%–70%.13 Thus the lack of intraoperative assessments of SLNs need not be a barrier to SLNB and, in those institutions where such assessments are available, it may be cost-effective to identify a subgroup of patients with a low probability of SLN involvement in whom intraoperative SLN assessment could be avoided.
When the SLN is positive, however, current guidelines recommend ALND to prevent axillary recurrence.14 The proportion of involved lymph nodes in an ALND appears to be an important prognostic factor for survival,15 and the results of the ALND may be used to plan further adjuvant therapy.16 When the presence of axillary metastases is documented preoperatively, either clinically or by image-guided biopsy,17 SLNB may be omitted and ALND performed directly. Since most axillary metastases are not detected preoperatively, we were interested in determining those factors associated with a positive SLN.
More recently, the necessity of ALND for staging in all patients with metastases to a sentinel node has been questioned, particularly if such staging information will not alter treatment recommendations.18 Furthermore, nonsentinel lymph nodes (NSLNs) are free of disease in 30%–60% of patients in which the SLN is involved.19 Since one of the tenets of SLNB is that prophylactic removal of uninvolved axillary lymph nodes does not improve outcome, the value of ALND in women whose NSLNs are free of disease has been questioned. In such women, ALND is unlikely to have any diagnostic, prognostic or therapeutic value, yet will predictably result in additional morbidity.20,21 This has led to an interest in predicting the probability of involvement of NSLNs in individual patients. A nomogram developed by the Memorial Sloan-Kettering Cancer Center (MSKCC) uses tumour factors, SLN factors and the method of detection of SLN metastases to determine the likelihood of NSLN metastasis.22 This nomogram has been shown to be more accurate in predicting NSLN metastasis than clinician estimation using the same data.23 The nomogram has been validated in patient populations in The Netherlands,24 Australia25 and the United States.26,27
We conducted this study to assist in the development of institutional guidelines for the conduct of SLNB. In particular, we sought to develop criteria for the intraoperative assessment of SLNs and to determine whether we could identify a subset of women with positive SLNs who could avoid ALND. The specific objectives were 1) to identify the factors predictive of an involved SLN to guide the intraoperative assessment of SLNs and 2) to determine the accuracy of the MSKCC nomogram for prediction of involved NSLNs in our patient population.
METHODS
We prospectively gathered data on the conduct and outcome of all SLNBs performed by 4 experienced breast surgeons at Sunnybrook and Women's College Health Sciences Centre (Sunnybrook) between July 1998 and June 2005.
Surgical technique
We performed SLNB using 99mTc-labelled sulfur colloid and isosulfan blue. Briefly, we injected unfiltered 99mTc-labelled sulfur colloid (1 mCi in 8 mL normal saline) peritumourally or in the subareolar region, and we injected 0.1 mCi in 0.05 mL normal saline intradermally 30 minutes to 4 hours preoperatively. We also injected 2–5 mL of isosulfan blue (Lymphazurin; United States Surgical) after induction of anesthesia. We massaged the breast for 5 minutes. We marked a zone of diffusion around the injection site and identified a hot spot using a gamma probe (Navigator; Johnson & Johnson). We defined sentinel nodes as nodes that contained 99mTc-labelled sulfur colloid and/or isosulfan blue or that were suspicious on palpation.
Pathological examination
We evaluated all sentinel nodes intraoperatively by touch prep or frozen section. After fixation, we sliced lymph nodes in 3-mm slices and embedded them in paraffin. We took 3 pairs of sections from each block at 50 μM intervals. We stained 1 of each pair with hematoxylin and eosin, and we stained the second for cytokeratin with the monoclonal anticytokeratin antibody (Clone CAM 5.2; BC Biosciences). We evaluated SLNs from 79 patients enrolled in the National Surgical Adjuvant Breast and Bowel Project B-32 protocol intraoperatively by touch prep; immunohistochemical staining for cytokeratin was performed only at the discretion of the pathologist after looking at the hematoxylin and eosin.
Variables
We collected data on patient age, tumour size, tumour type, nuclear grade, Bloom and Richardson grade, lymphovascular invasion, multifocality, estrogen receptor, progesterone receptor, Her2 neu overexpression, number of SLNs removed, number of SLNs positive, method of detection of SLN metastases and size of SLN metastases.
Statistical analysis
We compared patient or tumour characteristics between Sunnybrook and the MSKCC using a χ2 test. We determined factors predictive of a positive SLN by univariate and multivariate logistic regression. We considered factors that had a significance of p ≤ 0.15 in univariate models for entry into the multivariate model. We expressed odds ratios for each factor, and we set statistical significance at p = 0.05 for the multivariate model. To validate the MSKCC nomogram in the subset with a positive SLN, we recorded data to conform to the covariate structure of the nomogram. We used the online nomogram to estimate the probability of NSLN involvement. For both the derived multivariate model predicting the probability of SLN involvement and the nomogram predicting the probability of NSLN involvement in the subset of women with a positive SLN, we derived a receiver operating characteristic (ROC) curve from the resulting predicted values, and we calculated the area under the ROC curve using Hanley and McNeil's W statistic.28 We also produced calibration curves22 and Hosmer and Lemeshow's goodness-of-fit test29 for the multivariate model and the nomogram. We performed all analyses using SAS version 9.1.3 (SAS Institute Inc.).
RESULTS
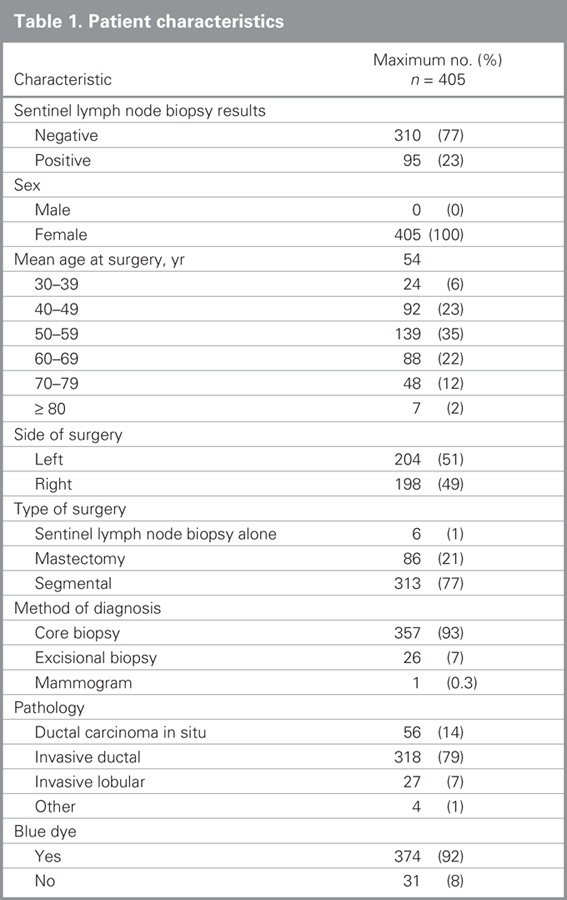
A total of 397 women, including 56 having mastectomies for extensive ductal carcinoma in situ, underwent 405 SLNBs during the study period. We retreived a median of 2 (interquartile range 1–3) SLNs for all women. The median and interquartile range was the same for those who had at least 1 positive SLN and those who had all negative SLNs. Descriptive characteristics of the study population can be found in Table 1. At least 1 SLN contained metastatic disease in 95 of 405 (23%) SLNBs performed; 92 of these women then underwent ALND. Three patients did not undergo ALND. One refused ALND even in the event of intraoperative detection of SLN metastases. One had 8 SLNs retrieved, 2 of which contained micrometastases identified on final pathology, thus we felt that ALND would add little to staging or local control and did not perform the procedure. There is no available information about why the third patient did not undergo ALND. One patient had extensive axillary involvement, and the SLNB was performed as part of the surgeon's learning curve rather than as a diagnostic test — the nomogram was not intended or validated for such application and we excluded this patient from our analysis. Complete data were available for 90 of 91 remaining patients who underwent a SLNB followed by ALND.
Table 1
Factors associated with SLN involvement
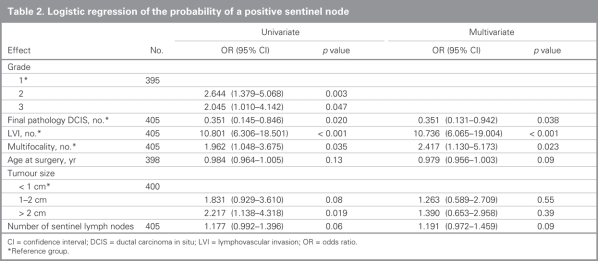
We performed univariate and multivariate analyses to determine factors predictive of SLN involvement (Table 2). We included factors significant or approaching significance on univariate analysis — grade (grade 2, p = 0.003; grade 3, p = 0.047), final pathology is ductal carcinoma in situ (p = 0.020), lymphovascular invasion (p < 0.001), multifocality (p = 0.036), age at surgery (per year, p = 0.13), tumour size (1–2 cm, p = 0.08; > 2 cm, p = 0.019) and number of SLNs (p = 0.06) — in our multivariate analysis. The exception was grade because it was highly correlated with lymphovascular invasion and multifocality (χ2, p = 0.004 and p < 0.001, respectively). Estrogen receptor, progesterone receptor and Her2 neu over-expression status and the year of diagnosis were nonsignificant on univariate analysis (data not shown). Ductal carcinoma in situ was negatively associated with SLN involvement (p = 0.038 on multivariate analysis), whereas multifocality (p = 0.023) and lymphovascular invasion (p < 0.001) were associated with higher rates of SLN involvement. In particular, lymphovascular invasion was highly significant: 12% of the patients without a positive SLN had lymphovascular invasion compared with 58% of the patients with a positive SLN. Age at surgery, tumour size and number of SLNs were not significant in the multivariate model.
Table 2
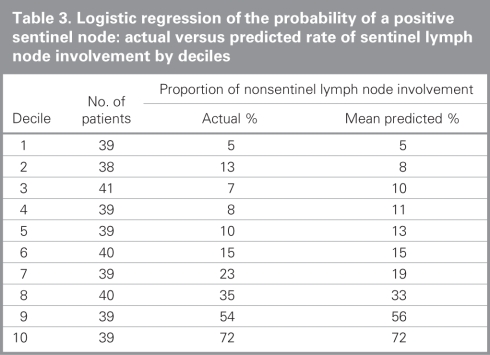
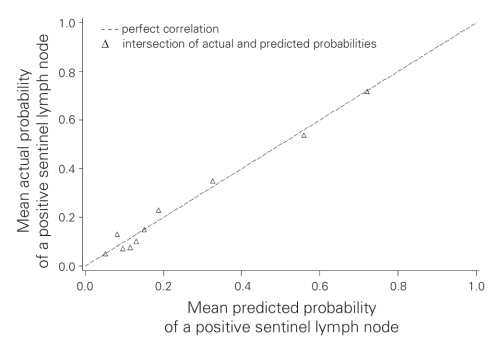
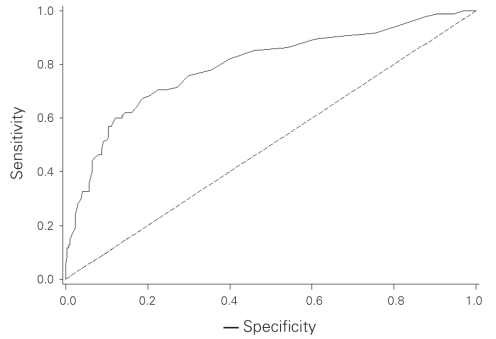
We subsequently grouped patients into deciles based on the predicted probability of a positive SLN to compare the mean predicted probability of a positive SLN for each decile with the actual proportion of patients with positive SLNs (Table 3). Correlation between the predicted and actual probabilities (correlation coefficient = 0.99) was found to be present when analyzed in a calibration plot (Fig. 1). We calculated the sensitivity and specificity of the model for prediction of SLN involvement in our data set, and we produced an ROC curve (Fig. 2). The estimated area under the curve was 0.80 with a Hosmer and Lemeshow goodness-of-fit χ2 statistic of 3.0 (p8 = 0.94), indicating that the derived model fit the observed data well. Application of the model predicted a positive SLN in 24% of SLNBs, compared with the 23% that we observed.
Table 3
Fig. 1. A calibration plot comparing actual and predicted probabilities of a positive sentinel lymph node (SLN). Correlation coefficient = 0.99.
Fig. 2. A receiver operating characteristic (ROC) curve assessing the model. The area under the ROC curve is 0.80.
Validation of the MSKCC nomogram
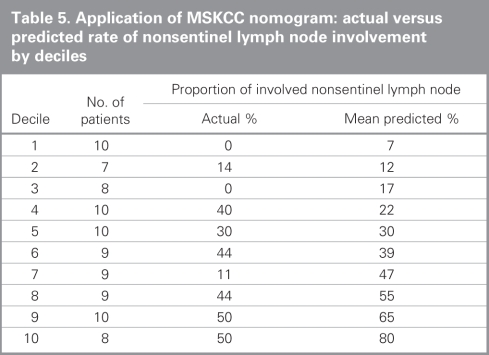
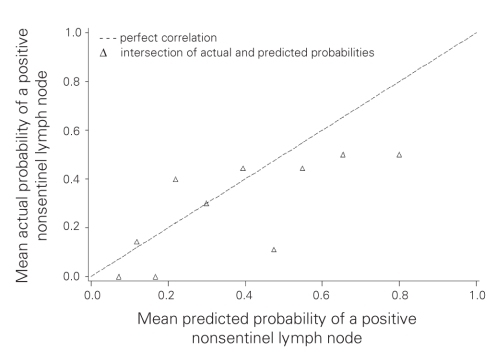
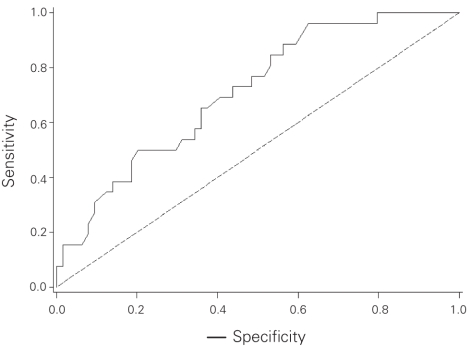
Compared with patients in the MSKCC population, our population with involved SLNs had some characteristics favouring a lower probability of NSLN involvement as predicted by the nomogram (Table 4). More of the Sunnybrook patients were Bloom and Richardson grade 1 (13% v. 3%), and fewer showed multifocality (18% v. 28%) or positive estrogen receptor status (71% v. 81%). In contrast, the presence of lymphovascular invasion was more frequent in the Sunnybrook cohort (58% v. 40%), and the absence of any uninvolved SLNs was also more frequent in the Sunnybrook cohort (50% v. 39%). There was a similar distribution of age, tumour size, number of positive SLNs and detection of SLN metastases by frozen section. We calculated the predicted probability of additional positive NSLNs using the MSKCC nomogram in the 90 patients with positive SLNs for whom complete data were available (nuclear grade was unavailable in 1 patient). The overall mean predicted probability was 38% (range 5%–95%), compared with the 29% of patients who actually had at least 1 positive NSLN. We grouped patients into deciles based on these predicted probabilities to compare the mean predicted probability of a positive NSLN for each decile with the actual proportion of patients with positive NSLNs (Table 5). Correlation between the predicted and actual probabilities (correlation coefficient = 0.74) was found to be present when analyzed in a calibration plot (Fig. 3). There was a tendency for the nomogram to over-predict NSLN involvement when compared with the actual data from the Sunnybrook population. We subsequently calculated the sensitivity and specificity of the nomogram for our data, producing an ROC curve (Fig. 4). The estimated area under the curve was 0.71 with a Hosmer and Lemeshow goodness-of-fit χ2 statistic of 15.1 (p8 = 0.60), indicating that the nomogram can reasonably predict but not closely fit the Sunnybrook data set.
Table 4
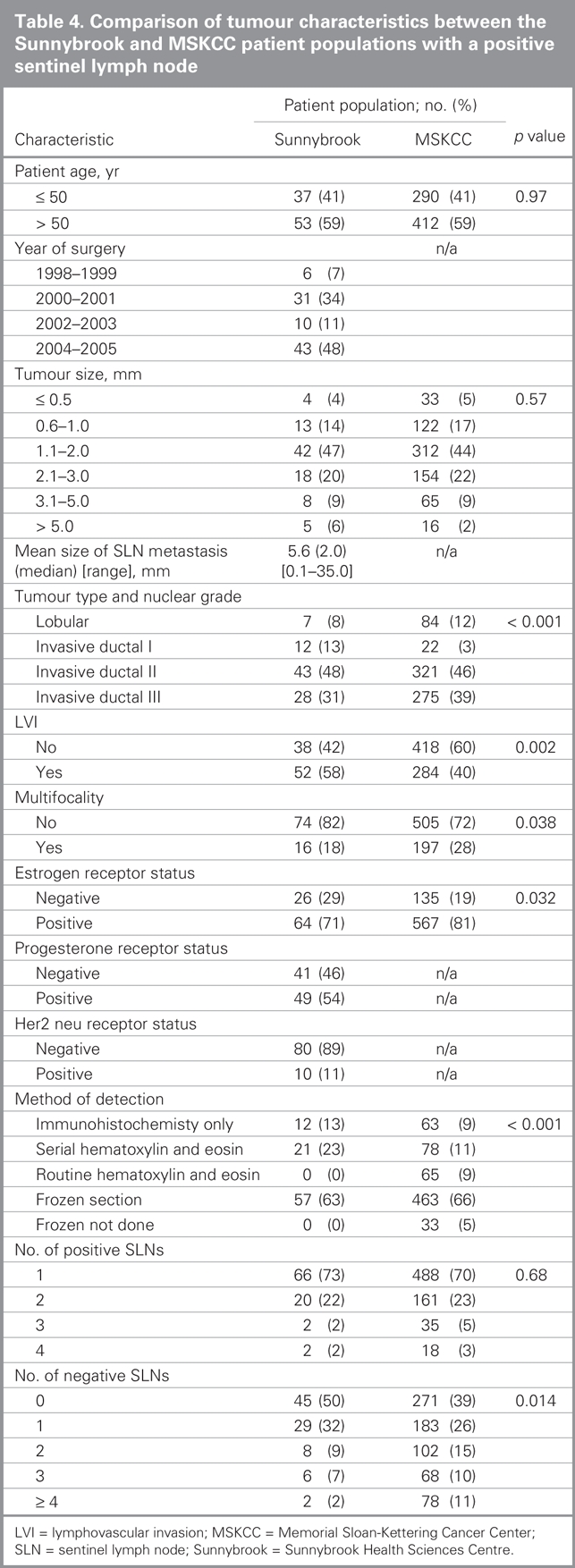
Table 5
Fig. 3. A calibration plot comparing actual and predicted probabilities of a positive nonsentinel lymph node. Correlation coefficient = 0.74.
Fig. 4. A receiver operating characteristic (ROC) curve assessing the nomogram. The area under the ROC curve is 0.71.
We attempted to derive a nomogram for the prediction of NSLN involvement using our data, but although the direction of contribution of each variable in our derived model was the same as in the MSKCC model, the small number of patients precluded the development of a reliable model (data not shown).
DISCUSSION
Our study showed that tumour grade 2 or 3, pure ductal carcinoma in situ, tumour size larger than 2 cm, lymphovascular invasion and multifocality were significantly associated with the probability of SLN involvement on univariate analysis (Table 2). These findings are similar to those of other groups.30–33 Ozmen and colleagues34 noted that patients whose tumours were associated with lymphovascular invasion were more likely to have positive SLNs on both univariate and multivariate analysis. Recently, Van den Eynden and colleagues35 determined that lymph vessel invasion and not blood vessel invasion was the only independent determinant of lymph node metastasis. Of these factors, only multifocality, lymphovascular invasion and a diagnosis of ductal carcinoma in situ were significant on multivariate analysis in our study (Table 3). The lack of significance for other covariates in the multivariate model may relate to the limited power of the data set, or the overwhelming effect of lymphovascular invasion as the dominant characteristic.
The multivariate model for prediction of SLN involvement fit closely with the data, and we were able to identify a population at low risk of SLN involvement who may not require routine intraoperative pathologic assessment of sentinel nodes. Women with small low-grade tumours are at low risk of SLN involvement, and these same factors also predict a low probability of NSLN involvement even if SLN metastases are present.22,36 Lymphovascular invasion often cannot be definitively determined to be absent on a core needle biopsy used for preoperative assessment, but as lymphovascular invasion was strongly correlated with grade in our study, selection of women with low-grade tumours may be a useful preoperative surrogate for those without lymphovascular invasion.
Ultrasound assessment of the axilla, with or without biopsy, is being increasingly used to identify women with axillary involvement preoperatively, thereby eliminating the need for SLNB before ALND.17,37,38 The specificity of this approach, reported as 73%–77%,17,37 is too low to warrant recommendation of axillary ultrasound as a substitute for SLNB. Most reported false-negative results on axillary ultrasound are generally associated with micrometastases,38 which similarly are associated with false-negative results on intraoperative assessment;39 thus axillary ultrasound may in future prove useful to guide selection of patients undergoing SLNB for intraoperative assessment. Currently, however, the technique itself is limited to specialized centres and is too operator-dependent to advocate a change in SLNB technique, including the use of intraoperative assessment, based on the results of preoperative axillary ultrasound examination. In contrast, we were unable to identify a group of women with a sufficiently high rate of SLN involvement to suggest that such women should not be offered SLNB. Although it would be reasonable to omit SLNB when the probability of the SLN being negative approached the false negative rate of SLNB, in our data set only 1% of patients had a 15% or less probability of a negative SLNB. The skewing of the data toward women with a lower probability of SLN involvement may be attributed to selective application of SLNB to lower-risk patients during the study period.
Although SLNB has been established as the procedure of choice for axillary staging among women who are node-negative, the risk of omitting completion ALND in women with an involved SLN has not been established in clinical trials. One trial comparing completion ALND versus axillary observation was closed owing to poor accrual (American College of Surgeons Oncology Group or ACOSOG Z0011 trial), whereas a phase III trial40 comparing ALND to axillary radiation is currently underway.Nonetheless, our current understanding of the biology of breast cancer is that prophylactic removal of uninvolved NSLNs is unlikely to have any direct therapeutic benefit. There is therefore considerable interest in decision aids to determine which women with positive SLNs are likely to benefit from ALND for residual disease.
We found that the MSKCC nomogram tended to overpredict NSLN involvement in our population. The area under the ROC curve was 0.71, which was lower than the value of 0.77 obtained when the nomogram was applied prospectively to the MSKCC population.22 Other groups that have undertaken the validation of the MSKCC nomogram within their patient population have calculated an area under the ROC curve of 0.72–0.86, suggesting our data are consistent with other published ROC curves.24–27
Use of immunohistochemistry for the evaluation of SLNs is widely used to minimize the risk of false-negative SLNBs, even if the significance of SLN metastases detectable only by immunohistochemistry remains controversial. Elimination of 12 patients in whom we detected SLN metastases with immunohistochemistry did not significantly alter the accuracy of the nomogram: the area under the curve was estimated at 0.70 with a 95% confidence interval of 0.57–0.83. The small difference in the area under the curve in our study compared with the MSKCC data may be related to differences in the underlying patient populations. Our population had a lower risk of NSLN involvement than the MSKCC population: the tumours tended to be of lower grade and more frequently unifocal and estrogen receptor–negative, all factors that predict a lower risk of NSLN involvement. In contrast, tumours in our data set more commonly had evidence of lymphovascular invasion, which has been associated with an increased risk of NSLN metastases in several studies.34,41–45 Although the area under the curve was reduced in our population compared with the MSKCC population, this does not directly relate to the clinical utility of the nomogram as reported by Degnim and colleagues.27 The reduction in accuracy of the nomogram in our population was predominantly related to a reduced specificity. As it was highly sensitive, application of the nomogram to our population would be more likely to result in ALND being performed when no NSLNs were involved, rather than omission of ALND in a significant proportion with residual axillary disease. Therefore, in our population with a low predicted probability of NSLN involvement, the risk of leaving residual disease is low. This finding contrasts the data of Kocsis and colleagues,46 who found that the nomogram underestimated the probability of NSLN involvement among low-risk women. With the threshold for performance of ALND set at a probability of NSLN involvement of 20%, the nomogram has 96% sensitivity in our population: in our cohort, 25 women, including 1 with NSLN involvement, would have been spared ALND. This analysis suggests a potential role for the MSKCC nomogram as a decision aid in our patient population, particularly if the predicted probability of NSLN involvement is low. As application of the nomogram requires information that is not available intraoperatively, selection of women with a low probability of SLN and NSLN involvement for ALND could await final pathologic examination with a low rate of repeat surgery. Decision analysis has shown that the use of intraoperative assessments of SLNs provides comparable quality of life to that associated with final pathologic assessment of the SLN.47 This implies that selective application of intraoperative assessment to those at greater risk of axillary disease may be a cost-effective strategy with little impact on patient satisfaction.
In conclusion, the presence of lymphovascular invasion and multifocality are associated with an increased risk of SLN involvement. Women with small low-grade tumours or ductal carcinoma in situ may not require intraoperative assessment of SLNs. The MSKCC nomogram tended to overpredict NSLN involvement in our population, suggesting that it would be most useful as a decision aid in selecting low-risk women in whom ALND may be omitted.
This work was presented at the 30th annual meeting of the American Society for Breast Disease, Las Vegas, Nevada, Apr. 27–29, 2006 (Abstract #2-19).
Contributors: Drs. Quan and Holloway designed the study. Drs. Ramjeesingh, Quan and Holloway acquired and analyzed the data. Dr. Gardner also analyzed the data. Drs. Ramjeesingh, Gardner and Holloway wrote the article, which all authors reviewed and approved for publication.
Competing interests: None declared.
Accepted for publication Sep. 22, 2007
Correspondence to: Dr. C.M.B. Holloway Department of Surgery Sunnybrook Health Sciences Centre University of Toronto Toronto ON M4N 3M5 fax 416 480-6002 claire.holloway@sunnybrook.ca
References
- 1.Recht A, Come SE, Gelman RS, et al. Integration of conservative surgery, radiotherapy, and chemotherapy for the treatment of early-stage, node-positive breast cancer: sequencing, timing, and outcome. J Clin Oncol 1991;9:1662-7. [DOI] [PubMed]
- 2.Chung MA, Steinhoff MM, Cady B. Clinical axillary recurrence in breast cancer patients after a negative sentinel node biopsy. Am J Surg 2002;184:310-4. [DOI] [PubMed]
- 3.Porter GA, McMulkin H, Lovrics GA. Sentinel lymph node biopsy in breast cancer: Canadian practice patterns. Ann Surg Oncol 2003;10: 255-260. [DOI] [PubMed]
- 4.Barone JE, Tucker JB, Perez JM, et al. Evidence-based medicine applied to sentinel lymph node biopsy in patients with breast cancer. Am Surg 2005;71:66-70. [PubMed]
- 5.Veronesi U. The sentinel node and breast cancer. Br J Surg 1999;86:1-2. [DOI] [PubMed]
- 6.Kennedy RJ, Kollias J, Gill PG, et al. Removal of two sentinel nodes accurately stages the axilla in breast cancer. Br J Surg 2003;90:1349-53. [DOI] [PubMed]
- 7.Palesty JA, Foster JM, Hurd TC, et al. Axillary recurrence in women with a negative sentinel lymph node and no axillary dissection in breast cancer. J Surg Oncol 2006;93:129-32. [DOI] [PubMed]
- 8.de Kanter AY, Menke-Pluymers MM, Wouters MW, et al. 5-Year follow-up of sentinel node negative breast cancer patients. Eur J Surg Oncol 2006;32:282-6. [DOI] [PubMed]
- 9.Khakpour N, Hunt KK, Kuerer HM, et al. Sentinel lymph node dissection provides axillary control equal to complete axillary node dissection in breast cancer patients with lobular histology and a negative sentinel node. Am J Surg 2005;190:598-601. [DOI] [PubMed]
- 10.Swenson KK, Mahipal A, Nissen MJ, et al. Axillary disease recurrence after sentinel lymph node dissection for breast carcinoma. Cancer 2005;104:1834-9. [DOI] [PubMed]
- 11.Zavagno G, Carcoforo P, Franchini Z, et al. Axillary recurrence after negative sentinel lymph node biopsy without axillary dissection: a study on 479 breast cancer patients. Eur J Surg Oncol 2005;31:715-7. [DOI] [PubMed]
- 12.Snider HC Jr, Rubin E, Henson R. Axillary ultrasonography to detect recurrence after sentinel node biopsy in breast cancer. Ann Surg Oncol 2006;13:501-7. [DOI] [PubMed]
- 13.Julian TB, Krag D, Brown A, et al. Preliminary technical results of NSABP B-32, a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer patients. Breast Cancer Res Treat 2004;88:S11-2.
- 14.Cantin J, Scarth H, Levine M. Hugi M and the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 13. Sentinel lymph node biopsy. CMAJ 2001;165:166-73. [PMC free article] [PubMed]
- 15.Voordeckers M, Vinh-Hung V, Van de Steene J, et al. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol 2004;70:225-30. [DOI] [PubMed]
- 16.Gemignani ML., Borgen PI. Is there a role for selective axillary dissection in breast cancer? World J Surg 2001;25:809-18. [DOI] [PubMed]
- 17.Nori J, Vanzi E, Bazzocchi M, et al. Role of axillary ultrasound examination in the selection of breast cancer patients for sentinel node biopsy. Am J Surg 2007;193:16-20. [DOI] [PubMed]
- 18.Calhoun KE, Hansen NM, Turner RR, et al. Nonsentinel node metastases in breast cancer patients with isolated tumour cells in the sentinel node: implications for completion axillary dissection. Am J Surg 2005;190:588-91. [DOI] [PubMed]
- 19.Chu KU, Turner RR, Hansen NM, et al. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel lymph node metastasis. Ann Surg Oncol 1999;6:756-61. [DOI] [PubMed]
- 20.Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol 2003;29:341-50. [DOI] [PubMed]
- 21.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer 1998;83:2776-81. [DOI] [PubMed]
- 22.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 2003;10:1140-51. [DOI] [PubMed]
- 23.Specht MC, Kattan MW, Gonen M, et al. Predicting nonsentinel node status after positive sentinel lymph biopsy for breast cancer: clinicians versus nomogram. Ann Surg Oncol 2005;12:654-9. [DOI] [PubMed]
- 24.Smidt ML, Kuster DM, van der Wilt GJ, et al. Can the Memorial Sloan-Kettering Cancer Center nomogram predict the likelihood of nonsentinel lymph node metastases in breast cancer patients in the Netherlands? Ann Surg Oncol 2005;12:1066-72. [DOI] [PubMed]
- 25.Soni NK, Carmalt HL, Gillett DJ, et al. Evaluation of a breast cancer nomogram for prediction of non-sentinel lymph node positivity. Eur J Surg Oncol 2005;31:958-64. [DOI] [PubMed]
- 26.Lambert LA, Ayers GD, Hwang RF, et al. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol 2006;13: 310-20. [DOI] [PubMed]
- 27.Degnim AC, Reynolds C, Pantvaidya G, et al. Nonsentinel node metastasis in breast cancer patients: assessment of an existing and a new predictive nomogram. Am J Surg 2005;190:543-50. [DOI] [PubMed]
- 28.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29-36. [DOI] [PubMed]
- 29.Hosmer D, Lemeshow S. Assessing the fit of the model. In: Applied logistic regression. 1st ed. Hoboken (NJ): John Wiley & Sons; 1989. p. 140-5.
- 30.Rivers AK, Griffith KA, Hunt KK, et al. Clinicopathologic features associated with having four or more metastatic axillary nodes in breast cancer patients with a positive sentinel lymph node. Ann Surg Oncol 2006;13:36-44. [DOI] [PubMed]
- 31.Andea AA, Bouwman D, Wallis T, et al. Correlation of tumour volume and surface area with lymph node status in patients with multifocal / multicentric breast carcinoma. Cancer 2004;100:20-7. [DOI] [PubMed]
- 32.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg 2005;200:516-26. [DOI] [PubMed]
- 33.Wada N, Imoto S, Yamauchi C, et al. Predictors of tumour involvement in remaining axillary lymph nodes of breast cancer patients with positive sentinel lymph node. Eur J Surg Oncol 2006;32:29-33. [DOI] [PubMed]
- 34.Ozmen V, Karanlik H, Cabioglu N, et al. Factors predicting the sentinel and non-sentinel lymph node metastases in breast cancer. Breast Cancer Res Treat 2006;95:1-6. [DOI] [PubMed]
- 35.Van den Eynden GG, Van der Auwera I, Van Laere SJ, et al. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer 2006;94:1643-9. [DOI] [PMC free article] [PubMed]
- 36.Sachdev U, Murphy K, Derzie A, et al. Predictors of nonsentinel lymph node metastasis in breast cancer patients. Am J Surg 2002;183:213-7. [DOI] [PubMed]
- 37.van Rijk MC, Deurloo EE, Nieweg OE, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol 2006;13:31-5. [DOI] [PubMed]
- 38.Nori J, Bazzocchi M, Boeri C, et al. Role of axillary lymph node ultrasound and large core biopsy in the preoperative assessment of patients selected for sentinel node biopsy. Radiol Med 2005;109:330-44. [PubMed]
- 39.Rutledge H, Davis J, Chiu R, et al. Sentinel node micrometastasis in breast carcinoma may not be an indication for complete axillary dissection. Mod Pathol 2005;18:762-8. [DOI] [PubMed]
- 40.Hurkmans CW, Borger JH, Rutgers EJ, et al.; EORTC Breast Cancer Cooperative Group. Radiotherapy Cooperative Group. Quality assurance of axillary radiotherapy in the EORTC AMAROS trial 10981/22023: the dummy run. Radiother Oncol 2003;68:233-40. [DOI] [PubMed]
- 41.Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: Can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol 1999;17:1720-6. [DOI] [PubMed]
- 42.Turner RR, Chu KU, Qi K, et al. Predictive factors for metastatic involvement of nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer 2000;89:574-81. [DOI] [PubMed]
- 43.Abdessalam S, Zervos EE, Prasad M, et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg 2001;182:316-20. [DOI] [PubMed]
- 44.Weiser MR, Montgomery LL, Tan LK, et al. Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol 2001;8:145-9. [DOI] [PubMed]
- 45.Viale G, Maiorano E, Masszrol G, et al. Histologic detection and clinical implications of micrometastases in axillary sentinel lymph nodes for patients with breast carcinoma. Cancer 2001;92:1378-84. [DOI] [PubMed]
- 46.Kocsis L, Svebis M, Boross G, et al. Use and limitations of a nomogram predicting the likelihood of non-sentinel node involvement after a positive sentinel node biopsy in breast cancer patients. Am Surg 2004;70:1019-24. [PubMed]
- 47.Chao C, Abell T, Martin RC II, et al. Intraoperative frozen section of sentinel nodes: a formal decision analysis. Am Surg 2004;70:215-20. [PubMed]