Abstract
Background
We sought to review the clinical presentation and outcomes of surgical management of gastrointestinal stromal tumours (GISTs).
Methods
We reviewed clinical and pathological records of 41 patients (23 men and 18 women) with GISTs. We performed survival analyses using the Kaplan– Meier method and evaluated long-term survival and the independent prognostic factors that affect survival using univariate analyses. We used the Cox proportional hazards regression model to estimate the simultaneous effect on overall survival.
Results
The stomach was the most common tissue of origin (n = 20, 48.8%). The mean tumour diameter was 8.3 cm. We detected advanced-stage tumours in 22 (53.7%) patients. We performed complete resection in 31 (75.6%) patients. Mitotic count was greater than 5/50 high-power field [HPF] in 22 (53.6%) patients. Immunohistochemical staining for CD117 was positive in 40 (97.6%) patients. Five patients (12.2%) died in the early postoperative period. The mean follow-up period was 38.7 months. The median length of survival was 53 months and the 5-year survival rate was 49.4%. Univariate analyses revealed significantly enhanced survival for the following variables: patient age less than 60 years (p = 0.011), male sex (p = 0.048), tumour diameter less than 5 cm (p = 0.029), low-risk tumour according to Fletcher classification (p = 0.022), complete resection (p < 0.001), and lack of local recurrence (p < 0.001) and/or metastasis (p < 0.001). Our Cox proportional hazards model revealed that complete tumour resection was the only factor to increase survival.
Conclusion
Overall survival is significantly affected by positive margins. A complete surgical resection with negative margins is the best method for definitive treatment of GISTs.
Abstract
Contexte
Nous voulions examiner la présentation clinique et les résultats du traitement chirurgical des tumeurs du stroma gastro-intestinal (TSGI).
Méthodes
Nous avons étudié les dossiers cliniques et pathologiques de 41 patients (23 hommes et 18 femmes) présentant une TSGI. Nous avons analysé leur survie au moyen de la méthode Kaplan–Meier et nous avons évalué la survie à long terme et les facteurs pronostiques indépendants ayant une incidence sur la survie en procédant à des analyses unidimensionnelles. Nous avons utilisé le modèle de régression des risques proportionnels de Cox pour estimer l'effet simultané sur la survie globale.
Résultats
L'estomac était le tissu d'origine le plus courant (n = 20; 48,8 %). Le diamètre moyen de la tumeur était de 8,3 cm. Nous avons détecté des tumeurs au stade avancé chez 22 (53,7 %) patients. Nous avons pratiqué une résection complète chez 31 (75,6 %) patients. La numération mitotique était de plus de 5/50 au fort grossissement chez 22 (53,6 %) patients. La coloration immunohistochimique pour CD117 a été positive chez 40 (97,6 %) patients. Cinq patients (12,2 %) sont morts au cours de la période postopératoire immédiate. La période de suivi moyenne s'est établie à 38,7 mois. La durée médiane de survie a atteint 53 mois et le taux de survie à 5 ans, 49,4 %. Des analyses unidimensionnelles ont révélé une survie bien meilleure correspondant aux variables suivantes : patient âgé de moins de 60 ans (p = 0,011), sexe masculin (p = 0,048), diamètre de la tumeur inférieur à 5 cm (p = 0,029), tumeur à faible risque selon la classification de Fletcher (p = 0,022), résection complète (p < 0,001) et absence de récidive locale (p < 0,001) ou de métastases (p < 0,001). Notre modèle des risques proportionnels de Cox a révélé que la résection complète de la tumeur constituait le seul facteur d'augmentation de la survie.
Conclusion
Une marge positive a un effet important sur la survie globale. La résection chirurgicale complète et des marges négatives constituent la meilleure méthode de traitement définitif des TSGI.
Gastrointestinal stromal tumours (GISTs) are rare tumours that originate from Cajal cells in the myenteric plexus. These cells are most commonly located in the stomach (39%–70%) and account for 0.1%–3% of all gastrointestinal system tumours.1–3 The tumours are most commonly observed after the fourth decade of life, and they have an equivalent male:female ratio. They may or may not cause symptoms, depending on their location, and they are often discovered incidentally.4
Surgical resection is currently the “gold standard” in the management of GISTs. Complete resection with negative margins is the main goal of surgery. Nevertheless, the survival rate in patients with GISTs is low, whereas the rates of local recurrence or metastasis are high.5
We sought to review data from the early postoperative period, investigate outcomes in the late postoperative preiod and determine factors that affect survival among patients who had surgery for GISTs.
METHODS
We retrospectively investigated patients who had surgery for GISTs in the first, third and fourth general surgery clinics of the Ataturk Training and Research Hospital between 1999 and 2005. We reviewed demographic data, clinical findings, diagnostic methods, tumour characteristics, intraoperative findings, surgical procedures, postoperative morbidity and mortality rates, histopathologic findings and data from follow-up studies by browsing hospital archives. In cases where postoperative evaluation was not possible, we telephoned the patients to obtain the data.
During the preoperative period, we obtained biopsy specimens from selected patients by endoscopic interventions, which helped diagnose GISTs after careful histopathologic evaluations. The percutaneous approach was never used to obtain biopsy specimens.
The purpose of surgery was to resect the tumour completely, including invaded adjacent tissues if any. We considered an absence of tumour tissue 1 mm or more from the edge of the specimen to be a clear margin; tumour tissue extension within less than 1 mm of the edge of the specimen was considered to be a positive margin. We considered resection to be complete if margins of the resected material were clear, whereas we considered the procedure to be incomplete if positive margins were detected.
We performed immunohistochemical analyses in all specimens in the postoperative period. We determined the number of mitoses in the tumour tissues. We classified tumours into 4 groups based on Fletcher classification:
1. very low-risk tumour (diameter < 2 cm and mitosis count < 5/50 high-power field [HPF]),
2. low-risk tumour (diameter 2–5 cm and mitosis count < 5/50 HPF),
3. intermediate-risk tumour (diameter < 5 cm and mitosis count 10/50 HPF or diameter 5–10 cm and mitosis count < 5 /50 HPF) and
4. high-risk tumour (diameter > 5 cm and mitosis count > 5/50 HPF or diameter > 10 cm and any mitotic rate).
We noted early postoperative morbidity and mortality. Postoperative mortality referred to death within 30 days after surgery. Patients who had incomplete resections were prescribed a 400 mg/day dose of an oral tyrosine kinase receptor inhibitor (STI-571/imatinib mesylate, known as Gleevec; Novartis). Patients who had complete resections did not receive any adjuvant therapy. We expressed survival as the number of months from the date of surgery to last follow-up or death.
Our statistical analyses included the χ2 test, Fisher exact test, Student t test, Kaplan–Meier method and log-rank test. We excluded patients who died in the early postoperative period from our survival analysis. We used the Kaplan– Meier method for our survival analyses, and we evaluated the differences using the log-rank test. We evaluated long-term survival and the independent prognostic factors that affect survival using univariate analyses. We performed simultaneous association of multiple variables using the Cox proportional hazards regression model to estimate the simultaneous effect on overall survival. We entered independent variables that showed statistical significance in the univariate analysis into our multivariate analysis. We considered p < 0.05 to be statistically significant. We used the SPSS 10.0 for Windows package (Microsoft) to perform our statistical analyses.
RESULTS
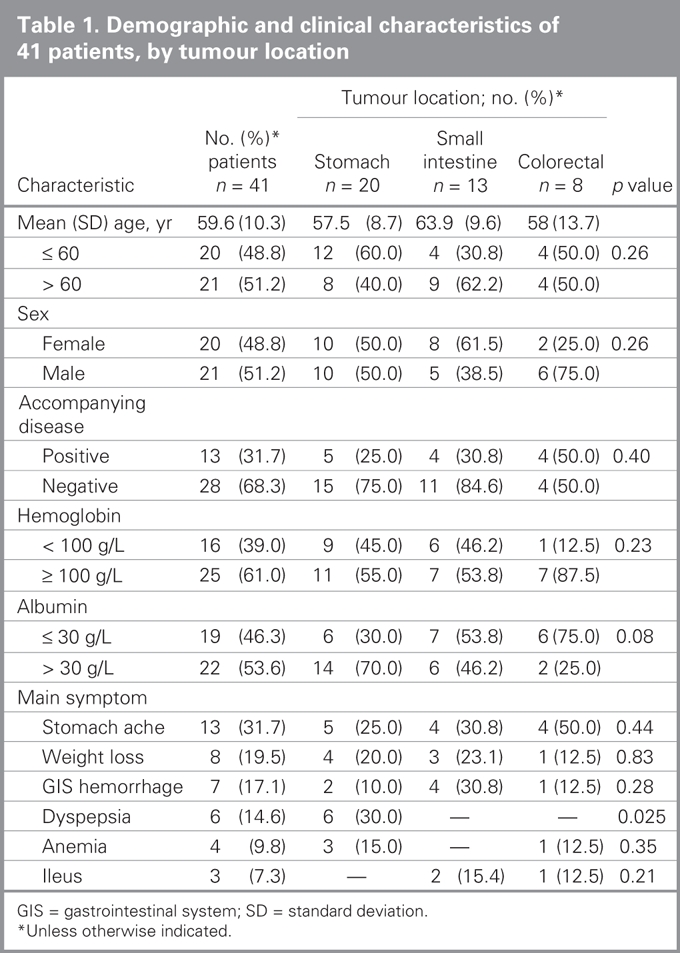
We included a total of 41 patients with available data in our study. We obtained postoperative data on 11 patients by telephone. The mean age was 59.6 (standard devision [SD] 10.3, range 32–76) years, and the female:male ratio was 18:23. Tumours were most commonly located in the stomach (n = 20, 48.8%), followed by the intestines (n = 13, 31.7%), colon (n = 7, 17%) and rectum (n = 1, 2.4%). We found no GISTs in the esophagus. The most common GIST symptoms were stomach ache (31.7%), weight loss (19.5%) and gastrointestinal hemorrhage (17%). At the time of diagnosis, the mean hemoglobin level was 108 (SD 21, range 72–140) g/L, and the mean albumin level was 30 (SD 4, range 23–39) g/L. A total of 13 patients (31.7%) had accompanying disorders, including hypertension (n = 5, 12.2%), congestive heart failure (n = 3, 7.3%), chronic obstructive lung disease (n = 3, 7.3%) and diabetes (n = 2, 4.9%). Table 1 lists demographic and clinical characteristics of patients based on GIST localizations.
Table 1
Methods for diagnosing GISTs included plain radiography (n = 41, 100%), double-contrast radiographic investigations (n = 24, 58.5%), ultrasonography (n = 41, 100%), computed tomography (n = 34, 89.9%), magnetic resonance imaging (n = 25, 61%), rectosigmoidoscopy (n = 31, 75.6%), gastroduodenoscopy (n = 30, 73.2%), colonoscopy (n = 25, 61%) and endorectal ultrasonography (n = 2, 4.9%). Endoscopic biopsy specimens were obtained in the preoperative period from all patients with stomach and colorectal tumours (n = 28, 68.3%), but not from those who had tumours in the small intestine. Of the 28 patients from whom specimens were obtained, 12 (42.8%) received a diagnosis of GIST after the biopsy. The rate of GIST diagnosis from biopsy specimens was 45% (9/20) in gastric and 37.5% (3/8) in colorectral stromal tumours.
Five patients (12.2%) underwent emergency surgeries owing to obstruction (n = 3, 60%), stomach ache mimicking acute abdominal pain (n = 1, 20%) and gastrointestinal system hemorrhage (n = 1, 20%).
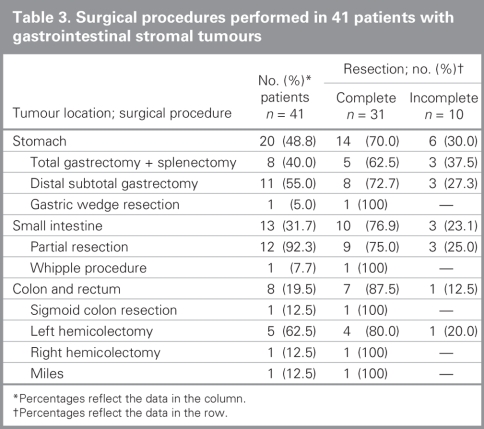
Surgical findings and postoperative outcomes based on tumour locations are listed in Table 2. The mean tumour diameter was 8.3 (SD 7.1, range 3–35) cm. We detected advanced-stage tumours in 22 (53.7%) patients; of these 17 (77.3%) had locally advanced tumours and 5 (22.7%) had metastatic (to peritoneum, n = 4, 80% and liver, n = 1, 20%) tumours. We performed macroscopic gross resection in 34 (82.9%) patients. Complete resection was achieved in 31 (75.6%) patients. Details on the surgical procedures are summarized in Table 3.
Table 2
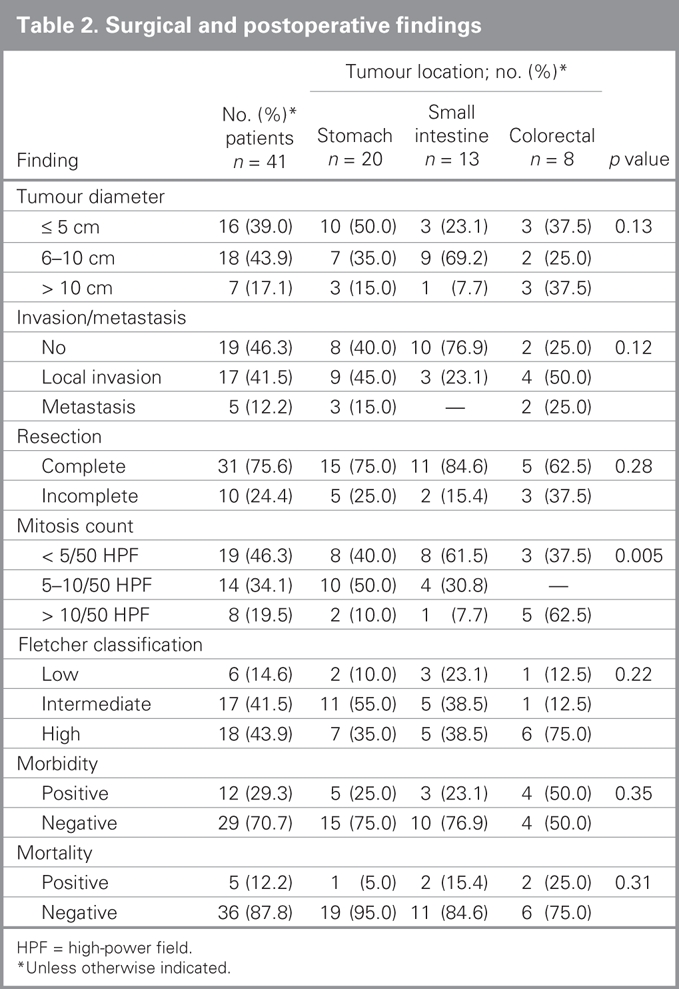
Table 3
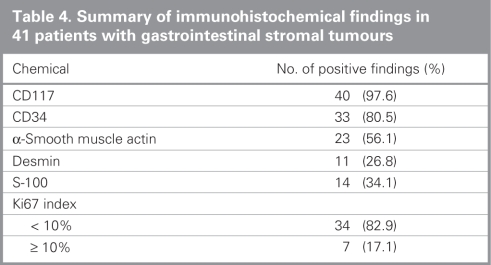
Histopathologic investigation of all resected specimens revealed fusiform-epitheloid cells originating from muscularis propria. Immunohistochemical staining for CD117 was positive in 40 (97.6%) patients (Table 4). The mean mitosis count was 6.3/50 (SD 3.6, range 2–14) HPF. We detected mitoses less than 5/50 HPF in 19 (46.3%) patients, and mitoses equal to or greater than 5/50 HPF were detected in 22 (53.7%) patients. We detected regional lymph node involvement in 1 patient (2.4%). Tumours were low-risk in 6 (14.6%) patients, intermediate-risk in 17 (41.5%) patients and high-risk in 18 (43.9%) patients according to Fletcher classification. No very low-risk tumours were detected.
Table 4
Twelve patients experienced postoperative complications: wound infection (n = 5, 41.7%), atelectasis (n = 2, 16.7%), pleural effusion (n = 2, 16.7%), anastomosis failure (n = 2, 16.7%) and hemorrhage (n = 1, 8.3%). One patient with a stomach tumour experienced hemorrhage due to total gastrectomy plus splenectomy and required re-exploration. One of the 2 patients with anastomosis failure had also received total gastrectomy plus splenectomy owing to a tumour in the stomach. Failure of esophagojejunostomy in this patient was managed using a conservative approach; the patient spontaneously recovered after 2 weeks of total parenteral nutrition. The other patient with anastomosis failure underwent segmental colon resection owing to a tumour in the left colon. The patient had a fistula, which we treated with a diverting colostomy and closed 8 weeks later.
Five patients (12.2%) died in the early postoperative period due to pulmonary thromboemboli (n = 2, 40%), myocardial infarction (n = 1, 20%), respiratory failure (n = 1, 20%) and heart failure (n = 1, 20%). Of these, 4 (80%) had complete resections and 1 (20%) had an incomplete resection.
The mean follow-up period was 38.7 (SD 19.1, range 6–83) months. After complete resection, we detected local recurrence in 6 (22.2%) of the 27 patients who survived within an average of 19 (SD 8.5, range 9–32) months, and liver metastases developed in 7 patients (25.9%) within an average of 22.4 (SD 9.9, range 13–37) months. Thirteen (48.1%) of the 27 surviving patients who had tumours with mitosis counts equal to or greater than 5/50 HPF had complete resection; 5 (38.5%) of these 13 patients had local recurrence. On the other hand, 14 of the patients who had tumours with mitosis counts less than 5/50 HPF had complete resection; we detected local recurrence in 1 patient (7.1% (p = 0.08). We detected liver metastasis in 2 (14.3%) of the 14 patients with mitosis counts less than 5/50 HPF who had complete resection and 5 (38.5%) patients with mitosis counts equal to or greater than 5/50 HPF who had complete resection (p = 0.21). We excised tumours in a repeat surgery in 2 of 6 patients (33.3%) with local recurrence, whereas patients with liver metastasis did not undergo repeat surgery. All patients with local recurrence and liver metastasis received tyrosine kinase receptor inhibitor medication. Among patients who had complete resections, we detected local recurrence in 4 (50%) of 8 patients with high-risk tumours and 2 (15.4%) of 13 patients with intermediate-risk tumours (p = 0.06). No local recurrence was detected in any of the 6 patients with low-risk tumours according to Fletcher classification.
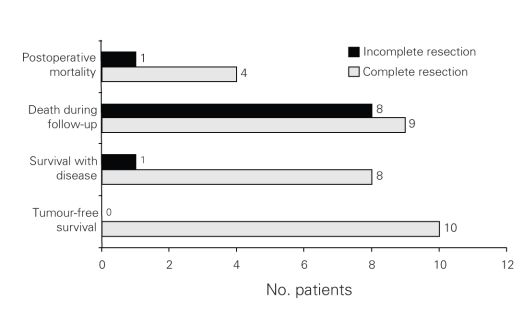
Seventeen of 36 patients (47.2%) died during postoperative long-term follow-up. Of these 17 patients, 9 (52.9%) had complete resections and 8 (47.0%) had incomplete resections. Sixteen (94.1%) died due to tumour-related reasons. Of the 9 patients who had complete resection, death related to the tumour occurred in 8 (88.9%), and death was not related to the tumour in 1 (11.1%) patient. Of the total number of patients who had complete resections, currently 10 (37%) patients are tumour-free, and 8 (29.6%) patients have recurrent or metastatic disease. Eight of 9 patients (88.9%) who had incomplete resection died; death was associated with the tumour in all patients (Fig. 1).
Fig. 1. Results of surgical resection in patients with gastrointestinal stromal tumours.
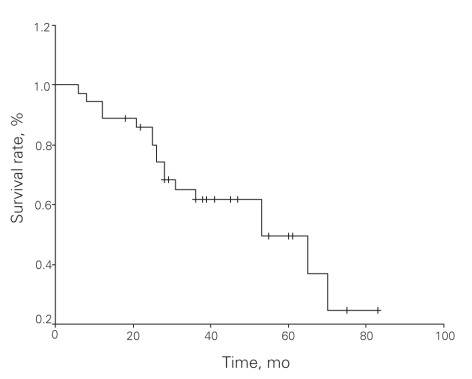
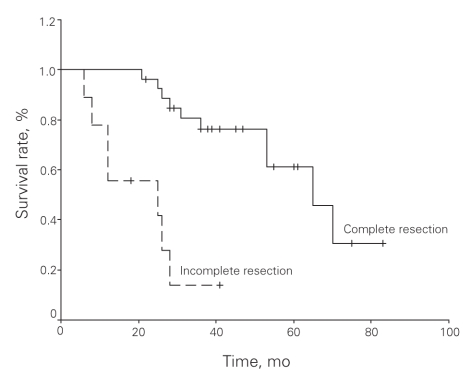
The median survival was 53 months and the median 5-year survival rate was 49.4%. Univariate analyses revealed significantly enhanced survival with the following factors: patient age younger than 60 years (p = 0.011), male sex (p = 0.048), tumour diameter less than 5 cm (p = 0.029), low-risk tumour according to Fletcher classification (p = 0.022), complete tumour resection (p < 0.001), and lack of local recurrence (p < 0.001) and/or metastasis after complete resection (p < 0.001). Multivariate analysis (Cox proportional hazards model) revealed that complete tumour resection was the only factor to increase survival (Table 5). An overall survival curve is shown in Figure 2 and survival curves by margin status are shown in Figure 3.
Table 5
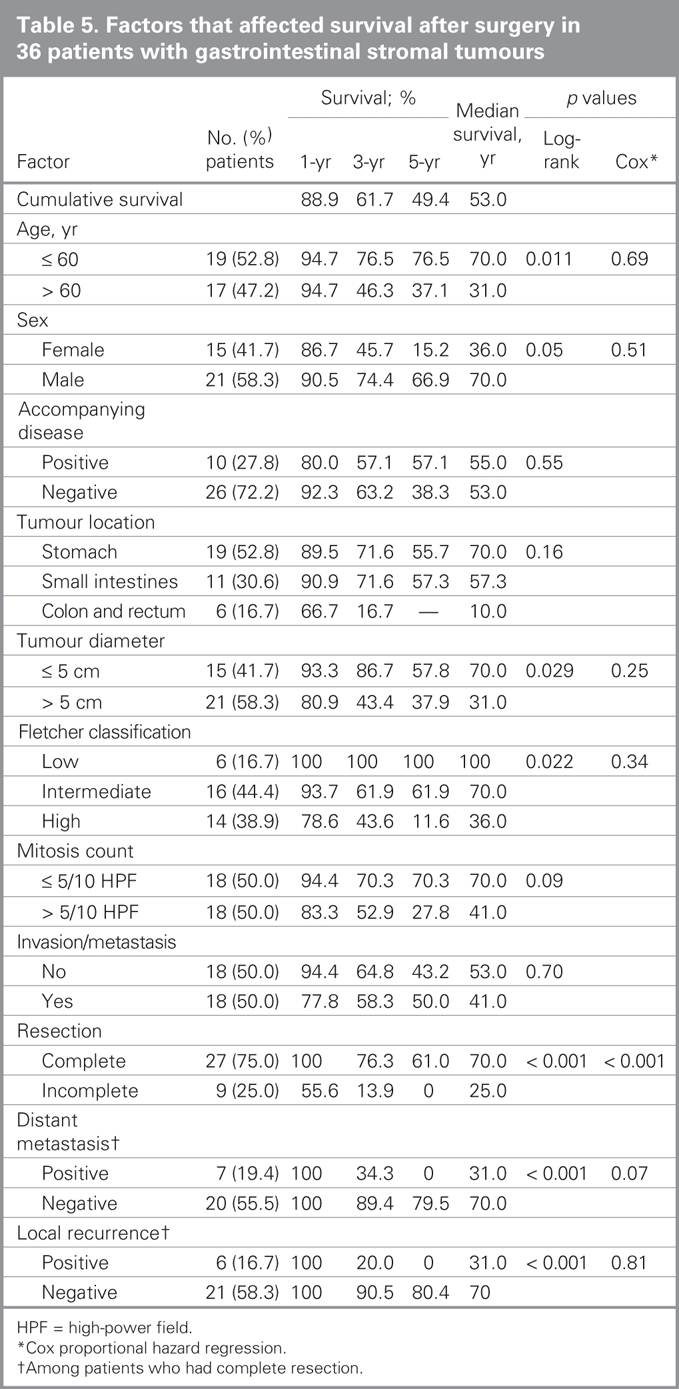
Fig. 2. Overall survival in patients with gastrointestinal stromal tumours (n = 36).
Fig. 3. Disease-specific survival by margin status in patients with gastrointestinal stromal tumours.
DISCUSSION
Gastrointestinal stromal tumours were first defined by Mazur and Clark6 after they detected a subgroup of gastrointestinal mesenchimal tumours that did not originate from smooth muscle or have neurogenic basis. In 1998, Kindblom and colleagues1 showed that these tumours originated from Cajal interstitial cells (pacemaker cells of myenteric plexus) or multipotent mesenchymal stem cells. Gastrointestinal stromal tumours make up 0.1%–3% of all gastrointestinal system tumours and 5% of all soft-tissue sarcomas.2 They most commonly are located in the stomach (39%–70%) and small intestine (20%–35%), whereas the colon and rectum (5%–12%) and esophagus (2%–5%) are less common locations.3,4,7–10 Consistently, we found in our study that GISTs were localized most commonly in the stomach (48.8%), followed by the small intestine (31.7%). However, the colorectal localization ratio was higher in our study than that reported in the literature (19.51%). That GISTs generally occur in people after the fourth decade of life, with most studies finding a mean age at diagnosis of about 60 years, and with an even distribution in both sexes is also consistent with our data.4
Gastrointestinal stromal tumours may not cause symptoms; they may cause nonspecific symptoms depending on the location of the tumour and they may be detected incidentally during investigation of these symptoms or during autopsy.11,12 Abdominal pain, melena and weight loss are the most common symptoms in patients with GISTs.13 Rarely, an abdominal mass is palpable. Likewise, the most common symptoms in our patients were abdominal pain (31.7%), weight loss (19.5%) and gastrointestinal system hemorrhage (17%).
No laboratory test can specifically confirm or rule out the presence of a GIST. Plain abdominal radiographs are useful only in terms of detecting intestinal obstruction and perforation. In double-contrast radiographic series (barium swallow, barium enema or both) tumours could be visualized as a sharp-edged filling defect; however, this method does not have a considerable diagnostic value.14 Detection of the tumour and defining its location with the enteroclysis method is not easy. The tumour can be detected using ultrasonography; however, this method may not be adequate in evaluating intestines filled with gas and fluid. Although CT, MRI and endoscopic ultrasonography have been reported to be helpful in the detection and staging of GISTs, definitive diagnosis can be established after histopathologic investigations.15–20 A “full-layer biopsy” should be obtained by an experienced endoscopist for true histopathologic diagnosis, because GISTs have submucosal localization. For this reason, some authors reported that GISTs had been diagnosed in only 27%–50% of the cases by endoscopic biopsy.10–13 In our study, endoscopic biopsies were obtained from 68.3% of the patients, and GISTs were diagnosed in 42.8% of the biopsy specimens in the preoperative period. Similar to El-Zohairy and colleagues11 and Gold and Dematteo,12 we do not favour performing a preoperative percutaneous biopsy because of the risk of peritoneal seeding or tumour rupture.
Gastrointestinal stromal tumours rarely cause acute gastrointestinal hemorrhage or obstruction. Obstruction can result from intraluminal growth of an endophytic tumour or from luminal compression of an exophytic lesion. Gastrointestinal bleeding is produced by pressure necrosis and ulceration of the overlying mucosa with resulting hemorrhage from disrupted vessels. In our study, 12.2% of patients with GISTs required emergency surgery owing most commonly to intestinal obstruction (60%). In these patients, GISTs were located in the small intestine (n = 4, 80%) and colon (n = 1, 20%).
Surgical resection is currently the “gold standard” in GIST management, although new-generation chemotherapy is widely used. Less than 10% of GISTs (generally 0%–2%) affect regional lymph nodes.21–24 Thus, lymphadenectomy is not a goal of GIST surgery; rather, complete resection with a negative margin should be the goal.5,11 We found a lymph node metastasis rate of 2.43% in our study.
In our study, 12.2% of the patients died due to extratumoural causes within the first month after surgery. We believe that the following factors contributed to postoperative mortality rates in our patients:
severe diseases accompanying the tumour in almost one-third of the patients (31.7%),
requirement for aggressive surgery (75.6%) for complete resection and
existence of postoperative complications in 29.3% of this relatively elderly (> 60 years; 51.2%) population.
The 5-year survival rate after surgical resection has been reported as 30%–65% among patients with GISTs.7,21,25–27 Similarly, we observed a 5-year survival rate of 49.4%.
Completeness of resection is an important factor that affects survival in patients with GISTs. Patients have a 5-year survival rate of 32%–93% following complete resection.21,26–29 We found a 5-year survival rate of 61% among patients who had complete resections, which our multivariate analyses have shown affects survival significantly. The effects of incomplete resection on survival rates among patients with advanced-stage GISTs are controversial. According to Wu and Bucher,30 debulking of large tumours could increase the effectiveness of chemotherapy even if negative margin was not maintained. On the contrary, Langer and colleagues31 concluded that incomplete resection only helped patients recover from such symptoms as pain or hemorrhage and that it did not affect survival. DeMatteo and colleagues26 and Crosby and colleagues32 reported 5-year survival rates after incomplete resection of 9% and 8%, respectively. The 5-year survival rate after complete resection was 42% in both studies. We found a median survival rate of 70 months and 25 months in patients who had complete resections with and without negative margins, respectively. Furthermore, no 5-year survival was recorded in patients who had complete resections without negative margins. Wu and colleagues30 reported that they could maintain negative margins in 49% of patients, whereas Besana-Ciani and colleagues5 reported a rate of 78.9%. However, maintaining a macroscopic negative margin with complete resection does not necessarily mean that microscopic negative margins are also maintained.12 In our study, 82.9% of all patients had macroscopic gross resections; the actual negative margin was maintained in 75.6% of these patients.
Whether or not the GIST is malignant is another factor that affects survival. Common histopathologic criteria are not inadequate in distinguishing between benign and malignant tumours. With a simple approach, a given tumour can be considered malignant when a mitosis count greater than 5/50 HPF is detected. In our study, tumours in 53.6% of patients had a mitosis count of greater than 5/50 HPF. Regardless of presentation, the disease-specific 5-year survival rate for patients with malignant GISTs is 29%–35%.26,33,34 We found 5-year survival rates of 27.8% among patients with malignant GISTs and 70.3% among patients with benign GISTs. Although the 5-year survival rate of patients with benign GISTs was more than twice that of those with malignant GISTs, we were unable to show a significant correlation between mitosis count and survival.
Longer survival has been reported for tumours smaller than 5 cm in diameter.26,35 Consistently, we found that patients with tumours smaller than 5 cm in diameter had a significantly higher 5-year survival rate compared with those who had tumours larger than 5 cm in diameter (57.8% v. 37.9%).
The relation between the location of the tumour and the patient's survival is controversial. DeMatteo and colleagues26 proposed that the patient's survival depended on the location of the tumour, whereas Lillemoe and Efron36 suggested the opposite. There is consensus, however, on fair prognosis of GISTs located in the stomach.7,27,28,35 We found a 5-year survival rate of 55.7% among patients with tumours located in the stomach; the rate increased to 57.3% among patients with GISTs in the small intestine. One of 8 patients with colorectal GISTs is still alive. However, the 5-year survival rate among patients with colorectal GISTs has not been determined because a 5-year follow-up period has not yet been completed. On the other hand, the 3-year survival rate in patients with colorectal GISTs was low compared with that in patients with GISTs located in the stomach and small intestine (16.7% v. 71.6%). Although a small number of patients have been included in our analysis, our results suggest that the survival rate is lower among patients with colorectal GISTs compared with those with GISTs in the stomach and small intestine.
Recurrence is one of the most important problems among patients with malignant GISTs even if complete resection has been performed. DeMatteo and colleagues26 and Pierie and colleagues34 reported recurrence rates of about 40%–52%. We found recurrence rates of 7.1% and 38.5% following complete resection in patients with benign and malignant GISTs, respectively. However, predicting the behaviour of the GIST based solely on its mitosis count may not always yield accurate results.27,35,37 For this purpose, Flacher and colleagues35 developed a scale that estimates aggressive behaviour risk of the tumour by evaluating both mitosis count and tumour diameter. When we evaluated our cases according to this scale, we did not observe local recurrence of tumours with low risk of aggressive behaviour; however, we detected local recurrence rates of 15.4% and 50% in tumours with intermediate and high risk of aggressive behaviour, respectively.
Recurrences usually occur locally or as liver metastases. We detected a local recurrence or liver metastasis rate of 48.1% in patients who had complete resections. The value of metastasectomy in local recurrence or liver metastasis is controversial. However, it has been reported that the median survival in patients with local recurrence was shorter compared with those with metastatic disease (9–12 mo v. 20 mo). Thus, complete resection of recurrent tumours would affect survival in a positive way.26,38,39 The fact that nonresectable or metastatic GISTs are resistant to conventional cytotoxic treatment and that radiotherapy is not effective in these cases renders repeat surgery necessary in patients with local recurrences.8,40–42 Consistently, using univariate analysis, we found a low rate of survival among patients with local recurrence or metastasis. Our multivariate analyses showed that local recurrence or metastasis did not affect survival. Our data showed that complete resection (in which no remnant tumour tissue was detected) was the only factor to affect survival. Thus, we suggest that recurrent or metastatic mass should be excised, if possible. Similarly, DeMatteo and colleagues26 proposed repeat surgery only in select patients who had complete resections but then experienced isolated local recurrence or isolated metastasis. On the other hand, surgical intervention bears a high risk of morbidity and mortality in these patients. Thus, we were able to perform repeat surgeries in only 33.3% of the patients with local recurrence. Recurrent tumours were widely dispersed in remaining patients. Multiple metastatic masses prevented resection in patients with liver metastasis. Three-year survival was achieved in patients who had repeat surgery owing to local recurrence, whereas other patients died less than 3 years after surgery. About one-third (34.3%) of the patients with liver metastasis survived 3 years, but died less than 5 years after surgery. On the other hand, 5-year survival rates were 80.4% and 79.5% in patients who had complete resections but did not experience local recurrence or metastasis, respectively.
Radiotherapy and conventional chemotherapy are known to be ineffective in the management of GISTs.41,42 However, imatinib mesylate is currently under investigation in clinical trials at several institutions. In one study, Duffaud and Blay43 reported that GISTs had been managed in more than 80% of patients and that objective responses had been obtained from 50%–60% of patients, whereas tumour progression was observed in only 10%–15%. Imatinib mesylate has not yet been tested as a neoadjuvant therapy in the treatment of potentially resectable GISTs.44 Moreover, imatinib mesylate is not recommended as adjuvant therapy in patients who have complete resections unless a recurrence is observed. Hence, in our study, consistent with the report by Kubota,45 we administered imatinib mesylate therapy after surgery only in patients with 1) incomplete tumour resection, 2) unresectable recurrent tumour or failure to perform repeat surgery for complete resection of the recurrent tumour and 3) unresectable metastatic disease. However, our small sample does not allow us to comment on the long-term consequences of this treatment.
In conclusion, our univariate analysis revealed that the following factors are associated with an increased survival rate: patient age younger than 60 years, male sex, tumour diameter less than 5 cm, low-risk tumour according to Fletcher classification, complete tumour resection and lack of local recurrence and/or metastasis after complete resection. On the other hand, multivariate analysis revealed that complete resection with negative margins was the only factor to affect survival. Thus we propose that aggressive surgeries that aim to remove the tumour completely by maintaining a negative margin be performed in patients with GISTs.
Contributors: Drs. Unalp and Onal designed the study. Drs. Derici and Kamer acquired the data, which Drs. Bozdag and Tarcan analyzed. Dr. Unalp wrote the article, which Drs. Derici, Kamer, Bozdag, Tarcan and Onal reviewed. All authors gave final approval for publication.
Competing interests: None declared.
Accepted for publication Sep. 22, 2007
Correspondence to: Assoc. Prof. H.R. Unalp Ataturk Egitim ve Arastirma Hastanesi 4 Genel Cerrahi Klinigi 35820 Izmir, Turkey fax 0090 232 243 48 48 halukunalp@gmail.com
References
- 1.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PMC free article] [PubMed]
- 2.Rossi CR, Mocellin S, Mencarelli R, et al. Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer 2003;107:171-6. [DOI] [PubMed]
- 3.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 2002;38 Suppl 5:S39-51. [DOI] [PubMed]
- 4.Sturgeon C, Chejfec G, Espat N. Gastrointestinal stromal tumors: a spectrum of diseases. Surg Oncol 2003;12:21-6. [DOI] [PubMed]
- 5.Besana-Ciani I, Boni L, Dionigi G, et al. Outcome and long term results of surgical resection for gastrointestinal stromal tumors (GIST). Scand J Surg 2003;92:195-9. [DOI] [PubMed]
- 6.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [DOI] [PubMed]
- 7.Trupiano JK, Stewart R, Misick C, et al. Gastric stromal tumors, a clinicopathological study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviour. Am J Surg Pathol 2002;26:705-14. [DOI] [PubMed]
- 8.Joensuu H, Fletcher C, Dimitrijevic S, et al. Management of malignant gastrointestinal stromal tumours. Lancet Oncol 2002;3:655-64. [DOI] [PubMed]
- 9.Lev D, Kariv Y, Issakov J, et al. Gastrointestinal stromal sarcomas. Br J Surg 1999;86:545-9. [DOI] [PubMed]
- 10.Bauer S, Corless CL, Heinrich MC, et al. Response to imatinib mesylate of a gastrointestinal stromal tumor with very low expression of KIT. Cancer Chemother Pharmacol 2003;51:261-5. [DOI] [PubMed]
- 11.El-Zohairy M, Khalil el-SA, Fakhr I, et al. Gastrointestinal stromal tumor (GIST)'s surgical treatment, NCI experience. J Egypt Natl Canc Inst 2005;17:56-66. [PubMed]
- 12.Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg 2006;244:176-84. [DOI] [PMC free article] [PubMed]
- 13.Miller TA. Leimyosarcoma: Not all gastric malignancies have a dismal prognosis. Gastroenterology 1993;104:940-1. [DOI] [PubMed]
- 14.Yamashita F, Sasatomi E, Kiyama M, et al. Radiographic observation of a case of gastrointestinal stromal tumor in stomach. Kurume Med J 2001;48:233-6. [DOI] [PubMed]
- 15.Ghanem N, Altehoefer C, Furtwangler A, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol 2003;13:1669-78. [DOI] [PubMed]
- 16.Tateishi U, Hasegawa T, Satake M, et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr 2003;27:792-8. [DOI] [PubMed]
- 17.Shojaku H, Futatsuya R, Seto H, et al. Malignant gastrointestinal stromal tumor of the small intestine: radiologic-pathologic correlation. Radiat Med 1997;15:189-92. [PubMed]
- 18.Stroobants S, Goeminne J, Seegers M, et al. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer 2003;39:2012-20. [DOI] [PubMed]
- 19.Chak A, Canto MI, Rosch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc 1997;45:468-73. [DOI] [PubMed]
- 20.Belloni M, De Fiori E, Mazzarol G, et al. Endoscopic ultrasound and computed tomography in gastric stromal tumours. Radiol Med (Torino) 2002;103:65-73. [PubMed]
- 21.Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215:68-77. [DOI] [PMC free article] [PubMed]
- 22.Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol 1995;2:26-31. [DOI] [PubMed]
- 23.Burkill GJ, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 2003;226:527-32. [DOI] [PubMed]
- 24.Sawaki A, Yamao K. Imatinib mesylate acts in metastatic or unresectable gastrointestinal stromal tumor by targeting KIT receptors — a review. Cancer Chemother Pharmacol 2004;54 Suppl 1:S44-9. [DOI] [PubMed]
- 25.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999;30:1213-20. [DOI] [PubMed]
- 26.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [DOI] [PMC free article] [PubMed]
- 27.Miettinen M, El-Rifai W, Sobin LH, et al. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002;33:478-83. [DOI] [PubMed]
- 28.Fujimoto Y, Nakanishi Y, Yoshimura K, et al. Clinico-pathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 2003;6:39-48. [DOI] [PubMed]
- 29.McGrath PC, Neifeld JP, Lawrence W Jr, et al. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg 1987;206:706-10. [DOI] [PMC free article] [PubMed]
- 30.Wu PC, Langerman A, Ryan CW, et al. Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era. Surgery 2003;134:656-65. [DOI] [PubMed]
- 31.Langer C, Gunawan B, Schuler P, et al. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg 2003;90:332-9. [DOI] [PubMed]
- 32.Crosby JA, Catton CN, Davis A, et al. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol 2001;8:50-9. [DOI] [PubMed]
- 33.Pidhorecky I, Cheney RT, Kraybill WG, et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000;7:705-12. [DOI] [PubMed]
- 34.Pierie JP, Choudry U, Muzikansky A, et al. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 2001;136:383-9. [DOI] [PubMed]
- 35.Fletcher C, Berman J, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459-65. [DOI] [PubMed]
- 36.Lillemoe KD, Efron DT. Gastrointestinal stromal tumors. In Cameron JL, editor. Current surgical therapy. Philadelphia: Mosby; 2001. p.112-7.
- 37.Bilimoria MM, Holtz DJ, Mirza NQ, et al. Tumor volume as a prognostic factor for sarcomatosis. Cancer 2002;94:2441-6. [DOI] [PubMed]
- 38.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 2002;1:117-23. [DOI] [PubMed]
- 39.Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer 2002;38 Suppl:S37-8. [DOI] [PubMed]
- 40.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [DOI] [PubMed]
- 41.Shiu MH, Farr GH, Papachristou DN, et al. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 1982;49:177-87. [DOI] [PubMed]
- 42.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;228:355-65. [DOI] [PMC free article] [PubMed]
- 43.Duffaud F, Blay JY. Gastrointestinal stromal tumors: biology and treatment. Oncology 2003;65:187-97. [DOI] [PubMed]
- 44.Bucher P, Villiger P, Egger JF, et al. Management of gastrointestinal stromal tumours: from diagnosis to treatment. Swiss Med Wkly 2004;134:145-53. [DOI] [PubMed]
- 45.Kubota T. Gastrointestinal stromal tumor (GIST) and imatinib. Int J Clin Oncol 2006;11:184-9. [DOI] [PubMed]