Abstract
Background
We sought to investigate the incidence of perioperative venous thromboembolism (VTE) — pulmonary embolism, superior mesenteris vein thrombosis and deep vein thrombosis — in patients with peritoneal carcinomatosis after cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy.
Methods
We performed cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on 60 consecutive patients with a mean age of 52 (range 24–76) years. We reviewed a prospective database recording complications and patient, tumour and surgical characteristics to determine the incidence of VTE. We reviewed hospital charts of patients with VTE to obtain clinical information including vital signs, risk factors, presence of comorbid conditions, VTE prophylaxis and subjective clinical symptoms.
Results
A total of 6 of 60 patients (10%) who had cytoreductive surgery and hyperthermic intraperitoneal chemotherapy experienced VTE. All patients with VTE had extensive peritoneal disease and long durations of surgery: the median duration was 431 (range 330–683) minutes. Tachycardia (mean 104 beats/min) was the only consistent abnormal vital sign recorded, with only 33% of patients experiencing clinical symptoms.
Conclusion
This prospective study demonstrates a high rate of VTE in this patient population. Unfortunately, clinical signs and symptoms are a poor predictor of VTE. Therefore, routine screening of this specific population at high risk for VTE is warranted.
Abstract
Contexte
Nous avons étudié l'incidence de la thromboembolie veineuse périopératoire (TVP), soit l'embolie pulmonaire, la thrombose de la veine mésentérique supérieure ou la thrombose veineuse profonde, chez des patients atteints de carcinomatose péritonéale après une chirurgie cytoréductrice alliée à une chimiothérapie hyperthermique intrapéritonéale.
Méthodes
Nous avons recouru à une chirurgie cytoréductrice et à une chimiothérapie hyperthermique intrapéritonéale chez 60 patients consécutifs âgés en moyenne de 52 ans (intervalle de 24 à 76 ans). Nous avons passé en revue une base de données prospective où se trouvaient consignées les complications, ainsi que les caractéristiques des patients, des tumeurs et des chirurgies, afin de mesurer l'incidence de la TVP. Nous avons examiné les dossiers hospitaliers des patients atteints de TVP afin de relever certaines données cliniques telles que signes vitaux, facteurs de risque, présence de comorbidités, prophylaxie anti-TVP et symptômes cliniques subjectifs.
Résultats
En tout, 6 patients sur 60 (10 %) soumis à la chirurgie cytoréductrice et à la chimiothérapie hyperthermique intrapéritonéale ont présenté une TVP. Chez tous les patients qui ont présenté une TVP, la maladie péritonéale était étendue et la chirurgie avait été longue : durée médiane de 431 minutes (intervalle de 330 à 683 minutes). La tachycardie (moyenne de 104 battements/min) a été le seul signe vital anormal commun à avoir été enregistré, seulement 33 % des patients ayant manifesté des signes cliniques.
Conclusion
Cette étude prospective fait état d'un taux de TVP élevé chez cette population de patients. Malheureusement, les signes et symptômes cliniques sont de piètres prédicteurs de la TVP. C'est pourquoi nous estimons qu'un dépistage systématique s'impose chez cette population particulièrement à risque de TVP.
It is well known that cancer is a risk factor for venous thromboembolism (VTE),1 which is defined as the presence of clotted blood in a vein, right cardiac chamber or in the pulmonary arterial tree, and comprises pulmonary embolism (PE), deep vein thrombosis (DVT) and superior mesenteric vein thrombosis. It is estimated that the risk of VTE among patients with cancer is 4–6 times greater than among patients without cancer.2 The incidence of VTE in patients with cancer after general surgery is also increased, ranging from 15% to 40%.1 Compared with patients without cancer who undergo the same surgical procedure, cancer patients have twice the risk of a postoperative DVT and more than 3 times the risk of a fatal PE.1 This is a reflection of the hypercoagulable state associated with malignancy, combined with the thrombogenic effects of many cancer therapies.3
The combination of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and postopertative intraperitoneal chemotherapy in select patients with gastrointestinal peritoneal carcinomatosis has been shown to increase long-term survival.4,5 However, it remains a procedure with significant perioperative morbidity and mortality.4–16 To our knowledge, there has been no publication specifically documenting the occurrence of VTE in patients who undergo these procedures. In studies that have examined the overall mortality and morbidity in patients who undergo cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, the incidence of PE ranges from 0.4% to 6% and that of DVT ranges from 3% to 5%, whereas the incidence of total VTE ranges from 5% to 10%.4–11 Three of these studies were prospective.5,6,11
We sought to investigate the incidence of perioperative VTE in patients with peritoneal carcinomatosis who have undergone cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy. Furthermore, we examined clinical parameters in each patient with a diagnosis of VTE to determine whether any were associated with the diagnosis.
METHODS
A total of 60 consecutive patients with peritoneal carcinomatosis had cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy between February 2000 and June 2006. All patients had a good functional status and were without evidence of distant metastases. We performed the procedures according to the protocol previously described by Sugarbaker and colleagues.12 Pathologic diagnoses included cancers of the appendix, colon or rectum, primary peritoneum, endocrine/small intestine or peritoneal mesothelioma.
One of us (L.M.) recorded clinical data, including patient and tumour characteristics, treatment factors, complications as classified by Dindo and colleagues,17 30-day mortality rates and disease-free survival in a prospective database. All patients received prophylactic pneumatic sequential compression stockings and subcutaneous unfractionated heparin. We performed computed tomography (PE protocol) or Doppler ultrasonography in all patients in whom we suspected VTE. We determined the incidence of VTE via the prospective database. Although we did not use a prospective screening protocol to detect VTE, we compiled retrospective data regarding negative radiologic investigations. We reviewed the hospital charts of the identified patients with VTE to obtain data on demographics, vital signs, risk factors, presence of comorbid conditions, prophylaxis, use of aprotinin and subjective clinical symptoms.
RESULTS
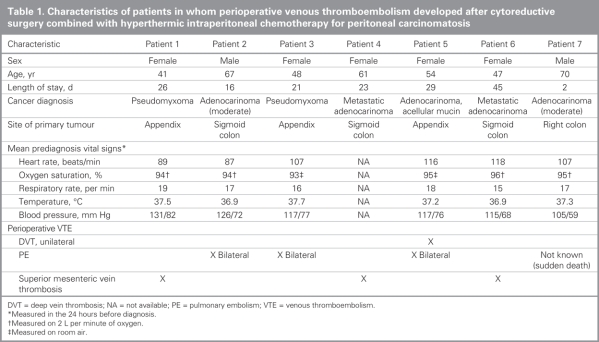
We found that a total of 6 out of 60 patients (10%) who underwent cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy had documented VTE. Characteristics of these patients are summarized in Table 1. In 5 patients, the VTE occurred postoperatively while the patients were in hospital. One patient received a diagnosis of VTE postoperatively after discharge from hospital. One additional patient died suddenly on postoperative day 2. We were unable to confirm the exact cause of death because the patient's family refused an autopsy; however, PE was highly suspected on the differential diagnosis. Three patients (5%) received a diagnosis of superior mesenteric vein thrombosis and 3 (5%) received a diagnosis of bilateral PEs. One patient (2%) with bilateral PEs was also found to have a unilateral DVT. As for negative investigations, computed tomography (CT) scans of the abdomen and pelvis were negative for intra-abdominal thrombosis in 30 patients, CT scans of the chest and PE protocol scans were negative in 10 patients, and bilateral leg Doppler ultrasounds were negative in 6 patients.
Table 1
Among the 6 patients with VTE (5 women and 1 man), the mean age was 53 (range 41–67) years compared with a mean age of 52 (range 24–76) years among those without VTE (p = 0.42). The primary tumour was an appendiceal neoplasm in 3 patients and colonic adenocarcinoma in 4 patients. All patients had extensive disease at the time of surgery, with 80% having a peritoneal cancer index score greater than 13. All had long durations of surgery; the median duration was 431 (range 330–683) minutes among patients with VTE and 380 (range (124–683) minutes for the entire cohort (p = 0.18). The median length of stay in hospital was 25 (range 16–45) days for patients with VTE compared with 21 (9–48) days for the entire cohort (p = 0.43). Patients with VTE had a median estimated blood loss of 1000 (range 700–3000) mL compared with 1654 (range 150–4000) mL among those without VTE. All but 1 patient (patient 4) with VTE received packed red blood cells. Comorbidities were relatively uncommon. One patient had a history of heart disease (patient 2), and another (patient 7) had a history of chronic obstructive pulmonary disease. Both conditions were stable for some time and not active at the time of surgery or VTE diagnosis.
The mean vital signs recorded in the 24 hours preceding the diagnosis of VTE are illustrated in Table 1. The mean heart rate was 104 (range 87–118) beats/min, the mean respiratory rate was 17 (range 15–19) breaths per minute, the mean temperature was 37.3° (range 36.9– 37.7°), the mean blood pressure was 119/72 (range 105/59–131/82) mm Hg and oxygen saturation ranged from 94% on 2 L per minute of oxygen to 95% on room air. We did not record vital signs for patient 5 because the VTE was detected on an outpatient CT scan. All patients who did not have VTE experienced tachycardia during their stays in hospital. Subjective symptoms experienced by patients in the 24 hours preceding VTE diagnosis included shortness of breath (patient 2) and leg swelling (patient 5). Therefore, one-third of patients with documented VTE experienced symptoms. The tests we ordered to diagnose VTE were generally based on patient symptoms and clinical intuition rather than specific signs; however, we detected superior mesenteric vein thrombosis incidentally on CT scans in all 3 patients who were being examined for nonspecific symptoms such as diarrhea or ileus.
Postoperatively, all patients received 5000 units of heparin subcutaneously 3 times daily, with the exception of patient 1 who was admitted to hospital before diagnosis and was started on the same dose of heparin at the time of admission. This treatment continued postoperatively. No other patient received heparin preoperatively as they were admitted on the day of surgery. All patients used pneumatic compression stockings postoperatively. The 1 patient (patient 4) in whom VTE developed after discharge from hospital was not discharged with any VTE prophylaxis. Patients 3, 5, 6 and 7 all received an intraoperative bolus and continuous intravenous infusion of aprotinin, a protease inhibitor that inhibits the action of plasmin and decreases breakdown of fibrin and intraoperative bleeding. There was no statistical correlation between aprotinin use and VTE.
DISCUSSION
The incidence of VTE in our study (10%) corresponds to the upper-end of the range described in the literature for this patient population. The incidence of PE was 5% and that of DVT was 2%. It is interesting to note that superior mesenteric vein thrombosis was diagnosed as often as PE. Superior mesenteric vein thrombosis has not been commonly described in this patient population.4–16 The reason(s) for the high incidence of VTE in our patients is worth examining. Previous studies of morbidity and mortality in patients with peritoneal carcinomatosis have tended to address isolated histological groups such as patients with appendiceal mucinous neoplasms or colorectal cancers. In contrast, our study pooled the type of surgery (cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy) and assessed a particular complication (VTE), regardless of the histological subtype of the tumour. In addition, the data recorded in our patient group have not previously been reported in the same detail in previous studies. Comparable studies include prospective and retrospective reviews. Prospective studies such as ours should more accurately reflect actual rates of VTE. Despite this, it is likely that our study, even though prospective, has underestimated the incidence of VTE because no study to date has uniformly used a screening protocol to determine the actual rate of VTE in this very high-risk patient population.
Patients with cancer who undergo major abdominal surgery are at high risk for postoperative development of VTE. This condition, specifically PE, is a major cause of postoperative deaths among patients admitted to hospital.1 Venous thromboembolism occurs in up to 30% of patients with cancer, compared with an annual incidence of 0.1% in the general population.3 Furthermore, significant risk factors associated with the development of VTE include advanced cancer stage at the time of diagnosis, prolonged hospital stay (immobilization), extensive surgical procedures with a prolonged general anesthetic, mucinous tumours of the gastrointestinal tract and the use of central venous catheters.1,3,18,19 Our patients had all the high-risk factors for VTE. In fact, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is one of the most extensive surgical procedures known. Stays in the intensive care unit with central catheter insertions and substantial postoperative immobility are the norm. Postoperative complications occur in most patients.8,16
The number of comorbidities present has been correlated with patients' increased risk for VTE,1,18 but this was not a factor in our study because candidates for cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy were strictly screened. Similarly, the presence of symptoms associated with VTE was inconsistent. The absence of clinical symptoms cannot necessarily be used to rule out PE. A study by Le Gal and Perrier20 concluded that regardless of patient age, no risk factor, clinical symptom or sign is sensitive or specific enough to rule in or out the diagnosis of PE. Detection of superior mesenteric vein thrombosis based on the typical symptom of midabdominal colicky pain may be difficult in the case of postoperative pain associated with a laparotomy. We found all superior mesenteric vein thromboses incidentally when we ordered CT scans of the abdomen for other indications. Overall, clinical observation alone likely underestimates the real incidence of VTE, even in an extremely high-risk patient population.
The only abnormal mean vital sign that we observed in patients with VTE was elevated heart rate, demonstrating tachycardia at 104 beats/min; unfortunately, nearly all patients in our cohort had periods of tachycardia. Tachycardia was also reported in a study examining the development of VTE in patients with gastrointestinal carcinomas.19 All other vital signs were within normal ranges. We did not draw arterial blood gases; however, oxygen saturation monitoring did not indicate a state of hypoxia in any patient, although this was not performed on the 1 patient in whom VTE developed after discharge. Therefore, tachycardia is a subtle yet nonspecific symptom that is important in the recognition of VTE.
Prophylaxis against VTE remains the most appropriate strategy to reduce morbidity and mortality.1 Current recommendations of the American College of Chest Physicians for high-risk general surgery patients with multiple risk factors include prophylactic low-dose unfractionated heparin (5000 units, 3 times daily) or low-molecular weight heparin (> 3400 units daily) combined with the use of pneumatic compression stockings.1 Furthermore, more recent recommendations include discharging these high-risk patients and continuing with prophylactic low-molecular weight heparin for 4 weeks.21 Although in our study all patients with VTE received prophylactic low-dose unfractionated heparin 3 times daily (with the exception of patient 1 who received this treatment twice daily), the patient in whom VTE developed after discharge (patient 4) was not prescribed prophylactic heparin. Both treatments have been directly compared, and no single study has demonstrated a difference in the rates of symptomatic VTE or bleeding rates. However, low-molecular weight heparin is associated with significantly fewer asymptomatic DVTs.1 Studies have been inconclusive and have shown both an increase and decrease in bleeding complications with low-molecular weight heparin compared with low-dose unfractionated heparin. Other clinical advantages of low-molecular weight heparin include 1 daily dose and a lower risk of heparin-induced thrombocytopenia; however, treatment with low-molecular weight heparin is more costly. Low-dose unfractionated heparin has been used primarily at our centre owing to an unproven perceived increased risk of bleeding with the higher doses of low-molecular weight heparin recommended for this patient population. However, based on the VTE rate that we observed, our group is currently reconsidering the ideal perioperative prophylaxis for VTE. Most prophylaxis trials also recommend low-dose unfractionated heparin or low-molecular weight heparin before surgery.1 Patients in our cohort did not receive preoperative heparin, with the exception of patient 1 who was previously admitted to hospital. Again, this is owing primarily to the concern of intraoperative bleeding with these extensive surgeries — a real concern in our patient group since 2 patients experienced severe diffuse intravascular coagulopathy, 1 succumbing to intraoperative hemorrhage. Blood transfusions are required in most patients undergoing this procedure.6–8,10,11,16 Mechanical prophylaxis with sequential compression stockings is recommended in patients at high risk for bleeding, although this approach is not as effective in preventing VTE.1 Sugarbaker and colleagues6 report using mechanical prophylaxis only and have achieved PE and DVT rates of 6% and 4% respectively; this may also be an underestimation of the true incidence of VTE.
We were concerned that aprotinin may have been implicated in the development of VTE. Aprotinin is a protease inhibitor that has been shown to decrease bleeding and erythrocyte transfusion during surgery as a result of its ability to inhibit fibrinolysis.22 In our study, 2 patients without VTE experienced complications related to diffuse intravascular coagulopathy; 1 died due to this complication, prompting the empiric use of intraoperative aprotinin. Four of the 6 patients who received diagnoses of VTE received aprotinin intraoperatively. However, basic science evidence as well as a meta-analysis and systematic review of the use of aprotinin in liver transplantation have not demonstrated an increased risk of VTE.23,24
Although routine screening for VTE among all surgical patients is not cost-effective, it is our opinion that routine screening of this specific high-risk population is warranted. Screening would include CT (PE protocol and abdomen, completed concurrently) and bilateral Doppler ultrasonography of the legs before discharge. Early detection of VTE will allow initiation of treatment before the development of symptoms or death. Further, a study of this nature is necessary to confirm our suspicion that the actual incidence of VTE is higher than previously reported in this patient population. There are a number of options for VTE prophylaxis including standard low-dose unfractionated heparin, low-molecular weight heparin (preoperatively, postoperatively and extended for 4 weeks), mechanical pneumatic sequential compression stockings and even full anticoagulation of these high-risk patients for 1– 3 months after discharge. However, a routine screening protocol needs to be implemented to determine the actual risk of VTE before considering the more aggressive prophylactic options.
Contributors: Drs. Mack and Temple designed the study. Dr. Lanuke wrote the article. All authors collected and analyzed data, reviewed the article and provided final approval for its publication.
Competing interests: None declared.
Accepted for publication Sep. 12, 2007
Correspondence to: Dr. L.A. Mack Tom Baker Cancer Centre Division of Surgical Oncology 1331–29th St. NW Calgary AB T2N 4N2 fax 403 283-1651 lloydmac@cancerboard.ab.ca
References
- 1.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126(Suppl):338S-400S. [DOI] [PubMed]
- 2.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case–control study. Arch Intern Med 2000;160:809-15. [DOI] [PubMed]
- 3.Negus JJ, Gardner JJ, Tann O, et al. Thromboprophylaxis in major abdominal surgery for cancer. Eur J Surg Oncol 2006;32:911-6. [DOI] [PubMed]
- 4.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [DOI] [PubMed]
- 5.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [DOI] [PubMed]
- 6.Sugarbaker PH, Alderman R, Edwards G, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol 2006;13: 635-44. [DOI] [PubMed]
- 7.Moran BJ, Mukherjee A, Sexton R. Operability and early outcome in 100 consecutive laparotomies for peritoneal malignancy. Br J Surg 2006;93:100-4. [DOI] [PubMed]
- 8.Smeenk RM, Verwaal VJ, Zoetmulder FAN. Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei — a report of 103 procedures. Eur J Surg Oncol 2005;32:186-90. [DOI] [PubMed]
- 9.Culliford AT IV, Brooks AD, Sharma S, et al. Surgical debulking and intraperitoneal chemotherapy for established peritoneal metastases from colon and appendix cancer. Ann Surg Oncol 2001;8:787-95. [DOI] [PubMed]
- 10.Ahmad SA, Kim J, Sussman JJ, et al. Reduced morbidity following cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion. Ann Surg Oncol 2004;11:387-92. [DOI] [PubMed]
- 11.Kusamura S, Younan R, Baratti D, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006;106:1144-53. [DOI] [PubMed]
- 12.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995;221:124-32. [DOI] [PMC free article] [PubMed]
- 13.Shen P, Levine EA, Hall J, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg 2003;138:26-33. [DOI] [PubMed]
- 14.Pilati P, Mocellin S, Rossi CR, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomastosis arising from colon adenocarcinoma. Ann Surg Oncol 2003;10:508-13. [DOI] [PubMed]
- 15.Cavaliere F, Perri P, Di Filippo F, et al. Treatment of peritoneal carcinomatosis with intent to cure. J Surg Oncol 2000;74:41-4. [DOI] [PubMed]
- 16.Verwaal VJ, van Tinteren H, van Ruth S, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol 2004;85:61-7. [DOI] [PubMed]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [DOI] [PMC free article] [PubMed]
- 18.Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol 2006;24:1112-8. [DOI] [PubMed]
- 19.Mandala M, Falanga A, Cremonesi M, et al. The extension of disease is associated to an increased risk of venous thromboembolism (VTE) in patients with gastrointestinal (GI) carcinoma. Thromb Haemost 2006;95:752-4. [PubMed]
- 20.Le Gal G, Perrier A. Contemporary approach to the diagnosis of non-massive pulmonary embolism. Curr Opin Pulm Med 2006;12:291-8. [DOI] [PubMed]
- 21.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery. The @RISTOS project. Ann Surg 2006;243:89-95. [DOI] [PMC free article] [PubMed]
- 22.Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology 2006;105:1034-46. [DOI] [PubMed]
- 23.Molenaar IQ, Legnani C, Groenland TH, et al. Aprotinin in orthotopic liver transplantation: evidence for a prohemostatic, but not a prothrombotic, effect. Liver Transpl 2001;7:896-903. [DOI] [PubMed]
- 24.Molenaar IQ, Warnaar N, Groen H, et al. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant 2007;7:185-94. [DOI] [PubMed]