Abstract
Background
By optimizing surgical and/or radiotherapy treatment, local recurrence rates of resectable rectal cancers have been reported to be less than 10% in both clinical trials and in some population-based studies. We examined patterns of care and local recurrence rates for rectal cancers in the province of Manitoba.
Methods
We used the provincial cancer registry to identify all rectal cancers diagnosed from 1994 to 1997. These dates allowed for a minimum of 5 years of follow-up. We obtained information on cancer staging through a retrospective chart review and information on surgical procedures from the cancer registry. We included in our study only those patients with stages I, II and III disease who had an anterior resection, Hartmann procedure or abdominal perineal resection with clear margins. We determined local recurrences by chart review and we reported the crude rate.
Results
We identified 333 patients among whom there was a relatively even distribution of stage I, II and III cancers. Fifty-three percent of patients received a stoma. Adjuvant radiotherapy was administered in 6%, 45% and 80% of patients with stage I, II and III cancers, respectively. Radiotherapy was only given preoperatively to 3 patients. We identified local recurrences in 13%, 16% and 24% of patients with stage I, II and III disease, respectively, with an overall rate of 17.4%. Recurrence rates by hospital ranged from 12% to 43%. Actual rates may be a few percentage points higher because 13% of patients had incomplete follow-up information and were assumed to have no recurrence.
Conclusion
Between 1994 and 1997, the management of resectable rectal cancer in Manitoba did not meet benchmarks for the period in terms of documentation, treatment and, most importantly, the outcome of local recurrence. Initiatives should be taken to ensure that current performance and outcomes have improved.
Abstract
Contexte
En optimisant le traitement chirurgical ou la radiothérapie, il a été possible d'obtenir des taux de récurrences locales de cancers rectaux résécables à moins de 10 % dans les 2 études cliniques et dans certaines études de populations. Nous avons étudié les schémas thérapeutiques et les taux de récurrences locales de cancers rectaux dans la province du Manitoba.
Méthodes
Nous avons utilisé le registre provincial sur les cancers pour recenser tous les cancers rectaux diagnostiqués entre 1994 et 1997. Ces dates ont permis d'effectuer un suivi d'une durée minimum de 5 ans. Nous avons obtenu des renseignements sur la stadification du cancer par un examen rétrospectif des dossiers et par des données sur les interventions chirurgicales obtenues du registre sur le cancer. Nous avons inclus dans notre étude uniquement les patients présentant un cancer de stade I, II et III qui avaient déjà subi une résection, une intervention de Hartmann ou une résection abdomino-périnéale avec marges saines. Nous avons déterminé le taux de récurrences locales en fonction de l'examen des dossiers et nous avons présenté un rapport sur les taux bruts.
Résultats
Nous avons recensé 333 patients parmi lesquels les cancers de stade I, II et III étaient distribués relativement également. Cinquante-trois pour cent des patients ont reçu une stomie. Une radiothérapie adjuvante a été administrée respectivement à 6 %, 45 % et 80 % des patients présentant des cancers de stade I, II et III. La radiothérapie n'a été administrée durant la période préopératoire qu'à 3 patients. Nous avons identifié les récurrences locales chez 13 %, 16 % et 24 % des patients porteurs d'un cancer de stade I, II et III, respectivement, avec un taux global de 17,4 %. Les taux de récurrences, selon les hôpitaux, ont varié de 12 % à 43 %. Les taux réels pourraient être supérieurs de quelques points de pourcentage, parce que 13 % des patients présentaient des données incomplètes au moment du suivi et on a présumé qu'ils ne présentaient pas de récurrence.
Conclusion
Entre 1994 et 1997, le traitement du cancer rectal résécable au Manitoba n'a pas atteint les normes pour la période sur les plans de la documentation, du traitement et plus important encore, de l'évolution de récurrences locales. Il faudra adopter des mesures pour veiller à l'amélioration du rendement actuel et des résultats.
Colorectal cancer is the third most common cancer in both sexes in terms of incidence and mortality,1 with rectal cancer representing about one-quarter of cases.2 A major issue in rectal cancer is local recurrence after a potentially curative resection, which almost guarantees death from disease3 and symptoms that are often difficult to palliate.
Strategies to achieve low recurrence rates include improved surgical technique4 and the use of adjuvant5 or neoadjuvant chemoradiotherapy6 or neoadjuvant radiotherapy.7,8 Local recurrence rates of less than 10% have been reported not only in clinical trials,6,9 but also on a population basis.3,10 In Manitoba, standard of care for rectal cancer has been relatively consistent with the 1991 National Institute of Health (NIH) chemoradiotherapy guidelines.11,12
The primary objective of our study was to determine the local recurrence rates after curative surgery for rectal cancer in the province of Manitoba. We also examined patterns of care to identify opportunities for quality improvement.
METHODS
Study population
We conducted a retrospective review of administrative databases and the charts of all patients with American Joint Commission on Cancer (AJCC) stage I–III rectal cancer treated with curative intent involving an abdominal approach in Manitoba between 1994 and 1997.
We identified patients with an invasive adenocarcinoma of the recto-sigmoid, rectum and unknown ano-rectum (International classification of diseases, 9th revision [ICD-9],13 codes 154.0, 154.1 and 154.8) using the Manitoba Cancer Registry. We defined a rectal cancer as a tumour of the colorectum with a lower border within 15 cm of the anal verge. We included only patients who had an anterior resection (ICD-9 codes 45.76, 48.63), Hartmann procedure (ICD-9 codes 45.75 after October 1989, 48.66 before October 1989, and 48.62) or abdominal-perineal resection (ICD-9 code 48.5). We excluded patients with stage 0 or stage IV disease, patients treated with an endoscopic or transanal resection and patients with residual disease in the pelvis after resection based on the surgical report and/or cancer at the radial margin based on the pathology report. The last year of diagnosis to be included in the study was 1997 to allow for a minimum of 5 years of follow-up; follow-up data were only available up to 2003, when we began our study. The first year of diagnosis to be included in the study was 1994 based on sample size considerations, the number of rectal cancers diagnosed per year in the province and the number of patients assumed to have undergone resection. The University of Manitoba Health Research Ethics Board approved our study. Consistent with the ethics approval, patient and surgeon identity remained anonymous.
Sample size considerations
We estimated the crude local recurrence rate in Manitoba to be 20%. Using the method by Bohning,14 the sample size required to estimate Manitoba's local recurrence rate with 95% confidence within 5% absolute percentage points was 246 patients.
Data sources
The Manitoba Cancer Registry, which is housed at CancerCare Manitoba, was started in 1937 and became population-based in 1956. Cancer reporting is mandated by law in Manitoba, and information on all potential new cases must be forwarded to the Manitoba Cancer Registry. Multiple sources of ascertainment of incident cases are used, including physician notifications; pathology and hematology reports; and hospital admission, mortality and autopsy records. In examining cases registered from 1991 to 1995, the North American Association of Central Cancer Registries estimated the Manitoba Cancer Registry to be 95%–98% complete in ascertaining all cancer cases.15 For every case, the cancer registry includes information on diagnosis according to the ICD-9 code (ICD-10 since 2002), date of diagnosis, tumour grade, morphology, topology, date of birth, sex, vital status and treatment information. Adjuvant (or neoadjuvant) radiotherapy refers to any locoregional radiotherapy occurring within 6 months of surgery. The Manitoba Vital Statistics department provides information on mortality. The cancer registry is part of a linked data resource that also incorporates administrative data maintained by Manitoba Health as part of the management of the provincial health care insurance plan.
Local recurrence
The Manitoba Cancer Registry does not have complete recurrence information. Most cancer patients in the province are referred to CancerCare Manitoba, the central cancer agency, for consideration of chemotherapy and/or radiotherapy. It has the only radiation facilities in the province. A chart is created for each patient seen at CancerCare Manitoba, and it was from these charts that we identified local recurrences by retrospective review. A cancer registrar noted any mention of a local recurrence from the progress notes, imaging reports, surgical reports and/or pathology reports. We then reviewed patients for study inclusion or exclusion, and one of us (S.L.) confirmed local recurrences. To be counted as an event, a local recurrence did not require pathological confirmation and was included regardless of the presence or absence of systemic metastases.
For patients without a Manitoba Cancer Registry chart or for whom follow-up information was considered to be incomplete, we planned to contact the surgeon, medical oncologist and/or family physician involved in the patient's care for information on local recurrence. Only 13% of patients did not have satisfactory follow-up information from the Manitoba Cancer Registry or chart information. Owing to the small number of patients and a more favourable stage distribution in this group than the overall cohort, we elected not to pursue the follow-up information and assumed that these patients did not experience local recurrence. Thus local recurrence estimates are conservative.
Statistical analysis
Descriptive analysis included patient, tumour and treatment characteristics for the cohort. We reported local recurrence as a crude rate. We calculated hospital-specific local recurrence 95% confidence intervals.
RESULTS
A total of 598 patients received diagnoses of rectal cancer in Manitoba between 1994 and 1997, of whom 339 (56.7%) had stage I–III disease. About 20% of the remaining patients had stage IV disease, and the rest had stage 0 or unstageable disease, possibly owing to a death certificate diagnosis, an unresected primary or a local excision with unknown nodal status. All but 6 of the 339 patients with stage I–III disease had an abdominal procedure, leaving 333 patients for inclusion in our study. Patient, tumour and treatment characteristics of the cohort are outlined in Table 1.
Table 1
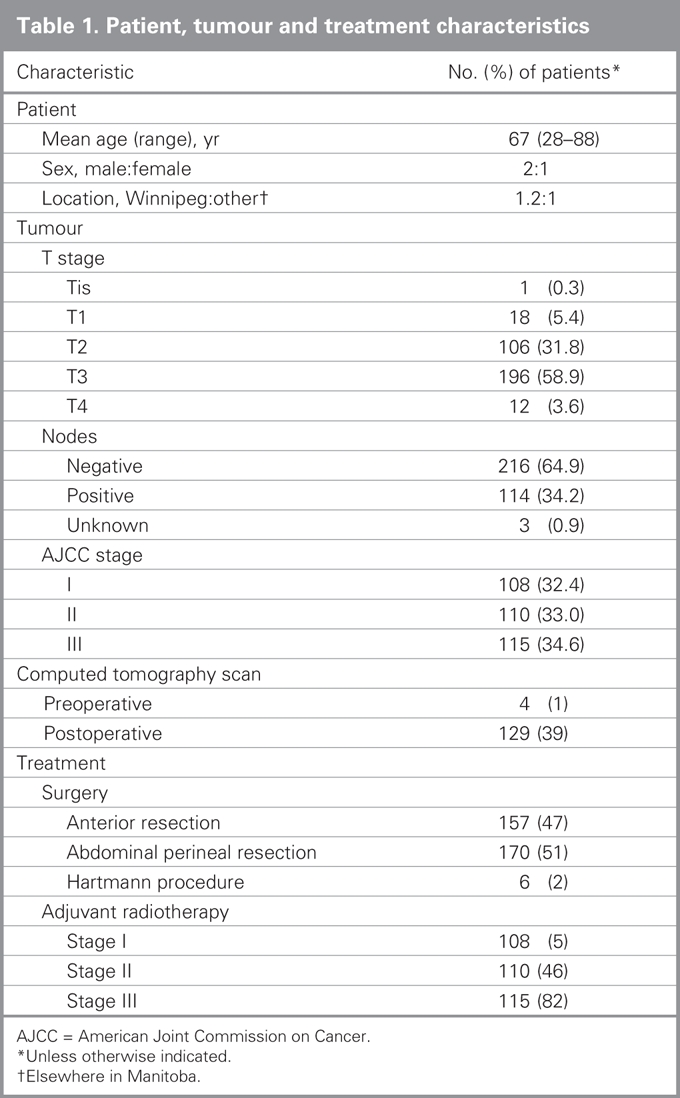
Documentation of precise tumour location was available in 50% of the patients' charts. Pathology reports were relatively complete (> 95%) for tumour penetration (T-stage), grade, nodal status and proximal and distal margins. The number of nodes was explicitly stated in 91% of reports, with a median of 7 nodes identified. Although our study protocol was to exclude patients with a positive microscopic margin, only 5% of pathology reports provided a specific statement regarding the status of the radial margins. Less than 1% of patients had a computed tomography (CT) scan preoperatively. From the surgical procedures recorded, the permanent ostomy rate for this cohort was estimated to be 53%. Patients were relatively evenly distributed by stage. Adjuvant radiotherapy was administered in 47% of patients, with only 3 patients receiving radiotherapy preoperatively. It appears that concomitant chemotherapy was used for more than 80% of patients who received radiotherapy.
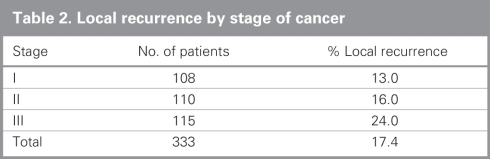
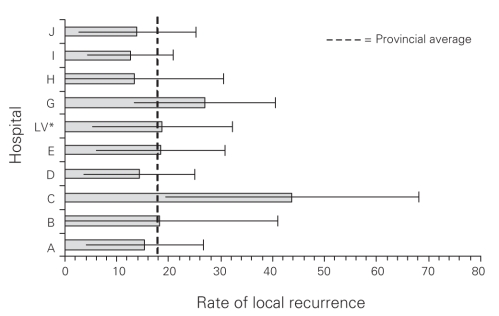
Local recurrence overall and by stage is presented in Table 2. The overall local recurrence rate was 17.4%. The local recurrence rates with and without radiotherapy for stage II disease were 18% and 14%, respectively; those for stage III disease were 22% and 25%, respectively. Local recurrence by hospital ranged from 13% to 44%, as shown in Figure 1.
Table 2
Fig. 1. Manitoba rectal cancer: local recurrence rate by hospital. *LV = low volume hospitals, fewer than 15 cases contributed to the study.
DISCUSSION
Between 1994 and 1997, the management of resectable rectal cancer in Manitoba did not meet benchmarks for the period in terms of documentation, treatment and, most importantly, the outcome of local recurrence.
Surgical and pathological reporting for rectal cancer in Manitoba was not ideal. A 50% documentation of tumour location is inadequate given its importance in terms of documenting the appropriateness of a stoma, the need for adjuvant radiotherapy and the risk of local recurrence. Although most pathology reports provided the essential information, there were notable deficiencies. The radial margin is important in terms of the risk of local recurrence16 but was only reported 5% of the time. The total number of nodes is important in node-negative disease to establish how certain a negative nodal status is, and thus complete reporting, instead of the 91% observed in our study, would be expected; a median of 7 nodes would, by current standards, be considered inadequate to correctly stage patients.17,18 This could be a problem with the adequacy of the surgical resection and/or pathological processing and identification. Staging inaccuracies create problems with regards to prognosis and the appropriate use of adjuvant therapy.
Although not a treatment in itself, CT scan results can have important treatment implications when more advanced disease is found.19 It is surprising that preoperative CT scanning was so rarely used. It is difficult to know what the appropriate use of CT scanning should have been during this period of time; however, current rectal cancer guidelines would support its use preoperatively.20 Ostomy rates of over 50% in Manitoba from 1994 to 1997 are considered excessive and remained relatively unchanged up to 2003 (data not shown). In similar population-based studies from Norway10 and Sweden,21 ostomy rates for essentially the same period were 35% and 36%, respectively. Inconsistent with NIH guidelines,11 about half of the patients in our study with stage II disease did not receive radiotherapy for unknown reasons. Most patients (82%) with stage III disease appropriately received radiotherapy. It is unlikely that this could be improved upon.
We found the local recurrence rate after a curative resection for rectal cancer in the province of Manitoba between 1994 and 1997 to be excessive at more than 17%. This is a conservative estimate given that 13% of patients had no follow-up information and were assumed to have no local recurrence. Review of these patients' tumour and survival characteristics compared with those of the overall cohort would suggest that this is a reasonable assumption. The true local recurrence rate in our study would unlikely exceed 19%, based on the application of a 17.4% local recurrence to the group with no follow-up information. It is important to note that we only included patients in whom tumours were resected; the surgical report stated that no tumour was left behind and the pathology report showed clear margins. Thus it is unlikely that the inclusion of locally advanced (fixed) tumours could explain this suboptimal recurrence rate; only 3.6% of patients had T4 disease, with few recurrences in that group. Local recurrence rates vary widely by hospital, likely representing variation at the level of the surgeon, which has been reported previously.22,23 Low-volume hospitals handled fewer than 15 cases, whereas the highest volume hospital handled 62 cases over the 4-year period, thus we doubt our data are appropriate to comment on high-versus low-volume. No hospital had a local recurrence rate of less than 10%. For the tertiary care centres, this may be owing to referral of more advanced or difficult tumours; however, stage of disease was similar in these centres and the rest of the province; we could not examine differences in tumour location owing to the limited reporting of that information. Overall local recurrence rates would only be improved by a few percentage points and could still be considered excessive if quality improvement initiatives were focused solely on improved compliance with radiotherapy among patients with stage II disease and if improvement initiatives focused on outlier hospitals alone.
How do Manitoba results compare and what benchmark should be used? Results of the NCCTG rectal cancer trial5 were the basis of the 1991 NIH consensus guidelines. In the NCCTG trial, the local recurrence rate among patients with stage II and III disease treated with chemoradiotherapy postoperatively was 13.5%. The average local recurrence rate of similar patients in Manitoba was 20%. It is difficult to reconcile this comparison further. However, NCCTG numbers have an advantage because local recurrences were not counted in the presence of distant recurrence and a disadvantage in that only one-third of the patients had stage II disease; these effects may balance out. A retrospective review of patients treated in the United States using the NCCTG chemoradiotherapy protocol showed a local recurrence rate of 16%.24 Similar to this study Phang and colleagues25 conducted a population-based study in British Columbia in 1994 and reported local recurrence rates almost identical to ours. Their results sparked a province-wide quality assurance initiative. Despite challenges related to differing patient populations and definitions of local recurrence, comparisons to other randomized trials6–9 that used different strategies for surgery and/or adjuvant radiotherapy suggest that the benchmark for local recurrence is less than 10%. Population-based studies, however, are the best evidence that Manitoba results are suboptimal. A population-based study by Wibe10 from Norway reported a local recurrence rate of less than 10% between 1997 and 1999. The low rate was attributed to a national initiative in surgical quality assurance started in the early 1990s. Fewer than 10% of patients received radiotherapy and/or chemotherapy. Similarly impressive results in the late 1990s have been reported in Sweden owing to surgical quality assurance and to 50% of patients receiving preoperative short course radiotherapy.3,21 From a quality-improvement perspective, steps to achieving this benchmark within a population have been published in Sweden and Norway.26,27 Implementation of such a program in Winnipeg, where two-thirds of Manitoba's population lives, would be a good start.
Limitations to our study include those biases that are inherent to the use of administrative data sets28 and retrospective studies. The Manitoba Cancer Registry has been shown to be of high quality in capturing all patients with cancers,15 and the capture of surgical procedures from administrative data sets has been shown to be relatively accurate.29 In terms of our primary outcome of local recurrence, the results could only be worse owing to missed local recurrences. The local recurrences that we identified were quite obvious from the documentation despite not being proven by biopsy results. Obtaining a more precise estimate of local recurrence is unnecessary from a quality-assurance perspective because results are poor enough to indicate that improvement is necessary.
We previously presented this information at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium30 and a provincial consensus conference for the treatment of rectal cancer. Attendance at the conference by surgeons, radiation oncologists and other health care providers involved in the treatment of rectal cancer was exceptional. Recent literature pertaining to the local management of rectal cancer as well as our study results were reviewed and discussed. A report including provincial treatment guidelines and recommendations for further work was provided to the administration at CancerCare Manitoba and the Department of Surgery at the University of Manitoba. There has been little activity on those recommendations to date. A repeat audit for patients who received diagnoses of rectal cancers from 2004 to 2005 is planned to examine whether patterns of care and local recurrence outcomes have changed with time. Results of a recently presented randomized trial of surgeon education and mentoring may indicate that improvements in local recurrence rates might have occurred serendipitously in the absence of any organized quality assurance program.31
Our study shows that between 1994 and 1997 in the province of Manitoba the management of resectable rectal cancer in terms of documentation, treatment and, most importantly, local recurrence fell below benchmarks for that period. Initiatives should be taken to ensure that performance meets current benchmarks.
Acknowledgments
We are grateful to the Health Sciences Centre Corporate Innovations and Opportunities Fund for an operating grant in support of this project. We would also like to acknowledge the support of the Manitoba Medical Services Foundation for a previous grant that provided a basis for much of the data used in this study. During the conduct of this study, D. Turner was supported in part by a Senior Research Fellowship from the Canadian Institutes of Health Research, and S. Latosinsky was provided with salary support through the Rudy Falk Clinician–Scientist Award. We would like to thank Dr. Ahmet Leylek for his initial work on rectal cancer in the Manitoba population that greatly facilitated this project. We would also like to thank X. Sun, M. Parisien and M. Stepushyn for assistance with staging, interpreting and reviewing Cancer Registry information and for assistance with administrative data extraction.
Presented at the American Society of Clinical Oncology 2005 Gastrointestinal Cancers Symposium: Latosinsky S, Turner D, Leylek A, Stepushyn M, Sun X, Parisien M. Local recurrence following rectal cancer treatment in Manitoba.
Contributors: Both Drs. Latosinsky and Turner designed the study, acquired and analyzed data, reviewed the article and approved its final publication. Dr. Latosinsky wrote the article.
Competing interests: None declared.
Accepted for publication Feb. 5, 2008
Correspondence to: Dr. S. Latosinsky Division of Surgical Oncology Department of Surgery University of Manitoba GF 434-820 Sherbrook St. Winnipeg MB R3A 1R9 fax 204 787-4837 slatosinsky@hsc.mb.ca
References
- 1.Canadian Cancer Society/National Cancer Institute of Canada. Canadian cancer statistics 2005. Pub. no. 111-227. Available: www.cancer.ca/Canada-wide/Publications/Publications%20on%20cancer%20statistics/Cancer%20statistics%20publications.aspx?sc_lang=en (accessed 2009 Jan. 16).
- 2.Cheng X, Chen VW, Steele B, et al. Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group in the United States, 1992–1997. Cancer 2001;92:2547-54. [DOI] [PubMed]
- 3.Palmer G, Martling A, Cedermark B, et al. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007;14:447-54. [DOI] [PubMed]
- 4.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998;133:894-9. [DOI] [PubMed]
- 5.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709-15. [DOI] [PubMed]
- 6.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [DOI] [PubMed]
- 7.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336:980-7. [DOI] [PubMed]
- 8.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [DOI] [PubMed]
- 9.van den Brink M, Stiggelbout AM, van den Hout WB, et al. Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol 2004;22:3958-64. [DOI] [PubMed]
- 10.Wibe A, Moller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer–implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002;45:857-66. [DOI] [PubMed]
- 11.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444-50. [PubMed]
- 12.Latosinsky S, Demers A, Sun X, et al. Trends in rectal cancer survival in relation to the local control strategy [abstract]. 2006 ASCO Annual Meeting Proceedings Part I. J Clin Oncol 2006;24(18 Suppl):13536.
- 13.International classification of diseases. 9th rev. Geneva: World Health Organization; 1978.
- 14.Bohning D. Confidence interval estimation of a rate and the choice of sample size. Stat Med 1988;7:865-75. [DOI] [PubMed]
- 15.Chen VW, Wu XC, Andrews PA. Cancer Incidence in North America, 1991–1995. In: Volume one: incidence. Sacramento (CA): North American Association of Central Cancer Registries; 1999.
- 16.Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:996-9. [DOI] [PubMed]
- 17.Wong JH, Severino R, Honnebier MB, et al. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999;17:2896-900. [DOI] [PubMed]
- 18.Goldstein NS, Sanford W, Coffey M, et al. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol 1996;106:209-16. [DOI] [PubMed]
- 19.Kerner BA, Oliver GC, Eisenstat TE, et al. Is preoperative computerized tomography useful in assessing patients with colorectal carcinoma? Dis Colon Rectum 1993;36:1050-3. [DOI] [PubMed]
- 20.Stelzner M. 2003 SSAT-AGA-ASGE Workshop on “Palliative Therapy of Rectal Cancer.” Summary statement. J Gastrointest Surg 2004;8:253-8. [DOI] [PubMed]
- 21.Nystrom L. Swedish Rectal Cancer Results [lecture]. BC Rectal Cancer Day; Sept. 2002.
- 22.Hermanek P, Wiebelt H, Staimmer D, et al. Prognostic factors of rectum carcinoma–experience of the German Multicentre Study SGCRC. German Study Group Colo-Rectal Carcinoma. Tumori 1995;81(3 Suppl):60-4. [PubMed]
- 23.Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998;227:157-67. [DOI] [PMC free article] [PubMed]
- 24.Stocchi L, Nelson H, Sargent DJ, et al. Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol 2001;19:3895-902. [DOI] [PubMed]
- 25.Phang PT, MacFarlane J, Taylor RH, et al. Practice patterns and appropriateness of rectal cancer management in British Columbia. BC Med J 2003;45:327-9.
- 26.Påhlman L, Karlbom U. Teaching efforts to spread TME surgery in Sweden. Recent Results Cancer Res 2005;165:82-5. [DOI] [PubMed]
- 27.Wibe A, Eriksen MT, Syse A, et al. Total mesorectal excision for rectal cancer — What can be achieved by a national audit? Colorectal Dis 2003;5:471-7. [DOI] [PubMed]
- 28.Malin JL, Kahn KL, Adams J, et al. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst 2002;94:835-44. [DOI] [PubMed]
- 29.Williams JI, Young W. A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Williams JI, Anderson G, et al, editors. Patterns of health care in Ontario. 2nd ed. Ottawa: Canadian Medical Association; 1996. p. 339-45.
- 30.Latosinsky S, Turner D, Leylek A, et al. Local recurrence following rectal cancer treatment in Manitoba [abstract]. Gastrointestinal Cancers Symposium; January 2005; Miami. Miami: American Society of Clinical Oncology; 2005.
- 31.Simunovic M, Coates A, Smith A, et al. Preliminary results from the Quality Initiative in Rectal Cancer (QIRC) Trial [abstract]. Can J Surg 2006;49(Suppl):24-5.