Abstract
Background
Higher hospital and surgeon volumes have been associated with improved outcomes following hepatic resection; however, there appear to be additional factors that also play a role. The objective of our study was to examine the outcomes following hepatic resection over the past 13 years in a large urban Canadian health region.
Methods
We used administrative procedure codes to identify all patients from 1991/92 to 2003/04 who underwent a hepatic resection in the Calgary health region, which has a referral base of about 1.5 million people. The primary outcome was operative mortality, defined as death before discharge.
Results
There were 424 hepatic resections performed in the stated time period. Annual volume was stable until 2000, when it increased substantially. This corresponded to the formation of a multidisciplinary group that provided care to these patients. There were 25 deaths over the study period for a mean mortality of 5.9%. The mean length of stay in hospital was 14.6 (median 10) days. Over time, however, mortality steadily decreased. This corresponded to a concomitant increase in the volume of hepatic resections performed.
Conclusion
Over the past 13 years, the number of hepatic resections performed has increased; there has been a corresponding improvement in mortality rates. The improved rates are likely the result of multiple factors.
Abstract
Contexte
On a démontré que les hôpitaux et les chirurgiens qui effectuent des nombres plus élevés de résections hépatiques obtiennent de meilleurs résultats de cette intervention, mais il semble toutefois que d'autres facteurs sont aussi en cause. Notre étude visait à déterminer les résultats des résections hépatiques effectuées au cours des 13 dernières années dans une grande région de santé urbaine du Canada.
Méthodes
Nous avons utilisé des codes administratifs d'intervention pour repérer tous les patients de 1991/92 à 2003/04 qui ont subi une résection hépatique dans la région de santé de Calgary, dont le bassin de référence compte environ 1,5 million de personnes. La mortalité opératoire, définie comme un décès survenu avant le congé de l'hôpital, a constitué le résultat principal.
Résultats
On a pratiqué 424 résections hépatiques au cours de la période à l'étude. Le volume annuel est demeuré stable jusqu'en 2000, et il a alors augmenté considérablement. L'augmentation a correspondu à la création d'un groupe multidisciplinaire de soin de ces patients. On a enregistré 25 décès au cours de la période à l'étude, pour une mortalité moyenne de 5,9 %. La durée moyenne du séjour à l'hôpital s'est établie à 14,6 jours (médiane de 10 jours). Au fil du temps, toutefois, la mortalité a diminué régulièrement, cette diminution correspondant à une augmentation du nombre de résections hépatiques pratiquées.
Conclusion
Au cours des 13 dernières années, le nombre de résections hépatiques pratiquées a augmenté et on constate une amélioration correspondante des taux de mortalité. L'amélioration des taux découle probablement de multiples facteurs.
The reported results for hepatic resection surgery have changed dramatically over the past 40 years. In 1977, a review of hepatic surgery was undertaken by Foster and Berman.1 They reviewed the outcomes of 621 hepatic resections and found an overall mortality of 13%, and a mortality rate of just over 20% for major hepatectomies. In the late 1980s, mortality rates hovered around 10%.2,3 Recent studies from high-volume expert centres have reported mortality rates below 5%.4–7 The impact of provider volume and surgeon training on outcomes following complex surgical procedures has been reported in many studies.8–21 Birkmeyer and colleagues10 highlighted this as an issue for many surgical procedures across the United States. However, there has been debate about the relative importance of volume versus other processes of care and structural factors that may impact outcomes.22–25 It seems very likely that volume or the “practice makes perfect” argument is in part responsible for the improved outcomes often observed at high-volume centres. There are likely other factors that contribute to these findings. This is highlighted well in an article by Dimick and colleagues26 in which they show a significant difference over time in mortality rates between high-and low-volume centres performing hepatic resections across the United States. In 1988–1989, mortality at both high-and low-volume centres was about 10%; by 1998–2000, mortality at low-volume centres was 8%, whereas that in high-volume centres dropped to almost half that value. Some of the other factors that may play a role in this improvement and cannot be attributed to volume effects have been highlighted by others.4,5 These include changes in patient selection, improvements in perioperative and anesthetic care, a better understanding of hepatic anatomy, the use of vascular occlusion techniques and the creation of hepatobiliary surgery as a specialty. In addition, the creation of a multidisciplinary treatment group that brings together individuals with different yet complementary skill sets may also contribute to improved outcomes.27,28 The multidisciplinary approach to oncology attempts to ensure that the complete therapeutic armamentarium is considered for all patients.
The objective of our study was to determine how the volume and outcomes following hepatic resection in the Calgary health region (CHR; Calgary, Alberta) have changed over the past 13 years. The CHR accounts for 36.1% of the residents of the province of Alberta and serves as a referral base for a population approaching 1.5 million, as the surrounding health regions in the province don't have a tertiary health care centre. Within the health region, all patients admitted to hospital have a record of their encounters in the form of discharge abstractions. We used administrative data from all hospitals in the CHR from 1991/92 to 2003/04 to determine the procedural volumes and outcomes for patients who underwent hepatic resection.
METHODS
Before April 2002, we identified all patients in the CHR who underwent a hepatic resection between 1991/92 and 2003/04 using the procedure codes for partial hepatectomy (the clinical modification of the International Classification of Diseases, 9th revision [ICD-9-CM] 50.22)29 and lobectomy (ICD-9-CM 50.3). After April 2002, we identified patients using the Canadian Classification of Health Interventions (CCI),30 code 1.OA.87. This administrative data set includes information about the urgency of admission, demographic data, date of admission and discharge and the status of the patient at discharge. We assessed comorbid disease using the Charlson comorbidity Index score with the original weights described by Charlson.31 The primary outcome was operative mortality, defined as death before discharge. Other outcomes that we examined included length of stay in hospital. The Conjoint Health Ethics Review Board at the University of Calgary approved our study.
Statistical analysis
We used descriptive statistics to report on the baseline characteristics of patients in the data set. Data are expressed as the mean and the standard deviation for continuous variables, and simple proportions for dichotomous variables. We performed univariate comparisons of baseline characteristics and crude in-hospital mortality rates using χ2 tests for categorical data and either analysis of variance (ANOVA) or the Kruskal–Wallis method for continuous data, depending on whether their distribution was most appropriate for parametric or nonparametric testing, respectively. We performed our analyses using SAS Software version 8.2 (SAS Institute Inc.).
RESULTS
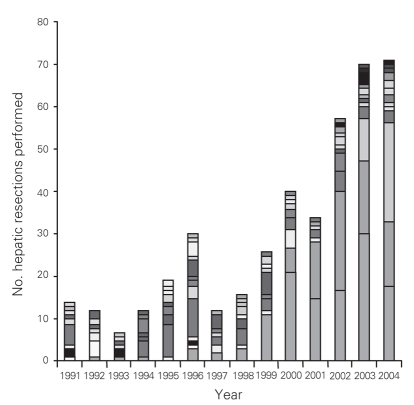
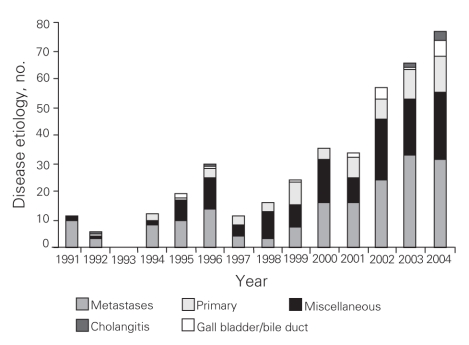
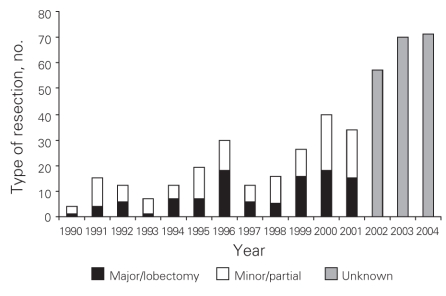
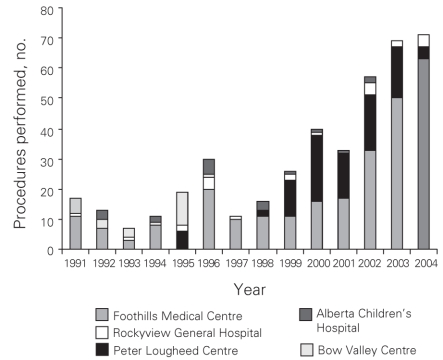
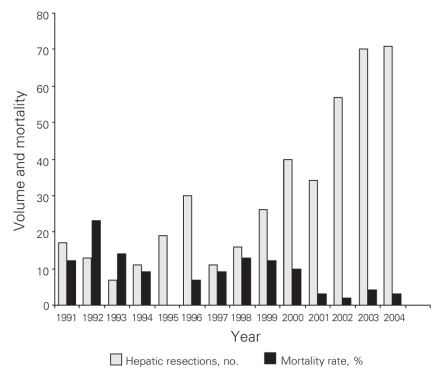
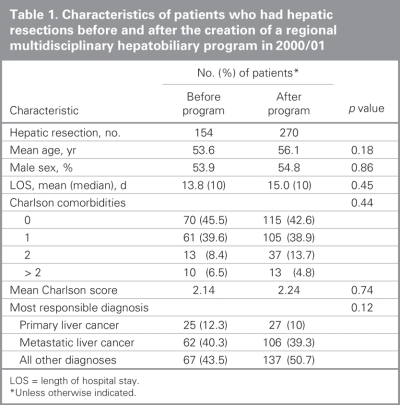
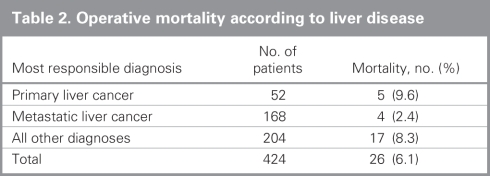
From 1991 to 2004 inclusive, 424 resections were performed within the CHR. Of these resections, 20 (5%) were performed at the Alberta Children's Hospital, the rest were performed at 4 adult hospitals: 275 at the Foothills Medical Centre (FMC, 65%), 90 at the Peter Lougheed Centre (PLC, 21%), 24 at the Rockyview General Hospital (RGH, 6%) and 15 at the Bow Valley Centre (BVC, 4%). The mean age of patients was 55.3 (range 1–86) years, and 55% were men. Figure 1 shows the number of hepatic resections performed per fiscal year for a given surgeon. The data demonstrate that annual volume was fairly stable until 2000/01, when there was a marked increase in surgical volume. After 2000/01, 3 surgeons performed most of the resections in the CHR. Figure 2 shows the disease etiology by fiscal year. The most common cause for hepatic resection was metastatic malignancy; followed by miscellaneous causes, including intrahepatic cholangiocarcinoma, benign tumours and traumatic injuries; hepatocellular carcinoma; biliary tract malignancy, including gallbladder cancer; and recurrent pyogenic cholangitis. Figure 3 depicts the annual breakdown by procedure type (minor/partial resection or major resection/lobectomy). However, this distinction could not be made after 2001 because, beginning in 2002, coding was done using the ICD-10-CA32 and its accompanying Canadian Classification of Health Interventions (CCI) index, and the newer coding system does not provide this detail. Figure 4 shows the number of procedures performed according to hospital site. After 1998/99, most procedures were performed at 2 adult hospitals that met the criteria for high-volume hepatic surgery12,26 (FMC, PLC); in 2003/04, most procedures were performed at the FMC. Figure 5 depicts the overall volume of hepatic surgery in the CHR along with the corresponding annual mortality rates. This depiction shows that over time there was a steady decrease in the annual mortality rate with a corresponding increase in the volume of hepatic resection surgery. The average length of stay in hospital was 14.6 (median 10) days. There were 26 deaths, for an in-hospital mortality rate of 6.1%. The mortality rate dropped significantly from 2000/01 onward. Prior to this time, the mean mortality rate was 9.7%, whereas it dropped to 4.1% from 2001/02 onward (p = 0.020). Table 1 demonstrates that the patient characteristics were not significantly different before or after 2000/01. Table 2 demonstrates that the mortality rates varied according to the pathologic diagnosis (p = 0.030).
Fig. 1. Number of hepatic resections performed per year, by surgeon. Each shade represents a different surgeon.
Fig. 2. Disease etiology by year.
Fig. 3. Type of resection performed by year. We defined major and minor resections as the removal of at least 2 or less than 2 segments, respectively. Unknown liver resections were coded using the ICD-10.
Fig. 4. Procedures performed per hospital.
Fig. 5. Volume and mortality by year.
Table 1
Table 2
DISCUSSION
The creation of a regional multidisciplinary hepatobiliary program (Box 1) in the CHR coincided with the point in time at which outcomes improved. This multidisciplinary group was created in 2000, which corresponds to a dramatic and statistically significant increase and decrease, respectively, in both the volume of hepatic resections performed and the operative mortality observed from the fiscal year 2000/01 onwards, as shown in Figure 5.
Box 1.
The formation of this regional hepatobiliary program resulted in several changes to the delivery of patient care in 2000 that may have contributed to these improved outcomes. The first of 3 subspecialty-trained surgeons (F.R.S.) began to practise in 1999 and was joined by a second subspecialty-trained surgeon (O.F.B.) in 2000. In the same year, a multidisciplinary liver clinic was created in the CHR. Multidisciplinary tumour board rounds were scheduled on a weekly basis beginning in 2001. In 2003, the third subspecialty-trained surgeon (E.D.) joined the program, and at the same time major hepatobiliary surg-ery became concentrated at a single hospital (FMC). In addition, the surgical volumes increased substantially around the time that operative mortality rates decreased. Thus there were a number of changes that may have contributed to the overall improvement in outcomes.
Surgeon training and surgeon volume are very likely contributing factors, but because they are so closely intertwined it can be very difficult to distinguish the relative importance of each. Although it is intuitive that subspecialty training would impact outcomes, this has not been previously studied in relation to outcomes following hepatic resection. Surgeon training has, however, been associated with improved outcomes in colorectal cancer surgery.33,34 Surgeon volume has been associated with improved mortality rates after other high-risk cancer surgery, most notably esophageal and pancreatic resective surgery.10
Our study does not have the ability to individually examine the influence of surgeon training and volume, but our results suggest that these factors and others play a role. For instance, the first subspecialty-trained surgeon began practice in the health region in 1999. This surgeon's caseload that year was 11 cases, which would be considered high-volume for an entire hospital.12,26 The drop in mortality was not observed for another 2 years. This suggests that there were other factors at play. Hospital volume is likely one of these other factors. The region saw a dramatic increase in the number of hepatic resections performed beginning in 2000/01, close to the time when mortality rates also dropped dramatically. Other studies have shown that hospital volume is associated with improved mortality after hepatic resection and after other high-risk cancer surgeries.11,12,26,35,36 After the creation of the regional hepatobiliary program, the procedures became increasingly concentrated at a single centre (Fig. 4). The creation of a single regional program in contrast to multiple competing programs (or surgeons) may have been an important factor in the increasing volumes noted. Although referral to the program is voluntary, the number of surgeons performing hepatic resection outside the program has decreased since its inception.
Still, there were likely other important factors, in addition to volume and training, that affected patient outcomes. This regional specialty program aimed to improve collaboration between different specialties, including surgeons, medical oncologists, diagnostic and interventional radiologists, hepatologists and gastroenterologists. All patients undergoing surgery were reviewed at weekly multidisciplinary tumour board rounds beginning in 2001/02. We believe this may be one of the factors that led to improved processes of care for these patients. It is possible that by centralizing surgery at a single centre, more patients have been exposed to these processes of care. The importance of such a multidisciplinary approach to the treatment of liver tumours has recently been emphasized,37,38 and current practice guidelines from several countries state that care by a multidisciplinary team should be the standard.39,40 Another important factor is likely concurrent improvements in surgical technique along with anesthetic and postoperative care.4 It is not possible to separate the relative contributions of each of these factors that have contributed to the improved outcomes noted.
Other studies have suggested that processes of care, in addition to volume and training, are also important. Birkmeyer and colleagues41 found that cancer centres, as designated by the National Cancer Institute (NCI), had lower 30-day mortality rates for colon resections, lung resections, gastrectomies and esophagectomies than did other centres, even when controlling for volume and patient characteristics. Urbach and Baxter23 found that for a low volume of high-risk surgical procedures performed at a given institution, improved outcomes were seen when the centre was considered to be high-volume for other procedures. This would suggest that higher-volume hospitals can achieve superior results with a wide variety of complex procedures and that perhaps these hospitals have adopted broadly applicable processes of care that are not specific to any one disease or procedure. Birkmeyer and colleagues36 have attempted to define the role of other processes of care. They found that high-volume hospitals for several cancer resections, including hepatic resections, had lower rates of operative mortality. Patients treated at these centres were also more likely to have undergone preoperative stress tests, been seen by medical and radiation oncologists and received perioperative invasive monitoring; however, these differences in processes of care were not enough to explain the differences in outcomes.
Another interesting observation was the timing of the increased surgical volume of hepatic resections. We observed this dramatic increase in volume after the multidisciplinary hepatobiliary program was created in 2000. Cases that may not have been performed in the past were performed as the program provided the structure to allow surgeon collaboration and the performance of technically demanding procedures within the supportive construct of the program.
Patients who are voluntarily referred to the multidisciplinary liver clinic are triaged by an administrative assistant. The referrals are divided equally among the 3 surgeons. This is a paradigm shift for most surgeons, who have historically practised separately. By dividing referrals equally between the physicians and avoiding differential referrals (and thereby long waiting lists), access to care improved. All new cancer referrals are seen within 2 weeks. This group practice pattern enhances patient access and creates a favourable scenario for referral among physicians.
There are limitations to our study, including the use of administrative data, which does not allow us to explore the relative importance of many crucial processes of care for patients undergoing complex oncologic surgery.36 Although the coding of patient data is thought to be accurate, this has not been validated, and the potential for error exists. Administrative data do not allow assessment of important outcomes beyond operative mortality such as survival and recurrence rates. In addition, a limitation of the newer ICD-10/CCI coding system was demonstrated in our study. After introduction of the ICD-10/CCI system in 2002, information on the type of hepatic resection performed was unavailable (Fig. 3). The ICD-10/CCI was implemented because these coding manuals have a richer amount of information compared with ICD-9-CM. For hepatic surgical procedures, however, there is a definite lack of useful information, and it is clearly inferior to the information in ICD-9-CM. Another limitation is that the intervention took place in a Canadian health region and, thus, the results may not be generalizable to other health care systems. Urbach and colleagues22 found that Canadian studies are less likely than American studies to identify volume–outcome relations, possibly owing to smaller sample sizes and to inherent differences in the financing and delivery of health care in the different systems.
In conclusion, the volume of hepatic resections performed increased over the study period; this corresponded with improved operative mortality rates in a geographically based Canadian health region. The reasons for the improved outcomes are likely multifactorial and possibly relate to improved processes of care seen with the creation of a multidisciplinary hepatobiliary program, in addition to the effects of increasing surgical volume and specialized surgical training.
Acknowledgments
Dr. Dixon is a Population Health Investigator with the Alberta Heritage Foundation for Medical Research (AHFMR) and is supported with a New Investigator grant from the Canadian Institutes for Health Research. This work was also supported through an Establishment Grant from the AHFMR and a grant from the Centre for the Advancement of Health in Calgary, Alberta.
This material was presented at the 13th Annual Scientific Meeting of the Canadian Society of Surgical Oncology in Toronto, Ont., on Apr. 27, 2007.
Contributors: Drs. Dixon, Dowden, Burak and Sutherland designed the study. Drs. Dixon, Bathe, Sadler, McKinnon and Miller acquired the data, which Drs. Dixon, Bathe and McKay and Ms. You analyzed. Drs. Dixon and McKay wrote the article, which all authors reviewed and approved for publication.
Competing interests: None declared.
Accepted for publication Oct. 29, 2007
Correspondence to: Dr. E. Dixon Departments of Surgery, Oncology and Community Health Sciences University of Calgary Tom Baker Cancer Centre 1331–29th St. NW Calgary AB T2N 4N2 fax 403 521-3744 elijah.dixon@calgaryhealthregion.ca
References
- 1.Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg 1977;22:1-342. [PubMed]
- 2.Savage AP, Malt RA. Elective and emergency hepatic resection. Determinants of operative mortality and morbidity. Ann Surg 1991;214:689-95. [DOI] [PMC free article] [PubMed]
- 3.Bozzetti F, Gennari L, Regalia E, et al. Morbidity and mortality after surgical resection of liver tumors. Analysis of 229 cases. Hepatogastroenterology 1992;39:237-41. [PubMed]
- 4.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406. [DOI] [PMC free article] [PubMed]
- 5.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46. [DOI] [PubMed]
- 6.Cherqui D, Alon R, Lauzet JY, et al. [Limitation of blood transfusions during hepatectomies. Study of 150 consecutive hepatic resections on healthy and pathological livers] [Article in French]. Gastroenterol Clin Biol 1996;20:132-8. [PubMed]
- 7.Brancatisano R, Isla A, Habib N. Is radical hepatic surgery safe? Am J Surg 1998;175:161-3. [DOI] [PubMed]
- 8.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [DOI] [PubMed]
- 9.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: potential benefits of the Leapfrog initiative. Surgery 2001;130:415-22. [DOI] [PubMed]
- 10.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346: 1128-37. [DOI] [PubMed]
- 11.Choti MA, Bowman HM, Pitt HA, et al. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg 1998;2:11-20. [DOI] [PubMed]
- 12.Dimick JB, Cowan JA Jr, Knol JA, et al. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg 2003;138: 185-91. [DOI] [PubMed]
- 13.Edwards EB, Roberts JP, McBride MA, et al. The effect of the volume of procedures at transplantation centers on mortality after liver transplantation. N Engl J Med 1999;341:2049-53. [DOI] [PubMed]
- 14.Finlayson EV, Birkmeyer JD. Effects of hospital volume on life expectancy after selected cancer operations in older adults: a decision analysis. J Am Coll Surg 2003;196:410-7. [DOI] [PubMed]
- 15.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg 2003;138:721-5. [DOI] [PubMed]
- 16.Glasgow RE, Showstack JA, Katz PP, et al. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg 1999;134:30-5. [DOI] [PubMed]
- 17.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 2000;232:786-95. [DOI] [PMC free article] [PubMed]
- 18.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364-9. [DOI] [PubMed]
- 19.McMillan RW, Uppot R, Zibari GB, et al. Can low volume liver transplant centers be successful? The Regional Transplant Center of Willis-Knighton & Louisiana State University Medical Center. The first 120 liver transplants. J La State Med Soc 1999;151:367-72. [PubMed]
- 20.Ruby ST, Robinson D, Lynch JT, et al. Outcome analysis of carotid endarterectomy in Connecticut: the impact of volume and specialty. Ann Vasc Surg 1996;10:22-6. [DOI] [PubMed]
- 21.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998;228:429-38. [DOI] [PMC free article] [PubMed]
- 22.Urbach DR, Austin PC. Conventional models overestimate the statistical significance of volume-outcome associations, compared with multilevel models. J Clin Epidemiol 2005;58:391-400. [DOI] [PubMed]
- 23.Urbach DR, Baxter NN. Does it matter what a hospital is “high volume” for? Specificity of hospital volume-outcome associations for surgical procedures: analysis of administrative data. Qual Saf Health Care 2004;13:379-83. [DOI] [PMC free article] [PubMed]
- 24.Urbach DR, Croxford R, MacCallum NL, et al. How are volume–outcome associations related to models of health care funding and delivery? A comparison of the United States and Canada. World J Surg 2005;29:1230-3. [DOI] [PubMed]
- 25.Berger DH, Ko CY, Spain DA. Society of University Surgeons position statement on the volume-outcome relationship for surgical procedures. Surgery 2003;134:34-40. [DOI] [PubMed]
- 26.Dimick JB, Wainess RM, Cowan JA, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg 2004;199:31-8. [DOI] [PubMed]
- 27.Licitra L, Cavina R, Cerrotta A. The multidisciplinary approach in clinical oncology. Tumori 1998;84:279-80. [DOI] [PubMed]
- 28.Raven RW. Oncology as a multidisciplinary subject. Clin Oncol 1978;4:307-8. [PubMed]
- 29.International classification of diseases, 9th revision (clinical modification). 5th ed. Washington: US Department of Health and Human Services; 1996.
- 30.Canadian classification of health interventions. Ottawa: Canadian Institute for Health Information; 2006.
- 31.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [DOI] [PubMed]
- 32.Quan H. ICD-10-CA/CCI coding algorithms for defining clinical variables to assess outcome after aortic and mitral valve replacement surgery. Can J Cardiol 2006;22:153-4. [DOI] [PMC free article] [PubMed]
- 33.Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998;227:157-67. [DOI] [PMC free article] [PubMed]
- 34.Prystowsky JB, Bordage G, Feinglass JM. Patient outcomes for segmental colon resection according to surgeon's training, certification, and experience. Surgery 2002;132:663-70. [DOI] [PubMed]
- 35.Simunovic M, Rempel E, Theriault ME, et al. Influence of hospital characteristics on operative death and survival of patients after major cancer surgery in Ontario. Can J Surg 2006;49:251-8. [PMC free article] [PubMed]
- 36.Birkmeyer JD, Sun Y, Goldfaden A, et al. Volume and process of care in high-risk cancer surgery. Cancer 2006;106:2476-81. [DOI] [PubMed]
- 37.Vauthey JN, Choti MA, Helton WS. AHPBA/SSO/SSAT Consensus Conference on hepatic colorectal metastases: rationale and overview of the conference. Ann Surg Oncol 2006;13:1259-60. [DOI] [PubMed]
- 38.Garden OJ, Rees M, Poston GJ, et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55(Suppl 3):iii1-8. [DOI] [PMC free article] [PubMed]
- 39.NHS Executive. Manual of cancer service standards. London: Department of Health; 2000.
- 40.Marcaccio M, Langer B, Rumble B, et al; Expert Panel on HPB Surgical Oncology. Hepatic, pancreatic, and biliary tract (HPB) surgical oncology standards. Toronto: Cancer Care Ontario; 2006
- 41.Birkmeyer NJ, Goodney PP, Stukel TA, et al. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer 2005;103:435-41. [DOI] [PubMed]