Abstract
Background
Soft-tissue sarcoma involving the popliteal fossa remains challenging because it is difficult to achieve wide margins with limb salvage in this location. Adjuvant therapy is frequently necessary, and limb function can be adversely affected. We reviewed our experience with these tumours.
Methods
Our prospective tumour database served to identify all patients with popliteal sarcomas treated at the McGill University Health Centre and the Maisonneuve-Rosemont Hospital between 1994 and 2005. We assessed oncologic and functional outcomes as well as complications.
Results
Our study included 18 patients (12 women and 6 men). The mean age was 54 (range 16–84) years. The mean duration of follow-up was 55 (range 4–126) months. Frequent histologic diagnoses were liposarcoma (n = 6), synovial sarcoma (n = 4) and leiomyosarcoma (n = 3). Tumour size varied from 2 to 21 cm (median 11 cm). American Joint Committee on Cancer staging was as follows: 4 patients had stage IIa disease, 3 patients had stage IIb, 10 patients had stage III and 1 patient had stage IV disease. Treatment consisted of limb salvage in 15 patients and amputation in 3. Fourteen patients had radiotherapy, 4 had chemotherapy, and 3 needed partial sciatic nerve resection. Margins were negative in 7 of 18 patients and microscopically positive in 11 patients. Complications included wound infections in 3 patients and thrombophlebitis in 2 patients. Of the patients undergoing limb-salvaging procedures, 1 experienced local recurrences after limb salvage (7%), and 5 experienced lung metastases (20%). Local recurrence was always associated with positive margins, whereas metastases occurred only in patients without local recurrence. The mean Musculoskeletal Tumor Society 1987 score was 33 (range 24–35). The mean Toronto Extremity Salvage Score results was 82.4 (range 63.8–100). At latest follow-up, 6 patients had died of disease, 1 was alive with disease, and 11 (61%) patients remained free of disease.
Conclusion
Despite the high rate of microscopically positive margins, the local recurrence rate was 7%. Amputation did not prevent death. We found function to be good to excellent in most patients who had limb-salvaging surgery.
Abstract
Contexte
Le sarcome des tissus mous qui atteint le creux poplité pose toujours un défi parce qu'il est difficile de produire des marges larges tout en épargnant le membre à cet endroit. Une thérapie adjuvante s'impose souvent et peut avoir un effet indésirable sur la fonction du membre. Nous avons passé en revue notre expérience de ces tumeurs.
Méthodes
Nous avons utilisé notre base de données prospective sur les tumeurs pour repérer tous les patients atteints d'un sarcome du creux poplité et traités au Centre universitaire de santé McGill et à l'Hôpital Maisonneuve-Rosemont entre 1994 et 2005. Nous avons évalué les résultats oncologiques et fonctionnels, ainsi que les complications.
Résultats
Notre étude a porté sur 18 patients (12 femmes et 6 hommes) qui avaient en moyenne 54 ans (intervalle de 16 à 84 ans). La durée moyenne du suivi s'est établie à 55 mois (intervalle de 4 à 126 mois). Le liposarcome (n = 6), le synovialome (n = 4) et le léiomyosarcome (n = 3) étaient les diagnostics histologiques fréquents. La grosseur des tumeurs a varié de 2 à 21 cm (médiane de 11 cm). Le stade selon l'American Joint Committee on Cancer était le suivant : la maladie était au stade IIa chez 4 patients, au stade IIb chez 3 patients, au stade III chez 10 patients et au stade IV chez 1 patient. Le traitement a consisté à épargner le membre chez 15 patients et à l'amputer chez 3 patients. Quatorze patients ont reçu une radiothérapie, 4, une chimiothérapie et 3 ont eu besoin d'une résection partielle du nerf sciatique. Les marges étaient négatives chez 7 des 18 patients et microscopiquement positives chez 11 patients. Les complications ont inclus une infection de la plaie chez 3 patients et une thrombophlébite chez 2 autres. Parmi les patients qui ont subi une intervention visant à épargner le membre, 1 a eu des récidives locales par la suite (7 %) et 5 ont eu des métastases aux poumons (20 %). La réapparition locale a toujours été associée à des marges positives, tandis que seuls les patients qui n'ont pas eu de récidive locale ont eu des métastases. Le score moyen de la Musculoskeletal Tumor Society 1987 s'est établi à 33 (intervalle de 24 à 35). Les résultats moyens sur l'échelle Toronto Extremity Salvage Score se sont établis à 82,4 (intervalle de 63,8 à 100). Au dernier suivi, 6 patients étaient morts de la maladie, 1 était vivant mais était malade, et 11 (61 %) étaient guéris.
Conclusion
En dépit du taux élevé de marges microscopiquement positives, le taux de récidive locale s'est établi à 7 %. L'amputation n'a pas évité la mort. Nous avons constaté que la fonction variait de bonne à excellente chez la plupart des patients qui ont subi une intervention chirurgicale visant à épargner le membre.
Soft-tissue sarcomas are a rare form of cancer, consisting of 2% of all malignancies.1,2 Their occurrence in the popliteal fossa accounts for 3%–5% of all extremity and trunk soft-tissue sarcomas.3–5 The popliteal fossa is defined as the space bordered by the hamstring tendons proximally, the gastrocnemius muscles distally, the posterior knee joint capsule anteriorly and the deep fascia posteriorly. It includes the popliteal vessels and the posterior tibial and common peroneal nerves. Intracompartmental soft-tissue tumours by definition are bordered in all directions by a natural barrier such as the fascia,6 known to be resistant to tumour transgression. Flexor areas such as the groin, the axilla, the elbow and the popliteal fossa are not considered to have compartmental barriers and thus are called extracompartmental.7 The importance of this anatomic location has been recognized through the surgical staging system adopted by the Musculoskeletal Tumor Society (MSTS).7,8 Extracompartmental soft-tissue sarcomas have been reported to have a poorer prognosis than intracompartmental tumours because of their proximity to the critical neurovascular bundle and their periarticular location, both of which usually preclude wide excision or negative margins.6,8–10 Historically, amputation was often recommended because close margins were to be expected.9,11 In addition, scarring and fibrosis induced by surgery or radiotherapy can result in stiffness and poorer functional outcome for this lower extremity location.4,9,12,13 Progress in diagnostic imaging methods such as computed tomography and magnetic resonance imaging have allowed for better staging and improved treatment planning. Advances in limb-salvaging techniques coupled with the use of neoadjuvant or adjuvant therapies have improved tumour control rates and functional outcomes.14,15 Some recent publications lend support to similar outcomes for extracompartmental tumours, including flexor fossa sarcomas.3,5,14,16–20 Although some studies have addressed limb salvage in carefully selected patients with flexor fossa sarcomas treated with a multidisciplinary approach3,9,19 and reported satisfactory clinical outcomes, it is only very recently that popliteal location was specifically addressed.5,20
Our working hypothesis was that patients with popliteal soft-tissue sarcomas who underwent limb-salvaging surgery combined with a multimodal approach had good oncologic and functional outcomes.
METHODS
Our prospective tumour patient database served to identify all eligible participants. We identified all patients with soft-tissue sarcomas of the popliteal fossa treated at the McGill University Health Centre or the Maisonneuve-Rosemont Hospital between 1994 and 2005. Eligibility criteria included patients with deep popliteal sarcomas with a minimum follow-up of 2 years for all surviving individuals. Tumours included those involving the popliteal space and those extending beyond its borders. We excluded patients with well-differentiated liposarcomas and popliteal sarcomas who did not have surgery. We obtained ethics approval from the review boards of the McGill University Health Centre and the Maisonneuve-Rosemont Hospital.
We conducted a retrospective chart review to obtain and update all the necessary information on each participant. We reviewed radiographs to ensure the involvement of the popliteal area. Data collected included demographic and tumour characteristics, procedures, complications, and oncologic and functional outcomes. We staged tumours in accordance with the American Joint Committee on Cancer (AJCC).21 We assessed function using the MSTS score, 1987 version, (MSTS 1987) and the Toronto Extremity Salvage Score (TESS). The MSTS 1987 is a well-known function survey completed by the clinician that consists of 7 items: range of motion, pain, stability, deformity, strength, activity level and emotional acceptance. Each item is rated on a scale from 1 to 5, for a best possible score of 35 points.22 The TESS is a validated questionnaire self-administered by the patients to rate their ability to perform daily living tasks.23,24 Scores are calculated out of a possible 100 points; a higher score means a better result.
We scheduled radiation therapy based on the possibility of minimizing the late effects of radiotherapy or on our expectations of obtaining adequate margins. Some patients included in this series were part of a prospective randomized study addressing preoperative versus postoperative radiotherapy in the treatment of soft-tissue sarcomas. Chemotherapy was either administered to downstage the disease or as part of prospective clinical trials.
RESULTS
We identified 23 patients eligible for inclusion in our study. Of these, we excluded 5 patients because 1 did not have surgery (preterminal condition at diagnosis) and 4 had well-differentiated liposarcomas. This left 18 patients who met our inclusion criteria: 6 men and 12 women. The mean age was 54 (range 16–84) years at the time of diagnosis. The mean duration of follow-up was 55 (range 4–126) months. All surviving patients, continuously free of disease, had a minimum follow-up of 2 years. The most common histological diagnoses were liposarcoma (n = 6), synovial sarcoma (n = 4) and leiomyosarcoma (n = 3). We also identified 1 malignant fibrous histiocytoma, 1 extraskeletal chondrosarcoma and 1 hemangiopericytoma. Two histological diagnoses were not specified. All tumours were primary. The median tumour size was 11 (range 2–21) cm. Histology revealed sarcomas to be of low grade in 5 patients (grade II/IV in 5) and of high grade in 13 patients (grade III in 9 and grade IV in 4). At the time of diagnosis, we identified pulmonary metastases in 1 patient (6%). The AJCC stages were as follows: 4 patients had stage IIa disease, 3 patients had stage IIb, 10 patients had stage III and 1 patient had stage IV disease.
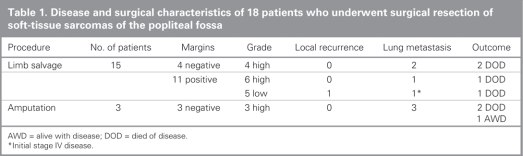
Surgical characteristics of the patients are summarized in Table 1. Treatment consisted of limb-salvaging surgery for 15 patients and primary above-knee amputation for 3 patients. Indications for amputation were direct neurovascular or joint involvement and inability to prevent gross residual disease. Surgical margins were negative in 7 patients, including the 3 whose limbs were amputated, and microscopically positive in 11 patients. Although we found the incidence of positive margins after limb-salvaging surgery to be high at 73%, no patients were left with gross residual disease. Chemotherapy was administered in 4 patients (preoperatively in 1, postoperatively in 2 and both pre-and postoperatively in 1 patient). Neoadjuvant chemotherapy was administered in 2 patients with unresectable lesions to reduce tumour volume to allow for limb salvage. Lack of tumour response in both patients led to amputation. Fourteen patients had radiotherapy: therapy was postoperative in 6 patients (63 Gy) and preoperative in 8 patients (50 Gy), among whom 6 had a postoperative boost (13 Gy). All patients with high-grade tumours but no amputation underwent radiotherapy. Three patients required minor sciatic nerve branches to be resected, but none invoved the posterior tibial or the common peroneal nerve trunks.
Table 1
We recorded local recurrence after limb salvage in 1 patient (7%). This patient had an extraskeletal chondrosarcoma with lung metastases at diagnosis and positive margins at surgery. Lung metastases developed in 5 patients (30%) after treatment. They were treated respectively with thoracotomy, thoracotomy and chemotherapy, pleurodesis and, for 2 patients, palliation. Postoperative complications included 3 wound dehiscences following limb salvage: 1 without associated radiotherapy, 1 with pre-and postoperative regimens and 1 with postoperative radiotherapy only. None required soft-tissue reconstruction, and 2 experienced deep vein thrombosis. Nine of the 15 patients who had limb-salvaging surgery completed the MSTS 1987; the mean score was 32.5 (range 24.0–35.0). The Toronto Extremity Salvage Score (TESS) was completed by 8 patients who had limb salvage; the mean result was 83.9 (range 63.8–100). The worst result belonged to a patient who had postoperative radiotherapy at 66 Gy and experienced severe stiffness of the knee with range of motion of only 20° (range 20°–40°). No patients needed amputation after limb salvage whether for oncologic issues or wound complication. At latest follow-up, 6 patients had died of disease, 1 was still alive with slowly progressing lung disease 5 years after amputation and 11 remained free of disease.
DISCUSSION
Popliteal soft-tissue sarcoma is rare even in the context of a specialized orthopedic oncology practice, and remains a difficult challenge. In our study, the popliteal location of any soft-tissue sarcoma accounted for only 2.7% of patients included in our prospective database of 848 patients with soft-tissue sarcomas. Popliteal involvement precludes achieving wide surgical margins in limb-sparing procedures. Risk of local recurrence is closely linked with surgical margins achieved at the time of surgery.14,17,25,26 Negative margins in the context of radiotherapy have been shown to be an important factor in the prediction of local recurrence.27 Radiation therapy has been demonstrated to improve the rate of local control28 but does not obviate the need to aim for adequate margins. Most local recurrences are usually detected within 2 years after surgery.29 Only 1 patient in our study experienced local recurrence; the tumour had positive margins and was a large lesion. This patient underwent debulking only of the recurrent tumour because his initial stage IV disease had progressed. The overall local recurrence rate after limb salvage in our study was 7%. Such a rate is low and compares favourably to recurrence rates reported for soft-tissue sarcomas in more conventional locations treated with surgery and radiotherapy.25,26 In tumours with positive margins we observed a recurrence rate of 9% (1/11), but we recorded no recurrence for the 4 patients who had tumours with negative margins. Limb salvage for popliteal sarcoma provides an acceptable rate of local recurrence with the combination of radiotherapy and the planning of positive margins. It remains unclear if local recurrence in soft-tissue sarcoma has a strong adverse impact on survival. Patients who underwent amputation all had high-grade and large tumours (≥ 7 cm), thus representing limited prognosis (Table 1). No recurrence was identified in the 3 amputees; nevertheless, lung metastases developed in all patients, leading to 2 deaths. Five of the 6 deaths involved large and deep high-grade tumours. The other involved extraskeletal chondrosarcoma that was metastatic at the time of diagnosis. Of the 6 patients in whom pulmonary metastases developed postoperatively, all had large (> 5 cm) and high-grade tumours but negative surgical margins. The mean event-free survival was 3.6 years.
Interestingly, studies that specifically addressed popliteal sarcomas have reported similarly limited numbers of cases (27 and 29 patients) and similar outcomes.5,20 Both studies reported that 7% of patients had metastatic disease at diagnosis. Amputation rates were 14% and 28%, respectively, and one of the studies reported no difference in survival among the amputees and the limb-salvage group.5 The rate of local recurrence was 10% in both studies. Wound complication rates were 30% and 23%, respectively.
Radiotherapy is known to impair wound healing, especially in a neoadjuvant setting.12 Wound complications have been reported to impair function. Reapproximating the remaining hamstrings and gastrocnemius has been suggested to minimize dead space and wound problems.5 Function can also be altered by radiation-induced fibrosis, lymphedema and joint stiffness that correlate with radiation dosage and field size.30,31 In our study, patients who had limb-salvaging surgery and who completed both the TESS and MSTS 1987 assessments expressed high levels of satisfaction, as evidenced by the mean scores of 82.4% and 33 out of a possible 35, respectively. We did not observe below-average functional scores when minor nerve branches of the sciatic nerve were sacrificed. Stiffness and swelling resulting from postradiation fibrosis and deep vein thrombosis explained the lowest scores. In our experience, the incidence of deep vein thrombosis and its negative impact on functional outcome support routine antithromboprophylaxis.
Our study, similar to others recently published,5,20 supports limb-salvaging surgery with adjuvant radiation therapy as treatment for most patients with soft-tissue sarcomas of the popliteal fossa. Microscopically positive margins do not appear to be a clear indication for amputation although they imply a substantial risk for local recurrence. Negative margins, whether from wide amputation or marginal limb-salvage resection, do not preclude metastatic disease. Our data support the crucial role of adjuvant radiation therapy for local control by providing a reasonably low incidence of local failure in the presence of microscopically positive margins. We recognize that a longer follow-up period may show a higher incidence of local failure.
The timing of radiotherapy remains a multidisciplinary decision that has to be evaluated individually for each clinical presentation. Postoperative irradiation might be favoured (because wound complications are reported to be substantially increased with preoperative treatments12), taking into account that in our study a postoperative boost of radiation was frequently needed to address the high percentage of patients with positive margins after surgery.
Contributors: All authors designed the study and acquired the data, which Drs. Turcotte, Ferrone and Isler analyzed. Drs. Turcotte, Ferrone and Isler wrote the article, which Dr. Isler and Ms. Wong reviewed. All authors gave final approval for publication.
Competing interests: None declared.
Accepted for publication Sep. 17, 2007
Correspondence to: Dr. R.E. Turcotte McGill University Health Centre 1650 Cedar Ave., Rm. B5 159.6 Montréal QC H3G 1A4 fax 514 934-8453 robert.turcotte@muhc.mcgill.ca
References
- 1.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg 1996;33:817-72. [PubMed]
- 2.Eilber FC, Eilber FR. Soft tissue sarcoma. In: Cameron JL, editor. Current surgical therapy. 7th ed. St. Louis (MO): Mosby; 2001. p. 1213-8.
- 3.Eilber FC, Eckardt JJ, Rosen G, et al. Large, deep, high-grade extremity sarcomas: treating tumors of the flexor fossae. Surg Oncol 1999;8:211-4. [DOI] [PubMed]
- 4.Gerrand CH, Wunder JS, Kandel RA, et al. The influence of anatomic location on functional outcome in lower-extremity soft-tissue sarcoma [see comment]. Ann Surg Oncol 2004;11:476-82. Comment in: Ann Surg Oncol 2004;11:453-4. [DOI] [PubMed]
- 5.Pritsch T, Bickels J, Winberg T, et al. Popliteal sarcomas, presentation, prognosis and limb salvage. Clin Orthop Relat Res 2007;455:225-33. [DOI] [PubMed]
- 6.Peabody TD, Simon MA. Principles of staging of soft-tissue sarcomas. Clin Orthop Relat Res 1993;289:19-31. [PubMed]
- 7.Enneking WF. A system of grading musculoskeletal neoplasms. Clin Orthop Relat Res 1986;204:9-24. [PubMed]
- 8.Enneking WF, Spanier SS, Goodman MA. Current concepts review: the surgical staging of musculoskeletal sarcoma. J Bone Joint Surg Am 1980;62:1027-30. [PubMed]
- 9.Shiu MH, Collin C, Hilaris BS, et al. Limb preservation and tumor control in the treatment of popliteal and antecubital soft tissue sarcomas. Cancer 1986;57:1632-9. [DOI] [PubMed]
- 10.Mandard AM, Petiot JF, Marnay J, et al. Prognostic factors in soft tissue sarcomas: a multivariate analysis of 109 cases. Cancer 1989;63:1437-51. [DOI] [PubMed]
- 11.Enneking WF, Spanier SS, Malawer MW. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer 1981;47:1005-22. [DOI] [PubMed]
- 12.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235-41. [DOI] [PubMed]
- 13.Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower extremity soft tissue sarcoma. J Surg Oncol 2000;73:206-11. [DOI] [PubMed]
- 14.Bell RS, O'Sullivan B, Liu FF, et al. The surgical margin in soft tissue sarcoma. J Bone Joint Surg Am 1989;71:370-5. [PubMed]
- 15.O'Connor MI, Pritchard DJ, Gunderson LL. Integration of limb-sparing surgery, brachytherapy, and external-beam irradiation in the treatment of soft-tissue sarcomas. Clin Orthop Relat Res 1993;(289):73-80. [PubMed]
- 16.Brant TA, Parsons JT, Marcus RB, et al. Preoperative irradiation for soft tissue sarcomas of the trunk and extremities in adults. Int J Radiat Oncol Biol Phys 1990;19:899-906. [DOI] [PubMed]
- 17.Rööser BO, Attwell R, Berg NO, et al. Prognostication in soft tissue sarcoma: a model with four risk factors. Cancer 1988;61:817-23. [DOI] [PubMed]
- 18.Rööser B, Attewell R, Berg NO, et al. Survival in soft tissue sarcoma: prognostic variables identified by multivariate analysis. Acta Orthop Scand 1987;58:516-22. [DOI] [PubMed]
- 19.Yang RS, Lane JM, Eilber FR, et al. High grade soft tissue sarcoma of the flexor fossae. Cancer 1995;76:1398-405. [DOI] [PubMed]
- 20.Rudiger HA, Beltrami G, Campanacci DA, et al. Soft tissue sarcomas of the popliteal fossa: outcome and risk factors. Eur J Surg Oncol 2007;33:512-7. [DOI] [PubMed]
- 21.Greene FL, Page DL, Fleming ID. AJCC Cancer Staging Manual. 6th ed. New York (NY): Springer; 2002.
- 22.Enneking W. Modification of the system for functional evaluation in the surgical management of musculoskeletal tumors. In: Limb salvage in musculoskeletal oncology. New York: Churchill-Livingston; 1987. p. 626–39.
- 23.Davis AM, Bell RS, Badley EM, et al. Evaluating functional outcome in patients with lower extremity sarcoma. Clin Orthop Relat Res 1999;358:90-100. [PubMed]
- 24.Davis AM, Wright JG, Williams JI, et al. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 1996;5:508-16. [DOI] [PubMed]
- 25.Sadoski C, Suit HD, Rosenberg A, et al. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol 1993;52:223-30. [DOI] [PubMed]
- 26.Trovik CS, Bauer HC, Berlin O, et al. Local recurrence of deep-seated, high-grade, soft tissue sarcoma: 459 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand 2001;72:160-6. [DOI] [PubMed]
- 27.Cormier JN, Ballo MT. The influence of anatomic location on functional outcome in lower-extremity soft-tissue sarcoma [comment]. Ann Surg Oncol 2004;11:453-4. Comment on: Ann Surg Oncol 2004;11:476-82. [DOI] [PubMed]
- 28.Wilson AN, Davis A, Bell RS, et al. Local control of soft tissue sarcoma of the extremity: the experience of a multidisciplinary sarcoma group with definitive surgery and radiotherapy. Eur J Cancer 1994;30A:746-51. [DOI] [PubMed]
- 29.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperarive versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005;75:48-53. [DOI] [PubMed]
- 30.Cantin J, McNerr GP, Chu FC, et al. The problem of local recurrence after treatment of soft tissue sarcoma. Ann Surg 1968;168:47-53. [DOI] [PMC free article] [PubMed]
- 31.Stinson SF, DeLaney TF, Greenberg J, et al. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int J Radiat Oncol Biol Phys 1991;21:1493-9. [DOI] [PubMed]