Abstract
Objective
Plasma high-molecular-weight kininogen (HK) is cleaved in inflammatory diseases by kallikrein to HKa with release of bradykinin (BK). We postulated a direct link between HKa and cytokine/chemokine release.
Methods and Results
HKa, but not BK, releases cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and chemokines IL-8 and MCP-1 from isolated human mononuclear cells. At a concentration of 600 nM, glutathione-S-transferase (GST) fusion proteins of kininogen domain 3 (D3), a fragment of domain 3, E7P (aaG255-Q292), HK domain 5 (D5), the D5 recombinant peptides HG (aa K420-D474) and HGK (aa H475-S626) stimulated secretion of IL-1β from mononuclear cells. Monoclonal antibodies (MAbs) specific for D5 or specific for D3 blocked release of IL-1β by HKa, supporting the importance of both domains. Antibodies to HK receptors on leukocytes including Mac-1, LFA-1, uPAR, and C1qR inhibited IL-1β secretion induced by tKa 98%, 89%, 85%, and 62%, respectively. Fractionation of mononuclear cells identified the responsible cell, a blood monocyte. Inhibitors of signaling pathways NFkB, JNK, and p38 but not extracellular signal-regulated kinase (ERK) decreased cytokine release from mononuclear cells. HKa increased the synthesis of IL-1β as deduced by an increase of IL-1β mRNA at 1 to 2 hours.
Conclusions
HKa domains 3 and 5 may contribute to the pathogenesis of inflammatory diseases by releasing IL-1β from human monocytes using intracellular signaling pathways initiated by uPAR, β2 integrins and gC1qR.
Keywords: chemokines, cytokines, kininogen, monocytes, uPAR
From the discovery of kininogen,1 the kallikrein–kinin system (KKS) has been intimately involved with inflammation. Plasma kallikrein cleaves HK to form BK and cleaved HK (HKa), which differs from HK because of major conformational changes.2 BK increases capillary permeability by opening the tight junctions between endothelial cells and directly stimulates nerve endings causing pain, and is a potent vasodilator directly relaxing smooth muscles by releasing PGI2. The sum of these effects of BK reproduces many but not all aspects of inflammation. Emphasis in the past decade has shifted from contributions of HK to the humoral aspects of inflammation to its cellular participation, particularly interactions of HKa with leukocytes and endothelial cells.3 Receptors on either or both of these cell types include selectins, which mediate leukocyte rolling and integrins, which mediate cell adhesion. Cellular proteases such as matrix metalloproteases degrade extracellular matrix protein in the basement membrane, facilitating neutrophil and mononuclear cell migration and emigration into tissues. The activation and participation of the KKS has been documented in inflammatory bowel disease4 and arthritis5 in rodents, which are models for human diseases such as rheumatoid arthritis and Crohns disease. Cytokines and chemokines released primarily but not exclusively from monocytes and tissue macrophages are known to play a central role in human inflammatory diseases.
A missing link in this pathophysiologic sequence is a direct link between the KKS and chemokine formation and secretion. Up until now, only factor XII was known to interact with monocytes to release IL-1.6 We have previously shown that HK binds specifically and saturably to human neutrophils with a Kd of 9 nM.7 The receptor involved is Mac-1 (CD11a/CD18).8 HKa can displace fibrinogen from Mac-1 because of its higher affinity and thus exerts an anti-adhesive effect.9 HKa has been shown to be at least 10-fold more effective on detaching neutrophils from negatively charged surfaces than HK. We demonstrated that HKa can inhibit the formation of a signaling complex involving uPAR, which binds to an integrin (αvβ3 or α5β), which in turn complexes with caveolin to activate Syk kinase.10 This enzyme then phosphorylates focal adhesion kinase which phosphorylates its substrate, paxcillin, a reaction that occurs at focal adhesion plaques. Thus, HKa is capable of inhibiting intracellular changes that modulate signaling of endothelial cells.
The monocyte is known to display uPAR, Mac-1, and when stimulated globular head of C1q receptor (gC1qR) on its surface. Therefore, we postulated that HKa might stimulate monocyte activation by binding to all 3 receptors. HKa contains 2 of its 6 domains involved with cell binding. Both D3 and D5 are needed for binding to Mac-111 but only D5, which is highly exposed in HKa, is needed for binding to uPAR. When monocytes are activated, they synthesize and/or secrete both cytokines and chemokines. The process of inflammation requires the participation of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. In addition, chemokines such as IL-8 and monocyte chemotactic peptide 1 (MCP-1) play a major role in the migration of monocytes.
Therefore, we tested the hypothesis that HKa would lead to secretion and synthesis of inflammatory cytokines by binding to monocytes. If this were true, it would provide the link between KKS activation and inflammation. We also assess the mechanisms by which this occurs. We determine which domains and peptides of HKa are required. We also define the monocyte receptors that are responsible for binding HKa as well as the signaling pathways involved in cytokine release.
Materials and Methods
Proteins and Antibodies
HK, HKa, and LK were purchased from Enzyme Research Laboratories (South Bend, Ind). HK was >95% a single band of 110 kDA on both nonreduced and reduced SDS electrophoresis. HK had been digested with plasma kallikrein (molar ratio of 100:1 of HK to plasma kallikrein) for 20 minutes at 37°C. The resulting HKa was composed of 2 bands. 62 kDa (amino acid 1 to 362) and 46 kDa (420–626) represent the heavy chain and light chain, respectively. Monoclonal antibody against uPAR (#3936) was purchased from American Diagnostica Inc (Stamford, Conn). Monoclonal antibody to Mac-1 (αM subunit), 2LPM19c, was purchased from DAKO (Carpentaria, Calif). Monoclonal antibody against LFA-1 (αL subunit), was purchased from Antigenix America Inc. (Huntington Station, NY). The monoclonal antibody 74.5.2 recognizes the HK binding site on gC1qR was prepared as previously described.12
Purification of Recombinant Proteins
The recombinant proteins fused to GST were produced as described earlier13 and were purified by the procedure of Smith and Johnson.14 The fractions containing the proteins GST, GST-D3, and GST-E7P the recombinant peptides HG and HGK were identified by A280 and by SDS-polyacrylamide gel, followed by Coomassie staining. The identity of GST-E7P was confirmed by protein sequencing after removal of GST by thrombin cleavage. On a reduced SDS-electrophoretic gel, each protein was a single band with the correct predicted molecular weight (supplemental Figure I, available online at http://atvb.ahajournals.org,). Endotoxin assayed using QCL-1000 Chromogenic Limulus Amebocyte lysate (LAL) kit from Bio Whittaker, Inc (Walkersville, Md) indicated that all recombinant proteins had less than 0.01 EU/mL.
Peripheral blood mononuclear cells were isolated from normal subjects on a Histopaque gradient (Sigma Chemical Co, St. Louis, Mo). Untouched monocytes were isolated from mononuclear cells by magnetic cell sorting (MACS)15 (supplemental Figure II). The cells were suspended in Hanks balanced salt solution, 0.1% bovine serum albumin (HBSSA).
Release of IL-1β From Human Peripheral Blood Mononuclear Cells or Monocytes
Lipopolysaccharide (LPS)-free HKa, GST-D3, GST-E7P, BK, GST-D5, GST-HG, and GST-HGK were incubated for 0, 5, 15, 30, 60, 90, and 180 minutes at 37°C with 2×106/mL mononuclear cells or 1×106/mL monocytes suspended in HBSSA. After this incubation, the cell suspension was centrifuged at 13 000g for 5 minutes and the supernatant was used for assay of IL-1β by enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems Inc, Minneapolis, Minn).
Preparation and Purification of MAbs C11C1 and 2B5
MAb C11C1 (IgGk) was produced in tissue culture supernatant. MAb 2B5 was isolated from ascites. Both were purified as previously described.16 The amount of endotoxin present in each was below the lowest detection levels (0.02 ng/mL).
Fluorescein Isothiocyanate Labeling of Recombinant Proteins
GST-E7P and GST (glutathione-S-transferase) were labeled with fluorescein isothiocyanate (FITC) labeled according to the procedure of Holmes et al17 with the following modifications. Briefly, 2 mg of each product at a concentration of 1.3 to 2 mg/mL were dialyzed into 50 mmol/L boric acid, 200 mmol/L NaCl pH 9.5 at 4°C. FITC was freshly prepared and solubilized with dry dimethyl sulfoxide (Sigma Aldrich, St. Louis, Mo) at 5 mg/mL, incubated for 2 hours at 25°C (0.1 mg FITC to 1 mg protein) and dialyzed into 50 mmol/L boric acid/borax, 200 mmol/L NaCl pH 7.5. FITC incorporation was measured for 1.0 second, EX 485 nm, EM 535 nm, in a Victor2 1420 multilabel counter (Wallac Oy, Turku, Finland) and resulted in 23 700 counts/μg protein or 0.5 residues FITC/mole protein.
GST-E7P Binding to Mononuclear Cells
The binding of GST-E7P to mononuclear cell was measured using a Millipore MultiScreen 0.65 μm plate filtration system (Millipore Corp., Bedford, Mass). After pretreatment of each well with 2 mg/mL albumin, 100 μL containing 2×106/mL mononuclear cell were added to the test wells. FITC GST-E7P or FITC-GST was added in increasing concentrations from 0 to 900 nM for each point in triplicate and incubated for 1 hour at 25°C. After incubation, the supernatant was removed by vacuum and the mononuclear cells retained in the filters were washed once with HBSSA buffer. The plate was again vacuumed to dryness and read on a Victor2 (as above) by removal of the backing funnel and inverting the plate. Bound FITC was detected using a top reading protocol using FITC windows for 1.0 second. The collected data were exported into SigmaPlot version. 9.0 (Systat Software, Inc, Point Richmond, Calif) for background subtraction, statistics, and graphing.
Signaling Pathway Inhibition Experiments
For pathway selective inhibition, 1, 10, and 100 μmol/L of MG-132 (Carbobenzoxyl-L-leucyl-L-leucinal;Z-LLL-CHO) was used as a selective inhibitor for NFkB, SP 600125 (anthra [1,9-cd]pyrazol-6(2H)-one) as a selective inhibitor for JNK, SB202190, FHPI (4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole) as a selective p38 inhibitor and U0126 (1,4-Diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene) as a selective inhibitor for ERK activation. All inhibitors were obtained from Calbiochem, (La Jolla, Calif). Mononuclear cells were preincubated with the respective inhibitors for 60 minutes before stimulation with GST-E7P 600 nM) for another 60 minutes at 37° C. After this incubation, the cell suspension was centrifuged at 13 000g for 5 minutes and the supernatant was used for assay of IL-1β (IL-1β) by ELISA.
IL-1β mRNA Formation by HKa
Detection of IL-1β mRNA from mononuclear cells was performed by reverse-transcription polymerase chain reaction (RT-PCR) using sequence-specific primers. Briefly, total RNA was prepared using Trizol® reagent (Invitrogen). Specific primers for human IL-1β and gC1qR were designed to anneal to sequence in exons on both sides of one intron of the mRNA to exclude amplification of potential contaminating genomic DNA. The following primer pairs were used: for IL-1β, forward, 5′-ACAGACCTTCCAGGAGAATG-3′, and reverse, 5′-GCAGTTCAGTGATCGTACAG-3′); for gC1qR, forward, 5′-CGGCCGGGCCTCCTGCGGCC PCR-3′, and reverse, 5′-TCAGGCTCCTGTTCTTCAAC-3′. RNA (a total of 100 ng) was used as template in a one-step RT-PCR reaction (SuperScript One-Step RT-PCR with Platinum® Taq, Invitrogen). RT for cDNA synthesis was accomplished in 30 minutes incubation at 50°C, which was followed by PCR cycling as follows: initial denaturation at 94°C for 3 minutes followed by 25 cycles of 94°C for 30 seconds, 30 seconds of annealing at 55°C, and 1 minute of extension at 72°C using 0.2 μmol/L primers. The RT-PCR reactions yielded a product of 127 bp for IL-1β, and a product of 320 bp for gC1qR, respectively, which were identified in 4% agarose gel electrophoresis.
Data Analysis
All experiments were performed in 3 to 5 different donors. Each condition in each donor was repeated 3 times (triplicates). All results were expressed as mean±SEM. All results were analyzed by unpaired Student t test. Because of the variability of monocytes response in different donors, all studies have their own positive standard (endotoxin) and negative controls: cell alone, GST and D6. When appropriate, paired Student t tests were used. The term significant in the results indicate P<0.001.
Results
HKa Releases Cytokines and Chemokines From Mononuclear Cells as a Function of Time
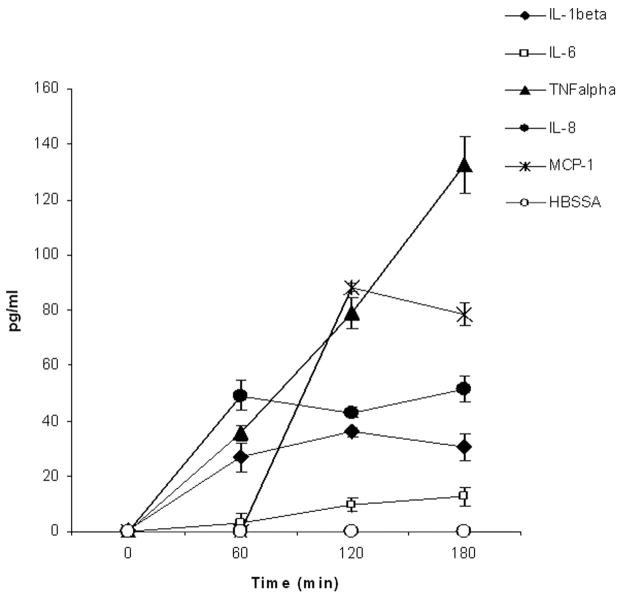
Mononuclear cells stimulated by HKa (600 nM) for 0 to 180 minutes (n=3) releasing increasing amount of cytokines IL-1β, IL-6, TNF-α, and chemokines IL-8 and MCP-1 (Figure 1).
Figure 1.
Time course of release of cytokines and chemokines by HKa from human blood mononuclear cells. Mononuclear cells (2.0×106/mL) were incubated with HKa (600 nM) or HBSSA for 0, 60, 120, and 180 minutes at 37°C. After incubation, the suspension was centrifuged at 13 000g for 5 minutes. The supernatant was used for assay of the IL-1β, IL-6, TNFα, IL-8, and MCP-1.
HKa Releases IL-1β From Mononuclear Cells as a Function of Concentration
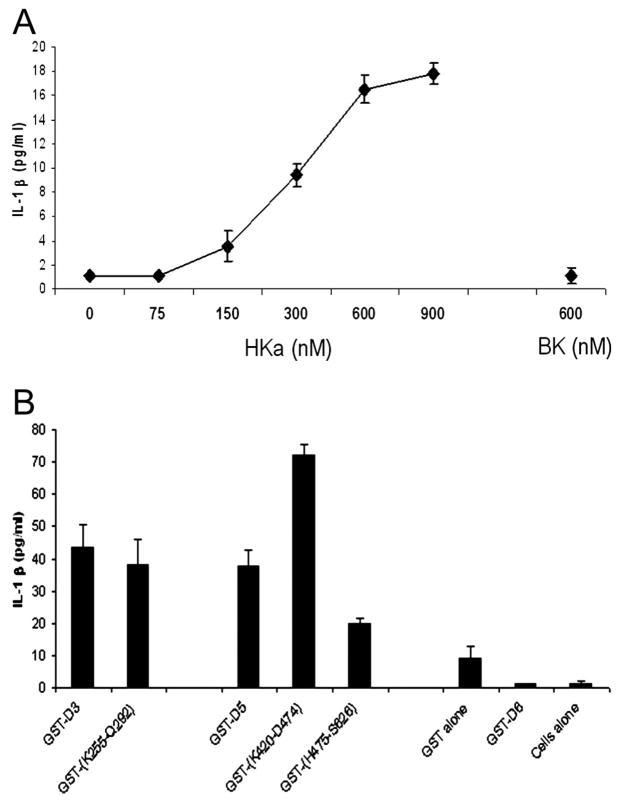
IL-1β was released from mononuclear cells by HKa in a concentration-dependent manner (9.4 at 300 nM, 16.5 at 600 nM, and 17.8 pg/mL at 900 nM) (n=3) (Figure 2A) but at 600 nM, IL-1β was not significantly released by BK.
Figure 2.
A, Concentration dependence of release of IL-1β from mononuclear cells by HKa. Mononuclear cells (2.0×106/mL) were incubated with HKa (0, 300, 600, and 900 nM), BK (600 nM) for 60 minutes at 37°C and processed as in Figure 1. B, Release of IL-1β from mononuclear cells incubated with recombinant kininogen domains and subdomains. Mononuclear cells (2.0×106/mL) were incubated with GST-D3, GST-E7P (K255-Q292), GST-D5, GST-(K420-D474), GST-(H475-S626), GST-D6 or GST, or HBSSA for 60 minutes at 37°C and processed as in Figure 1.
Subdomains of HKa D3 and D5 Release IL-1β From Mononuclear Cells
Recombinant kininogen were tested for release of IL-1β from mononuclear cells (n=3) (Figure 2B). GST-D3 and its subdomain, GST-E7P, released 43.5 and 38.3 pg/mL, respectively. GST-D5 and its subdomains, GST-HG and GST-HGK, released 37.6, 72.1, and 19.8 pg/mL, respectively. GST, GST-D6, or cells alone did not release significant concentrations of IL-1β.
Antibodies to Domain 3 and 5 of HK Inhibited IL-1β Release From Mononuclear Cells
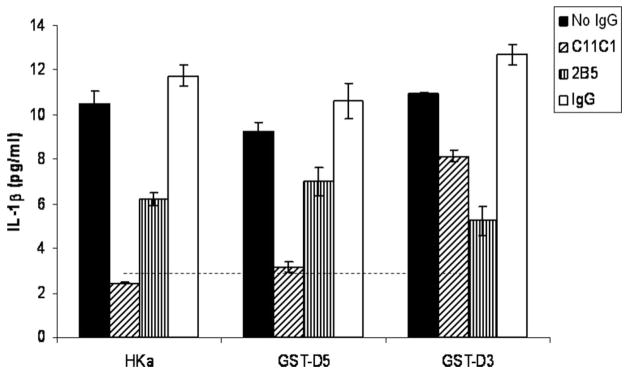
Antibodies to kininogen C11C1 (anti-D5) and 2B5 (anti-D3) significantly inhibited the release of IL-1β from mononuclear cells when stimulated by HKa, GST-D5, and GST-D3 (600 nM) (n=3) (Figure 3).
Figure 3.
Effect of monoclonal antibodies to HKa light chain and heavy chain on release of IL-1β by HKa, GST-D5 and GST-D3. Mononuclear cells (2.0×106/mL) were incubated with 1.2 μmol/L of C11C1, or 2B5 for 30 minutes at 37°C. HKa, GST-D5, GST-D3, or IgG were then added and incubated for 60 minutes at 37°C and processed as in Figure 1. Dotted line indicates IL-1β concentration at 0 minutes with monocytes alone.
GST-E7P Binds to Mononuclear Cells
For the remaining studies involving domain 3 we chose GST-E7P. Because all forms of kininogen except HKa and E7P were used as GST fusion proteins we tested whether GST-E7C would bind to monocytes. Fluorosceinated GST-E7P from 100 to 900 nM show an increasing amounts of binding approaching but not reaching saturation at 900 nM (supplemental Figure III).
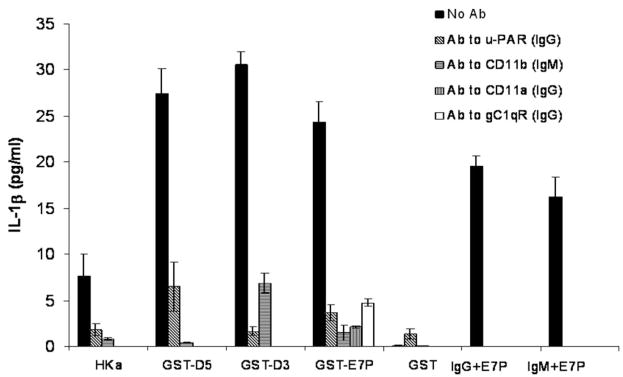
Antibodies to Kininogen Receptors uPAR, Mac-1, LFA-1(CD11a), and gC1qR Inhibited IL-1β Release From Mononuclear Cells
Antibodies to human uPAR (CD 87) and Mac-1 (CD11b/18) significantly inhibited the release of IL-1β from mononuclear cells when stimulated by HKa, GST-D5 GST-D3, GST-E7C, but not GST alone (600 nM) (n=3) (Figure 4). Anti LFA-1 (CD11a/18) significantly inhibited release of IL-1β from GST-E7C stimulated mononuclear cells and anti-gC1qR significantly inhibited release of IL-1β (Figure 4).
Figure 4.
Effect of monoclonal antibodies to uPAR, Mac-1, LFA-1 ((CD11a/CD18) and gC1qR on release of IL-1β by HKa, GST-D5, GST-D3, and GST-E7P. Mononuclear cells (2.0×106/mL) were incubated with antibodies to uPAR (31 nM), Mac-1 (62 nM), LFA-1 (31 nM), and gC1qR (10, 30,100 nM) for 10 minutes at 37°C. HKa, GST-D5, GST-D3, or GST-E7P (600 nM) were then added and incubated for 60 minutes at 37°C and processed as in Figure 1.
HKa Releases IL-1β From Purified Monocytes
The release of IL-1β from mononuclear cells, monocytes isolated by MACS, and nonmonocyte mononuclear cells were then tested (supplemental Figure II). Mononuclear cells (1×106/mL) incubated with GST-E7P released 11.3 pg/mL IL-1β; monocytes alone (1×106/mL) generated 10.1 pg/mL IL-1β; and nonmonocyte cells (1×106/mL) secreted 0.2 pg/mL IL-1β (n=3). GST and cells alone released 2 to 4 pg/mL from mononuclear cells and monocytes and none from nonmonocytic cells.
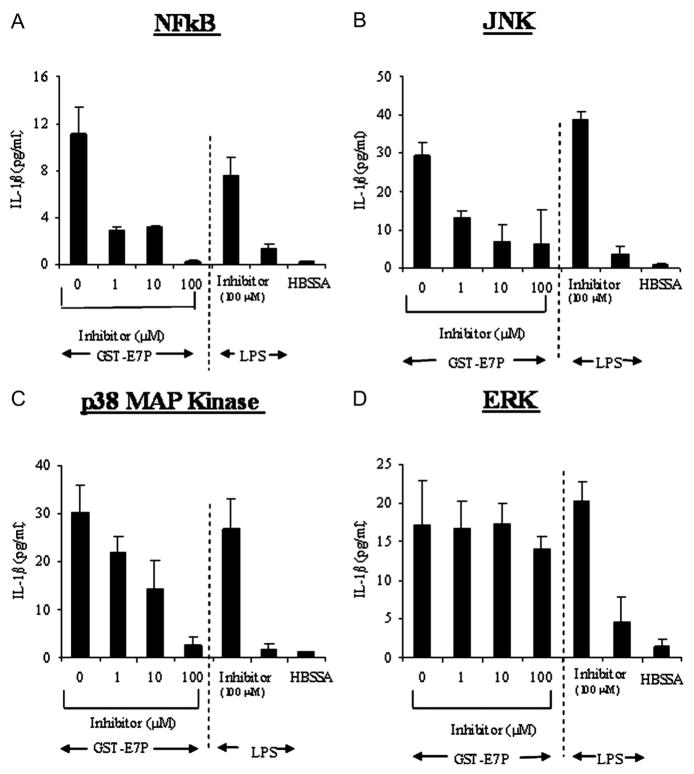
NFkB, p38, JNK, but not ERK, Are Required for Release of IL-1β by GST-E7P
The inhibition of NFkB by MG-132 with 1, 10, and 100 μmol/L significantly reduced IL-1β release from mononuclear cells by 69.7%, 67.3%, 88.5%, respectively, when stimulated by GST-E7P (Figure 5A). The release of IL-1β with LPS (10 μg/mL) was blocked by 83.4%.
Figure 5.
Effect of signaling pathway (NFkB, JNK, P38 and ERK) inhibitors on release of IL-1β by GST-E7P. The inhibition of of NFkB, p38, JNK, and activation significantly reduced IL-1β release from mononuclear cells stimulated by GST-E7P (A, B, and C). In contrast, the inhibition of ERK did not significantly reduce IL-1β release from mononuclear cells (D).
The inhibition of JNK by SP 600125 with 1, 10, and 100 μmol/L significantly reduced IL-1β release from mononuclear cells by 55.7%, 76.3%, 78.9%, respectively, when stimulated by GST-E7P (Figure 5B). The release of IL-1β with LPS (10 μg/mL) was blocked by 90.23%.
The inhibition of p38 by SB202190 with 1,10, and 100 μmol/L significantly reduced IL-1β release from mononuclear cells by 27.8%, 14.2%, 91.0%, respectively, when stimulated by E7P (Figure 5C). The release of IL-1β with LPS (10 μg/mL) was blocked by 76.8%.
In contrast the ERK activation inhibitor U0126 (1, 10, and 100 μmol/L) failed to inhibit GST-E7P-induced release of IL-1β from mononuclear cells (Figure 5D). In contrast, the release of IL-1β with LPS (10 μg/mL) was blocked by 77.4% with U0126.
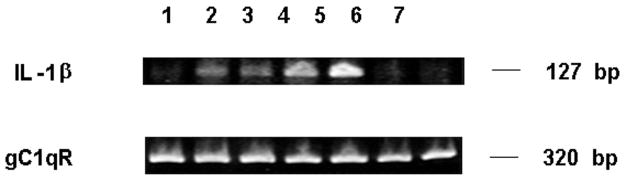
Induction of mRNA Expression for IL-1β by HKa in Mononuclear Cells
After mononuclear cells (4×106/mL) were treated with HKa (600 nM) or LPS (10 ng/mL), total RNA was extracted and RT-PCR reactions for expression of IL-1β and gC1qR mRNA were performed as described in Materials and Methods. PCR product was separated in 4% ethidium bromide-stained agarose gels and photographed. HKa induces expression of mRNA for IL-1β in mononuclear cells within an hour (Figure 6) similar to LPS (positive control).
Figure 6.
HKa induces mRNA expression for IL-1β in mononuclear cells. All experiments contain mononuclear cells (4×106/mL) additions were HKa (600 nM) or LPS (10 ng/mL). Lanes (1–7) are indicated as follows: 1, HBSSA 0 hour; 2, HKa 1 hour; 3, HKa 2 hours; 4, LPS 1 hour; 5, LPS 2 hours; 6, HBSSA 1 hour; 7, HBSSA 2 hours. In contrast to IL-1β mRNA, the mRNA of gC1qR is constitutive (present without stimulation at all time points) with both HKa and LPS.
Discussion
In this study, we have shown that HKa is capable of releasing both inflammatory cytokines TNFα, IL-1β, and IL-6, and chemokines IL-8 and MCP-1 from human blood mononuclear cells over a 3-hour period of observation (Figure 1). As expected, TNF-α rises linearly to high levels (130 pg/mL) by 180 minutes. In vivo, the cytokines are rapidly cleared but this does not occur in vitro. Because TNF-α probably contributes to the formation and secretion of both IL-1β and IL-8, the time course for these is similar, reaching a maximum value between 60 and 120 minutes of 30 to 40 pg/mL. In contrast, MCP-1 shows a lag with no release by 60 minutes but a maximum at 230 minutes, suggesting that HKa is stimulating the synthesis of MCP-1. IL-6, in accordance with its proven behavior, shows very low levels, which seem to be slowly increasing in accordance with its slower formation over a number of hours. Thus, HKa can stimulate a wide variety of inflammatory cytokines and chemokines. Whether HKa can also stimulate or suppress anti-inflammatory cytokines such as IL-10 and IL-12 will be a subject of future study.
We decided to focus on IL-1β as a representative and responsive cytokine (Figure 2A). We show a concentration-dependent response to HKa, reaching a maximum at a concentration of HK (600 to 900 nM) that is present in normal plasma. In contrast, BK cannot release IL-1β. Although it is well known that BK can stimulate the formation of prosta-glandins and nitric oxide (NO), little evidence exists that cytokines can be released. This observation is the first evidence that HKa can activate leukocyte secretion and/or release because most previous studies have concentrated on the inhibition of extracellular events such as adhesion,18 migration, and angiogenesis.3
As a first step to localizing the structural requirements of HKa to stimulate mononuclear cells, we found that both the recombinant fusion proteins, GST-D3 and GST-D5, stimulate mononuclear cells to secrete cytokines and chemokines (Figure 2B). This finding was not totally unexpected because both bound to neutrophils which have similar receptors on their surfaces.
We buttressed these findings by showing that the corresponding domain-specific monoclonal antibodies could block the effects of HKa (Figure 3). MAb C11C1 has been shown be directed to an epitope including the amino acid sequence, aa441 to 502, which makes up most of D519 and inhibits the coagulant activity of HK which requires both D5 and D6. Previous studies have shown that C11C1 inhibits the binding of HK/HKa to neutrophils.11 MAb 2B5 recognizes both D2 and D3 of HK on immunoblots and inhibits the ability of D2 and D3 to inhibit cysteine proteases.16 In this study, mAb C11C1 completely inhibited the ability of HKa and GST-D5 but was weaker on GST-D3. MAb-2B5 showed weaker inhibition on HKa and GST-D3 but none on GST-D5. These results were consistent with the specificity of these antibodies and indicate that HKa exerts most of its effect via D5 but with some contribution by D3. These findings are consistent with similar results of the mAbs on neutrophil secretion of elastase.11 Interestingly, either GST-D3 or GST-D5 was able to stimulate IL-1β from mononuclear cells to an equal degree (Figure 3), indicating that both domains play a role in HKa agonist activity.
We further localized the sequences responsible on D3 and D5 to stimulate secretion of IL-1β (Figure 4). Our previous studies have identified a polypeptide from D3 coded for by the 3′ region of exon 7, which, when expressed as a fusion protein GST-G255-Q292 (E7P), inhibited the binding of HK to neutrophils.11 Similarly, 2 contiguous peptides, from D5 G422-K458 and The459-Lys478, each inhibited the binding of HK to neutrophils. We tested a similar overlapping recombinant polypeptide from D5, GST-K420-D474 (GST-HG), which also inhibited the binding of HK to neutrophils. GST-HG was a strong stimulant of IL-1β release for human mononuclear cells. In another study,20 we demonstrated that peptides containing the sequence H475-G497 had inhibitory activity on the adhesion of human monocyte cells to immobilized Mac-1. We therefore tested a peptide, GST-H475-S626, which contained this sequence. No significant stimulatory effect was found. However, the peptide contained the entire D6 domain and it is possible that the C-terminal portion of D5 must be free or that D6 inhibits the stimulation.
We then proceeded to identify which receptors on mononuclear cells were responsible for the stimulation of IL-1β release (Figure 4). Three receptors have been implicated for HK on leukocytes. We have shown that HKa binds directly to Mac-1.21 However, we have also shown that Mac-1–mediated adhesion of HEK 293 cells to fibrinogen and was upregulated by transfection with uPAR, initiating a signaling pathway mediated by focal adhesion kinase and MAP kinase.22 Therefore, we tested whether antibodies to Mac-1, and/or uPAR were capable of inhibiting the secretion of IL-1β by mononuclear cells stimulated with HKa, GST-D5, GST-D3, and GST-E7P. Both antibodies inhibited 76% to 95%, indicating that either antibody could prevent the action of HKa. These results are consistent with the observation that Mac-1 and uPAR bind to each other in purified systems and on the cell surface23 as well as our observation that uPAR upregulates Mac-1 on the cell surface.22 It is likely that uPAR, gC1qR, and Mac-1 exist as a trimolecular complex on monocytes similarly to the co-localization of uPAR, gC1qR, and cytokeratin 1 on endothelial cells.24 In the later case, antibodies to each of the endothelial receptors were found significantly inhibit binding when used separately.
Previous studies demonstrated that HKa could disrupt a uPAR-integrin signaling pathway.10 These results are the first to indicate that HKa after binding initiates several interrelated signaling cascades. Inhibitors of each pathway initiated by NFκB, JNK, and p38 MAP (all of which are related to cytokine synthesis and/or secretion) prevent the stimulation of the monocytes by HKa.
The discovery that HKa is an agonist which can release a variety of inflammatory cytokines and chemokines from monocytes fills an important gap in our knowledge. The finding that rats deficient in kininogen have impaired angiogenic responses25 is easily explained by the lack of BK, a potent angiogenic stimulus. But the decrease in the response of HK-deficient Lewis rats to the ability of proteoglycan-polysaccharides to induce inflammatory arthritis26 and inflammatory bowel disease27 is difficult to rationalize based on the known actions of BK. These diseases depend on the cytokines and chemokines stimulated by bacterial products through the innate immune system in addition to adaptive immunity mediated by B- and T-lymphocytes. The participation of the kallikrein-kinin system in inflammation through effects on monocytes is now much more cogent. These findings should reveal novel approaches to down-regulating inflammatory diseases and new targets including kininogen itself as well as its receptors, Mac-1, uPAR, and gC1qR.
Acknowledgments
We thank Princess Graham for her help in editing as well as manuscript and graphics preparation.
Source of Funding
Dr Khan was supported in part by NIH training grant T32 HL07777. This article was supported in part by NIH grants R01 CA083121 and R01 AR051713 (R.W.C.).
Footnotes
Disclosures
None
References
- 1.Jacobsen S, Kriz M. Some data on two purified kininogens from human plasma. Br J Pharmacol. 1967;29:25–36. doi: 10.1111/j.1476-5381.1967.tb01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisel JW, Nagaswami C, Woodhead JL, DeLa Cadena RA, Page JD, Colman RW. The shape of high molecular weight kininogen: organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy. J Biol Chem. 1994;269:10100–10106. [PubMed] [Google Scholar]
- 3.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–550. [PubMed] [Google Scholar]
- 4.Sartor RB, DeLa Cadena RA, Green KD, et al. Selective kallikrein-kinin system activation in inbred rats differentially susceptible to granulomatous enterocolitis. Gastroenterology. 1996;110:1467–1481. doi: 10.1053/gast.1996.v110.pm8613052. [DOI] [PubMed] [Google Scholar]
- 5.DeLa Cadena RA, Stadnicki A, Uknis AB, et al. Inhibition of plasma kallikrein prevents peptidoglycan-induced arthritis in the Lewis rat. FASEB J. 1995;9:446–452. doi: 10.1096/fasebj.9.5.7896018. [DOI] [PubMed] [Google Scholar]
- 6.Toossi Z, Sedor JR, Mettler MA, Everson B, Young T, Ratnoff OD. Induction of expression of monocyte interleukin 1 by Hageman factor (factor XII) Proc Natl Acad Sci U S A. 1992;89:11969–11972. doi: 10.1073/pnas.89.24.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafson EJ, Schmaier AH, Wachtfogel YT, Kaufman N, Kucich U, Colman RW. Human neutrophils contain and bind high molecular weight kininogen. J Clin Invest. 1989;84:28–35. doi: 10.1172/JCI114151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wachtfogel YT, DeLa Cadena RA, Kunapuli SP, et al. High molecular weight kininogen binds to Mac-1 on neutrophils by its heavy chain (domain 3) and its light chain (domain 5) J Biol Chem. 1994;269:19307–19312. [PubMed] [Google Scholar]
- 9.Gustafson EJ, Lukasiewicz H, Wachtfogel YT, et al. High molecular weight kininogen inhibits fibrinogen binding to cytoadhesins of neutrophils and platelets. J Cell Biol. 1989;109:377–387. doi: 10.1083/jcb.109.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao DJ, Guo YL, Colman RW. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ Res. 2004;94:1227–1234. doi: 10.1161/01.RES.0000126567.75232.46. [DOI] [PubMed] [Google Scholar]
- 11.Khan MM, Kunapuli SP, Lin YZ, Majluf-Cruz A, DeLa Cadena RA, Cooper SL, Colman RW. The binding sites on high molecular weight kininogen for attachment to activated neutrophils are three noncontiguous peptides. Am J Physiol. 1998;275:H145–H150. doi: 10.1152/ajpheart.1998.275.1.H145. Heart Circ Physiol 44. [DOI] [PubMed] [Google Scholar]
- 12.Ghebrehiwet B, Lu PD, Zhang W, Lim B-L, Eggleton P, Leigh LEA, Reid KBM, Peerschke EIB. Identification of functional domains on gC1q-R, a cell surface protein which binds to the globular “heads” of C1q, using monoclonal antibodies and synthetic peptides. Hybridoma. 1996;15:333–342. doi: 10.1089/hyb.1996.15.333. [DOI] [PubMed] [Google Scholar]
- 13.Kunapuli SP, Bradford HN, Jameson BA, et al. Thrombin-induced platelet aggregation is inhibited by the heptapeptide Leu271-Ala277 domain 3 in the heavy chain of high molecular weight kininogen. J Biol Chem. 1996;271:11228–11234. doi: 10.1074/jbc.271.19.11228. [DOI] [PubMed] [Google Scholar]
- 14.Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 15.Ryan EJ, Marshall AJ, Magaletti D, et al. Dendritic cell-associated lectin-1: a novel dendritic cell-associated, C-type lectin-like molecule enhances T cell secretion of IL-4. J Immunol. 2002;169:5638–5648. doi: 10.4049/jimmunol.169.10.5638. [DOI] [PubMed] [Google Scholar]
- 16.Schmaier AH, Schutsky D, Farber A, Silver LD, Bradford HN, Colman RW. Determination of the bifunctional properties of high molecular weight kininogen by studies with monoclonal antibodies directed to each of its chains. J Biol Chem. 1987;262:1405–1411. [PubMed] [Google Scholar]
- 17.Holmes K, Lants LM, Fowlkes BJ, Schmid I, Giorgi JV. Current Protocols in Immunology. Sup 44. John Whiley & Sons, Inc; 2001. Section 5.3.5. [DOI] [PubMed] [Google Scholar]
- 18.Guo YL, Wang S, Colman RW. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1427–1433. doi: 10.1161/hq0901.095277. [DOI] [PubMed] [Google Scholar]
- 19.DeLa Cadena RA, Colman RW. The sequence HGLGHGHEQQHGLGHGH in the light chain of high molecular weight kininogen serves as a primary structural feature for zinc-dependent binding to an anionic surface. Prot Sci. 1992;1:151–160. doi: 10.1002/pro.5560010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavakis T, Kanse SM, Pixley RA, et al. Regulation of leukocyte recruitment by polypeptides derived from high molecular weight kininogen. FASEB J. 2001;15:2365–2376. doi: 10.1096/fj.01-0201com. [DOI] [PubMed] [Google Scholar]
- 21.Sheng N, Fairbanks MB, Heinrikson RL, et al. Cleaved high molecular weight kininogen binds directly to the integrin CD11b/CD18 (Mac-1) and blocks adhesion to fibrinogen and ICAM-1. Blood. 2000;95:3788–3795. [PubMed] [Google Scholar]
- 22.Zhang H, Colman RW, Sheng N. Regulation of CD11b/CD18 (Mac-1) adhesion to fibrinogen by urokinase receptor (uPAR) Inflamm Res. 2003;52:86–93. doi: 10.1007/s000110300006. [DOI] [PubMed] [Google Scholar]
- 23.Simon DI, Rao NK, Xu H, et al. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- 24.Mahdi F, Shariat-Madar Z, Todd RF, 3rd, Figueroa CD, Schmaier AH. Expression and colocalization of cytokeratin 1 urokinase plasminogen activator receptor on endothelial cells. Blood. 2001;97(8):2342–2350. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi I, Amano H, Yoshida S, et al. Suppressed angiogenesis in kininogen-deficiencies. Lab Invest. 2002;82:871–880. doi: 10.1097/01.lab.0000018885.36823.d6. [DOI] [PubMed] [Google Scholar]
- 26.Sainz IM, Isordia-Salas I, Castaneda JL, Agelan A, DeLa Cadena RA, Pixley RA, Adam A, Sartor RB, Colman RW. Inflammation is modulated by kininogen deficiency in a rat model of inflammatory arthritis. Arthritis Rheum. 2005;52:2549–2552. doi: 10.1002/art.21202. [DOI] [PubMed] [Google Scholar]
- 27.Isordia-Salas I, Pixley RA, Li F, Sainz IM, Sartor RB, Adam A, Colman RW. Kininogen (HK) deficiency modulates chronic intestinal inflammation in genetically susceptible rats. Am Journal of Physiology: Gastrointestinal and Liver Physiology. 2002;283:G180–G186. doi: 10.1152/ajpgi.00514.2001. [DOI] [PubMed] [Google Scholar]