Abstract
The complement system comprises a series of proteases and inhibitors that are activated in cascade-like fashion during host defense (Makrides 1998). A growing body of evidence supports the hypothesis that immune mechanisms, including complement activation, are involved in inflammatory conditions associated with vascular injury (Acostan, Qin, and Halperin 2004; Giannakopoulos, Passam, Rahgozar, and Krillis 2007), and disseminated intravascular coagulation associated with massive trauma (Huber-Lang, this volume). We propose that platelets and platelet derived microparticles focus complement to sites of vascular injury where regulated complement activation participates in clearing terminally activated platelets and microparticles from the circulation, and dysregulated complement activation contributes to inflammation and thrombosis. Given the central role of platelets in hemostasis and thrombosis, it is not surprising that activated complement components have been demonstrated in many types of atherosclerotic and thrombotic vascular lesions (Torzewsjki, Bowher, Wlatenberger, and Fitzsimmons 2007; Niculescu, Niculescu, and Rus 2004).
Complement Activation on Platelets
Evidence for direct activation of both classical and alternative pathways of complement on platelets is emerging (Del Conde, Cruz, Zhang, Lopez, and Afshar-Kharghan 2005; Peerschke, Yin, Grigg, and Ghebrehiwet 2006). For example, P-selectin, a platelet alpha granule membrane protein that contains a short consensus repeat domain common to many complement binding proteins (Kansas 1996), has been identified as an activator of the alternative complement cascade on platelets (Del Conde et al. 2005). In addition, platelet alpha granules contain Factor D, the serine protease that cleaves Factor B of the alternative pathway to its active form (Davis 3rd, and Kenney 1979).
Platelets also express binding sites for classical complement components, most notably for C1q (Peerschke and Ghebrehiwet 1997; Peerschke, Reid, and Ghebrehiwet 1994. C1q interactions with platelets trigger a variety of cellular and biochemical responses that may contribute to inflammation and thrombosis. For example, the C-terminal collagen like domain of C1q binds to the 60kDa platelet calreticulin (CR) homologue, cC1qR (Peerschke et al. 1997). This interaction is associated with induction of GPIIb-IIIa, platelet aggregation, P-selectin expression, and generation of platelet procoagulant activity (Peerschke, Reid, and Ghebrehiwet 1993).
In contrast, the amino terminal, globular domain of C1q binds the 33kDa gC1qR/p33 (gC1qR) (Ghebrehiwet, Lim, Peerschke, Willis, and Reid 1994). gC1qR is a ubiquitously expressed cellular protein (Ghebrehiwet and Peerschke 1998; Ghebrehiwet, Lim, Kumar, Feng, and Peerschke 2001). Although its 73 amino acid presequence contains a mitochondrial targeting motif (Dedio, Jahnen-Dechent, Bachmann, and Muller-Esterl 1998), the expression of gC1qR in other cellular compartments, including the cell surface of platelets and endothelial cells, has been confirmed (Ghebrehiwet et al. 1994; Mahdi, Mader, Figueroa, and Schmaier 2002; Peerschke, Murphy, and Ghebrehiwet 2003).
Mature gC1qR exhibits a noncovalent trimeric structure (Ghebrehiwet et al. 1998; Jiang, Zhang, Krainer, and Xu 1999). Multimerization is an essential process that increases the affinity of gC1qR for multivalent ligands such as C1q (Ghebrehiwet et al. 1998). The geometry and topography of the three dimensional structure of gC1qR indicates that gC1qR could engage C1q via at least two, if not three, of its globular heads. This could induce the subtle conformational change that is necessary to trigger C1 activation (Ghebrehiwet, Cebada Mora, Tantral, Jesty, and Peerschke 2006). Indeed, recombinant gC1qR has been shown recently to activate C1 (Peerschke et al. 2006).
The expression of C1q binding sites on human blood platelets (Peerschke et al. 1994; Peerschke et al. 1997), combined with the ability of gC1qR to engage the globular domain of C1q (Ghebrehiwet et al. 1994), suggests that platelets may possess an intrinsic capacity to initiate the classical complement pathway. Indeed, platelet mediated activation of C4 has been demonstrated recently using both solid phase and flow cytometric approaches (Peerschke et al. 2006).
Deposition of C3b and C5b-9 on activated platelets was detected also. Platelet mediated complement activation did not require thrombin generation (Peerschke et al. 2006). Thrombin has the potential to directly activate C5 (Huber-Lang, Sarma, Zetoune, Rittirsch, Neff, McGuire, Lambris, Warner, Flierl, Hoesel, Gebhard, Younger, Drouin, Wetsel, and Ward 2006) and other complement components (Huber-Lang, this volume), including C3. C3 activation on platelets was reduced in the absence of C1 or Factor B, suggesting a role, instead, for classical and alternative pathway C3 convertases (Peerschke et al. 2006).
Data from our laboratory (Peerschke et al. 2006) show that platelet mediated classical pathway C4 activation occurs in the presence of purified C1 and C4, and in diluted plasma or serum. Dilution is necessary, presumably to alter the balance between complement components and their circulating inhibitors. This is of interest, as it may have implications for clinical situations, such as massive trauma or cardiopulmonary bypass, in which significant hemodilution occurs.
C4 activation on platelets further requires divalent cations and platelet stimulation. C4 activation appears particularly enhanced following platelet exposure to shear stress (1800 s -1, 60 min), and correlates with gC1qR expression (Peerschke et al. 2006). Antibodies directed against gC1qR and exposure of platelets to plasmin, a protease previously shown to degrade gC1qR (Peerschke, Petrovan, Ghebrehiwet, and Ruf 2004), inhibit C4 activation. Thus, plasmin generation at sites of inflammation or vascular injury may regulate platelet mediated classical complement activation. Further regulation of classical complement pathway activation may be achieved by secretion of platelet alpha granule C1 inhibitor (C1 INH) (Schmaier, Amenta, Xiong, Heda, and Gewirtz 1993).
Requirements for platelet mediated complement activation are summarized in Table 1.
Table 1.
Platelet Mediated Complement Activation
| Positive Regulators | Negative Regulators |
|---|---|
| Platelet activation | EDTA |
| Platelet granule secretion | C1q deficiency |
| gC1qR/p33 | C4 deficiency |
| Platelet associated IgG or immune complexes | Plasmin |
| P-Selectin | Factor B depletion |
Complement Activation on Platelet Microparticles (PMP)
PMP are a storage pool for disseminating blood borne bioactive effectors (Morel, Toti, Hugel, and Freyssinet 2004) such as tissue factor, procoagulant phospholipids, and inflammatory mediators. Compared to activated platelets, PMP can express 50–100 fold higher procoagulant activity (Sinauridze, Kireev, Popenko, Pichugin, Panteleev, Krymskaya, and Ataullakhanov 2007). They retain selected platelet membrane constituents, including glycoproteins Ib, IIb-IIIa, and P-selectin (Gawaz, Ott, Reininger, Heinzmann, Neumann 1996; George, Pickett, Saucerman, McEver, Kunicki, Kieffer, and Newman 1986) which support vascular inflammation by participating in heterotypic communication with leukocytes and vascular endothelial cells (Martinez, Tesse, Zobairi, and Andriantsitohaina 2005; Barry, Pratico, Savani, and FitzGerald 1998).
Increasing evidence is emerging to support cross-talk between coagulation and complement systems (Markiewski and Lambris 2007; Markiewski, Nilsson, Ekdahl, Mollnes, and Lambris 2007). In this regard, PMP present a surface for the assembly and interaction of complement and coagulation cascades. PMP were recently shown to express gC1qR and to activate C4 in the absence of immune complexes (Yin, Ghebrehiwet, and Peerschke 2008). PMP further supported C3 activation and deposition of C5b-9. Amplification of complement activation on PMP may be achieved via exposure of P-selectin and engagement of the alternative pathway (Del Conde et al. 2005). Although the extent of complement activation on PMP was low compared to platelets, normalization of complement activation for PMP size suggests that PMP may be several orders of magnitude more active than platelets, and likely present concentrated activated complement components to vascular targets.
In addition, negatively charged phospholipids such as cardiolipin and phosphatidyl serine (PS) are expressed on PMP and have been shown to activate C1 (Kovacsovics, Tschopp, Kress, and Isliker 1985). Since PS is present on early apoptotic cells (Vermes, Haanen, Steffen-Nakken, and Reutelingsperger 1995), we propose that complement activation on microparticles may be involved in physiologic clearance mechanisms. Indeed, complement components C1q, Factor B, and C3 have all been shown to participate in the phagocytosis of apoptotic cells (Mevorach, Mascarenhas, Gershoev, and Eldon 1998).
Pathophysiology of Platelet Mediated Complement Activation
Potential pathophysiologic consequences of platelet mediated complement activation are summarized in Table 2. Activation of the complement system is associated with generation of potent proinflammatory peptides, C3a and C5a (Makrides 1998). C3a has been reported to enhance platelet stimulation by subthreshold concentrations of traditional agonists such as ADP or thrombin (Polley and Nachman 1983). In addition, both C3a and C5a bind receptors on endothelial cells and stimulate upregulation of interleukins 8 and 1β, in addition to RANTES, and strongly activate the MAP kinase signaling pathway (Monsinjon, Gasque, Chan, Ischenko, Brady, and Fontaine 2003). Thus, the association of PMP with endothelial cells (Mause, von Hundelshausen, Zernecke, Koenen, and Weber 2005) may carry activated complement components along the vasculature and accelerate endothelial responses to vascular injury. In addition, C5a is known to induce tissue factor expression by endothelial cells and leukocytes in vitro (Ikeda, Nagasawa, Horiuchi, Nishizaka, and Niho 1997; Muhlfelder, Miemetz, Kreutzer, Beebe, Ward, and Rosenfeld 1979).
Table 2.
Pathophysiologic consequences of platelet and PMP mediated complement activation
| Complement Component | Pathophysiologic Effect |
|---|---|
| C3a, C5a, C4a | Inflammatory cell recruitment |
| C3a | Enhanced platelet response to agonists (e.g. ADP, thrombin) |
| C5a | Endothelial cell activation, including tissue factor expression |
| C3b | Recognition and phagocytosis of apoptotic platelets and microparticles |
| C5b-9 | Platelet activation and expression of procoagulant activity; Endothelial cell activation |
When complement activation goes to completion, the terminal complement complex (TCC) (C5b-9) is formed. This complex is responsible for lysis of host pathogens and may contribute to tissue damage. Moreover, sublytic quantities of TCC have been reported to activate platelets and endothelial cells, leading to expression of procoagulant activity (Wiedmer, Esmon, and Sims 1986). Thus, inhibition of potential injurious/inflammatory effects of complement activation by infusion of specific C5a antagonists (Allegretti, Moriconi, Beccari, Di Bitondo, Bizzarri, Bertini, and Colotta 2005) is under intense investigation.
In addition, the complement cascade is linked to other protease activated cascades of the blood including the coagulation (Polley and Nachman 1978), kinin (Kaplan, Silverberg and Ghebrehiwet 1986), and fibrinolytic systems (Schaiff and Eisenberg 1997). Generation of a novel thrombin dependent C5 convertase (Polly et al. 1978; Polley and Nachman 1979; Zimmerman and Kolb 1976) has been described during blood coagulation, and direct activation of C5 and other complement components by thrombin has been reported (Huber-Lang 2006 and this volume).
Deposition of complement components, C1q, C3, and C4, and generation of the terminal complement complex C5b-9 has been shown in human atherosclerotic lesions (Niculescu and Rus 1999). The extent of C5b- 9 deposition has been correlated with severity of the vascular lesion (Vlaicu, Niculescu, Rus and Cristea 1985). Moreover, iC3b deposition appears to be highest in vulnerable and ruptured plaques (Laine, Pentikainen, Wurzner, Penttila, Paavonen, Meri, and Kovanen 2002). Moreover, elevations in circulating C5a have been associated with increased cardiovascular risk in patients with advanced atherosclerosis (Seidl, Exner, Amighi, Kastl, Zorn, Maurer, Wagner, Huber, Minar, Wojta, and Schillinger 2005).
However, the role of complement in atherogenesis in animal models remains controversial. Deficiency of C5 fails to protect Apo E−/− mice from atherosclerosis (Patel, Thelander, Hernandez, Montenegro, Hassing, Burton, Mundt, Hermanowski-Vosatka, Wright, Chao, and Detmers 2001). Although these results suggest that C5 activation and by extension C5b-9 generation are not required for atherogenesis, they do not rule out important contributions by complement components upstream of C5, including C3a, C3b, iC3b and C1q.
A role for C1q in the pathogenesis of inflammatory tissue injury in a murine stroke model is supported by accumulation of C1q in brain lesions within 3–6 hours post ischemia (Mack, Sughrue, Ducruet, Mocco, Sosunov, Hassid, Silverberg, Ten, Pinsky, and Connolly 2006). Further studies demonstrate C1q mediated amplification of ischemic cerebral injury in immature mice. Adult C1q deficient (−/−) mice, however, are not protected from stroke. In contrast, mature C3 deficient (−/−) mice demonstrated a marked resistance to cerebral injury. These observations are consistent with neuroprotective effects observed in mice treated with a C3a receptor antagonist.
Complement activation and deposition on platelets may also contribute to ongoing thrombosis. C4 fragments have been detected on platelets from a subset of patients with systemic lupus erythematosus (SLE), and this correlated with a history of neurologic events and the presence of antiphospholipid antibodies (Navratil, Manzi, Kao, Krishnaswami, Liu, Ruffing, Shaw, Nilson, Dryden, Johnson, and Ahearn 2006). These observations are consistent with preliminary data from our laboratory which suggest a statistically significant correlation between classical complement pathway C1q and C4d deposition on platelets and arterial thrombosis in SLE patients with anti phospholipid antibody syndrome (APS) (Peerschke, Yin, Alpert, Salmon, Roubey and Ghebrehiwet 2007). Given the association of APS with circulating autoantibodies, the thrombogenic potential associated with APS may involve activation of the classical complement pathway on platelets and PMP.
Regulation of complement activity on platelets
Platelets are armed with a variety of mechanisms to prevent in situ complement activation. Platelets contain C1 INH in their alpha granules and express C1 INH on their surface following granule secretion (Schmaier et al. 1993). C1 inhibitor is an alpha globulin that blocks the esterolytic activity of the first component of the classical complement cascade. The mechanism whereby C1 INH is expressed on the activated platelet surface is not yet known, but recent work with endothelial cells demonstrates C1 INH binding to P-selectin (Shenghe and Davie 2003). This interaction does not interfere with CI INH activity, but prevents leukocyte rolling on endothelial cells (Shenghe et al. 2003; Buerke, Prufer, Dahm, Oelert, Meyer, and Darius 1998).
Platelet alpha granules also contain releasable Factor H (Devine and Rosse 1987). Factor H binds cell associated C3b and acts as a cofactor for the cleavage of C3b by Factor I. Factor H can bind directly to platelets via GPIIb-IIIa and indirectly via thrombospondin-1 (Vaziri-Sani, Hellwage, Zipfel, Sjoholm, Iancu, and Karpman 2004).
In addition to soluble inhibitors, platelets express a number of surface membrane anchored complement regulatory proteins. Under physiologic conditions, platelet damage or lysis by C5b-9 assembly is regulated by surface membrane CD55, CD59, and clusterin. Apparently clusterin is more highly expressed on platelets than on other mammalian cell types (Gnatenko, Dunn, McCorkle, Weissmann, Perrotta, and Bahou 2003). If stimulated platelets are indeed actively engaged in the initiation and propagation of both classical and alternative pathways, the arming of platelets with potent complement regulatory mechanisms would be necessary to prevent uncontrolled cytolysis and production of inflammatory mediators.
Summary and Conclusion
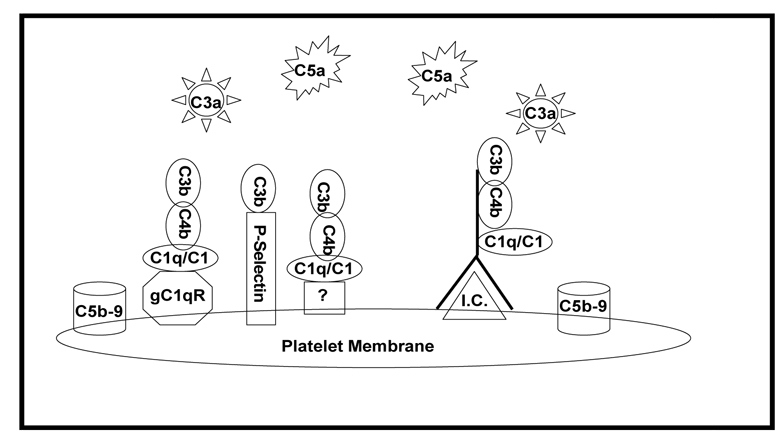
Potential mechanisms contributing to platelet mediated complement activation are summarized in Figure 1. Evidence for both immune complex independent and dependent classical complement pathway activation on platelets and PMP has been reported, as well as amplification of complement activation via engagement of the alternative pathway. These observations provide strong support for the involvement of platelets in the generation of complement derived inflammatory mediators and complement induced platelet and vascular endothelial cell activation. We hypothesize that platelets focus complement activation to sites of vascular injury, and that interactions between platelets and the complement system contribute to acute and chronic inflammation and thrombosis. Thus, understanding mechanisms of complement activation on platelets will have a significant impact on identifying novel therapeutic targets for treating patients with thrombotic complications associated with atherosclerosis, ischemia/reperfusion injury, and APS.
Figure 1. Proposed mechanisms for complement activation on and by platelets and PMP.
Complement activation may occur directly via expression of gC1qR, P-selectin, and as yet unidentified constituents expressed by or secreted from activated platelets. In autoimmune diseases, complement activation may occur via assembly of immune complexes (I.C.) on platelets. Complement activation leads to generation of inflammatory C3a and C5a peptides, as well as assembly of the C5b-9 membrane attack complex.
( = IgG)
= IgG)
Acknowledgements
This work was supported in part by grants HL67211 (EIBP) and AI060866 (BG) from the National Institutes of Health, and an American Heart Association Heritage Affiliate postdoctoral award # 0625900T (WY).
Contributor Information
Ellinor I. B. Peerschke, Department of Pathology, The Mount Sinai School of Medicine, New York, NY
Wei Yin, Department of Mechanical and Aerospace Engineering, Oklahoma State University, Stillwater, OK
Berhane Ghebrehiwet, Departments of Medicine and Pathology, Stony Brook University, Stony Brook, NY
References
- Acostan J, Qin X, Halperin J. Complement and complement regulatory proteins as potential molecular targets for vascular diseases. Curr. Pharm. Dis. 2004;10:203–211. doi: 10.2174/1381612043453441. [DOI] [PubMed] [Google Scholar]
- Allegretti M, Moriconi A, Beccari AR, Di Bitondo R, Bizzarri C, Bertini R, Colotta F. Targeting C5a: recent advances in drug discover. Curr. Med. Chem. 2005;12:217–236. doi: 10.2174/0929867053363379. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Shields IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerke M, Prufer D, Dahm M, Oelert H, Meyer J, Darius H. Blocking of classical complement pathway inhibits endothelial adhesion molecules expression and preserves ischemic myocardium from reperfusion injury. J. Pharmacol. Exp. Ther. 1998;286:429–438. [PubMed] [Google Scholar]
- Davis AD, 3rd, Kenney DM. Properdin factor D; effects on thrombin-induced platelet aggregation. J. Clin. Invest. 1979;64:721–728. doi: 10.1172/JCI109515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedio J, Jahnen-Dechent W, Bachmann M, Muller-Esterl W. The multiligand binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J. Immunol. 1998;160:3534–3542. [PubMed] [Google Scholar]
- Del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DV, Rosse WF. Regulation of the activity of platelet-bound C3 convertase of the alternative pathway of complement by platelet factor H. Proc. Natl. Acad. Sci. USA. 1987;84:5873–5877. doi: 10.1073/pnas.84.16.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M, Ott I, Reininger AJ, Heinzmann U, Neumann FJ. Agglutination of isolated platelet membranes. Arterioscler. Thromb. Vasc. Biol. 1996;16:621–627. doi: 10.1161/01.atv.16.5.621. [DOI] [PubMed] [Google Scholar]
- George JN, Pickett EB, Saucerman S, McEver RP, Kunicki TJ, Kieffer N, Newman PJ. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J. Clin. Invest. 1986;78:340–348. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B, Cebada Mora C, Tantral L, Jesty J, Peerschke EIB. gC1qR/p33 serves as a molecular bridge between the complement and contact activation systems and is an important catalyst in inflammation. Adv. Exp. Med. Biol. 2006;586:95–105. doi: 10.1007/0-387-34134-X_7. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EIB. gC1qR/p33 a member of a new class of multifunctional and multicompartmental cellular proteins, is involved in inflammation and infection. Immunol. Rev. 2001;180:65–77. doi: 10.1034/j.1600-065x.2001.1800106.x. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B, Lim BL, Peerschke EIB, Willis AC, Reid KBM. Isolation of cDNA cloning, and overexpression of a 33-kDa cell surface glycoprotein that binds to the globular heads of C1q. J. Exp. Med. 1994;179:1809–1821. doi: 10.1084/jem.179.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B, Peerschke EIB. Structure and function of gC1qR a multiligand binding membrane protein. Immunobiol. 1998;199:225–238. doi: 10.1016/S0171-2985(98)80029-6. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos B, Passam F, Rahgozar S, Krillis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109:422–430. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WE. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101:2285–2293. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetse RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Nagasawa K, Horiuchi T, Nishizaka H, Niho Y. C5a induced tissue factor activity on endothelial cells. Thromb. Haemost. 1997;77:394–398. [PubMed] [Google Scholar]
- Jiang J, Zhang Y, Krainer A, Xu RM. Crystal structure of p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc. Natl. Acad. Sci. USA. 1999;96:3572–3577. doi: 10.1073/pnas.96.7.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- Kaplan AP, Silverberg M, Ghebrehiwet B. The intrinsic coagulation/kinin pathway – the classical complement pathway and their interactions. Adv. Exp. Med. Biol. 1986;198:1311–1325. doi: 10.1007/978-1-4757-0154-8_2. [DOI] [PubMed] [Google Scholar]
- Kovacsovics T, Tschopp J, Kress A, Islike H. Antibody independent activation of C1, the first component of complement by cardiolipin. J. Immunol. 1985;135:2695–2700. [PubMed] [Google Scholar]
- Laine P, Pentikainen MO, Wurzner R, Penttila A, Paavonen T, Meri S, Kovanen PT. Evidence for complement activation in ruptured coronary plaques in acute myocardial infarction. Am. J. Cardiol. 2002;90:404–408. doi: 10.1016/s0002-9149(02)02498-0. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Sughrue MW, Ducruet AF, Mocco J, Sosunov SA, Hassid BG, Silverberg JZ, Ten VS, Pinsky DJ, Connolly ES., Jr . Temporal pattern of C1q deposition after transient focal cerebral ischemia. Wiley InterScience; 2006. www.interscience.wiley.com. [DOI] [PubMed] [Google Scholar]
- Mahdi F, Mader ZS, Figueroa CD, Schmaier AH. Factor XII interacts with multiprotein assembly of urokinase plasminogen activator receptor, gC1qR and cytokeratin 1 on endothelial cell membranes. Blood. 2002;15:3585–3596. doi: 10.1182/blood.v99.10.3585. [DOI] [PubMed] [Google Scholar]
- Makrides SC. Therapeutic inhibition of the complement system. Pharmacol. Rev. 1998;150:59–87. [PubMed] [Google Scholar]
- Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Martinez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1004–H1009. doi: 10.1152/ajpheart.00842.2004. [DOI] [PubMed] [Google Scholar]
- Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arteriscler. Thromb. Vasc. Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- Mevorach D, Mascarenhas JO, Gershoev D, Eldon KB. Complement dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. F.A.S.E.B. J. 2003;17:1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr. Opin. Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- Muhlfelder TW, Miemetz J, Kreutzer D, Beebe D, Ward PA, Rosenfeld SI. C5 chemotactic fragment induces leukocyte production of tissue factor activity: a link between complement and coagulation. J. Clin. Invest. 1979;63:147–150. doi: 10.1172/JCI109269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil JS, Manzi S, Kao AH, Krishnaswami S, Liu CC, Ruffing MJ, Shaw PS, Nilson AC, Dryden ER, Johnson JJ, Ahearn JM. Platelet C4d is highly specific for systemic lupus erythematosus. Arthritis and Rheum. 2006;54:670–674. doi: 10.1002/art.21627. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Niculescu T, Rus H. C5b-9 terminal complement complex assembly on apoptotic cells in human arterial wall with atherosclerosis. Mol. Immunol. 2004;36:949–955. doi: 10.1016/j.yexmp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. Complement activation and atherosclerosis. Mol. Immunol. 1999;36:949–955. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- Nilsson B. 2007 this volume. [Google Scholar]
- Patel S, Thelander EM, Hernandez M, Montenegro J, Hassing H, Burton C, Mundt S, Hermanowski-Vosatka A, Wright SD, Chao YS, Detmers PA. ApoE−/− mice develop atherosclerosis in the absence of complement component C5. Biochem. Biophys. Res. Commun. 2001;286:164–170. doi: 10.1006/bbrc.2001.5276. [DOI] [PubMed] [Google Scholar]
- Peerschke EI, Ghebrehiwet B. C1q augments platelet activation in response to aggregated Ig. J. Immunol. 1997;159:5594–5598. [PubMed] [Google Scholar]
- Peerschke EIB, Murphy TK, Ghebrehiwet B. Activation-dependent surface expression of gC1qR/p33 on human blood platelets. Thromb. Haemost. 2003;90:331–339. [PubMed] [Google Scholar]
- Peerschke EIB, Petrova RJ, Ghebrehiwet B, Ruf W. Tissue factor pathway inhibitor-2 (TFPI-2) recognizes the complement and kininogen binding protein gC1qR/p33 (gC1qR): implications for vascular inflammation. Thromb. Haemost. 2004;92:811–819. doi: 10.1160/TH04-03-0188. [DOI] [PubMed] [Google Scholar]
- Peerschke EIB, Reid KBM, Ghebrehiwet B. Platelet activation by C1q results in the induction of alpha IIb/beta 3 integrins (GPIIb/IIIa) and the expression of P-selectin and procoagulant activity. J. Exp. Med. 1993;178:579–587. doi: 10.1084/jem.178.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke EIB, Reid KBM, Ghebrehiwet B. Identification of a novel 33-kDa C1q-binding site on human blood platelets. J. Immunol. 1994;152:5896–5901. [PubMed] [Google Scholar]
- Peerschke EIB, Yin W, Alpert DR, Salmon JE, Roubey RAS, Ghebrehiwet B. Enhanced serum complement activation on platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus (SLE) and antiphospholipid antibodies (aPL) Blood. 2007 doi: 10.1177/0961203308099974. (abstract) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerschke EIB, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J Thromb Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- Perez-Pujol S, Marker PH, Key NS. Platelet microparticles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry. 2007;71A:38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- Polley MJ, Nachman RL. The human complement system in thrombin-mediated platelet function. J. Exp. Med. 1978;147:1713–1726. doi: 10.1084/jem.147.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley MJ, Nachman RL. Human complement in thrombin-mediated platelet function: uptake of the C5b-9 complex. J. Exp. Med. 1979;150:633–645. doi: 10.1084/jem.150.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley MJ, Nachman RL. Human platelet activation by C3a and C3a des-arg. J. Exp. Med. 1983;158:603–615. doi: 10.1084/jem.158.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaiff WT, Eisenberg PR. Direct induction of complement activation by pharmacologic activation of plasminogen. Coron. Artery Dis. 1997;8:9–18. doi: 10.1097/00019501-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Schmaier AH, Amenta S, Xiong T, Heda GD, Gewirtz AM. Expression of C1 Inhibitor. Blood. 1993;82:465–474. [PubMed] [Google Scholar]
- Seidl WS, Exner M, Amighi J, Kastl SP, Zorn G, Maurer G, Wagner O, Huber K, Minar E, Wojta J, Schillinger M. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Europ. Heart. J. 2005;26:2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
- Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membrane have 50 – 100 fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- Shenghe C, Davie AE., III Complement regulatory protein C1 inhibitor binds to selectins and interferes with endothelial-leukocyte adhesion. J. Immunol. 2003;171:4786–4791. doi: 10.4049/jimmunol.171.9.4786. [DOI] [PubMed] [Google Scholar]
- Torzewsjki J, Bowher DE, Wlatenberger J, Fitzsimmons C. Processes in atherogenesis: complement activation. Atherosclerosis. 2007;132:131–138. doi: 10.1016/s0021-9150(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Vaziri-Sani F, Hellwage J, Zipfel PF, Sjoholm AG, Iancu R, Karpman D. Factor H binds to washed human platelets. J. Thromb. Haemost. 2004;3:154–162. doi: 10.1111/j.1538-7836.2004.01010.x. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffen-Nakken H, Reutelingsperger C. A novel assay of apoptosis : flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluoresceinlabeled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Vlaicu R, Niculescu F, Rus HG, Cristea A. Immunohistochemical localization of the terminal C5b-9 complement complex in human aortic fibrous plaque. Atherosclerosis. 1985;57:163–177. doi: 10.1016/0021-9150(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68:875–880. [PubMed] [Google Scholar]
- Yin W, Ghebrehiwet B, Peerschke EIB. Expression of complement components and inhibitors on platelet microparticles. Platelets. 2008 doi: 10.1080/09537100701777311. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman TS, Kolb NP. Human platelet-initiated formation and uptake of the C5-9 complex of human complement. J. Clin. Invest. 1976;57:203–211. doi: 10.1172/JCI108261. [DOI] [PMC free article] [PubMed] [Google Scholar]