Abstract
Several studies have demonstrated the effectiveness of arginine analog nitric oxide synthase (NOS) inhibitor therapy in preventing and treating murine lupus nephritis. However, MRL/MpJ-FASlpr (MRL/lpr) mice lacking a functional NOS2 (inducible NOS [iNOS]) gene (NOS2−/−) develop proliferative glomerulonephritis in a fashion similar to their wild-type (wt) littermates. This finding suggests that the effect of arginine analog NOS inhibitors is through a non-iNOS–mediated mechanism. This study was designed to address this hypothesis.
NOS2−/− mice were given either vehicle or a NOS inhibitor (SD-3651) to determine if pharmacological NOS inhibition prevented glomerulonephritis, using wt mice as positive controls. Urine was collected fortnightly to measure albumin. At the time of full disease expression in wt mice, all mice were killed, and renal tissue was examined for light, immunofluorescence, and electron microscopic evidence of disease. Serum was analyzed for anti–double-stranded DNA antibody production.
NOS2−/− mice had higher serum anti–double-stranded DNA antibody antibody levels than those of wt mice. SD-3651 therapy reduced proteinuria, glomerular immunoglobulin G deposition, and electron microscopic evidence of podocytopathy and endothelial cell swelling without affecting proliferative lesions by light microscopy.
These studies confirm that genetic iNOS deficiency alone is insufficient to prevent proliferative glomerulonephritis and suggest that iNOS activity may inhibit autoantibody production. These results also suggest that SD-3651 therapy acts via a non–iNOS-mediated mechanism to prevent endothelial cell and podocyte pathology. Studies that elucidate this mechanism could provide a useful drug target for the treatment of nephritis.
Keywords: lupus nephritis, nitric oxide, animal models, nitric oxide synthase, enzyme inhibitors
INTRODUCTION
Systemic lupus erythematosus is a prototypic autoimmune disease marked by autoantibody production and immune complex deposition in affected tissues. Reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates (RNIs) are implicated in the pathogenesis of both the onset of humoral autoimmunity and the innate immune response after immune complex deposition.1 Numerous studies have also demonstrated the overproduction of ROIs and RNIs both systemically and in the kidney of patients with lupus and lupus nephritis. Serum levels of RNIs correlated with disease activity and are being pursued as possible new biomarkers of lupus activity.2
In murine models of lupus, RNI production was significantly increased, and treatment with nitric oxide synthase (NOS) inhibitors, including a moderately selective inducible NOS (iNOS) inhibitor, N6-(1-iminoethyl)-L-lysine dihydrochloride, both prevented3,4 and treated5 glomerulonephritis and prolonged survival. However, MRL/MpJ-FASlpr (MRL/lpr) mice lacking the NOS2 gene (for iNOS) still developed proliferative nephritis that was similar in severity to that of their MRL/lpr wild-type (wt) littermates.6 These seemingly contradictory results would suggest either that iNOS is not the only target of NOS inhibitors responsible for their treatment effect or that mice deficient in NOS2 develop compensatory mechanisms that result in similar disease expression. The following study was performed to determine potential non–iNOS-mediated effects of a moderately selective iNOS inhibitor (SD-3651, a lysine-based analog selective competitive inhibitor and prodrug of L-NIL) on the lupus disease phenotype in MRL/lpr NOS2−/− mice.
The original derivation of the MRL/lpr NOS2−/− mice was lost because of colony contamination. We thus derived a new line of MRL/lpr NOS2 mice by breeding C57Bl/6 NOS2−/− mice to MRL/lpr mice over 9 generations to produce MRL/lpr NOS2−/− mice (NOS2−/−) and MRL/lpr NOS2+/+ littermates (wt) for study. NOS2−/− mice were treated before and during the ages of active disease with either placebo or SD-3651 using sustained release pellets deposited subcutaneously. The mice expressing the NOS2−/− genotype developed the same immune complex proliferative glomerulonephritis as that of the wt mice, confirming our original report in an independently derived line. Surprisingly, the NOS2-deficient mice produced significantly higher levels of double-stranded DNA (dsDNA) antibodies, an effect not seen in the original study. Treatment of NOS2-deficient MRL/lpr mice with SD-3651 significantly reduced proteinuria and immunoglobulin (Ig) G deposition in the glomerulus as well as capillary endothelial cell swelling seen on electron microscopy (EM). These EM findings occurred despite similar glomerular disease by light microscopy. Reactive oxygen intermediate and RNI production by spleen cells was not affected by SD-3651 therapy in the NOS−/− mice. These observations suggest that SD-3651 therapy prevents endothelial cell and podocyte pathology in this model of lupus nephritis via non–iNOS-mediated mechanisms but does not impact the development of proliferative renal disease.
MATERIALS AND METHODS
Mice and Sample Collection
The Ralph H. Johnson Veterans Affairs Institutional Animal Care and Use Committee approved all procedures. MRL/MpJ-FASlpr (MRL/lpr) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions in the animal research facility at the Ralph H. Johnson Veterans Affairs Medical Center in Charleston, SC. Mice were serologically tested for common murine pathogens on a random basis. MRL/lpr NOS2−/− mice were generated as described below. Eight NOS2+/+, 8 NOS2−/−, and 8 NOS2−/− SD-3651–treated MRL/lpr mice were included in the analysis.
Every 2 weeks, before and during treatment, individual mice were placed on a 24-hour low nitrate + nitrite (NOx) diet (Zeigler Brothers, Gardners, PA) with distilled water and placed in metabolic cages for an additional 24 hours on the same diet/water for urine collection. Throughout the remainder of the experiment, they were fed standard mouse chow and tap water. At 24/25 weeks of age, mice were anesthetized and bled by retro-orbital bleeding immediately before killing and harvest of renal and spleen tissue for analysis.
Generation of the NOS2−/− MRL/lpr Mice
NOS2tm1Lau mice on the C57BL/6 background genetically deficient in NOS2 (C57Bl/6 NOS2−/−) were generously donated from the laboratory of Victor E. Laubach, PhD, at Glaxo Wellcome Inc. This line was developed as previously described.7 The C57Bl/6 NOS2−/− mice were backcrossed 9 times to MRL/lpr mice in our laboratory. To reduce the number of backcrosses necessary to generate NOS2−/− mice on the MRL/lpr background, speed congenics techniques were used as previously described.8 All 12 genetic susceptibility loci known were present in the MRL/lpr mice used in these studies. MRL/lpr NOS2+/− mice were bred to generate wt MRL/lpr (NOS2+/+) and MRL/lpr NOS2−/− (NOS2−/−) littermates.
Treatment
Treatment was begun at 10 weeks of age as MRL/lpr mice begin producing increasing amounts of nitric oxide (NO) at approximately 12 weeks of age.3 Mice were divided into 3 groups: (1) NOS2+/+ littermates allowed to develop spontaneous disease, (2) NOS2−/− mice administered 60-day slow release subcutaneous pellets containing only vehicle (Innovative Research, Sarasota, FL), and (3) NOS2−/− mice given SD-3651 (a selective competitive iNOS inhibitor, a gift from Pfizer Global Research and Development) 30 mg/kg/d by 60-day slow release subcutaneous pellets from 10 to 24/25 weeks of age. The 30 mg/kg/d dose was determined by a dose-ranging study in MRL/lpr wt mice with active disease and demonstrated to suppress NO production to levels similar to those of predisease mice. Mice were administered SD-3651 subcutaneously at 0, 3, 10, and 30 mg/kg/d in twice-daily divided doses. The 30 mg/kg/d dose was the lowest effective dose that suppressed urine NOx production after 2 weeks of therapy (data not shown). This subcutaneous dosing was used in the spleen reactive oxygen and nitrogen intermediates experiments in which 8 mice were treated with vehicle and 8 with SD-3651 30 mg/kg/d for 2 weeks.
Urine NOx Assay
Twenty-four–hour urine collections were obtained from individual mice by placing them in metabolic cages. Samples were collected into 1 mL of an antibiotic solution containing 25 μg/mL ampicillin, 50 μg/mL gentamicin, and 200 μg/mL chloramphenicol to prevent bacterial growth. The samples were centrifuged at 2500g to remove debris and filtered through a 0.22-μm centrifugal filter (Millipore, Bedford, MA). Nitrate + Nitrite was assayed using a chemiluminescence detector (Model 280i; Sievers, Boulder, CO), with vanadium chloride at 95°C as a reducing agent as described.9 Unknown sample concentrations were interpolated from curves generated from nitrate standards (Sigma-Aldrich, St. Louis, MO) by the manufacturer’s specifications. All samples were assayed in duplicate or triplicate and reported as micromoles per mouse per day.
Urine Albumin Enzyme-Linked Immunosorbent Assay
Standard enzyme-linked immunosorbent assay techniques were used to detect urine albumin using the published methods of Sekine et al.8 and reported as milligrams of urine albumin per mouse per day.
Antioxidant Capacity
Total plasma antioxidant potential was determined by the ferric-reducing ability of plasma according to the methods of Benzie and Strain10 that were modified by McA-nulty et al.11 Working solutions were prepared daily. The ferric-reducing ability of serum (FRAS) was expressed as iron equivalents from a standard curve made with iron sulfate hexahydrate from 0 to 2000 μM (Sigma).
Qualitative Glomerular Scores
At the end of treatment, mice were killed for harvest of tissue. One kidney was removed from each mouse and immediately fixed in paraformaldehyde and stained with hematoxylin and eosin before examination by a pathologist (P.R.) who was masked to the treatment group. Pathological changes of renal disease activity were graded qualitatively as previously published (8). Glomeruli were graded for hypercellularity (0–4), mesangial expansion (0–4), hyperlobulation (0–4), chronic inflammation (0–4), dilatation (0–4), necrosis (0–8), hyperlobularity (0–4), crescent formation (0–8), epithelial cell reactivity (0–4), thrombi in loops (0–4), vasculitis (0–4), thickened membranes (0–4), and neutrophil debris (0–4). Scores were additive, with the most common elements seen in this model being hypercellularity, neutrophils, thickened membranes, epireactivity, mesangial hypercellularity, and necrosis.
Immunofluorescence Detection of IgG and C3 in Glomerular Tissue
At the time of killing, the remaining kidney was removed and snap frozen in liquid nitrogen. Frozen tissue was fixed in acetone, washed in phosphate-buffered saline (PBS), incubated in 3% hydrogen peroxide for 10 minutes, and rinsed in PBS. Slides were incubated in fluorescein isothiocyanate–conjugated monoclonal IgG antibody to C3 or IgG (Cappel; MP Biomedicals, Solon, OH) at a 1:50 dilution in PBS for 1 hour in the dark at room temperature. Slides were then rinsed in PBS and distilled water and mounted with a cover slip using Vectashield hard set medium (Vector H-1400, Vector Laboratories, Burlingame, CA). Slides were scored (0–3) in a masked fashion by an experienced renal pathologist (S.E.S.) for the intensity and coverage of immunofluorescence in the glomerulus (0, no fluorescence; 0.5, trace, just detectable above background; 1, fluorescence scattered and light; 2, bright but not diffuse; and 3, bright and diffuse). Intensities were scored separately for basement membrane and mesangial deposits.
Electron Microscopy of Glomerular Tissue
Electron microscopy was performed to determine glomerular ultrastructural changes associated with NOS2 genotype and therapy. Paraformaldehyde-fixed tissue was prepared for EM as described.12 The extents of subendothelial, subepithelial, and mesangial electron-dense deposits; podocyte foot process effacement; and capillary loop endothelial cell swelling were qualitatively scored on a scale of 0 to 3 by an experienced renal pathologist (S.E.S.) adept with EM semiquantitative measures (0, not present; 1, small scattered; 2, medium-sized deposits but not diffuse; and 3, diffuse and prominent).
Qualitative Determination of Reactive Intermediate Production in Spleen Cells by Flow Cytometry
Spleen cells were isolated, and red blood cells were lysed as previously described.13 Cells were then incubated with 1 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF) or 0.2 μM 5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate acetyl ester (CM; Invitrogen, Carlsbad, CA) in PBS for 45 minutes at 37°C according to the manufacturer’s recommendation. Cells were then washed with PBS and incubated for 30 minutes at 36°C to allow for deacetylation and reaction with RNIs or ROIs to develop fluorescence. Cells were then analyzed by flow cytometry, using forward scatter and side scatter to gate on single lymphocytes. With an excitation wavelength of 495 nm, fluorescence intensity was determined at 515 nm. Flow cytometry data were then used to analyze the percentage of cells positive for DAF or CM fluorescence.
STATISTICAL METHODS
For comparison of data between groups, Student t test was used for normally distributed data; otherwise, a Mann-Whitney U test was used. For qualitative assays performed across multiple experiments, data were factor corrected to reduce the effect of interexperiment variations.14 All data were expressed as mean ± SE, and all associations were considered significant if P values were less than or equal to 0.05.
RESULTS
Urine NOx Levels Are Lower in NOS−/− Mice
To confirm that the NOS2−/− mice had a low NO-producing phenotype and that iNOS inhibitor therapy did not lower markers of systemic NO production below the NOS2−/− baseline, urine was collected before and every other week during treatment and analyzed for urine NOx as a surrogate marker of systemic NO production. As expected, there was a significant difference (P < 0.05) in the level of urinary NOx during the week of killing between the NOS2+/+ mice and the NOS2−/− mice treated with vehicle (2.4 ± 0.9 vs. 0.6 ± 0.3 μmol/mouse/d). No significant difference in urine NOx level was observed between the NOS2−/− mice treated with vehicle compared with that of the ones treated with SD-3651 (0.4 ± 0.2 μmol/mouse/d). Thus, SD-3651 treatment did not seem to significantly impact NO production by the constitutive NOS isoforms eNOS or nNOS.
Urine Protein Excretion Is Less in NOS−/− Mice Treated With SD-3651
To determine the effect of the NOS2−/− genotype and iNOS inhibitor therapy on proteinuria as a marker of glomerulonephritis, the following experiment was performed. Urine collected every other week was analyzed for albumin content. During active disease (days 160 to 180), urine albumin levels were significantly higher among the NOS2+/+ and vehicle-treated NOS2−/− mice compared with those of the NOS2−/− SD-365–treated mice (P < 0.05 compared with the remaining groups; Fig. 1). This difference likely represented a non–iNOS-mediated effect of SD-3651 treatment on glomerular function.
FIGURE 1.
Urine albumin with nitric oxide synthase (NOS)2 knockout and SD-3651 therapy over time. Urine albumin (milligram per mouse per day) was determined every 2 weeks from 24-hour urine collections of individual mice. The x axis depicts mouse age in days, whereas the y axis depicts the average level of proteinuria for each group at each time point. Urine albumin levels were significantly less in the older NOS2−/− SD-3651–treated mice when compared with those of the NOS2+/+ and the NOS2−/− mice with active disease of the same age. *P < 0.05 versus NOS2+/+ and NOS2−/−.
Glomerular Pathology Is Similar Between NOS2+/+, NOS2−/−, and SD-3651–Treated NOS2−/− Mice
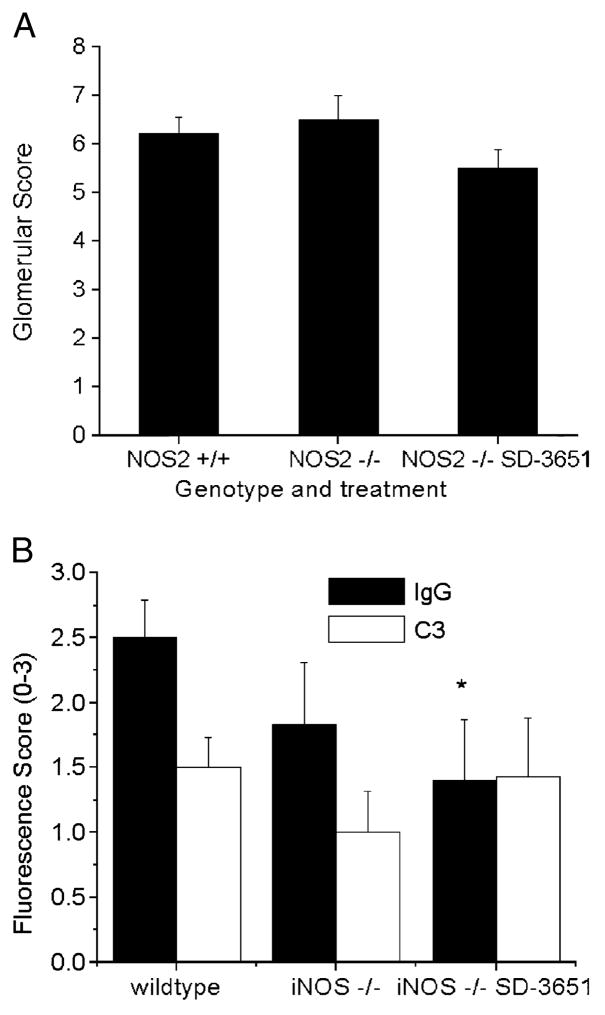
To determine if the effect of the NOS2−/− genotype and SD-3651 therapy on proteinuria was secondary to effects on glomerular pathology, kidneys were harvested at the time of killing, sectioned, stained with hematoxylin and eosin, and scored for pathological changes. Glomerular pathological scores were not significantly different between the 3 groups of mice (Fig. 2A). Thus, despite the difference in urinary albumin excretion observed in the SD-3651–treated mice, proliferative renal disease was not impacted. This lack of impact of the NOS2−/− genotype is consistent with our prior report.
FIGURE 2.
Effect of genetic and pharmacological manipulation of inducible nitric oxide synthase (iNOS) on renal pathology. (A) Kidney sections from mice in each group were stained and scored for features of lupus nephritis (0–60). The x axis depicts the genotype and treatment group, whereas the y axis depicts the total score for each group. There were no significant differences in histological scores (P > 0.05). (B) Immunofluorescence scores for glomerular IgG and C3 deposition by group. Frozen kidney sections obtained at the time of killing (24/25 weeks) were immunostained for IgG and C3 and scored for the intensity and extent of coverage of fluorescence on a qualitative scale (0–3). The x axis depicts the genotype and treatment group, whereas the y axis depicts the qualitative score. The SD-3651–treated mice had significantly lower scores than those of the wt mice. *P < 0.05 for NOS2+/+ versus NOS2−/− SD-3651–treated mice.
Glomerular IgG and C3 Immunofluorescence
To determine the effect of the NOS2−/− genotype and SD-3651 therapy on IgG and complement glomerular deposition, kidneys were removed after the killing, snap frozen, cryosectioned, fixed, and immunostained as above. Immunohistochemical grading for glomerular IgG and C3 was performed. Glomerular IgG staining was greatest in the NOS2+/+ group, with the NOS2−/− vehicle-treated and the NOS2−/− SD-3651–treated groups showing intermediate and low levels (Figs. 2B and 5A, B). Statistically significant differences were only seen between the NOS2+/+ and NOS2−/− SD-3651–treated groups. There were no significant differences between groups in C3 staining.
FIGURE 5.
Representative immunoglobin G (IgG) immunofluorescence and electron microscopy. Representative IgG immunofluorescence micrographs of glomeruli from wild-type (A) and SD-3651–treated nitric oxide synthase (NOS)2−/− mice (B) as described in Figure 2B. Representative electron micrographs of glomeruli from vehicle-treated NOS2−/− (C) and SD-3651–treated NOS2−/− mice (D) as described in Figure 4.
Anti-dsDNA Antibody (Anti-dsDNA) Production Is Increased in MRL/lpr NOS2−/− Mice
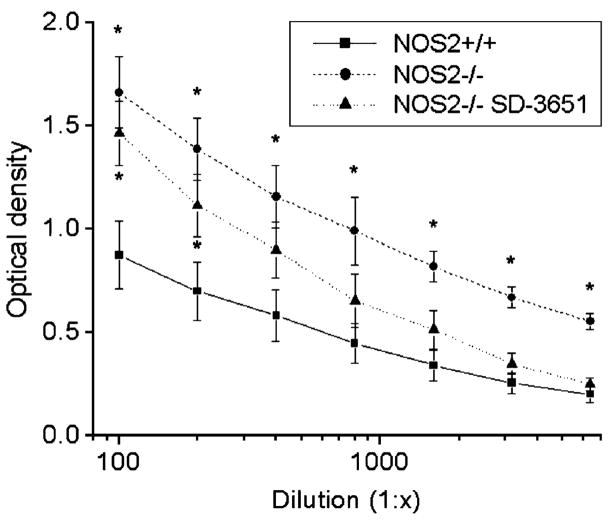
To determine the effect of the NOS2−/− genotype and SD-3651 therapy on systemic lupus erythematosus–specific autoimmunity, serum anti-dsDNA antibody levels were determined from blood collected at the time of killing. Anti-dsDNA antibody levels were less in the wt than those in either of the NOS2−/− groups (P < 0.05, Fig. 3), suggesting that either genetic manipulation of NOS2 leads to increased autoantibody production either directly or indirectly through compensatory mechanisms. This finding was not previously noted in MRL/lpr NOS2−/− mice or in mice treated with iNOS inhibitors.
FIGURE 3.
Anti-dsDNA antibody titration curves for nitric oxide synthase (NOS)2+/+, NOS2−/−, and NOS2−/− SD-3651–treated mice. Serum dsDNA antibody levels were determined at the time of killing (24/25 weeks). The x axis depicts the dilution of the serum before the analysis assay, and the y axis depicts the optical density at each dilution. The levels were significantly higher in the NOS2−/− compared with those of the NOS2+/+ mice and for the NSO2−/− SD-3651–treated mice at lower dilutions (1:100 and 1:200). *P < 0.05 versus NOS2+/+.
Glomerular EM
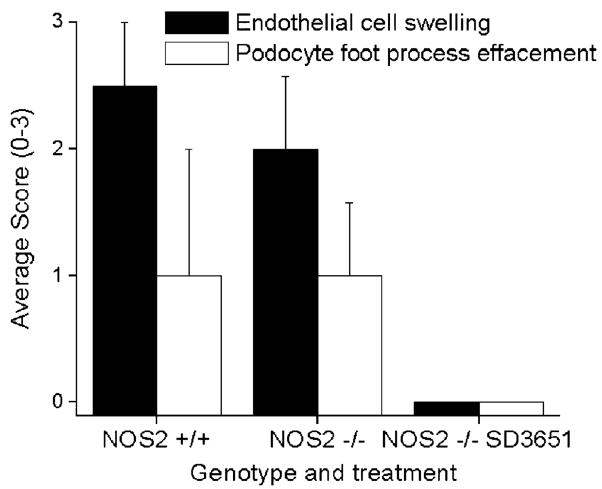
Because differences in levels of albuminuria between groups could not be explained by light microscopic changes, EM was performed on glomerular tissue from a subset of mice in each group (n = 3). There were similar levels of significant podocyte foot process effacement and capillary loop endothelial cell swelling in the NOS2+/+ and NOS2−/− mice, yet these changes were absent in the NOS2−/− SD-3651–treated mice (Figs. 4 and 5C, D). Although the amount of electron-dense deposits in the subendothelial and subepithelial spaces seemed similar in all 3 groups (data not shown), the ability to quantitatively assess immune complex deposition by EM was limited.
FIGURE 4.
Electron micrograph (EM) scoring of endothelial cell swelling and podocyte effacement. Fixed kidney sections from a subset of mice (n = 3) were examined by EM and scored (0–3) by a renal pathologist for the extent of endothelial cell swelling and podocyte effacement. Both features were less in the SD-3651–treated mice, but differences were statistically significant only for endothelial cell swelling. P < 0.05 versus the remainder of the groups. NOS, nitric oxide synthase.
Reactive Oxygen Intermediate and RNI Production in Spleen Cells
Increasing evidence suggests that intracellular NO production impacts lymphocyte function, although which NOS isoforms are responsible is not clear. To determine if intracellular spleen cell RNIs production was impacted by iNOS inhibitor therapy, spleen cells were isolated and stained for DAF, which fluoresces in the presence of NO. There were significant differences neither in the percentage of DAF + spleen cells nor the mean fluorescence intensity of cells between NOS2+/+ (47% ± 8%), NOS2−/− (49% ± 10%), or NOS2−/− (47% ± 9%) mice (n = 8) given SD-3651. A similar experiment was conducted by staining spleen cells with CM to detect ROI production. CM-H2DCFDA staining was the same in the same 3 groups (16% ± 3%, 17% ± 3%, and 16% ± 3%). These results suggest that ROI and RNI production among all spleen cells was not impacted by the NOS2−/− genotype or SD-3651 therapy and would suggest that the effect of SD-3651 therapy was not mediated by the inhibition of constitutive NOS isoforms.
Serum Antioxidant Capacity
To determine if differential endogenous antioxidant capacity may occur with SD-3651 therapy, the antioxidant capacity of serum from each of the 3 groups was measured using the FRAS assay. The FRAS iron equivalents were similar in the wt, NOS2−/−, and SD-3651 groups (716 ± 53 vs. 590 ± 32 vs. 692 ±55 iron equivalents, respectively). These results suggest that the difference in proteinuria seen in the SD-3651 treated NOS2−/− mice was not due to changes in antioxidant capacity.
DISCUSSION
Earlier investigations reporting a protective and therapeutic effect of the amino acid–based class of nonspecific and specific competitive iNOS inhibitors suggested that iNOS was an essential pathogenic mediator in lupus nephritis. In these studies, NOS inhibitors reduced proteinuria and distal glomerular inflammatory disease but did not change measures of humoral autoimmunity or immune complex deposition in the glomerulus. The hypothesis that iNOS is pathogenic in lupus nephritis was supported by observational human studies reporting increases in markers of systemic NO production and increases in iNOS staining in glomerular tissue among patients with active proliferative lupus nephritis. In contrast, earlier studies of MRL/lpr mice lacking an effective NOS2 gene reported a similar progression of clinical and pathological glomerulonephritis among the NOS2−/− and wt littermates, despite improvements in signs of rheumatoid factor–associated cryoglobulinemic vasculitis. These studies together inspired the hypothesis that the therapeutic and preventative effect of amino acid analog iNOS inhibitors on lupus nephritis occurs through a mechanism independent of specific competitive inhibition of iNOS. Alternatively, there may be compensatory increases in other inflammatory mediators that can induce proliferative nephritis in the setting of NOS2 deficiency.
The current study confirms that genetic deficiency of the NOS2 gene is insufficient to prevent inflammatory glomerulonephritis in MRL/lpr mice. There are 2 potentially important differences between the prior study and our current study. In the earlier study, NOS2−/− mice were backcrossed to the MRL/lpr background for only 4 generations, and thus there was likely heterogeneity in the genetic background of the mice in the first study. Furthermore, the mice in the earlier study were not housed under specific pathogen-free conditions. Both of these genetic and environmental differences may explain the differences in anti-dsDNA antibody levels between the MRL/lpr NOS2−/− mice in the 2 studies. In the current study, we demonstrated that anti-dsDNA antibody production was enhanced by genetic ablation of NOS2. This finding may have been masked by the genetic and/or environmental differences between the 2 studies.
The results of the current study suggest that iNOS may inhibit anti-dsDNA antibody activity. One potential mechanism for this inhibitory action is through modulation of redox signaling in T cells. The experiments to measure reactive intermediate production in the 3 groups failed to show a significant or meaningful difference in the RNI production between groups, but spleen cells from the NOS2−/− mice produced 40% more ROIs than did their wt counterparts. This result was not statistically significant, but the techniques used to measure ROIs used in these experiments were more qualitative than quantitative. Mitochondria in lupus T cells have chronically increased membrane hyperpolarization, adenosine triphosphate depletion, and size in association with altered T-cell receptor signaling and Ca2+ flux.15 This study did not address whether these changes in spleen cell ROI production reflect changes in mitochondrial function leading to altered T-cell activation or apoptosis. However, 1 hypothesis to address is that iNOS activity modulates mitochondrial respiration in this model.
The design of this study does not permit determination of the mechanism behind the differential effect of SD-3651 on anti-dsDNA antibody production and glomerular IgG deposition. These results can lead to several hypotheses: (a) the level of anti-dsDNA antibodies in this experiment does not equate to pathogenicity, (b) nonspecific NOS inhibition may lead to autoantibody production without affinity maturation or epitope spreading to pathogenic glomerular antigens, (c) nonspecific effects of SD-3651 could affect glomerular immune complex clearance, or (d) the more qualitative nature of the immunofluorescent scoring system has lead to an error detecting a difference in glomerular IgG deposition when there was none.
Because these effects occurred in mice lacking iNOS, they were independent of a direct effect of competitive inhibition of iNOS catalytic activity. Potential mechanisms for this effect include nonspecific competitive inhibition of other NOS isoforms such as eNOS or nNOS as the selectivity of L-NIL, for which SD-3651 is a prodrug, for iNOS activity (lipopolysaccharide-induced nitrate production) versus eNOS activity (increase in blood pressure) in rats is only 5.4-fold. Earlier studies showing a therapeutic effect of NOS inhibitors used either nonspecific NOS inhibitors3 or iNOS selective inhibitors at doses that may not have been selective for iNOS.4,16 There is a growing body of evidence that eNOS is important in T-cell activation in human lupus. Lupus T cells are marked by sustained mitochondrial hyperpolarization and increased T-cell activation via the T cell receptor that is dependent on NO production.15 The results of this and the prior studies inspire the hypothesis that the therapeutic effects of nonspecific NOS inhibition in earlier studies are likely multifactorial and possibly due to an effect on mitochondrial biogenesis.
Another potential action of SD-3651 is the reduction of arginine substrate for other NOS isoforms. Intracellular arginine levels are regulated by uptake via the cationic amino acid transporter isoforms 1 to 3, synthesis through the urea cycle, and hydrolysis by arginase to urea and ornithine.17 The impact of each of these phases of arginine metabolism varies between cell types and different stages of cell activation. Arginine availability in the cell is limited by the arginine analog competitive inhibitors of iNOS such as the NG-monomethyl arginine used in earlier studies3 as they compete for the same y+ transporter for ingress into the cell.18 L-NIL, a lysine analog that is the active metabolite of SD-3651, also reduces arginine flux into activated cells.19 The role for this mechanism in mediating the effects of SD-3651 was not addressed in this study but would seem unlikely given the lack of an effect of SD-3651 on urinary NOx production or intracellular NO production.
In this study, SD-3651 therapy in NOS2−/− mice suppressed proteinuria and IgG deposition in the mesangium without significantly changing C3 deposition in the glomerulus. This would suggest that the effect of iNOS inhibitor therapy was distal to complement activation. This interpretation is supported by the improvement in endothelial cell swelling and podocyte effacement in treated mice with SD-3651. Glomerular endothelial cell swelling is a recognized feature of nephritis.20 Immune complex–mediated activation of endothelial cells is one possible mechanism for this phenomenon as endothelial cells can bind directly or indirectly with anti-dsDNA antibody immune complexes.21 There is precedent in the diabetes literature for endothelial cell activation through increased reactive intermediate production and subsequent endothelial cell damage.22 The mechanism through which SD-3651 might interfere with this activation is unknown; however, it is logical to consider effects on reactive intermediate production. Endogenous NO production can interfere with mitochondrial cytochrome C oxidase activity, leading to increased superoxide production in endothelial cells. This process is inhibited by L-NMMA and eNOS siRNA.23 If SD-3651 inhibits eNOS, this would be a plausible hypothesis worth testing, although our current data provide no evidence to support effects of SD-3651 on eNOS.
There is similar evidence in the diabetes literature for increased reactive oxygen production as a common final pathway for podocyte damage in diabetic nephropathy.24 Podocyte ROI production is stimulated by hyperglycemia, and signs of podocyte activation such as increased vascular endothelial growth factor production are ameliorated by antioxidants.25 In diabetes, there are also angiotensin II–mediated increases in podocyte NADPH oxidase activity.26 In human lupus nephritis, the EM finding that correlated the most with nephrotic range proteinuria in patients with nonproliferative nephritis was the extent of podocyte effacement.27,28 This would suggest that improvement in podocyte effacement by SD-3651 therapy may have more bearing on the extent of proteinuria in these mice than the extent of endothelial cell swelling. The mechanism of podocyte activation in lupus nephritis is less well studied, but in models of membranous nephropathy, sublytic levels of C5-9 complement activation lead to upregulation of podocyte genes that produce oxidant injury and increase protease and extracellular matrix production.29 One might hypothesize that redox signaling is the final common pathway toward podocyte pathology in lupus nephritis as it is in diabetic nephropathy.
These experiments reveal seemingly antithetical effects of genetic and pharmacological manipulation of iNOS on autoimmunity in the MRL/lpr mouse model of lupus nephritis. On one hand, genetic deficiency of the NOS2 gene leads to increased humoral autoimmunity without affecting inflammatory glomerular lesions or proteinuria. The mechanism of this modulatory role of iNOS on humoral autoimmunity is unknown. The effect of SD-3651 therapy in NOS2-deficient MRL/lpr mice is to prevent glomerular endothelial cell swelling and podocyte effacement, an effect that is independent of competitive inhibition of iNOS activity. These studies challenge the notion that iNOS alone is sufficient for the progression of lupus nephritis in this model and increase the likelihood that the effectiveness of amino acid-based iNOS inhibitor therapy in murine lupus nephritis also results from non–iNOS-mediated mechanisms. The effects of SD-3651 on proteinuria in the face of continued proliferative disease isolate the importance of podocyte and/or endothelial cell pathology on proteinuria in this model of mixed proliferative, mesangial, and membranous glomerulonephritis. Pursuit of these non–iNOS-mediated effects of SD-3651 may lead to novel therapies to treat lupus nephritis.
Acknowledgments
The work of Drs Oates and Gilkeson was supported by grants K08AR002193 and R01AR045476 from the National Institutes of Health, Bethesda, MD, and Career Development, Research Enhancement Award Program, and Merit Review grants from the Medical Research Service, Ralph H. Johnson Veterans Affairs Medical Center, Charleston, SC.
The flow cytometry studies were made possible through the kind assistance of Dr Hai Qun Zheng, MD, at the Ralph H. Johnson Veterans Affairs Medical Center flow cytometry facility, and the FACSCalibur flow cytometer (Beckton Dikenson, San Jose, CA) was purchased by the Ralph H. Johnson Veterans Affairs Medical Research Service. The SD-3651 was made available through a gift from Pfizer Global Research and Development, Groton, CT. The NOS2−/− mice were a kind gift from Victor E. Laubach, PhD, at GlaxoSmithKline, Middlesex, UK.
References
- 1.Oates JC, Gilkeson GS. Nitric oxide in SLE. In: Tsokos GC, Gordon C, Smolen JS, editors. Systemic Lupus Erythematosus: A Companion to Rheumatology. 1. Philadelphia, PA: Mosby Elsevier; 2007. [Google Scholar]
- 2.Oates JC, Gilkeson GS. The biology of nitric oxide and other reactive intermediates in systemic lupus erythematosus. Clin Immunol. 2006;121:243–250. doi: 10.1016/j.clim.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg JB, Granger DL, Pisetsky DS, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly CM, Farrelly LW, Viti D, et al. Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase. Kidney Int. 2002;61:839–846. doi: 10.1046/j.1523-1755.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Oates JC, Ruiz P, Alexander A, et al. Effect of late modulation of nitric oxide production on murine lupus. Clin Immunol Immunopathol. 1997;83:86–92. doi: 10.1006/clin.1997.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilkeson GS, Mudgett JS, Seldin MF, et al. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997;186:365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laubach VE, Shesely EG, Smithies O, et al. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci U S A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekine H, Graham KL, Zhao S, et al. Role of MHC-linked genes in autoantigen selection and renal disease in a murine model of systemic lupus erythematosus. J Immunol. 2006;177:7423–7434. doi: 10.4049/jimmunol.177.10.7423. [DOI] [PubMed] [Google Scholar]
- 9.Braman RS, Hendrix SA. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Anal Chem. 1989;61:2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- 10.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 11.McAnulty SR, McAnulty LS, Nieman DC, et al. Influence of carbohydrate ingestion on oxidative stress and plasma antioxidant potential following a 3 h run. Free Radic Res. 2003;37:835–840. doi: 10.1080/1071576031000136559. [DOI] [PubMed] [Google Scholar]
- 12.Hayat MA. Principles and techniques of electron microscopy; biological applications. New York, NY: Van Nostrand Reinhold Co; 1970. [Google Scholar]
- 13.Oates JC, Gilkeson GS. Nitric oxide induces apoptosis in spleen lymphocytes from MRL/lpr mice. J Investig Med. 2004;52:62–71. doi: 10.1136/jim-52-01-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruijter JM, Thygesen HH, Schoneveld OJ, et al. Factor correction as a tool to eliminate between-session variation in replicate experiments: application to molecular biology and retrovirology. Retrovirology. 2006;3:2. doi: 10.1186/1742-4690-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy G, Perl A. The role of nitric oxide in abnormal T cell signal transduction in systemic lupus erythematosus. Clin Immunol. 2006;118:145–151. doi: 10.1016/j.clim.2005.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boer R, Ulrich WR, Klein T, et al. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- 17.Mori M, Gotoh T. Regulation of nitric oxide production by arginine metabolic enzymes. Biochem Biophys Res Commun. 2000;275:715–719. doi: 10.1006/bbrc.2000.3169. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt K, Klatt P, Mayer B. Uptake of nitric oxide synthase inhibitors by macrophage RAW 264.7 cells. Biochemical J. 1994;301:313–316. doi: 10.1042/bj3010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz D, Schwartz IF, Gnessin E, et al. Differential regulation of glomerular arginine transporters (CAT-1 and CAT-2) in lipopolysaccharide-treated rats. Am J Physiol Renal Physiol. 2003;284:F788–F795. doi: 10.1152/ajprenal.00221.2002. [DOI] [PubMed] [Google Scholar]
- 20.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 21.Chan TM, Cheng IK. Identification of endothelial cell membrane proteins that bind anti-DNA antibodies from patients with systemic lupus erythematosus by direct or indirect mechanisms. J Autoimmun. 1997;10:433–439. doi: 10.1006/jaut.1997.9998. [DOI] [PubMed] [Google Scholar]
- 22.Kruger AL, Peterson SJ, Schwartzman ML, et al. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 23.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 24.Marshall SM. The podocyte: a potential therapeutic target in diabetic nephropathy? Curr Pharm Des. 2007;13:2713–2720. doi: 10.2174/138161207781662957. [DOI] [PubMed] [Google Scholar]
- 25.Lee EY, Chung CH, Kim JH, et al. Antioxidants ameliorate the expression of vascular endothelial growth factor mediated by protein kinase C in diabetic podocytes. Nephrol Dial Transplant. 2006;21:1496–1503. doi: 10.1093/ndt/gfl022. [DOI] [PubMed] [Google Scholar]
- 26.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 27.Han TS, Schwartz MM, Lewis EJ. Association of glomerular podocytopathy and nephrotic proteinuria in mesangial lupus nephritis. Lupus. 2006;15:71–75. doi: 10.1191/0961203306lu2264oa. [DOI] [PubMed] [Google Scholar]
- 28.Kraft SW, Schwartz MM, Korbet SM, et al. Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2005;16:175–179. doi: 10.1681/ASN.2004050350. [DOI] [PubMed] [Google Scholar]
- 29.Couser WG, Nangaku M. Cellular and molecular biology of membranous nephropathy. J Nephrol. 2006;19:699–705. [PubMed] [Google Scholar]