Summary
The purinergic receptor P2X7 is involved in cell death, inhibition of intracellular infection and secretion of inflammatory cytokines. The role of the P2X7 receptor in bacterial infection has been primarily established in macrophages. Here we show that primary gingival epithelial cells, an important component of the oral innate immune response, also express functional P2X7 and are sensitive to ATP-induced apoptosis. Porphyromonas gingivalis, an intracellular bacterium and successful colonizer of oral tissues, can inhibit gingival epithelial cell apoptosis induced by ATP ligation of P2X7 receptors. A P. gingivalis homologue of nucleoside diphosphate kinase (NDK), an ATP-consuming enzyme, is secreted extracellularly and is required for maximal suppression of apoptosis. An ndk-deficient mutant was unable to prevent ATP-induced host-cell death nor plasma membrane permeabilization in the epithelial cells. Treatment with purified recombinant NDK inhibited ATP-mediated host-cell plasma membrane permeabilization in a dose-dependent manner. Therefore, NDK promotes survival of host cells by hydrolysing extracellular ATP and preventing apoptosis-mediated through P2X7.
Introduction
Bacteria that initiate infections at host epithelial surfaces are, in many instances, capable of entry into and survival within in host cells. Intracellular bacterial pathogens have evolved sophisticated mechanisms that enable them to prevail over host defences, replicate and successfully colonize host tissues (Rhoades and Ullrich, 2000; Amieva et al., 2002; Phalipon and Sansonetti, 2007). Many of these pathogens, including Helicobacter pylori, Salmonella enterica serovar Typhimurium and Mycobacterium and Chlamydia species are also known to prolong the integrity of their host-cell environment by inhibiting host-cell death. The relationship between bacterial microorganisms and apoptotic processes is a regulated and complex phenomenon, many aspects of which are yet to be explored. Epithelial cells, which are often the first line of defence and target sites of microbial infections, can show an elevated or repressed apoptotic response depending on the nature of the microbial challenge (Monack and Falkow, 2000; Byrne and Ojcius, 2004; Knodler et al., 2005).
Porphyromonas gingivalis, a Gram-negative anaerobe, is a major pathogen in severe and chronic forms of periodontal disease and has recently been identified as a risk factor in severe systemic conditions such as coronary heart disease (Socransky and Haffajee, 1992; Herzberg and Weyer, 1998; Kuramitsu et al., 2001). P. gingivalis can replicate, survive and later disseminate intercellularly within and through the epithelial cells that line host gingival tissues (Lamont et al., 1995; Yilmaz et al., 2004; 2006). Gingival epithelial cells (GECs), an important arm of the innate immune response of oral tissues, are among the first host cells colonized by P. gingivalis (Schroeder and Listgarten, 1997; Weinberg et al., 1998; Nisapakul-torn et al., 2001; Lamont and Yilmaz, 2002). The organism modulates a variety of signalling pathways and phenotypic properties of the epithelial cells, including inhibition of interleukin-8 secretion, modulation of intracellular calcium concentrations, and activation of integrin receptor-associated signalling pathways (Darveau et al., 1998; Yilmaz et al., 2002; Zhang et al., 2005). More importantly, P. gingivalis, which replicates to high levels intracellularly, does not induce host-cell death; instead, the infected GECs are resistant to apoptosis induced by potent pro-apoptotic agents (Yilmaz et al., 2004; Mao et al., 2007). Previously, it has been shown that P. gingivalis promotes host-cell survival by inhibiting mitochondrion-dependent apoptosis, activating the phosphatidylinositol 3-kinase (PI3K)/AKT (protein kinase B) pathway, inhibiting caspase-3 activation through upregulation of JAK/Stat signalling, and balancing the expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 (Nakhjiri et al., 2001; Yilmaz et al., 2004; Mao et al., 2007). P. gingivalis thus uses diverse mechanisms to modulate cell death pathways to ensure its intracellular survival. Nevertheless, the physiological upstream modulators of inhibition of apoptosis in the infected cells remain to be characterized.
The purinergic receptor, P2X7, has emerged recently as an important mediator of apoptosis, in addition to its role in initiation of inflammatory responses, control of infection by intracellular bacteria and stimulation of cell proliferation (Chen and Brosnan, 2006; Lister et al., 2007). Thus, extracellular ATP (ATPe) ligation of P2X7 receptors on macrophages produces a variety of cellular effects, including activation of an inflammasome, maturation and release of interleukin-1β, and induction of macrophage death by apoptosis and/or necrosis. ATP released from infected cells or stressed cells at sites of inflammation is thus viewed as a generic ‘danger signal’ that can alert the innate immune system to the presence of infection (Surprenant et al., 1996; Schwiebert and Zsembery, 2003; Khakh and North, 2006; Mariathasan and Monack, 2007).
A number of studies have shown that some intracellular bacterial pathogens such as Mycobacterium tuberculosis and Chlamydia psittaci that can cause persistent infections have the ability to inhibit P2X7-mediated apoptosis of their host cells (Lammas et al., 1997; Zaborina et al., 1999; Coutinho-Silva et al., 2001; Fairbairn et al., 2001; Franchi et al., 2007). In most cases, the bacterial factors responsible for inhibition of host-cell death have not been identified. In addition, the studies were conducted with infected macrophages, which are not the preferred host cells for many pathogens. The ability of infection to modulate P2X7 activity in epithelial cells remains poorly characterized, partly due to a functional P2X7 deficiency in the favourite epithelial cell line used to study many infections, the cervical carcinoma HeLa (Feng et al., 2006).
The present report shows for the first time that primary GECs express purinergic P2X receptors, including a functional P2X7, and are sensitive to ATPe-induced apoptosis. Consistent with the need of P. gingivalis to proliferate within the same cells, ATPe-mediated GEC apoptosis is inhibited by infection with P. gingivalis. No P. gingivalis factors that can inhibit apoptosis had been identified until now. This study demonstrates that a putative P. gingivalis ecto-nucleoside diphosphate kinase (NDK), which can scavenge ATPe, is involved in inhibition of P2X7-mediated apoptosis. An ndk-deficient mutant was no longer able to inhibit ATP-induced apoptosis of infected GECs and a recombinant P. gingivalis-NDK significantly inhibits one of the early events of ATPe-mediated P2X7 activation, plasma membrane permeabilization, in uninfected GECs and GECs infected with ndk-deficient mutant.
Results
Expression of the P2X7 receptor in primary GECs
To determine whether GECs express the ionotropic purinoreceptor family of receptors (P2X1−7), particularly the P2X7 receptor, the cells were analysed by reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence microscopy. The RT-PCR analysis showed that amplicons of the expected sizes were present for at least five P2X receptors: P2X2, P2X4, P2X5, P2X6 and P2X7 (Fig. 1A). Amplification of P2X6 gave an additional smaller band, previously shown to be the result of alternative splicing (Coutinho-Silva et al., 2005a). P2X1 and P2X3 were not expressed in GECs, indicating tissue specificity for these receptors. Immunofluorescence microscopy confirmed the uniform surface expression of the P2X7 receptor throughout the GEC plasma membrane (Fig. 1B), although a large proportion of the receptors appeared to be distributed in granular compartments, reminiscent of endocytic or secretory vesicles. Thus, the GECs, which comprise the initial lining of the gingival mucosa, express most P2X receptor family members, and particularly P2X7.
Fig. 1. Detection of P2X receptor(s) in GECs.
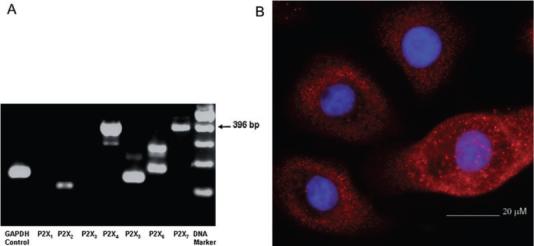
A. RT-PCR measurement of receptor mRNA. mRNA expression of P2X7 and other P2X receptors was verified by RT-PCR. Amplicons with predicted sizes were present for P2X2, P2X4, P2X5, P2X6 and P2X7. The arrow indicates the predicted 396 bp transcript for P2X7 receptor. B. Localization of P2X7 by immunofluorescence microscopy. GECs were fixed and incubated with anti-P2X7 antibody followed by Alexa-Fluor 594 conjugated secondary antibody (red). DAPI (blue) was used for staining the nucleus. The staining displayed strong and uniform expression of P2X7 receptors in the GECs, along with some punctuate staining consistent with lysosomes or endosomes.
Apoptosis of GECs Due to treatment with extracellular nucleotides
Ionotropic P2X receptors, such as P2X7, are linked to pores that switch their conformation from closed to open on binding of ATP, allowing ions such Ca++ and K+ to flow into cells. In macrophages, prolonged ATPe treatment leads to P2X7-dependent necrosis or apoptosis (Lemaire et al., 2006; Lister et al., 2007). In order to evaluate whether the P2X7 receptor may be functional, we examined the sensitivity of GECs to ATPe-mediated apoptosis. GECs were incubated with control buffer or 5 mM of the indicated nucleotides for 1 h in Mg-free buffer phosphate-buffered saline (PBS). The buffer was then removed and cells incubated in GEC culture-medium for an additional 24 or 48 h. Apoptosis was quantified by Annexin-V/propidium iodide (PI) staining and analysed by cytofluorimetry (Fig. 2A). Treatment with the P2X7 agonist ATP caused a significant increase in the level of apoptosis compared with control samples, whereas nucleotides such as ADP and UTP had no effect on the level of apoptosis (Fig. 2A). These initial results indicate that GECs are sensitive to ATPe-induced apoptosis and suggest a role for the P2X7 receptor. However, in the light of a recent report (Solini et al., 2007) demonstrating that the P2X4 receptor could also mediate ATP-induced apoptosis, we examined P2X4 protein expression in GECs by immunofluorescence microscopy and detected very low levels of P2X4 protein (not shown). Thus, we can not exclude the possibility that P2X4, although expressed at much lower levels than P2X7 protein, could contribute to some degree to ATP-induced apoptosis of GECs.
Fig. 2. Effect of P2X7 agonists and antagonists on GEC apoptosis.
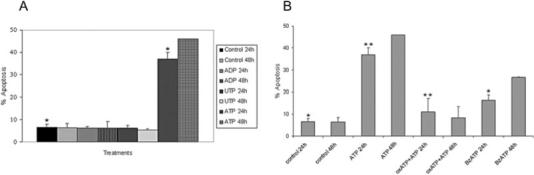
A. Effect of ATP and other nucleotides on apoptosis. GECs were incubated with control buffer or 5 mM of the indicated nucleotides for 1 h in Mg-free buffer (PBS). The buffer was then removed and cells were incubated in GEC culture medium for an additional 24 or 48 h. Apoptosis was quantified by cytofluorimetry using AnnexinV/PI staining. Error bars represent the standard deviations of at least two independent measurements (*P = 0.02 Student t-test).
B. Effect of oxATP and BzATP on apoptosis. GECs were incubated with the P2X7 agonist, BzATP (2 mM), for 1 h or alternatively with the antagonist oxidized ATP, oxATP (0.3 mM), for 2 h. Then, the antagonist was removed and ATP (5 mM) in Mg-free buffer (PBS) added for 1 h, followed by 24 or 48 h incubation with GEC culture medium. Apoptosis was quantified by cytofluorimetry using AnnexinV/PI staining. Error bars represent the standard deviations (± SD) of at least two independent measurements (**P = 0.01 and *P < 0.1 Student t-test).
Effect of P2X7 receptor agonists and antagonists on GEC apoptosis
The specificity of the receptor for ATP was further evaluated by measuring the effect of various P2X7 agonists and antagonists on apoptosis. ATP-induced apoptosis of GECs could be significantly blocked by pretreatment with the irreversible P2X7 antagonist oxidized (ox) ATP (0.3 mM); and incubation with the selective P2X7 agonist, benzoylbenzoyl (BzATP, 2 mM), stimulated a large level of apoptosis in GECs (Fig. 2B). Thus, the results suggest that P2X7 receptors in GECs are functional and are likely to be responsible for most of the apoptosis mediated by ATPe.
Inhibition of ATP-induced apoptosis by P. gingivalis infection
In macrophages, infection with mycobacteria or chlamydiae inhibits partially P2X7-mediated host-cell death (Lammas et al., 1997; Coutinho-Silva et al., 2001). In order to determine whether infection of epithelial cells with an intracellular pathogen may have a similar effect, we examined ATPe-induced apoptosis in GECs infected with P. gingivalis. Hence, the epithelial cells were pre-infected with P. gingivalis for 12 h before 1 h treatment with 5 mM ATPe followed by a 24 or 48 h infection. The percentage of apoptotic cells was measured by Annexin-V/PI staining. Consistent with previous findings (Yilmaz et al., 2004), infection by itself for 24 or 48 h induced only a low level of apoptosis (Fig. 3), but P. gingivalis infection suppressed considerably the pro-apoptotic effect of ATP (Fig. 3).
Fig. 3.
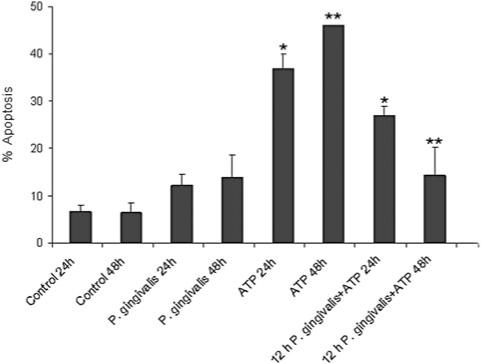
Porphyromonas gingivalis infection inhibits ATP-induced apoptosis in GECs. GECs were preinfected with P. gingivalis for 12 h before they were treated with 5 mM ATP in Mg-free buffer (PBS) for 1 h, followed by a 24 or 48 h infection period in GEC culture medium. Apoptosis was quantified by cytofluorimetry using AnnexinV/PI staining. Error bars represent the ± SD of at least two independent measurements. Asterisks (* and **) denote statistical significance (P < 0.05 Student t-test) between 12 h P. gingivalis + ATP treated and samples treated with ATP alone, respectively, for 24 and 48 h.
Identification of a P. gingivalis protein with homology to NDK
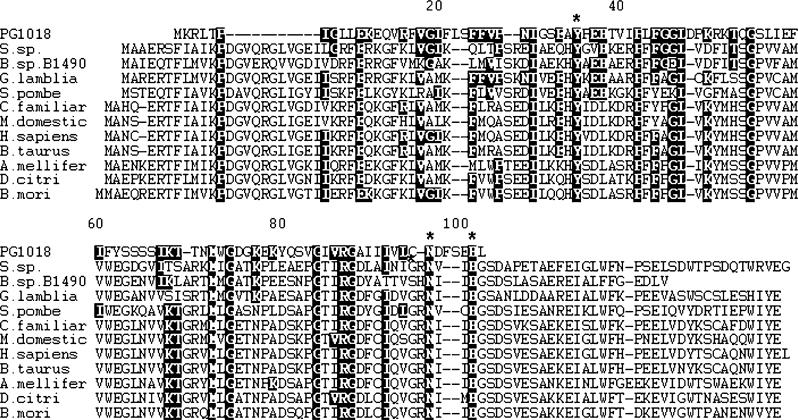
A BLAST similarity search of the P. gingivalis W83 proteome (Nelson et al., 2003), using the Bacillus anthracis NDK protein NP843987 as query, revealed a weak similarity (e = 0.002) to an unannotated hypothetical protein PG1018. A subsequent BLAST search of the non-redundant protein database using PG1018 as query revealed additional weak similarities to NDK homologues from a number of other bacillus strains and a putative protein from Giardia lamblia that has strong similarities to NDK proteins. The 102 amino acid sequence of PG1018 was aligned with a number of NDK proteins from organisms ranging from bacteria to humans (Fig. 4). The known NDK proteins, each containing ∼150 amino acids, showed strong sequence similarity to each other, with only a small amount of heterogeneity at the N- and C-termini. Optimal alignment of the PG1018 sequence required several gaps to be inserted into the alignment. However, significant amino acid conservation between the PG1018 and NDK proteins was observed. Importantly, Tyr34, Asn96 and His101 were conserved with residues in the NDK family of proteins that have been shown to be critical for kinase function (Schneider et al., 2001) (Fig. 4). This analysis suggests that PG1018 could be classified as a distantly related kinase belonging to the NDK family of kinases. In addition, recombinant PG1018 protein derived from P. gingivalis was able to hydrolyse ATP with a Km of 8.9 mM and a Kcat/Km equal to 1.62 min−1 μM−1 (not shown), similar to the values reported for M. tuberculosis (Kumar et al., 2005) and reinforcing its classification as an NDK enzyme.
Fig. 4.
Sequence comparison of the putative P. gingivalis-NDK with NDK from different organisms. In the sequences an asterisk indicates residues implicated in NDK kinase activity: Y35, N96 and H101. Highly conserved residues are indicated by dark background and gaps are indicated by dashes. Accession numbers for the sequences are P. gingivalis PG1018 NP905239.1; G. lamblia XP771399.1; Bacillus sp. B14905ZP01725465.1; B. mori ABF51506.1; B. taurus NP991387.1; Schizosaccharomyces pombe NP592857.1; D. citri ABG81980.1; P. pinaster CAC84493.1; A. mellifera XP393351.2; Synechococcus sp. CC9311 YP731929.1; Heliobacillus mobilis AAC84038.1; C. familiaris NP001019809.1; Mus domestica XP001363771.1; Homo sapiens NP000260.1. Sequence alignment of the PG1018 protein and representative NDK proteins from organisms ranging from bacteria to humans was performed using ClustalW.
Inhibition of ATP-mediated cell death by the P. gingivalis homologue of NDK
Supernatants from M. tuberculosis, another successful persistent intracellular bacterium, have been shown to contain the ATP-scavenging enzyme, NDK, and to inhibit ATP-induced death of macrophages (Zaborina et al., 1999). To establish a role for the P. gingivalis NDK homo-logue more directly, we therefore constructed an isogenic ndk mutant strain of P. gingivalis 33277 by allelic exchange, naming the ndk-deficient strain ΔPG1018. To ensure that any difference in the apoptotic properties of the mutant are not the result of changes in invasion efficiency, intracellular levels of ΔPG1018 were quantified by fluorescence microscopy, as we previously described (Yilmaz et al., 2006). Infection by both the wild-type and mutant strains showed similar numbers of intracellular bacteria at both early time points (1 h, 6 h, 12 h and 24 h) post infection (data not shown) and later time points (48 h) (Fig. 5A), confirming that invasion and infection efficiency was similar for both strains. Subsequently, the culture supernatants and pellets from the wild-type and ΔPG1018 strains were assayed for ATPase activities of the secreted and intracellular forms of the P. gingivalis NDK protein, using ATP as a substrate. The rate of ATP hydrolysis was found to be significantly lower in the mutant strain, compared with the wild-type strain (Fig. 5B), demonstrating that the P. gingivalis NDK homologue is secreted and consumes ATP.
Fig. 5. Infection and ATPase Activity of wild-type and ndk-deficient P. gingivalis strains.
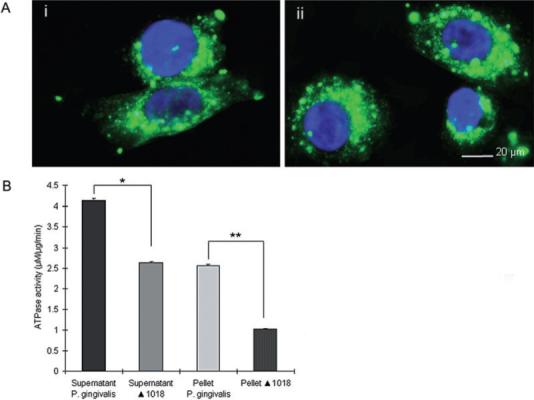
A. Immunofluorescence microscopy of invasion (48 h) by the wild type (i) and the mutant ΔPG1018 (ii) strains. GECs were infected with wild type or ΔPG1018 (ndk-deficient mutant) at an moi of 100. The samples were fixed and stained with P. gingivalis antibody and then incubated simultaneously with Oregon Green 488 secondary antibody (green) and DAPI (blue) to visualize the bacterial infection. Results are representative images of 150 cells studied per sample from at least two individual experiments performed in duplicate. B. ATPase activity of the wild-type and mutant strains. The enzymatic activities were measured in the samples containing 0.01 mg ml−1 protein from the supernatants and 1 mg ml−1 protein from the pellets of the wild-type and mutant strains by calculating the μM Pi generated per minute. (*P < 0.01; and **P < 0.001 Student t-test, n = 4).
Next, in order to assess the relevance of P. gingivalis-NDK for protection against apoptosis induced by ATPe, GECs were infected with the wild-type or ΔPG1018 strain in the presence of ATP or control buffer (no ATP) for 24 or 48 h. Uninfected cells were also treated with ATP, UTP or ADP for 24 or 48 h (not shown). Induction of apoptosis by ATP was confirmed using the TUNEL technique, which measures single-strand breaks in DNA. In agreement with the level of apoptosis measured by Annexin-V/PI staining, we found that incubation with ATP, but not UTP or ADP, leads to apoptosis in > 45% of the uninfected cells (Fig. 6A), and 12 h preinfection with the wild-type strain protects cells against apoptosis induced by subsequent incubation with ATP for 24 or 48 h. In contrast, ΔPG1018 infection by itself induced a low level of apoptosis after 24 or 48 h of infection, and 12 h preinfection with the mutant strain did not protect GECs against apoptosis following subsequent incubation with ATP (Fig. 6B). In fact, GECs preinfected with ΔPG1018 were more sensitive to ATP-induced apoptosis than uninfected cells as observed by the larger amount of cells dying after infection with the mutant in the presence of ATP (Fig. 6B). In order to verify the specific effect of the mutagenesis, the mutant strain was complemented by the wild-type ndk allele in trans. GECs that were infected with the complemented strain and incubated with ATP displayed similar levels of apoptosis induced by the wild strain (Fig. 6B). Thus, the results suggest a major role for the NDK homologue in the survival of ATP-treated host cells.
Fig. 6. DNA fragmentation of cells infected with the wild type, ndk-deficient and complemented strains.
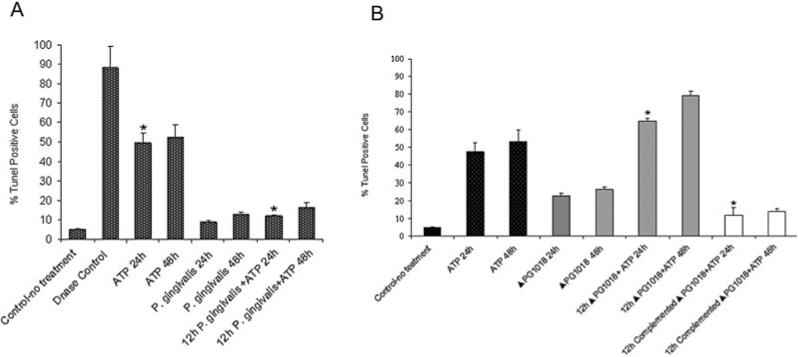
A. TUNEL assay determining the level of DNA fragmentation following treatment of wild-type P. gingivalis-infected cells with and without ATP. GECs were fixed and stained with TUNEL reagents and visualized by fluorescent microscopy to determine the level of DNA fragmentation. Samples incubated with DNAse and without any treatment, and without TUNEL enzyme (not shown) served as controls (*P < 0.05, Student t-test, n = 2).
B. TUNEL assay determining the level of DNA fragmentation following treatment of ndk-deficient mutant (ΔPG1018) and the complemented strain-infected cells with ATP. DNA fragmentation in GECs was measured as described in Fig. 6A. For both A and B, results represent means ± SD of approximately 150 cells studied per sample from at least two individual experiments performed in duplicate (*P < 0.005, Student t-test, n = 2).
Effect of ndk mutation on ATP-induced membrane permeabilization
The P. gingivalis-NDK homologue could either inhibit a P2X7-dependent apoptotic pathway in the cell or deplete extracellular ATP before ligation of P2X7. To discriminate between these possibilities, we therefore measured the effects of infection by wild-type and ndk-deficient strains on an early event following P2X7 ligation, namely plasma membrane permeabilization. In this regard, P2X7 ligation leads to opening of ion channels that are selective for small ions, followed by the opening of larger non-specific pores that are permeable to molecules smaller than 900 Da (Surprenant et al., 1996; Khakh and North, 2006). We investigated whether P. gingivalis infection could block P2X7-mediated permeabilization by using a 457 Da membrane-impermeant fluorescent dye, Lucifer Yellow (LY). As shown in Fig. 7A, cells treated with ATP showed a significant time dependent increase in LY uptake, whereas cells infected with the wild-type strain showed no significant increase in LY uptake and also demonstrated a dramatic ability to decrease LY uptake in response to ATP treatment. Approximately 83% of cells were stained with LY upon 1 h ATPe treatment followed by 48 h incubation, whereas the wild type-infected cells were only 20% positive for dye uptake after treatment with ATPe (similar to basal levels). Conversely, cells infected with the ΔPG1018 strain and incubated with ATP under the same conditions exhibited a significantly reduced level of inhibition of permeabilization (approximately 59% of the cells were positive for LY uptake after treatment with ATP). Accordingly, the results indicate that the NDK homologue of P. gingivalis interferes at an early step of P2X7-dependent signalling, suggesting that it acts before downstream apoptotic signalling events.
Fig. 7. Plasma membrane permeabilization of GECs infected with wild-type P. gingivalis and the ndk mutant strain following treatment with rNDK.
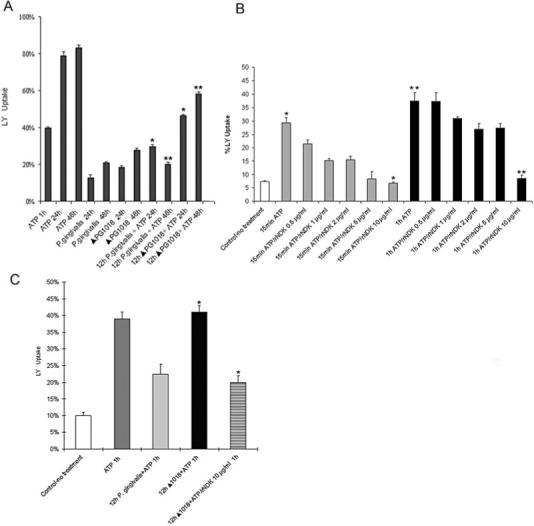
A. Differential internalization of LY (457 Da) by GECs during infection by P. gingivalis and ndk-deficient mutant (ΔPG1018) was visualized by fluorescence microscopy. The threshold of fluorescence intensity was determined with use of a sample that was uninfected and untreated. Values represent means ± SD of approximately 300 cells studied per sample from at least two individual experiments performed in duplicate (*P < 0.005; and **P < 0.01, Student t-test).
B. Differential internalization of LY by GECs following treatment with ATP and rNDK. LY uptake in GECs was measured as described in Fig. 7A. GECs were treated with 5 mM ATP and the varying concentrations of rNDK for 15 min or 1 h in Mg-free buffer (PBS). The starting concentration of rNDK was determined from the ATPase activity assay of the enzyme (*P = 0.01; and **P = 0.001, Student t-test).
C. Differential internalization of LY by ndk-deficient strain (ΔPG1018) infected GECs following treatment with ATP and rNDK. The results were obtained as described in Fig. 7A. GECs were infected with ndk-deficient strain for 12 h and then incubated with rNDK in the presence of 5 mM ATP for 1 h in Mg-free buffer (PBS) (*P = 0.03, Student t-test).
The ability of NDK to interfere with P2X7 ligation was confirmed by examining the effect of recombinant NDK (rNDK) on the P2X7-mediated dye uptake in uninfected cells. ATP-evoked LY uptake was significantly inhibited in a concentration dependent manner within 15 min and 1 h exposure to rNDK in uninfected GECs (Fig. 7B). Moreover, incubation of rNDK with the ndk-deficient strain (ΔPG1018) infected cells during the ATPe treatment greatly inhibited the ATP-induced dye uptake in the infected cells (Fig. 7C). The results decisively support the concept that the NDK homologue of P. gingivalis serves as a critical molecule for the inhibition of ATP-induced cell death in GECs.
Discussion
Epithelial cells represent a major line of initial defence against pathogenic bacteria that colonize mucosal tissues, but at the same time represent an initial site for host invasion (Ganz, 2002). An increasing number of intracellular bacterial pathogens are now known to modulate apoptotic pathways in epithelial cells to establish persistent infection (Monack and Falkow, 2000). P. gingivalis, a successful oral colonizer, is capable of intracellular replication and remains viable for extended periods in primary GECs (Rudney et al., 2001; Yilmaz et al., 2006). Interestingly, despite the burden of large numbers of intra-cellular bacteria, infected GECs do not undergo cell death and are instead resistant to chemically induced apoptotic cell death. P. gingivalis infection modulates several anti-apoptotic pathways in addition to interfering with other cell-signalling pathways and disseminating intercellularly through actin-based membrane protrusions (Yilmaz et al., 2004; 2006; Mao et al., 2007).
The P2X7 receptor has been studied primarily in cells of haemopoietic origin (Lister et al., 2007), but recent studies indicate that it is also expressed in some epithelial cells (Schwiebert and Zsembery, 2003; Pastore et al., 2006), although its function in epithelial cells remains largely uncharacterized. In macrophages, the receptor is involved in a number of essential cellular events including regulation of apoptosis, control of intracellular bacterial infection, and modulation of inflammatory immune responses (Coutinho-Silva et al., 2001; Fairbairn et al., 2001; Chen and Brosnan, 2006; Mariathasan and Monack, 2007). P2X7 receptor activation requires high concentrations of extracellular ATP (e.g. 100 μM-10 mM), but these concentrations can be attained at sites of bacterial infection and inflammation (Khakh and North, 2006; Idzko et al., 2007). P2X7-deficient mice are resistant to development of anticollagen-induced arthritis (Labasi et al., 2002) and accumulate lower levels of IL-1β in inflamed foot pads induced by injection of Freund's complete adjuvant (Chessell et al., 2005), suggesting that ATP concentrations sufficiently high to stimulate P2X7 can be produced in vivo under physiological conditions. ATP can also be released extracellularly in a regulated manner, and its release into the extracellular space from necrotic cells is expected to result in the activation of P2X7 receptors (Khakh and North, 2006). ATP released into the external milieu can thus serve as a ‘danger signal’ for other components of immune system and initiate a prompt innate immune response by triggering secretion of IL-1β, generating reactive oxygen species, inducing apoptosis and killing of intracellular pathogens following ligation of the danger signal receptor, P2X7. Conversely, these special signalling events can be modulated by intracellular bacteria to persist in the host cells (Coutinho-Silva et al., 2001; Fairbairn et al., 2001; Franchi et al., 2007). Several intracellular pathogens including Chlamydia and Mycobacterium species can enhance their survival by partially inhibiting host-cell apoptosis. In the case of M. tuberculosis, supernatant from the mycobacteria containing the NDK protein decreases the activity of the P2X7 receptor (Zaborina et al., 1999).
In the present study, we demonstrate for the first time that an intracellular pathogen can interfere with P2X7-dependent apoptosis of epithelial cells, and identify a likely mechanism. Primary GECs, which form an initial interactive interface with colonizing oral bacteria, express functional P2X7 purinergic receptors and are sensitive to apoptosis induced by the P2X7 agonists ATP and BzATP. Moreover, treatment of GECs with control nucleotides such as ADP and UTP did not induce apoptosis, while the specific P2X7 antagonist, oxATP, blocked ATP-induced death. Collectively these results further corroborate the role of P2X7 in ATP-induced apoptosis and establish the functionality of this mechanism in epithelial cells.
The host-adapted pathogen P. gingivalis suppresses P2X7-ATP-mediated apoptosis. This effect is mediated by a putative NDK homologue of P. gingivalis, as evidenced by a significant reversal of apoptosis suppression in cells infected with an ndk-deficient strain and the ability of rNDK to inhibit significantly ATP-induced plasma membrane permeabilization in both uninfected cells and cells infected with the ndk-deficient strain. In addition, analysis of the levels of intracellular bacteria during the 48 h infection period of GECs in the absence of ATPe treatment for wild-type versus ndk-deficient strain demonstrates comparable values, suggesting that the main role of the. NDK homologue of P. gingivalis is to interfere with host-cell apoptosis due to stimulation with extracellular ATP. In a similar manner, previous work on macrophages infected with the Mycobacterium bovis Bacille Calmette-Guérin has shown that induction of apoptosis with a Fas ligand (H2O2) did not kill the intracellular bacterium even though it caused the death of the host cells. However, promoting of apoptosis with ATP through P2X7-signalling stimulated the death of both the bacteria and the host cells (Molloy et al., 1994; Fairbairn et al., 2001). These results highlight the need of intracellular bacteria such as mycobacteria and P. gingivalis to evolve specialized mechanisms for interfering with P2X7 ligation.
In conclusion, our results suggest that NDK is secreted by P. gingivalis and consumes ATPe, which is released at sites of infection, thus preventing activation of P2X7 receptors by ligation with ATP. The consequent inhibition of P2X7-mediated apoptosis and extension of GEC viability would allow P. gingivalis to survive for prolonged periods in the gingival epithelium and contribute to disease when other host and bacterial factors are conducive to the processes of tissue destruction. Moreover, a study (Xia et al., 2007) examining the quantitative proteomics of intracellular P. gingivalis demonstrates the secretion of the NDK homologue during P. gingivalis infection in GECs. Interestingly, in the absence of exogenous ATPe, infection with the mutant lacking NDK results in a higher level of GEC apoptosis than infection with the wild-type strain, suggesting that some ATP may be released from the infected cell. It had been previously reported that ATP is released from macrophages infected with mycobacteria (Sikora et al., 1999), implying that the presence of ATPe may be a general phenomenon associated with many infections with intracellular pathogens. It is thus tempting to speculate that other intracellular bacteria may also produce secreted ATP-scavenging enzymes as a strategy for protecting the host cell. Although future investigations remain to elucidate the exact mechanisms and outcomes of the interaction of NDK with host cells, the enzyme may represent a novel virulence factor of P. gingivalis, and a potential target for therapeutic intervention.
Experimental procedures
Culture of primary GECs
Primary cultures of GECs were generated as described previously (Lamont et al., 1995). Briefly, healthy gingival tissue was obtained after oral surgery, and surface epithelium was separated by overnight incubation with 0.4% dispase. Cells were cultured as monolayers in serum-free keratinocyte growth medium (KGM) (Lonza) at 37°C in 5% CO2. GECs were used for experimentation at 75−80% confluence and cultured for 48 h before infection with bacterial cells or exposure to other test reagents in KGM.
Bacteria, growth conditions, mutant and complementation strain construction
Porphyromonas gingivalis ATCC 33277, the nucleoside diphosphate kinase (ndk)-deficient mutant and the complementation strain were cultured anaerobically for 24 h at 37°C in trypticase soy broth supplemented with yeast extract (1 mg ml−1), haemin (5 μg ml−1) and menadione (1 μg ml−1). Erythromycin (10 μg ml−1) was added to the media for culture of the mutant strain. The media of the complemented strain was supplemented with erythromycin (10 μg ml−1) and tetracycline (3 μg ml−1). All bacteria were grown for 24 h, harvested by centrifugation at 6000 g and 4°C for 10 min, washed twice, and resuspended in Dulbecco's Phosphate-buffered saline (Sigma) pH 7.3 before they were incubated with host cells. The number of bacteria was determined using a Klett-Summerson photometer.
Construction of mutant strain: Mutation in the PG1018 gene was obtained by allelic replacement, using a PCR fusion technique. A DNA sequence containing 1000 bp upstream of the PG1018 initiation codon was amplified from P. gingivalis ATCC 33277 chromosomal DNA using primers PG1018A (5′ACG GCTCTTCGGTTCAATTTGATCTTCTGT3′) and PG1018B (5′AT TTGCGGATAATCATATAGAACCATGCTA3′). A 1000 bp region downstream of the PG1018 stop codon was amplified using primers 1018C (5′TGTATATCAGGCCAAGCAAAGCCACCA CAT3′) and 1018D (5′TCAGAGAAAAAAGTATACTTAGGAAAG AAG3′). To replace the PG1018 gene, an ermF cassette was constructed from pVA3000 using primers ermF-f (5′CATGGTTC TATATGATTATCCGCAAATATGACAAAAAAGAAATTGCCCGTT CG3′) and ermF-r (5′ATGTGGTGGCTTTG CTTGGCCTGATATA CACTACGAAGGATGAAATTTTTCAGGGAC3′). A fusion PCR amplicon was produced using the technique previously described (Kuwayama et al., 2002). The final fusion product was cloned into the pCR2.1-TOPO vector (Invitrogen) and sequenced through the fusion region using the ermF start (5′ATGAACAGTAA GAAACCCCT3′) and ermF stop (5′CTGTCAAATCAGCCCT GTTA3′) sequencing primers. Once the construct was confirmed, the plasmid was linearized with BamHI and introduced into P. gingivalis by electroporation (Simionato et al., 2006). A double crossover recombination event was selected with erythromycin (10 μg ml−1). Insertion of the replacement allele was confirmed by PCR and Southern hybridization.
Construction of complementation strain: A DNA sequence containing 1500 bp upstream of the PG1018 initiation codon and PG1018 gene was amplified from P. gingivalis ATCC 33277 chromosomal DNA using primers, NdkUp (5′-GCGCGGATCCGA TGGACTGCGAGAGCATCTG-3′) and Nd kDaown 3′-CGCGTC GACTCAGAGAGGCAGAGGGCGGTCAATC-5′) which introduced SalI and BamHI sites. The PCR product was digested with SalI and BamHI and ligated into the corresponding sites of shuttle vector plasmid pT-COW (kindly provided by N. Shoemaker, University of Illinois), to create pT-1018, which was introduced into Ndk mutant by conjugation. Erythromycin (10 μg ml−1) and tetracycline (3 μg ml−1)-resistant transconjugants were selected, and the presence of plasmid was confirmed by PCR.
Reverse transcription PCR analysis for P2X receptors in primary GECs
Total RNA was isolated using RNeasy kit (Qiagen) following the manufacturer's instructions. Total RNA was converted into cDNA by standard reverse transcription with M-MLV-Reverse Transcriptase (Promega). cDNAs were amplified using the MJ, Mini (BIO-RAD laboratories) in a 50 ml reaction mixture containing one-fifteenth of the cDNA generated from reverse transcription reaction, 1 × PCR buffer, 2.5 mM MgCl2, 0.25 mM (each) dNTPs, 0.5 μM forward and reverse primers, and 1 U GoTaq DNA polymerase (Promega). The sequences of the primers used for GAPDH, P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 and P2X7 are listed in Table 1 (Coutinho-Silva et al., 2005a). The PCR cycling protocol for all primers was 94°C at 45 s, 60°C at 45 s, 72°C at 45 s. The protocol was conducted for 40 cycles and included an initial 10 min enzyme activation step at 95°C and a final 10 min extension step at 72°C. PCR products were electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining. The intestinal epithelial cells were used as positive controls for the primers for P2X1 and P2X3, which we previously detected in the intestinal cells (Coutinho-Silva et al., 2005b). The PCR products were sequenced to confirm authenticity.
Table 1.
Primers used P2X receptor amplification.
| Gene | Primer sequence, forward (5′-3′) | Primer sequence, reverse (3′-5′) |
|---|---|---|
| P2X1 | GCTGGTGCGTAATAAGAAGGTG | ATGAGGCCGCTCGAGGTCTG |
| P2X2 | AGGTTTGCCAAATACTACAAGATC | GCTGAACTTCCCGGCCTGTC |
| P2X3 | CTTCACCTATGAGACCACCAAG | CGGTATTTCTCCTCACTCTCTG |
| P2X4 | GATACCAGCTCAGGAGGAAAAC | GCATCATAAATGCACGACTTGAG |
| P2X5 | GGCATTCCTGATGGCGCGTG | GGCACCAGGCAAAGATCTCAC |
| P2X6 | AGCACTGCCGCTATGAACCAC | AGTGAGGCCAGCAGCCAGAG |
| P2X7 | TGATAAAAGTCTTCGGGATCCGT | TGGACAAATCTGTGAAGTCCATC |
| GAPDH | AACGGATTTGGTCGTATTGGGC | CTTGACGGTGCCATGGAATTTG |
Surface expression analysis of the p2x7 and p2x4 receptor by immunofluorescence microscopy
Gingival epithelial cells were grown on 4-well chambered glass slides (Nalge-Nunc International), washed with ice-cold PBS, and fixed with 10% neutral buffered formalin for 1 h at room temperature. After washing twice with PBS, the cells were treated with permeabilization solution (0.1% Triton X-100) for 15 min. Samples were then washed twice with PBS and incubated with antibody against an extracellular domain of the human P2X7 receptor (Chemicon) or antibody against human P2X4 (Santa Cruz Biotechnology) and detected with Alexa-Fluor 594 secondary antibody (Invitrogen). Samples with no primary antibody incubation were included as control. The samples were treated with 4,6-diamidino-2-phenylindole (DAPI) 1 μg ml−1 (Sigma) to visualize the nuclei. Finally, the samples were washed twice with PBS and analysed using a Zeiss Axio imager A1 fluorescence microscope equipped with band pass optical filter sets appropriate for imaging of the dyes and a cooled CCD camera (Qimaging). Single exposure images were captured sequentially and saved by Qcapture software v.1394.
Infection of primary GECs with P. gingivalis and treatment with extracellular nucleotides
Gingival epithelial cells were infected at a multiplicity of infection (moi) of 100 with P. gingivalis for 24 or 48 h at 37°C in a 5% CO2 incubator. All time points for the infections were carried backwards, so that all incubations could be stopped and assayed at the same time at the end of 48 h. For measurement of nucleotide-induced apoptosis and inhibition, GECs were incubated with 5 mM concentrations of ATP, ADP, UTP, 2 mM benzoylbenzoyl ATP (BzATP) (Sigma) for 1 h or 0.3 mM oxidized ATP (oxATP) (Sigma) in Mg++ free PBS for 2 h at 37°C in a 5% CO2 incubator. The medium was then removed and replaced with cell culture medium. The cells were incubated for the indicated time points (24 and 48 h). For some assays, cells were infected 12 h with P. gingivalis before ATP treatment in cell culture medium for the additional 24 or 48 h incubations.
Analysis of apoptosis by annexin-v and PI staining by cytofluorimetry
Early apoptotic changes were identified using fluorescein isothiocyanate (FITC)-conjugated Annexin-V-fluos (Roche), which binds to PS exposed on the outer leaflet of apoptotic cell membranes. PI (Sigma) was used for discrimination of necrotic cells from the annexin-V-positively stained cell cluster (Yilmaz et al., 2004). Briefly, GECs were grown on six-well plates (Falcon) at a density of 2 × 105 per well, incubated with the indicated treatments as described above, and dissociated using 0.05% trypsin/0.53 mM EDTA (Gibco BRL). Since apoptotic and necrotic cells could be present in the supernatant, both adherent GECs and cells in suspension were collected. The samples were then washed in cold phosphate-buffered saline (PBS), and resuspended in 100 μl Annexin-V-Fluos binding solution containing 20 μl Annexin-V-Fluos labelling reagent per 1000 ml Hepes buffer (10 mM Hepes/NaOH, pH 7.4, 140 mM NaCl, 5 mM CaCl2) and 1 μg ml−1 PI. After 15 min incubation in the dark at room temperature, 400 μl incubation buffer was added to each sample and the cells were analysed by flow cytometry using 488 nm excitation, a 515 nm band pass filter for fluorescein detection, and a 585 nm filter for PI detection. Cells incubated in the binding buffer with only Annexin-V or PI separately served as controls. For each dye, appropriate electronic compensation of the instrument was performed to avoid overlapping of the two emission spectra.
DNA fragmentation assay by TUNEL
DNA strand breaks induced during apoptosis were identified using a terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay kit following the manufacturer's protocol (Roche Applied Science). Briefly, GECs were grown on 4-well chambered slides, washed with ice-cold PBS, and fixed with 10% neutral buffered formalin for 1 h at room temperature. After washing twice with PBS, the cells were treated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. Samples were then washed twice with PBS and incubated with the TUNEL reaction mixture, containing FITC-labelled dUTP and terminal deoxynucleotidyl transferase, for 1 h at 37°C. Control cells were incubated in the absence (negative control) or presence (positive control) of DNAse (Roche Applied Science), in addition to control samples incubated without deoxynucleotidyl transferase. After incubation, the cells were washed twice with PBS and analysed using the fluorescence microscope system described above. An average of 150 cells per sample was studied from at least 2 separate experiments per condition.
Measurement of Lucifer Yellow (LY) uptake by fluorescence microscopy
Cell membrane permeabilization was visualized by the differential uptake of LY (457 Da) in uninfected GECs or cells infected with the wild-type P. gingivalis or the mutant strain (ΔPG1018) grown on 4-well chambered slides that were treated with 0.25 mg ml−1 LY with and without 5 mM ATP at 37°C for 10 min. The samples were then washed 3 times with PBS and examined under the Zeiss Axio imager A1 microscope described above. LY uptake in the acquired images was then quantified with NIH ImageJ analysis software. Samples treated with 5 mM ATP for 10 min and 2 h served as positive controls. The threshold of fluorescence intensity was determined with sample that was uninfected and untreated. At least 300 cells per sample from 2 separate experiments were analysed to determine the percentage of cells positively stained for LY.
Analysis of P. gingivalis and ndk mutant (ΔPG1018) invasion efficiency by immunofluorescence microscopy
Immunofluorescence labelling and microscopy for determining the level of infection were performed as previously described (Yilmaz et al., 2003; 2006). Briefly, GECs cultivated on the 4-well chambered cover-glass slides were infected with P. gingivalis, ΔPG1018, and the complemented strain at an moi of 100 at 37°C for 1, 6, 12, 24 or 48 h. The samples were incubated with anti-P. gingivalis 33277 antibody and reacted simultaneously with Oregon Green 488 secondary antibody (Invitrogen), and DAPI 1 μg ml−1 (Sigma). The samples were visualized using the fluorescence microscope system described above. Acquired images were analysed for the intensity of fluorescence emitted from the infected samples with NIH ImageJ analysis software. An average of 150 cells per sample was studied from at least 2 separate experiments.
Purification of recombinant NDK
Recombinant P. gingivalis-NDK (rNDK) was produced by cloning the PCR-amplified putative ndk coding sequences from P. gingivalis into the pET30 expression system (Novagen). After induction in E. coli, rNDK was purified by chromatography over a Ni+2 metal chelating resin and eluted with imidazole. The eluted samples were subjected to SDS-PAGE, and staining with Coomassie blue (not shown) indicated greater than 95% purity.
ATPase activity assay of P. gingivalis strains and rNDK
ATPase activity was measured using an ATPase colorimetric assay kit (Innova Biosciences). Samples were prepared from log phase P. gingivalis cells in addition to purified rNDK. Briefly, bacteria were centrifuged at 6000 g for 10 min. Both the supernatant and bacterial pellet were collected. The unused bacterial growth media used as control. Supernatants were filtered (0.22 μm pore size) whereas the pellets were lysed in 50 mM Tris-HCl buffer (pH 7.5) containing 0.15 M NaCl, 0.1% SDS and 1% Triton X-100. Protein levels were determined (BIO-RAD) before the ATPase activity assay. The assay contained purified Pi –free ATP to ensure lowest possible background signals. Inorganic phosphate (Pi) standards provided by the company were used to calculate a standard curve of the enzymatic activity. The results were determined by calculating the amount of enzyme that catalyses the reaction of 1 μmol ATP substrate per minute.
Acknowledgements
This study supported by NIDCR R01DE16593 and R01DE11111 grants.
References
- Amieva MR, Salama NR, Tompkins LS, Falkow S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol. 2002;4:677–690. doi: 10.1046/j.1462-5822.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- Byrne GI, Ojcius DM. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol. 2004;2:802–808. doi: 10.1038/nrmicro1007. [DOI] [PubMed] [Google Scholar]
- Chen L, Brosnan CF. Regulation of immune response by P2X7 receptor. Crit Rev Immunol. 2006;26:499–513. doi: 10.1615/critrevimmunol.v26.i6.30. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X (7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Ojcius DM, Gorecki DC, Persechini PM, Bisaggio RC, Mendes AN, et al. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005a;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005b;288:G1024–G1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X (7) -dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001;167:3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006;281:17228–17237. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intra-cellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- Ganz T. Epithelia: not just physical barriers. Proc Natl Acad Sci USA. 2002;99:3357–3358. doi: 10.1073/pnas.072073199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg MC, Weyer MW. Dental plaque, platelets, and cardiovascular diseases. Ann Periodontol. 1998;3:151–160. doi: 10.1902/annals.1998.3.1.151. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- Kumar P, Verma A, Saini AK, Chopra P, Chakraborti PK, Singh Y, Chowdhury S. Nucleoside diphosphate kinase from Mycobacterium tuberculosis cleaves single strand DNA within the human c-myc promoter in an enzyme-catalyzed reaction. Nucleic Acids Res. 2005;33:2707–2714. doi: 10.1093/nar/gki568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu HK, Qi M, Kang IC, Chen W. Role for periodontal bacteria in cardiovascular diseases. Ann Periodontol. 2001;6:41–47. doi: 10.1902/annals.2001.6.1.41. [DOI] [PubMed] [Google Scholar]
- Kuwayama H, Obara S, Morio T, Katoh M, Urushihara H, Tanaka Y. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 2002;30:E2. doi: 10.1093/nar/30.2.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Yilmaz O. In or out: the invasiveness of oral bacteria. Periodontol 2000. 2002;30:61–69. doi: 10.1034/j.1600-0757.2002.03006.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, Adinolfi E, et al. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol. 2006;177:7257–7265. doi: 10.4049/jimmunol.177.10.7257. [DOI] [PubMed] [Google Scholar]
- Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D, Falkow S. Apoptosis as a common bacterial virulence strategy. Int J Med Microbiol. 2000;290:7–13. doi: 10.1016/S1438-4221(00)80096-X. [DOI] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, Chung WO, Watanabe K, El-Sabaeny A, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Fleischmann RD, DeBoy RT, Paulsen IT, Fouts DE, Eisen JA, et al. Complete genome sequence of the oral pathogenic Bacterium porphyromonas gingivalis strain W83. J Bacteriol. 2003;185:5591–5601. doi: 10.1128/JB.185.18.5591-5601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun. 2001;69:4242–4247. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Girolomoni G. The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur J Dermatol. 2006;16:125–131. [PubMed] [Google Scholar]
- Phalipon A, Sansonetti PJ. Shigella's ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol. 2007;85:119–129. doi: 10.1038/sj.icb7100025. [DOI] [PubMed] [Google Scholar]
- Rhoades ER, Ullrich HJ. How to establish a lasting relationship with your host: lessons learned from Mycobacterium spp. Immunol Cell Biol. 2000;78:301–310. doi: 10.1046/j.1440-1711.2000.00938.x. [DOI] [PubMed] [Google Scholar]
- Rudney JD, Chen R, Sedgewick GJ. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–2707. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B, Babolat M, Xu YW, Janin J, Veron M, Deville-Bonne D. Mechanism of phosphoryl transfer by nucleoside diphosphate kinase pH dependence and role of the active site Lys16 and Tyr56 residues. Eur J Biochem. 2001;268:1964–1971. doi: 10.1046/j.1432-1327.2001.02070.x. [DOI] [PubMed] [Google Scholar]
- Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom BR. Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- Simionato MR, Tucker CM, Kuboniwa M, Lamont G, Demuth DR, Tribble GD, Lamont RJ. Porphyromonas gingivalis genes involved in community development with Streptococcus gordonii. Infect Immun. 2006;74:6419–6428. doi: 10.1128/IAI.00639-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- Solini A, Santini E, Chimenti D, Chiozzi P, Pratesi F, Cuccato S, et al. Multiple P2X receptors are involved in the modulation of apoptosis in human mesangial cells: evidence for a role of P2X4. Am J Physiol Renal Physiol. 2007;292:F1537–F1547. doi: 10.1152/ajprenal.00440.2006. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extra-cellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Medical. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- Xia Q, Wang T, Taub F, Park Y, Capestany CA, Lamont RJ, Hackett M. Quantitative proteomics of intracellular Porphyromonas gingivalis. Proteomics. 2007 doi: 10.1002/pmic.200700543. in press. doi: 10.1002/pmic.200700543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002;4:305–314. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Young PA, Lamont RJ, Kenny GE. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology. 2003;149:2417–2426. doi: 10.1099/mic.0.26483-0. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;31:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang T, Chen W, Yilmaz O, Park Y, Jung IY, et al. Differential protein expression by Porphyromonas gingivalis in response to secreted epithelial cell components. Proteomics. 2005;5:198–211. doi: 10.1002/pmic.200400922. [DOI] [PMC free article] [PubMed] [Google Scholar]