Abstract
The impact of estrogen exposure in preventing or treating cardiovascular disease is controversial. But it is clear that estrogen has important effects on vascular physiology and pathophysiology, with potential therapeutic implications. Therefore, it is the goal of this review to summarize, using an integrated approach, current knowledge of the vascular effects of estrogen, both in humans and in experimental animals. Aspects of estrogen synthesis and receptors, as well as general mechanisms of estrogenic action are reviewed with an emphasis on issues particularly relevant to the vascular system. Recent understanding of the impact of estrogen on mitochondrial function suggests that the longer lifespan of women compared to men may depend in part on the ability of estrogen to decrease production of reactive oxygen species in mitochondria. Mechanisms by which estrogen increases endothelial vasodilator function, promotes angiogenesis and modulates autonomic function are summarized. Key aspects of the relevant pathophysiology of inflammation, atherosclerosis, stroke, migraine and thrombosis are reviewed concerning current knowledge of estrogenic effects. A number of emerging concepts are addressed throughout. These include the importance of estrogenic formulation and route of administration and the impact of genetic polymorphisms, either in estrogen receptors or in enzymes responsible for estrogen metabolism, on responsiveness to hormone treatment. The importance of local metabolism of estrogenic precursors and the impact of timing for initiation of treatment and its duration are also considered. While consensus opinions are emphasized, controversial views are presented in order to stimulate future research.
I. Introduction
Scientific investigation into the non-reproductive cardiovascular actions of estrogen has waxed and waned over several decades. However, the field has been rejuvenated by a number of governmental initiatives, controversial outcomes of several large clinical trials (Hulley et al., 1998; Nair and Herrington, 2000; Rossouw et al., 2002), the growing public interest in “safer” more “bio-identical” hormones and the interest in personalized, sex-based medicine (pharmacogenomics). Furthermore, validation of controversial mechanisms of action of sex steroids, identification of novel effects of estrogen such as a regulator of mitochondrial function, and development of new theories of treatment efficacy based on further analyses of data from various observational and clinical trials (Clarkson, 2007; Grodstein et al., 2006; Hsia et al., 2006b; Manson et al., 2007; Salpeter et al., 2006; Salpeter et al., 2004) support the possibility that hormonal therapies may be viable options to prevent some chronic conditions of aging.
With these emerging areas of science in mind, this review will take an integrative approach toward understanding effects of estrogen on regulation of vascular reactivity, angiogenesis, atherosclerosis and thrombosis in an aging population. Information regarding steroid synthesis and receptors will be discussed briefly only to provide sufficient background information upon which to build the other discussion. Interactions of estrogen with other hormones, while an important consideration, are insufficiently understood and will not be included. Effects of estrogen on cardiac function, a growing field of investigation, also will not be included as the topic is sufficiently complex as to warrant a separate review. In other areas, such as changes in vascular function during hormonal transitions in puberty, information is scant. In all sections, consensus of understanding will be emphasized, and areas requiring more research will be identified.
II. Estrogen Synthesis and Receptors
A. Estrogenic/Androgenic Balance
The biosynthesis of gonadal steroids is understood well and explicated clearly in textbooks (Loose-Mitchell and Stancel, 2001). Only a few key points relevant to the current discussion of the vascular effects of estrogen merit mention here. Testosterone is a key intermediate in both women and men, being converted to estrogen by the action of aromatase and to the more potent androgen, dihydrotestosterone, by 5-alpha reductase. In women estradiol is the main form of circulating estrogen, and circulating levels of testosterone are relatively low. In men, testosterone is the principal circulating androgen, and circulating estrogen levels are much lower than in women.
A key point, though, is that circulating levels of hormones may not reflect those at the tissue level, as both aromatase and 5-α reductase can be found in a number of tissues, including blood vessels (Gonzales et al., 2007). For example, in bone, testostosterone is converted to 17β-estradiol by aromatase; estradiol then acts locally to promote mineralization and prevent osteoporosis. In fact, mutations of genes encoding either aromatase or estrogen receptor alpha result in altered bone phenotype in men (Carani et al., 1997; Smith et al., 1994). 5-α reductase in the prostate converts testosterone to the more potent androgen, dihydrotestosterone, a critical step for effective promotion of prostate growth and function (Steimer, 2003). Administration of an aromatase inhibitor to young men resulted in a decrease in endothelial vasodilator function, assessed by flow-mediated dilation of the brachial artery (Lew et al., 2003), providing evidence that conversion of testosterone to estradiol may contribute to regulation of the peripheral circulation in men. In women, evidence suggests that the relationship among circulating concentrations of free 17β estradiol, free testosterone and sex hormone binding globulin may be more predictive of changes in carotid intimal thickening than concentrations of any of these hormones alone (Karim et al., 2008). Despite these few examples, the complexities of gonadal steroid hormone metabolism and local variation are still not well understood, particularly with respect to the non-reproductive effects of gonadal steroids, including vascular effects. However, with the growing therapeutic use of inhibitors of gonadal steroid metabolism including aromatase inhibitors and inhibitors of 5-α reductase, particularly in women with a history of breast cancer, a better understanding of possible side effects, especially with long-term use, is essential (Nabholtz and Gligorov, 2006; Pritchard and Abramson, 2006). Nevertheless, more information is needed to illuminate how local balance between levels of androgens and estrogens influences cardiovascular function in both males and females and how imbalances may contribute to sex differences in the pathophysiology of cardiovascular disease.
B. Receptors for Estrogen
Evidence suggests that there are at least three, and possibly four, distinct receptors for estrogen: two ligand-activated transcription factors (estrogen receptor (ER)α and ERβ), one G-protein coupled receptor, GPER (also referred to as GPR 30), and a third, less well defined putative receptor, termed ER-X (Toran-Allerand, 2004). Most evidence for the existence of ER-X comes from studies of the brain; therefore, this putative receptor will not be further discussed in this review focusing on the vasculature.
1. Ligand activated transcription factors
The first estrogen receptor was discovered and described in the late 1950s; this remained the status quo until the discovery of a second estrogen-sensitive, ligand-activated transcription factor, named ERβ (Kuiper et al., 1996). The initially described ER was then termed ERα. Both ER forms have been localized to the vasculature, in both endothelial and smooth muscle cells (Mendelsohn and Karas, 1999). A single case of a man with a disruptive mutation of ERα has given some insight into the non-reproductive effects of this receptor (Sudhir et al., 1997a). This 31-year-old man was of tall stature due to incomplete epiphyseal closure and had decreased bone mineral density. It is interesting that this phenotype is similar to that seen in men with mutations resulting in aromatase deficiency, as mentioned above (Jones et al., 2006; Rochira et al., 2002). This individual also had early coronary arterial atherosclerosis and endothelial dysfunction, with no detectable flow-mediated dilation in the brachial artery (Sudhir et al., 1997b), thus providing additional support to the hypothesis that estrogen through receptor operated mechanisms regulates peripheral arterial function.
ERα and ERβ are both members of the nuclear hormone receptor superfamily and are encoded by distinct genes with different chromosomal locations (Dahlman-Wright et al., 2006). These receptors function as ligand-activated transcription factors to produce so-called genomic effects but may also act through additional mechanisms (see below). Like other members of this superfamily, one gene may result in multiple proteins and diverse responses (Zhou and Cidlowski, 2005). Mechanisms for this diversity include epigenetic changes, specifically methylation, of the genes encoding these receptors, multiple isoforms of each receptor as a consequence of alternative RNA splicing and multiple sites of translation initiation of receptor mRNA (Hirata et al., 2003; Kim et al., 2007; Lewandowski et al., 2002; Post et al., 1999; Ying et al., 2000). In addition, post-translational modifications may lead to alterations in both protein stability and function. Methylation of genes for both ERα and β are associated with atherosclerotic tissue, and methylation of the gene for ERβ increases with passage of isolated smooth muscle and endothelial cells, thus implicating this process in receptor responsiveness with aging (Kim et al., 2007; Post et al., 1999; Ying et al., 2000).
There is emerging evidence that types of receptor isoforms vary from tissue to tissue and from species to species. This may account for considerable functional diversity, but this emerging field has not yet matured enough to give clear insights into implications for the actions of estrogen on a particular organ system, such as the vasculature.
Estrogen receptor null mice have provided insights into the distinct roles of ERα and ERβ (Couse and Korach, 1999; Dupont et al., 2000). Two distinct ERα disrupted mice have been developed. The first, αERKOCH, involved disruption of key domains in the receptor protein; however, a transcriptionally active form of ERα truncated for the A/B domain can still be found in these mice in low amounts (Couse et al., 1997; Lubahn et al., 1993). Subsequently, a second mutant mouse (αERKOST) was generated, fully lacking ERα (Dupont et al., 2000). Disruption of ERα is not lethal; instead animals develop normally, with a life span comparable to wild-type littermates (Lubahn et al., 1993). Females and males of both αERKO types are infertile (Couse and Korach, 1999; Dupont et al., 2000). Consistent with observations in humans, endothelium-dependent vasodilatation is reduced in these animals (Rubanyi et al., 1997).
Knockouts of ERβ (βERKO) (Krege et al., 1998) also survive to adulthood and exhibit distinct phenotypes compared to αERKO. Knockouts of both ERα and ERβ have also been developed (Dupont et al., 2000). As with any scientific method, a number of caveats must be taken into account when interpreting results from study of transgenic animals, especially animals such as the ER knockouts available to date that are not conditional mutants. Non-conditional knockout mice go through the full process of development in the absence of ER, so phenotypic changes may be caused either by a change in developmental processes or by the absence of the receptor in the mature animal. These and other issues have been well summarized elsewhere (Couse and Korach, 1999). Nevertheless, when used appropriately and together with other complementary approaches, it is clear that genetically-modified mice can be very useful tools in understanding the distinct roles of the two ERs in cardiovascular function especially with aging and with fluctuations in endogenous hormonal milieu.
Levels of ERα and ERβ appear to be differentially regulated by estrogen itself. However, regulation of estrogen receptor may be dependent upon the tissue and duration of estrogen treatment. In cultured ovine endothelial cells, short treatment (2 hr) down-regulated but longer exposure for 6 hr increased expression of ERα while down-regulating ERβ (Ihionkhan et al., 2002). Chronic in vivo treatment with physiological levels of 17β-estradiol also up-regulates ERα protein in cerebral blood vessels (Stirone et al., 2003b). Compared to blood vessels from ovariectomized rats, levels of several ERα isoforms are higher in vessels from intact females and ovariectomized rats treated with estrogen. In contrast, expression of ERα, β and GPR30 are reduced by 17β-estradiol in endothelium-denuded arteries but not veins derived from humans with atherosclerosis (Haas et al., 2007). ERα is upregulated in endothelial cells of pigs following ovariectomy and down-regulated following treatment with oral estrogenic products. However, ERβ is somewhat more resistant to regulation by these manipulation (Okano et al., 2006). If, indeed estrogen receptor levels are regulated by estrogen itself, this could have major implications for interpretation of human studies of the cardiovascular effects of hormone therapy (Arnal and Bayard, 2002).
Other hormones and growth factors can regulate ER as well. In vascular cells growth factors have been shown to activate ERα in the absence of ligand, an effect which occurs via a MAP kinase-independent pathway (Karas et al., 1998). Progesterone can also affect levels of ER progesterone receptor A has been shown to function as a ligand-dependent trans-repressor of other steroid receptors, including ER (Edwards, 2005). The physiological and pathophysiological implications of these effects related to changes in the ratio of expression of ERα to ERβ have not yet been fully clarified.
A number of polymorphic sites of both ERα and ERβ gene loci have been identified in humans (Dahlman-Wright et al., 2006; Gennari et al., 2005; Rosenkranz et al., 1998). In the case of ERβ, two tightly linked polymorphisms have been associated with risk of myocardial infarction in women, with the rs1271572 polymorphism variant T allele associated with increased risk and the rs1256049 variant associated with decreased risk (Rexrode et al., 2007). There were no significant relationships found in men. In the case of ERα, several polymorphisms have been associated with an increased ability of hormone replacement therapy to increase levels of high density lipoprotein cholesterol in postmenopausal women (Herrington et al., 2002a). Associations between ERα polymorphisms have also been shown for risk of myocardial infarction and stroke in men (Schuit et al., 2004; Shearman, 2006; Shearman et al., 2006; Shearman et al., 2003). Systolic and mean arterial pressures of older men were higher in the TC and C/C genotypes in 30T/C compared to TT (Hayashi et al., 2007).
Studies of women have not been as consistent in showing these relationships, as a variety of confounding factors, including menopausal status and use of drugs for contraception and hormone therapy, make it more difficult to analyze data in women, requiring larger numbers of subjects (Shearman, 2006). However, in one study of older women, significant differences in arterial stiffness as reflected in brachial-ankle pulse-wave velocity were found among 401T/C and 301T/Cpolymorphisms of ERα (Hayashi et al., 2007). These findings underscore the impact of estrogen on cardiovascular function. They also highlight the likelihood that estrogen may play a protective role against cardiovascular disease in men as well as in women (Shearman, 2006). However, more work is needed to explore the physiological and pathophysiological impact of estrogen receptor polymorphisms with the etiology of diseases in aging animals and humans.
2. G protein-coupled estrogen receptor
Besides acting via ERα and ERβ, there is a long history of observations demonstrating that estrogen also acts via plasma membrane receptors (Hasbi et al., 2005). While considerable controversy remains concerning the mechanism of these so-called non-genomic actions of estrogen (see below), one candidate receptor is the G Protein-coupled Estrogen Receptor, GPER. Originally identified as an orphan G-protein coupled receptor, GPR30; this protein was later shown to be localized to the endoplasmic reticulum and to specifically bind estrogen. This receptor was then named GPER. Binding of estrogen results in intracellular calcium mobilization and synthesis of nuclear phosphatidylinositol 3,4,5-triphosphate when GPER is expressed in COX7 cells (Hasbi et al., 2005; Revankar et al., 2005) or in a breast cancer cell line (Revankar et al., 2005; Thomas et al., 2005). Activity of adenyl cyclase was also increased in HEK293 cells transfected with GPER (Thomas et al., 2005). As far as the relevance of GPER to vascular function, the jury remains out although the receptor has been identified in human internal mammary arteries and saphenous veins (Haas et al., 2007). As discussed below, there are a number of competing mechanisms to account for non-genomic actions of estrogen, and those relevant to vascular function have not yet been clearly elucidated.
III. General Mechanisms of Action
A. Effects on Gene Transcription
ER, members of the nuclear receptor superfamily, use a conserved DNA binding domain to interact with specific hormone response elements in the genome and influence gene transcription. Such effects, often referred to as “genomic” were those originally described for nuclear receptors, although, as discussed below, there is considerable evidence for other (“non-genomic”) mechanisms of action for many members of this receptor family, including ERα and ERβ. A major emerging theme in understanding the diverse actions attributed to these proteins involves their ability to adopt multiple states dependent on the nature of the bound ligand. Each ligand can induce a different conformation of the receptor; as a consequence distinct sets of co-activators and co-receptors may be recruited to the receptor-transcription complex, resulting in distinct effects (Heldring et al., 2007).
Similar to other members of this receptor family, ERs include structurally and functionally distinct domains, which are highly conserved during evolution (Nilsson et al., 2001). The most conserved of these domains is the DNA-binding domain, which is involved in DNA recognition and binding. A second domain, the ligand-binding domain occurs in the COOH-terminal. Two distinct transcriptional activation functions, AF1 and AF2, recruit a variety of co-regulatory proteins to the DNA-bound receptor (Matthews and Gustafsson, 2003). AF1 is localized to the N-terminal region while AF2 is localized to the conserved ligand-binding domain and relies on an agonist ligand-induced protein conformation. Depending on the cellular and promoter context, AF1 and AF2 act either independently or synergistically in regulating gene expression. Adding to the complexity of estrogen action, the pattern of genes modulated by ERα and ERβ also depend on the status of other cellular signaling pathways (Heldring et al., 2007).
Unlike other members of the nuclear receptor family, the ligand binding cavity of ERs accommodates a wide range of structurally different compounds, including metabolites of estrogen and even environmental contaminants referred to as endocrine disruptors (Heldring et al., 2007). Several different types of ER antagonists have been distinguished (Hall and McDonnell, 2005). ICI 182,780 (fulvestrant) opposes the actions of estrogen in all tissues and does not distinguish between the two types of ER. In contrast selective estrogen receptor modulators (SERMS) show tissue-specific actions, acting either as antagonist or agonist, depending on the cell type. An early example was the use of tamoxifen for treatment of estrogen-dependent breast cancer. The therapeutic use of tamoxifen in the tumor cell is as an antagonist of the estrogen receptor; however, tamoxifen also increases bone density, acting like an estrogen agonist (Love et al., 1992). While agonists, such as 17β-estradiol, induce a conformation of the ligand binding domain that promotes co-activator binding, the bulky side chains of SERMS prevent the agonist-induced conformation (Dahlman-Wright et al., 2006). Thus, by blocking AF-2, SERMS act as antagonists in cells depending mainly on this route for activity. However, in some tissues the second transcriptional activation function, AF-1, may be active, and SERMS may act as agonists in this case. Another contributor to the tissue specificity of estrogen receptor ligands appears to be variation from tissue to tissue of other ER-interacting proteins, termed co-activators and co-repressors (Hall and McDonnell, 2005). Although we have some understanding of the complex mechanisms by which ERs act, their effects vary significantly depending on the tissue context, suggesting considerable potential for further development of selective therapeutic agents.
As mentioned above, a number of SERMS have been developed, including tamoxifen, raloxifene, and others still in clinical development. Recently, a large study investigated the effect of raloxifene on cardiovascular disease in postmenopausal women (mean age: 67.5 years) (Barrett-Connor et al., 2006). As compared with placebo, raloxifene had no effect on the risk of primary coronary events, but was associated with an increased risk of fatal stroke and venous thromboembolism. As with large human trials or hormone replacement therapy, the advanced age of this patient population could alter the response to estrogenic compounds (Harman et al., 2005b). However, it also may be that other SERMS might have a more positive cardiovascular impact.
In contrast to the non-selective ER agonist, 17β estradiol, selective synthetic agonists like propylpyrazole triol (PPT) and diarylpropionitrile (DPN) have been developed (Harrington et al., 2003). These compounds distinguish between ERα and ERβ and may help to distinguish actions of distinct ERs (α, β, nuclear, non-nuclear) in experimental settings. PPT shows 400-fold selectivity in binding to ERα compared to ERβ (Stauffer et al., 2000). In contrast, DPN is selective for ERβ, although its selectivity is not as great as that of PPT (Meyers et al., 2001). Only a very few studies have used these selective compounds to investigate the nature of estrogen receptors mediating vascular effects (Bolego et al., 2006). Even fewer have used concentrations of these substances which are truly selective, that is in the nanomolar range (Harrington et al., 2003). For example, only the selective ERα agonist, DPN, induces acute NO-dependent vasodilation (Bolego et al., 2005). Similarly, in small mesenteric arteries from female mice, PPT increased flow-mediated relaxation; interestingly, there was no effect of PPT in arteries from males (Douglas et al., 2008). These studies using selective ER agonists support the conclusion that ERα is the principal form involved in mediating the actions of estrogen on vascular function. However, much more work on the roles of estrogen receptor sub-types, including a more in depth investigation of all vascular estrogenic actions, is clearly warranted.
B. Rapid Effects
In contrast to actions of estrogen mediated by the genomic mechanism described above, estrogen can also produce effects within a time span of seconds or minutes, too short to be mediated by the “classical” mechanism involving transcriptional activation of genes (Hammes and Levin, 2007; Revelli et al., 1998). These rapid, extranuclear actions have also been described for a number of other steroid hormones, including progesterone and aldosterone (Wehling, 1997). Activation of signaling pathways, besides modulating protein function, can also influence gene expression and thus protein levels. Therefore, the term, non-genomic, does not accurately describe such extranuclear actions; “membrane-initiated steroid signaling” and “nuclear-initiated steroid signaling” have been suggested as alternatives (Hammes and Levin, 2007). Interestingly, studies of evolution suggest that, in ancient lineages, an ER homolog is not responsive to estrogen but, instead, acts in a constitutive manner to activate transcription, even though estrogen also has important effects on reproduction in these animals (Keay et al., 2006; Thornton et al., 2003).
Although extensive investigations have focused on uncovering the mechanism of these rapid effects of estrogen, a consensus has yet to be reached. Two of the major alternatives include effects of classical ERs at the plasma membrane or a distinct membrane-associated receptor (Hammes and Levin, 2007; Hasbi et al., 2005; Revankar et al., 2005). Some of the evidence for and against these two mechanisms has been detailed elsewhere (Hammes and Levin, 2007; Moriarty et al., 2006). Key points of the controversy will be summarized here. There is considerable evidence that ERs can associate with the plasma membrane, although the particular isoform(s) of ERs remain in doubt, and there may be variability in expression among cell types. In several cell types, ERs associate with caveolae and large protein complexes. This association with caveolae, where a number of other signaling molecules also are found, is thought to promote efficient signaling. By these associations, estrogen appears to trigger a number of intracellular signaling pathways, including MAPK and phosphatidylinositol 3-kinase (PI3K)/Akt, activation of ion channel fluxes, generation of G-protein coupled receptor mediated second messengers and stimulation of growth factor receptors (Moriarty et al., 2006).
Much of the current investigation of a distinct membrane receptor has focused on GPER (GPR30; see above and (Hasbi et al., 2005)). This receptor is widely distributed in the brain as well as in peripheral tissues (Owman et al., 1996), but there is, as yet, little evidence for a functional role in the vasculature. In COS7 cells and some cancer cell lines, GPR30 was exclusively localized to the endoplasmic reticulum (Revankar et al., 2005). Activation by estrogen caused mobilization of intracellular calcium and increased synthesis of phosphatidylinositol 3, 4, 5-trisphosphate in the nucleus. Others have reported that GPR30 is localized to the plasma membrane (Hasbi et al., 2005). Alternatively, it has been suggested that GPR30 functions only in collaboration with ERα, perhaps serving to assemble a signal complex essential to rapid estrogen signaling (Hammes and Levin, 2007). At any rate, an understanding of the possible role of G-protein coupled receptors in estrogen effects on the vasculature awaits further investigation.
One of the best-described rapid actions of estrogen is the ability to stimulate endothelial NO synthase (eNOS) in vascular endothelial cells. Current knowledge of the mechanism of this response also has recently been reviewed (Hisamoto and Bender, 2005; Moriarty et al., 2006), so only key points will be summarized here. In general, ERs associated with the plasma membrane interact with a variety of scaffolding proteins, perhaps varying among cell types. These molecules include striatin (Lu et al., 2004) and Src-homology and collagen homology adapter protein (Shc). Furthermore, lipid modifications of ER appear to be important, including palmitoylation (Acconcia et al., 2005). ERs are targeted to lipid rafts; in endothelial cells, ER-centered protein complexes associate with caveolae. ERα, in particular, interacts with caveolin-1, an important structural protein in caveolae, and this interaction is essential for localization of ER to the plasma membrane in endothelial cells (Chambliss et al., 2000). Through this mechanism, estrogen activates eNOS via PI3K/Akt, leading to phosphorylation of eNOS on serine 1177, enhancing NO production. This mechanism leads to the well-described rapid effect of estrogen to enhance endothelial-dependent vasodilator responses mediated by NO, an effect that has been demonstrated both in vitro and in vivo (Li et al., 2007; Stirone et al., 2005a; Williams et al., 1992).
The complexity of understanding mechanisms of estrogen signaling has become even more apparent by recent investigations into the relationships among multiple signaling pathways initiated by membrane estrogen receptors and changes in transcription mediated by estrogen response element-containing genes (Edwards, 2005; Vasudevan and Pfaff, 2007). Studies in several different cell types have demonstrated that membrane-initiated cell signaling by estrogen can potentiate nuclear-initiated estrogen signaling. A number of kinase cascades as well as calcium channels appear to be implicated in this transcriptional potentiation. Furthermore, the participation of these different intracellular signaling pathways may occur either in parallel or in series, and the convergence of membrane-initiated estrogen effects to influence transcription may involve protein-protein interactions, protein translocation as well as phosphorylation of proteins (Vasudevan and Pfaff, 2007). As stated most clearly by (Vasudevan and Pfaff, 2007), “The novel idea that genomic transcription by hormones, i.e, ligand-dependent transcription at hormone response elements, can be affected by membrane-initiated signal transduction events initiated by cognate or noncognate ligands is a paradigm shift in nuclear receptor biology”. Future studies will be necessary to clarify the functional impact of these complex interactions among signaling pathways, both membrane- and nuclear-initiated, in vascular function.
Intracellular pathways also are activated by acute application of estrogen to isolated vascular smooth muscle. The physiological consequence in most cases is relaxation of vascular rings and inhibition of proliferation in cultured smooth muscle cells (see Section V). In arterial smooth muscle, relaxations result from increased efflux of calcium involving activation of cyclic guanylate cyclase and inhibition of ATP sensitive K+ channels and Ca++-activated K+ channels (Kleppisch and Nelson, 1995; Prakash et al., 1999; Quayle et al., 1995; White et al., 1995). One caveat of these experiments is the high concentrations of estrogen often needed to elicit a relaxation. However, these studies reinforce the concept that there are immediate cellular effects of estrogen in either endothelial or smooth muscle cells that alter the internal milieu of the cell resulting in altered responsiveness to subsequent stimuli (Haas et al., 2007; Miller et al., 2002).
C. Post-transcriptional and translational modulation of proteins/enzymes
In addition to direct estrogen-receptor regulated gene transcription, estrogenic substances may facilitate the transport of RNA from the nucleus to the cytoplasm (Jacob et al., 2006; Thampan, 1985), may influence protein expression indirectly through regulation of mRNA stability in the cytoplasm and may regulate the rate of gene transcription of enzymes required for post-translational modification of proteins by glycosylation, phosphorylation or methylation. Therefore, post-transcriptional regulation of gene expression by estrogen modifies the cellular proteome and phenotype at all levels of protein processing.
1. RNA stability
Concentrations of mRNA in a cell represent the sum of production through gene transcription and degradation, providing a local and rapid (non-genomic) mechanism to control protein concentration. That is, decreased stability of mRNA provides a mechanism for rapid termination of production of a protein; whereas increased stability provides a means to prolong the expression of a gene. The biochemical details of modulation of mRNA stability are reviewed elsewhere (Ing, 2005; Kracht and Saklatvala, 2002) and involve, in part, transcriptional regulation of estrogen-regulated mRNA stabilizing factor (Kawagoe et al., 2003). For the purpose of this review, it is important to emphasize that estrogen may auto-regulate the stability of mRNA for its own receptor in some tissues (Adams et al., 2007; Saceda et al., 1989). Therefore, differences in efficacy of SERMS may reflect differences in the ability of the SERM-bound receptor complex to alter estrogen receptor expression. Furthermore, within a single tissue, estrogen may stabilize some mRNA while destabilizing others. Some of the anti-inflammatory effects of glucocorticoids are explained by their effects on mRNA stability (Kracht and Saklatvala, 2002). However, the anti-inflammatory effects of estrogen on mRNA stability have not been investigated in the same way and may provide insight into regulation of growth factors and cytokines involved with estrogenic modulation of angiogenesis (Fieber et al., 2006; Kracht and Saklatvala, 2002), infection-induced inflammation (Batty et al., 2006; Zhong et al., 2006), glucose metabolism (Totary-Jain et al., 2005), lipoproteins (Srivastava et al., 1992), hypoxia (Fieber et al., 2006; Fish et al., 2007), shear-stress (Sokabe et al., 2004) and immunity (Mestas et al., 2005).
2. Post-translational modification of proteins
Signaling cascades and phosphorylation of mitogen kinases and Akt initiated by binding of estrogen to membrane receptors as outlined in Section III.B. is not usually discussed in terms of mechanisms by which estrogenic substances affect post-translational modification of proteins. Yet regulation of enzymes which in turn affect biological half-life of other enzymes, co-factors, or receptors may represent a more integrated approach to understanding how estrogen, and perhaps other sex steroids as well, influence vascular responses to cytokines, hormones or environmental stimuli such as hypoxia. For example, reversible, covalent attachment of small ubiquitin-like modifiers (SUMO), a process known as sumoylation, or phosphorylation of steroid receptor co-activators affects estrogen receptor binding and subsequently estrogen-receptor mediated gene transcription. Added to cells in culture, 17β-estradiol co-ordinates both phosphorylation and sumoylation of some co-activators through non-genomic mechanisms yet to be determined. Thus, post-translational modification of proteins, in this case, co-regulators by estrogen affects the ability of estrogen to initiate transcription of genes with estrogen receptor response elements (Wu et al., 2006). Some other post-translational modifications of enzymes by estrogen identified in non-vascular tissue are presented in Table 1. Except for work assessing regulation of nitric oxide synthase/nitric oxide by estrogen in endothelial cells (Hayashi et al., 1995; Kleinert et al., 1998; Okano et al., 2006; Sumi et al., 2001) and superoxide dismutase in vascular smooth muscle cells (Strehlow et al., 2003a), other evidence supporting these concepts is derived from studies of non-vascular cells such as neurons, glia, cancer cell lines and liver cells (for review see (Ing, 2005)). Therefore, the ability of estrogen to regulate degradation of mRNA and inactivate proteins causal to vascular injury or to stabilize mRNA and maintain proteins required for vascular repair requires further study in vascular cells of male and female animals.
Table 1.
Post-translational actions of estrogen
| Action | Physiological Consequences | Reference |
|---|---|---|
| Decreased turnover of growth factor-induced ornithine decarboxylase | Increased cell proliferation | (Huber and Poulin, 1996) |
| Activated secretion of MMP-7 | Increased paracellular permeability | (Gorodeski, 2007) |
| Altered synthesis of glycosyltransferases | Altered half-life of glycoprotein hormones | (Ulloa-Aguirre et al., 2001) |
| Increased phosphorylation of telomerase | Increased cell proliferation | (Kawagoe et al., 2003) |
| Increased expression of propyl hydroxylase domain 1(PHD1) | Decreased cellular sensitivity to hypoxia | (Tian et al., 2006) |
| Increased protein binding to mRNA for AT1 receptors | Decreased expression of AT1 receptors | (Wu et al., 2003) |
| Co-ordination of phosphorylation and sumoylation of steroid receptor co-activators | Cell specific control of ligand-dependent nuclear transcription | (Wu et al., 2006) |
Abbreviations: AT1, angiotensin receptor 1; MMP, matrix metalloproteinase
IV. Mitochondria
Estrogenic actions on mitochondrial function may contribute to the ability of estrogen to modulate a variety of age-related diseases, including endothelial and vascular dysfunction. Mitochondrial reactive oxygen species (ROS) are produced as a by-product of oxidative phosphorylation. ROS can affect mitochondrial lipids, proteins and DNA (Wallace, 2005). In particular, accumulation of ROS-induced mitochondrial DNA mutations over the lifespan is thought to contribute to the pathophysiology of a number of age-related diseases and to be a major cause of aging itself. Thus, an impact of estrogen on mitochondrial function might explain the longer lifespan of women as well as contribute to the ability of estrogen to protect against a variety of age-related diseases (Duckles et al., 2006; Wallace, 2005).
Estrogen can profoundly impact mitochondrial function in vascular endothelium (Stirone et al., 2005b) as well as in other cell types (Kim et al., 2006; Pedram et al., 2006; Yager and Chen, 2007). An important cellular target is the cerebral microvasculature, which comprises the blood brain barrier. Due to the relatively high energy demands of these specialized endothelial cells, cerebral vascular endothelium contains more mitochondria than endothelium in other vascular beds (Nag, 2003). Tissues with high metabolic activity would be predicted to produce higher levels of mitochondrial superoxide as a by-product of oxidative phosphorylation (Wallace, 2005) and be particularly subject to age-related disease. Indeed, mitochondrial ROS production may contribute to neurodegenerative diseases such as Parkinson’s, Alzheimer’s and Huntington’s (St-Pierre et al., 2006). Functional changes in the blood-brain barrier or other aspects of cerebrovascular function would also contribute to the pathophysiology of these age-related diseases of the brain.
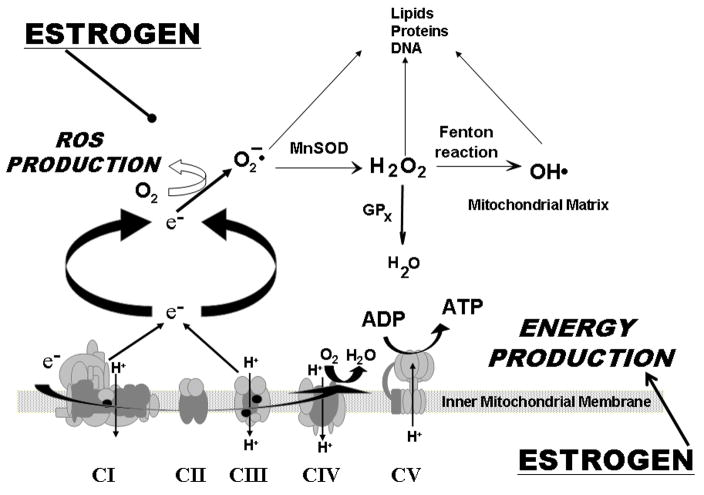
Estrogen impacts mitochondrial function through increasing oxidative phosphorylation, while at the same time decreasing mitochondrial superoxide production (Duckles et al., 2006; St-Pierre et al., 2006; Stirone et al., 2005a) (Figure 1). Exposure of ovariectomized rats to estrogen increased activities of both citrate synthase and complex IV, key rate limiting steps in the tricarboxylic acid cycle and electron transport chain, respectively, in cerebrovascular mitochondria. Corresponding increases in key related proteins after estrogen treatment include cytochrome c, and subunits I and IV of complex IV. Surprisingly, these indices of increased capacity for oxidative phosphorylation were associated with decreased ROS production after estrogen treatment. Levels of mitochondrial production of both superoxide and hydrogen peroxide were decreased after estrogen exposure. Chronic in vivo exposure to estrogen also increased levels of Mn superoxide dismutase, but did not affect levels of glutathione peroxidase or catalase (Stirone et al., 2005b).
Figure 1.
Schematic of the current hypothesis concerning the impact of estrogen treatment on mitochondrial function. Estrogen appears to promote energy production (oxidative phosphorylation) while decreasing mitochondrial generation of reactive oxygen species (ROS). The oxidative phosphorylation system is composed of five enzyme complexes, within the inner mitochondrial membrane. Activity of this system generates an electrochemical gradient across this membrane which leads to production of ATP. At the same time, electrons leaking into the mitochondrial matrix interact with oxygen, resulting in superoxide production. Superoxide is then metabolized by Mn superoxide dismutase (MnSOD); the resulting H2O2 is reduced to water by glutathione peroxidase-1 (GPx1). H2O2 can also be converted by the Fenton reaction to the highly reactive hydroxyl radical (OH·). ROS in the mitochondrial matrix target lipids, proteins and mtDNA. Adapted from (Duckles et al., 2006).
The mitochondrial targets for estrogen are not known but estrogen could act by influencing either nuclear or mitochondrial-encoded genes, or both. Indeed, levels of nuclear respiratory factor-1, a key master regulator of nuclear-encoded mitochondrial genes, increased after estrogen treatment (Stirone et al., 2005b). However, this mechanism does not rule out a direct effect of estrogen on the mitochondrial genome as estrogen receptors are found in mitochondria (Chen et al., 2004; Stirone et al., 2005b; Yang et al., 2004). Improved understanding of mechanisms by which mitochondrial and nuclear genomes are coordinated to maximize mitochondrial function will provide a better understanding of the impact of estrogen on energy production.
Consistent with the effect of estrogen on mitochondrial function described above, a number of genes for mitochondrial proteins encoded by either nuclear or mitochondrial DNA are regulated by either ERα or ERβ (O’Lone et al., 2007). In aorta from wild type ovariectomized female mice, estrogen treatment both up and down regulated a number of genes involved in mitochondrial function. However, in ERα knockout mice, a larger number of genes involved in mitochondrial function were down-regulated by estrogen treatment, implying that ERα predominately down-regulates genes involved in the electron transport chain (O’Lone et al., 2007), and enhanced expression of antioxidants. However, as noted by the authors, these findings in aorta are not necessarily consistent with studies of other tissues, vascular endothelial cells or even other blood vessels (O’Lone et al., 2007). Clearly, more studies of the effects of estrogen treatment on mitochondrial function will be essential to sort out these important and complex effects.
By decreasing mitochondrial production of ROS even while sustaining robust oxidative phosphorylation, estrogen would decrease the rate of accumulation of mitochondrial DNA mutations over the lifespan. By this mechanism, estrogen would protect against age-related disease, but one would not predict that estrogen would be able to reverse accumulated mutations of mitochondrial DNA. This mechanism of estrogen’s effects has important consequences for the timing of estrogen treatment. Thus, administration of estrogen after a significant period without estrogen exposure, would only protect against future mitochondrial damage, but would not reverse accumulated damage during estrogen-free periods. Such a mechanism might have contributed to the lack of effect of estrogen on cardiovascular disease in recent large trials of estrogen replacement therapy, where subjects entered the study an average of 10 years past menopause (Harman et al., 2005a; Harman et al., 2005b).
Mitochondrial production of ROS also plays a key role in oxidative stress (Madamanchi and Runge, 2007), so one would predict that estrogen may also have an important impact on vascular oxidative stress. Besides mitochondria, ROS can emanate from a number of sources, including nicotinamide adenine dinucleotide oxidize, xanthine oxidase, lipoxygenase or nitric oxide synthase uncoupling (Madamanchi et al., 2005) Although excess ROS production is proposed to be an initiating factor in vascular pathophysiology, lower levels of ROS can also serve important signaling functions in the vasculature. When ROS production remains low enough that mechanisms of ROS destruction are not overwhelmed, controlled activation of signaling pathways by ROS may be maintained (Gutierrez et al., 2006; Lyle and Griendling, 2006).
In addition to the mitochondria, estrogen also suppresses ROS through other mechanisms. For example, estrogen-treatment reduces angiotensin II-induced free radical production in vascular smooth muscle cells (Strehlow et al., 2003a) and decreases NADPH-stimulated superoxide production by mouse cerebral arteries (Miller et al., 2007a). Estrogen also suppresses strain-increased NADPH oxidase activity and intracellular generation of ROS in human umbilical vein endothelial cells (Juan et al., 2004). Furthermore, in vascular smooth muscle cells estrogen treatment increases protein levels of both Mn superoxide dismutase (SOD) and extracellular SOD by increasing transcription rate. There was no effect of estrogen on copper-zinc SOD, glutathione peroxidase or catalase. Similarly, treatment of ovariectomized rats with estrogen increased levels of MnSOD protein in cerebral blood vessels, but did not change levels of catalase or glutathione peroxidase (Stirone et al., 2005b).
Oxidative stress may influence blood flow in humans. For example, in estrogen-deficient postmenopausal women, whole leg blood flow was reduced compared to pre-menopausal women. Because blood flow increased only in the post-menopausal women following administration of an anti-oxidant, ascorbic acid, it was concluded that different levels of oxidative stress contributed to differences in blood flow between the two groups (Moreau et al., 2007). However, the disparate ages of the pre- and post-menopausal groups and the lack of direct demonstration of an effect of estrogen per se cast some doubt on the results of this study; nevertheless, it is clear that much more remains to be learned about the impact of estrogen on vascular oxidative stress and the implications for pathophysiology.
V. Physiological Consequences
In previous sections, intracellular mechanisms by which estrogenic compounds affect gene transcription and translation, protein synthesis and oxidative metabolism were described. In this section, the integrated consequences of these activities will be discussed.
A. Vascular Responsiveness
1. Arteries
In general, the most consistent effect of estrogen treatment on vascular responsiveness reported from a large number of studies conducted on isolated arteries, experimental animals and humans is vasodilation or suppression of vascular tone. Evaluation of these effects of estrogen need to consider that sex differences, per se, may not simply be reflective of differences in levels of circulating hormones. As described above, tissue localization of key enzymes responsible for testosterone metabolism may result in local tissue levels of estrogen or dihydrotestoserone that exceed circulating levels. Thus, the best way to evaluate effects of estrogen in intact organisms is to administer the hormone in gonadectomized animals. Indeed, cardiovascular effects of estrogen can be seen in both male and female gonadectomized animals (Bolego et al., 2005; Geary et al., 2000; McNeill et al., 1999), and, as discussed above, there is evidence in humans that estrogen may play an important role in regulating vascular function in males (Lew et al., 2003).
The most prominent effects of estrogen on vascular reactivity are mediated through direct effects on endothelial function (Miller and Mulvagh, 2007), but studies of very high concentrations of estrogen may show additional, non-physiological effects. A plethora of studies in humans have clearly demonstrated that estrogen promotes vasodilation through an eNOS-dependent mechanism (Miller and Mulvagh, 2007). These include demonstration of an estrogen-stimulated increase in plasma concentrations of NO, increases in reactive hyperemia after estrogen treatment and changes through the menstrual cycle reflective of an estrogenic effect. Interestingly, age influences flow-mediated vasodilation in women. In one study acute responses of postmenopausal women to estrogen (18 hr after placement of a transdermal patch) declined with age (Sherwood et al., 2007). Similarly, postmenopausal women receiving either acute estrogen (within 1 hr of sublingual administration) or chronic estrogen (3 months oral administration) all demonstrated increases in flow-mediated dilation, but this increase was significantly greater in women who were less than 5 years past menopause compared to women more than 5 years past menopause (Vitale et al., 2008). Furthermore, for women more than 5 years past menopause, flow-mediated vasodilation increased significantly more in women who had received estrogen treatment in the past compared to those who had not. These findings support the idea that, in the absence of estrogen, endothelium-dependent release of NO is reduced, and the ability of estrogen to increase this response is abrogated the longer an individual is without estrogen exposure. Whether this abrogation involves epigenetic regulation of estrogen receptors (see Section II. B.1.) or other mechanisms remains to be determined.
In both coronary and cerebral vascular beds and in the aorta, chronic exposure to estrogen, either endogenous or by estrogen replacement in ovariectomized female rodents, decreases vascular tone in an endothelium dependent manner (Duckles and Krause, 2007; Geary et al., 1998; Wellman et al., 1996; Widder et al., 2003). These effects have been shown to depend on an increase in NO production resulting from a genomic effect to increase levels of eNOS (McNeill et al., 1999; Stirone et al., 2003a) as well as more rapid effects to increase NO production (Knot et al., 1999; Stirone et al., 2005a). Because the ability of estrogen to alter vascular reactivity and increase eNOS levels are absent in ERα knock out mice (Geary et al., 2001) and are mimicked by selective estrogen receptor agonists (Widder et al., 2003), modulation of NO is most likely through ERα. Similar findings have been made in skeletal muscle arterioles, where flow-induced dilation was greater in female than male rats, and increased by estrogen in ovariectomized females (Huang et al., 1998). This effect of estrogen to modulate the regulation of wall shear stress was also shown to be dependent on enhanced endothelial NO release.
An endogenous substance, 27-hydroxycholesterol, inhibits the ability of estrogen to increase endothelial release of NO (Umetani et al., 2007). This inhibition occurred for both transcription-mediated and non-transcription-mediated effects of estrogen on NO production. The importance of this endogenous factor was demonstrated by measuring changes in vascular NO synthase after various manipulations that altered circulating levels of 27-hydroxycholesterol. Interestingly, 27-hydroxycholesterol had estrogenic effects on non-vascular cells. Thus, this substance exhibits a SERM-like effect, acting as an antagonist in the vasculature, but an agonist in other tissues.
In addition to effects of estrogen on endothelial production of NO, there is substantial evidence that estrogen affects production of other endothelial factors including products of cyclooxygenase. For example, chronic treatment with estrogen increased prostacylin synthesis in small caliber cerebral arteries and ovine fetal pulmonary arterial endothelium by elevating levels of cyclooxygenase-1 as well as prostacylin synthase (Jun et al., 1998; Ospina et al., 2002; Sherman et al., 2002), resulting in a shift from cyclooxygenase dependent vasoconstriction to vasodilation after estrogen treatment (Ospina et al., 2003). In rat mesenteric arteries, estrogen suppressed vasoconstriction which was dependent on activity of prostaglandin H-synthase (Davidge and Zhang, 1998). Using transfected cultured ovine endothelial cells; estrogen activated the human COX-1 promoter, a response mediated by either ERα or ERβ (Gibson et al., 2005). Interactions between NOS- and cyclooxygenase-dependent pathways and the effects of estrogen have been highlighted by several studies. Comparison of rat mesenteric arteries after ovariectomy or ovariectomy with estrogen treatment showed that estrogen increased the NO component of endothelium-dependent dilation, while decreasing the cyclooxygenase component (Case and Davison, 1999). Interactions among endothelial factors are highlighted by studies of cerebral vessels from mice with dysfunctional NOS. In cerebral vessels from control mice treated with estrogen, eNOS was upregulated, but there were no effects of estrogen treatment on cyclooxygenase-1, production of prostacylin or constriction to indomethacin. In contrast, in animals with dysfunctional NOS, either eNOS knockouts or after chronic treatment with a NOS inhibitor (Li et al., 2004), all three parameters were enhanced after estrogen treatment. Emphasizing the diversity of endothelial function in different vascular beds, in arteries from skeletal muscles of rats treated with a NOS inhibitor, estrogen treatment increased vasodilatation mediated by endothelium-derived hyperpolarizing factor (EDHF) (Huang et al., 2001).
The myriad of effects of estrogen on vascular function are highlighted by changes in gene expression in aorta of ovariectomized wild-type or ER knockout mice following treatment with estrogen (O’Lone et al., 2007). Four clusters of genes were identified, showing that ERα and ERβ regulate distinct sets of genes with little overlap between the two receptor types. ERα was responsible for most of the increases in gene expression caused by estrogen in wild-type aortae, while ERβ generally decreased expression of a different set of genes. As mentioned above, one of the most striking effects of estrogen was to modulate sets of genes involved in mitochondrial function, with both ERα and ERβ modulating different genes. Estrogen treatment also modulates cellular ROS production, by regulating both proteins involved in mitochondrial respiratory chain complexes and oxidoreductase gene sets. As pointed out by the authors, findings in mice aortae may only reflect vascular effects of estrogen in this specialized large artery, dominated by smooth muscle cells with estrogen acting mainly to reduce cell proliferation.
While much basic science work has been directed toward understanding how 17β estradiol affects vascular function, a common clinically prescribed product, conjugated equine estrogen, contains metabolites of estrogen, estrone and estrone sulfate (Kikuchi et al., 2000). Estrone sulfate must be hydrolyzed to estrone to enter cells. Estrone increases production of nitric oxide and prostacyclin in endothelial cells (Kikuchi et al., 2000; Lippert et al., 2000; Rauschemberger et al., 2008) and also increases proliferation of cultured rat smooth muscle cells (Rauschemberger et al., 2008). However, both estrone sulfate and estrone have a null effect on proliferation and migration of cultured human aortic smooth muscle cells (Dubey et al., 2000) but suppress transcription of pro-mitogenic factors like platelet-derived growth factor, interleukin-1 and -6 (Kikuchi et al., 2000). Reasons for these discrapencies among studies and between functional assays and molecular tests are not clear. However, efforts to better define conditions which affect responsiveness of various tissues to metabolites of estrogen are warranted given that the relationship among estradiol, free estradiol and estrone may relate to changes in development of carotid intimal hyperplasia (Karim et al., 2008). There is considerable genetic variation in expression of human hydroxysteroid sulfotransferase, and the biological activity of the enzyme may relate to the number of copies of the gene (Hebbring et al., 2007; Ji et al., 2007).
2. Veins
In contrast to what is known about effects of estrogen on arteries, information is scant in regard to estrogenic effects on veins. This lack of information is somewhat surprising in light of the well known adverse side effect of venous thrombosis in women using estrogenic treatments. As is observed in arteries, acute application of 17β-estradiol in vitro caused concentration-dependent, endothelium-dependent decreases in tone in rings of femoral veins derived from female pigs. These endothelium-dependent relaxations to 17β-estradiol were mediated by NO, but potassium channel activation seemed to contribute to the relaxation only in veins derived from gonadally intact females (Bracamonte et al., 2002a). These relaxations were not inhibited by the estrogen receptor antagonist ICI, 182,780 suggesting perhaps involvement of receptor(s) or mechanisms other than the classical ERα and ERβ. In support of this concept, 17α-estradiol also caused relaxation of veins in the presence and absence of the endothelium as did the SERM raloxifene (Bracamonte et al., 2002a; Bracamonte et al., 2002b). Hormonal status of the animal (gonadally intact or ovariectomized) influences the relative contribution of endothelium-derived NO and potassium channels as causal to the relaxations to both 17β-estradiol and raloxifene. In contrast to these results, are observations that acute application of 17β-estradiol does not cause endothelium-independent relaxations of human saphenous veins derived from persons with atherosclerosis (Haas et al., 2007). Several factors may contribute to these discrepancies. First, the saphenous vein is a muscular cutaneous, thermoregulatory, innervated vein compared to deep veins which are less muscular and not innervated in the same way. Most of the veins were derived from older (64 years of age) males and since hormonal status affects both expression of estrogen receptors and signaling cascades may not be representative of veins from women or individuals without atherosclerosis. Finally, although, thrombosis may occur in these superficial veins, it is not usually associated with estrogen treatments but with other conditions such as cancer. Clearly, additional information is needed in regard to differences in estrogen responsiveness of veins from various anatomical locations and how these responses relate to development of venous embolitic disease among individuals of differing ages, hormonal status or other disease conditions.
Using venous occlusion plethysmography, infusion of bradykinin caused a greater increase in diameter of dorsal hand veins in post-menopausal women after six months of treatment with oral conjugated equine estrogen (CEE) and progestin compared to untreated women (Ceballos et al., 2000). This effect was lost when treatment was stopped. Therefore, chronic menopausal hormoneal treatment seems to increase endothelium-dependent responses in cutaneous veins of women as in arteries. Monitoring changes in venous responsiveness using this technique has not been assessed in other clinical hormone treatment trials and may be useful in assessing differences among women in response to such treatments or perhaps to evaluate endothelial function in other populations as they age.
Critical to the development of thrombus is interaction of platelets with the venous wall. The in vitro response of porcine veins to autologous platelets was dependent upon the sex and hormonal status of the animal such that addition of platelets caused greater contraction of veins from ovariectomized animals than those with intact ovaries (Lewis et al., 2001). These contractions to the autologous platelets reflect both hormonal modulation of the venous wall and the platelets themselves. However, if one platelet-derived product, ADP, was added to the veins, endothelium-dependent relaxations were not reduced by indothethacin in veins from ovarectomized animals as they were from those derived from gonadally intact animals (Lewis et al., 2001). These observations suggest that the presence of ovarian hormones affects endothelial production of inhibitory prostanoids in veins as in arteries. Indeed if ovariectomized animals were treated for 4 weeks with either oral 17β-estradiol or raloxifene, endothelium-dependent relaxations to ADP were increased compared to ovariectomized animals but these relaxations were mediated by both NO and an inhibitory prostanoid only in veins from estradiol-treated animals. In contrast, endothelium in veins from raloxifene-treated animals produced a contractile prostanoid, most likely thromboxane (Lewis et al., 2006). Thromboxane stimulates platelets to aggregate and thus, may contribute to a procoagulant phenotype in response to this SERM which is known to increase the incidence of venous thrombosis in women (Barrett-Connor et al., 2002; Barrett-Connor et al., 2006). As the venous wall is a key component of Virchow’s triad required for the initiation of the thrombus (Bracamonte and Miller, 2001), more work is needed to understand how various estrogenic products affect both the endothelium and smooth muscle of veins in order to develop products with arterial protection but limited venous risks. Varicose veins represent a venous disorder that is associated with increases in circulating estrogen (Ciardullo et al., 2000; Vin et al., 1992). However, causality of this condition is complicated by various genetic components and physical factors such as obesity. In spite of the numerous studies of estrogen modulation of collagen formation in skin (Verdier-Sevrain et al., 2006), little is known about how estrogens affect extracellular matrix of the venous wall which leads to formation of the tortuosities.
B. Angiogenesis
The formation of new blood vessels from existing blood vessels, angiogenesis, requires several steps including degradation of existing vascular basement membrane, proliferation and migration of endothelial cells into tubular structures in the tissue and formation of new matrix around neovessels. In ovulating women, estrogenic regulation of these processes is evidenced by neovascular development in the uterus. However, these processes are essential in non-reproductive tissue for wound healing, repair of damaged organs, restoration of blood supply to ischemic tissue and tumor growth (Cid et al., 2002; Rubanyi et al., 2002). Estrogen regulates enzymes involved in formation of matrix including the matrix metalloproteinases and plasminogen activators which may be responsible for rendering complex atherosclerotic plaques unstable (Cid et al., 2002; Jones et al., 2003). Growth factors and adhesion molecules necessary for angiogenesis include fibroblast growth factor-2, vascular endothelial growth factor, nitric oxide and various integrins required for cell attachment (Cid et al., 2002; Rubanyi et al., 2002). Although estrogenic compounds increase proliferation of endothelial cells in vitro and in vivo in the vicinity of an endothelial lesion (Banerjee et al., 1997; Garnier et al., 1993; Krasinski et al., 1997), circulating endothelial-progenitor cells derived from the bone marrow may be a critical source of endothelial cells involved in maintaining and repairing damaged vascular lining, in angiogenesis to ischemic tissue, as well as in the formation of new blood vessels or vasculogenesis (Quraishi and Losordo, 2007; Takahashi et al., 1999).
There is little information regarding how the number and characteristics of colony forming progenitor cells in circulating blood change across the life span in both males and females. In reproductively competent individuals, the number of hematopoietic progenitor cells was greater in males compared to females but the variability in number of colony forming cells was higher in females than in males, suggesting that sex-steroids modulate hematopoiesis and perhaps other progenitor cells in the bone-marrow (Horner et al., 1997). Indeed, loss of ovarian hormones in animals and humans reduced the number of circulating bone-marrow-derived endothelial progenitor cells while estrogenic treatment of ovariectomized animals and post-menopausal women increased their number (Bulut et al., 2007; Goldschmidt-Clermont, 2003; Strehlow et al., 2003b). Estrogens slowed the senescence of these cells through increased telomerase activity and increased their proliferation through activation of ERα (Hamada et al., 2006; Imanishi et al., 2005a; b; Imanishi et al., 2005c; Masuda et al., 2007). Collectively, these effects would lead to rapid repair of vascular wounds by increasing endothelial regrowth with release of endothelium-derived factors, such as nitric oxide, which are inhibitory to smooth muscle proliferation, therefore reducing development of intimal hyperplasia (Krasinski et al., 1997; Schmidt-Lucke et al., 2005; Strehlow et al., 2003b). The pivotal contribution of ERα in the formation and regulation of endothelial-progenitor cells may explain in part the accelerated formation of atherosclerosis and adverse outcomes in men with disruption and/or polymorphism of the ERα (ESR1) gene in men (Ferrero et al., 2003; Schuit et al., 2004; Sudhir et al., 1997a; Sudhir et al., 1997b). In the future, it will be important to identify how populations of bone marrow-derived progenitor cells change with age and with gonadal hormone treatments in men and women. Studies also are needed to better understand how other progenitor and pleiotropic cells in the bone marrow, adventitia and adipose tissue are influenced by aging and hormonal interventions (Hong et al., 2007; Lewis et al., 1997; Mao et al., 2005; Oparil et al., 1999; Stringer et al., 2007) in order to develop cell-based therapies for revascularization of ischemic tissue and for tissue engineering.
C. Vascular consequences of estrogenic modulation of autonomic function
The autonomic nervous system is essential for homeostatic control of heart rate and blood pressure. ERα and ERβ are distributed throughout the central nervous system except in the cerebellum, of both male and female animals (Herbison et al., 2000; Kelly et al., 2005; Vanderhorst et al., 2005). ERα seems to be the predominant receptor subtype in the brain, although there are some differences in expression of each receptor between the sexes and between ovariectomized female animals and those treated with estrogen (Vanderhorst et al., 2005). Identification of ERβ in some earlier studies, however, may have been influenced by the specificity of the antibody used for immunological localization (Razandi et al., 2004).
Ligand stimulation of estrogen receptors in neurons activates non-genomic and genomic intracellular pathways similar to those described for endothelial and smooth muscle cells (Kelly et al., 2005; McEwen, 2001) (see Section III). Estrogenic immunoreactive fibers co-localize with adrenergic (tyrosine hydroxylase positive), cholinergic (vesicular acetylcholine transporter positive) and serotonergic positive cells. In addition to affecting the pituitary/gonadal axis controlling reproductive function, behavior, response to stress and body temperature, estrogenic mediated neuronal activity affects heart rate, blood pressure and sleep. Therefore, it is not surprising that disturbances in heart rate variability (palpitations) and hypertension, temperature regulation, and sleep are symptoms of the estrogen deplete state of menopause. Furthermore, central effects on appetite and activity may promote weight gain and lethargy associated with menopause in some women and, thus could be considered as a potential physiological component of “life-style risk factors” for cardiovascular disease.
1. Hypertension, sympathetic tone, and stress
Hypertension is a major cause of cardiovascular morbidity and mortality in postmenopausal women (Thom et al., 2006). Estrogen should reduce development of hypertension through peripheral actions such as up regulation of endothelium-derived vasodilator factors with simultaneous down regulation of vasoconstrictor factors, such as endothelin-1 (Barber and Miller, 1998; Barber et al., 1996; Best et al., 1998; Dubey et al., 2001), inhibition of the renin angiotensin system by reducing transcription of angiotensin converting enzyme in endothelial cells (Brosnihan et al., 1994; Gallagher et al., 1999) and down regulation of angiotensin 1 receptors (Nickenig et al., 1998). Another potential pathway involved in the etiology of hypertension is the production of 20-hydroxyarachidonic acid (20-HETE) by cytochrome P450a monooxygenase which shows an androgen sensitivity (Capdevila et al., 2007; Holla et al., 2001). Depletion of estrogen with a concomitant increase in androgens would, therefore, reduce local inhibitory signals while increasing pro-contractile signals at the vascular wall leading to increased peripheral resistance and blood pressure in the absence of concomitant decreases in sympathetic tone. In addition to these direct effects on the vascular wall, in general, withdrawal of estrogen increases sympathetic tone as measured by increases in peripheral sympathetic neuronal activity and circulating levels of norepinephrine resulting in increased blood pressure, especially in the presence of a stressor (Fernander et al., 2004; Liu et al., 2003; Owens et al., 1993; Saab et al., 1989; Vongpatanasin et al., 2001; Wyss and Carlson, 2003). Whether estrogen, when injected directly into the brain, increases or decreases sympathetic tone depends upon the specific nuclei which are stimulated (Saleh and Connell, 2007). Increased sympathetic tone may result from reduction of inhibitory effects mediated by ERβ as deletion of the gene for this receptor in mice resulted in a hypertensive phenotype (Zhu et al., 2002). In the brain, ERβ seems to be localized in cardiovascular centers with inhibitory neurons (Blurton-Jones and Tuszynski, 2002). Thus, estrogen depletion would be associated with withdrawal of inhibitory tone such as that imparted by the parasympathetic system thereby increasing peripheral resistance and lowering heart rate variability. Decreased heart rate variability was observed in women after oophorectomy compared to age-matched women who underwent hysterectomy with conservation of the ovaries. Heart rate variability was restored in the oophorectomized women after three months of estrogen therapy, although the type of estrogen therapy was not identified in this study (Mercuro et al., 2000). It remains to be resolved whether the type of treatment (oral or transdermal) or formulation (conjugated equine estrogen, 17β estradiol, estrone, or estrone in combination with testosterone or progestogens) would be critical in defining the overall effectiveness of modulating autonomic activity (Liu et al., 2003; Matthews et al., 2001; Matthews et al., 2005; Vongpatanasin et al., 2001).
The type of estrogenic treatment and parameters measured to ascertain estrogenic effects on blood pressure may be important in explaining discrepancies in changes in blood pressure reported in various clinical trials (Ashraf and Vongpatanasin, 2006; Felmeden and Lip, 2000). To date, none of the large scale clinical trials have evaluated blood pressure responses or heart rate variability with estrogen treatments relative to an individual’s ability to metabolize or respond to a particular estrogen. Genetic variability in estrogen receptors and the ability to metabolize estrogen may be a critical factor which could help to differentiate central autonomic effects of estrogen from those responses occurring at the level of the blood vessel wall in the systemic circulation. In clinical trials of hormone treatment blood pressure is usually measured under resting conditions. However, variation in blood pressure, as would occur over the course of 24 hours in response to various stimuli (exercise and stress), may be critical in evaluating estrogenic effects on sympathetic control (Kaplan et al., 1996; Mercuro et al., 2000; Saab et al., 1989; Vongpatanasin et al., 2001) as estrogenic treatment of menopausal women reduced increases in pituitary-adrenal hormones, blood pressure and pulse pressure induced by mental task induced stress (Matthews et al., 2001). Because postmenopausal women are at risk for hypertension (Felmeden and Lip, 2000; Thom et al., 2006), in the future, it will be critical to evaluate effects of various combination products and their route of administration on blood pressure control.
2. Metabolism of adrenergic neurotransmitter and regulation of adrenergic receptors
In the periphery, the sympathetic nervous system with adrenergic transmission comprises a major innervation of arteries, arterioles and veins. In addition to modulating neuronal activity through binding to estrogen receptors, estrogen also regulates adrenergic neurotransmission through effects on catecholamine re-uptake at the synaptic cleft (Ball and Knuppen, 1990; Hamlet et al., 1980; Herbison et al., 2000), genomic regulation of α-adrenergic receptors (Colucci et al., 1982; Herbison et al., 2000; Paden et al., 1982), and competition with norepinephrine for adrenergic binding sites (Ball and Knuppen, 1990; Hiemke and Ghraf, 1982; Paden et al., 1982; Parvizi and Wuttke, 1983). Furthermore, catecholestrogens (2-hydroxyestradiol and 4-hydroxyestradiol) show binding affinity for tyrosinase, affecting catecholamine synthesis, and for catechol-O-methyltransferase (COMT), affecting catecholamine degradation (Ball and Knuppen, 1990; Zhu, 2002) (Figure 2). Inhibition of catecholamine reuptake at the synaptic cleft and inhibition of degradation would have the net effect of prolonging the impact of an adrenergic neuronal signal.
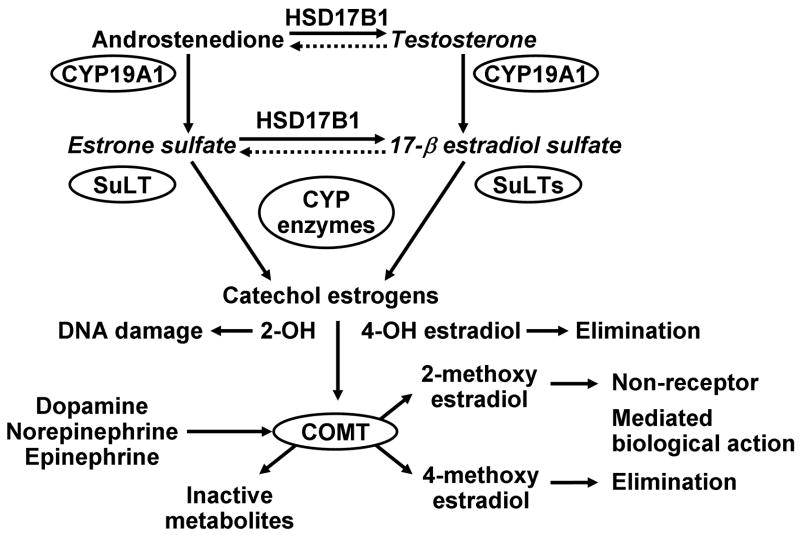
Figure 2.
Schematic of metabolic pathways involved in the synthesis and biotransformation of estrogen in the liver and extravascular tissue. Estrogen and some metabolites each have specific binding affinities for estrogen receptors. In addition, other metabolites of estrogen also have biological activities that do not require binding to the classically defined receptors. Identification of differences in copy numbers of genes and polymorphisms in CYP enzymes will affect the efficacy of a particular estrogenic treatment as well as impact the biological consequence of that treatment depending upon the rate of metabolism and the end product (see Table 2). Competition of the catechol estrogens with adrenergic transmitters for catechol O-methyltransferase (COMT) will affect the rate at which adrenergic neuronal signaling is sustained. Abbreviations: CYP, cytochrome P450 enzymes designated by numbers; HSD17B1, 17 β-hydroxysteroid dehydrogenase; SuLT, sulfatases; SULTs, sulfotransferases. Modified from Figure 4 of (Miller and Mulvagh, , 2007).
Furthermore, one catecholestrogen, 2-hydroxyestradiol, binds irreversibly to proteins and nucleic acids causing damage to DNA possibly providing an initiating step in breast and uterine cancers (Cavalieri et al., 1997). Thus, conversion of 2-hydroxyestradiol by COMT to 2-methoxyestradiol is considered a detoxifying step in the metabolism of estrogen. These metabolic products of estradiol also have important, but concentration-dependent effects on vascular smooth muscle and endothelial cells. At low concentrations, metabolic products of estradiol inhibit smooth muscle proliferation and endothelial proliferation and thus reduce vascular response to injury while at higher concentrations they are anti-angiogenic (del Pozo et al., 2004; Klauber et al., 1997). Thus, when the conversion of 2-hydroxyestradiol to 2-methoxyestradiol is absent as in cells derived from COMT knockout mice, antimitotic effects of 2-hydroxyestradiol are prevented (Zacharia et al., 2003; Zacharia et al., 2001).
Because COMT is a ubiquitous enzyme, it might be expected that genetic variation in COMT would affect circulating levels of estrogens in postmenopausal women and perhaps serve as a risk factor for stress-induced cardiovascular disease. The polymorphism at codon 158 in COMT (Val158Met) decreases the methylation activity of the enzyme (see (Weinshilboum, 2006)). In postmenopausal women genotyped as homozygous for the valine/valine codon (or high metabolizers COMTHH), serum levels of 17β-estradiol were lower three hours following an oral dose of estradiol valerate than women with either the heterozygous genotype or homozygous for the methionine/methionine codon (Worda et al., 2003). Although there was no increase in overall mortality associated with COMT polymorphisms, in a population based study of 2,979 non-diabetic individuals, there was an increase in non-ischemic heart disease among individuals with the met/met and met/val genotypes. These authors cautiously state that these findings may be incidental (Hagen et al., 2007).
In another population study of 2,507 peri- and post-menopausal women referred to a clinic for initiating estrogenic treatment, polymorphisms in COMT were associated with breast cancer occurrence but not with cardiovascular pathologies (Tempfer et al., 2004). As provocative as these results might be, without details regarding the phenotype of these women including other environmental risk factors (i.e. smoking status), medications including type and duration of hormonal therapy, years of follow-up or criteria for recording an adverse event, the clinical relevance of these observations remains to be delineated. Clearly, additional analyses are needed to better understand relationships between estrogen metabolism and cardiovascular disease including interactions with other metabolic risk factors like homocysteine and environmental estrogenic compounds like the phytoestrogens (Zhu, 2002).
3. Variation in vasomotor and neuronally-mediated symptoms of menopause – genetic considerations
Menopausal symptoms are reported in populations of women from around the world (Freeman and Sherif, 2007). In spite of the commonality of this occurrence, there is not universal presentation in all women of a single cadre of symptoms: sleep disturbances, night sweats, hot flashes, heart palpitations, irritability and depression. Presentation of each symptom can be absent in some women and range from mild to severe in others. There is some belief that menopausal symptoms may be associated with a culturally driven negative attitude about menopause in women (Freeman and Sherif, 2007). However, it seems just as likely that the negative impact on quality of life of women experiencing symptoms may influence their attitude about menopause. As discussed above, receptors for estrogen are widely distributed within the central nervous system in areas of the brain controlling body temperature (preoptic hypothalamus), sleep (raphe nuclei) and heart rate (solitary tract). Furthermore, there is anatomical evidence that menopausal symptoms are autonomically driven physiological responses. Thus, a probable explanation for variation in menopausal symptoms among women might be genetic variation in genes directing estrogen metabolism (synthesis and catabolism) or estrogen receptors. Indeed, data are beginning to emerge that provide insight into the genetic variations contributing to various menopausal phenotypes.
The most extensive investigation to date into polymorphisms of genes encoding enzymes needed for estrogen metabolism and estrogen receptors as well as SNP associations with vasomotor symptoms and cardiovascular risk parameters is the Study of Women Across the Nation (SWAN). SWAN is a longitudinal observational study of women in the United States between the ages of 42–52 years of age who were still menstruating, not using exogenous hormones and who were followed for six years. A strength of this study is that genotypes were analyzed from women of four ethnic/racial groups: African American, Caucasian, Chinese-American, and Japanese-American. While vasomotor symptoms (hot sweats, cold sweats and night sweats) were reported in all ethnic groups, associated genetic polymorphisms differed by race/ethnicity. In Caucasian women, vasomotor symptoms were associated more with polymorphisms in the gene for 17HDS responsible for conversion of estrone to 17β-estradiol (Figure 2). In this case, lower conversion would decrease variability in 17β-estradiol levels and therefore symptoms (Crandall et al., 2006)(Table 2).
Table 2.
Potential physiological consequences of single nucleotide polymorphisms associated with estrogen receptors and estrogen metabolism in Caucasian womena
| Gene | SNPs | Consequence |
|---|---|---|
| ESR1 | rs2234693 | Ovarian aging Cognitive function |
| rs9340799 | Cognitive function | |
| rs3798577 | Ovarian aging ApoA-1 |
|
|
| ||
| ESR2 | rs1256030 | Lumbar spine BMD |
| rs1271572 | Myocardial Infarction | |
|
| ||
| 17HSD | rs2830 | Vasomotor symptoms |
| rs592389 | Vasomotor symptoms | |
| rs615942 | Vasomotor symptoms | |
|
| ||
| CYP1A1 | rs2606345 | Depressive symptoms |
|
| ||
| CYP 19 | rs2414096 | Diabetes mellitus |
| rs2446405 | Insulin sensitivity | |
|
| ||
| COMT | VAL158Met | Cancer/non-ischemic heart disease ? |
Derived from (Hagen et al., 2007; Rexrode et al., 2007; Sowers et al., 2006; Tempfer et al., 2004).
Studies have yet to be conducted which link menopausal symptoms and genetic phenotypes to cardiovascular disease progression or outcomes. However, additional information should be forthcoming with continued analysis of DNA collected from participants in large trials such as SWAN, Women’s Health Initiative (WHI) and the ongoing Kronos Early Estrogen Prevention Study (KEEPS) (Harman et al., 2005a). Linking menopausal symptoms to progression of occlusive cardiovascular disease and/or hypertension and risk for adverse outcomes such as stroke, myocardial infarction or thrombosis is important as the current guidelines of the United States Food and Drug Administration (FDA) for use of hormonal treatment products is for relief of menopausal symptoms and not for prevention of chronic diseases. However, evidence from women who participated in the CEE-only arm of the WHI suggests that estrogenic treatments may provide vascular protection even to women who are asymptomatic for menopausal symptoms (Manson et al., 2007). In the WHI women who had undergone a hysterectomy were randomized to placebo or CEE-alone. Following cessation of the trial, women were contacted to participate in evaluation of coronary arterial calcium by computed tomography. Coronary calcification was lower in women who had been randomized to CEE compared to placebo (Table 3). Since coronary arterial calcification is considered a significant risk factor for future myocardial infarction (Budoff et al., 2005; Hecht et al., 2006; Raggi et al., 2003), these data support a protective vascular action of estrogen. Because about 75% of these women did not report having menopausal symptoms at the time they initially enrolled in the study, these data support the conclusion that menopausal estrogen treatments may benefit all women regardless of symptomatology.
Table 3.
Coronary Calcification in women participating in the CEEonly – arm of WHIa
| Number of Participantsc | |||
|---|---|---|---|
| CEE n=537 | Placebo n = 527 | Odds Ratiod (95%CI) | |
| CAC Scores (Agatston Units) | |||
| 0 (referent)b | 299 | 266 | 1.00 |
| >0 | 238 | 261 | 0.81 (0.64–1.03) |
| <10 (referent)b | 348 | 302 | 1.00 |
| ≥ 10 | 189 | 225 | 0.73 (0.57–0.93) |
| <100 (referent)b | 448 | 408 | 1.00 |
| ≥100 | 89 | 119 | 0.68 (0.50–0.93) |
|
| |||
|
Vasomotor Symptomse | |||
| Yes | 23.3% | 26.5% | |
| No | 76.7% | 73.5% | |
Derived from Tables 1 and 2 of (Manson et al., 2007).
Referent groups are all participants within the stated CAC range.
Numbers of individuals who were at least 80% adherent to CEE or placebo for at least 5 years.
Odds ratios were calculated for the CEE group compared to the Placebo group.
Percentage of women reporting moderate-to-severe vasomotor symptoms in each assigned group prior to initiation of treatment (baseline).
Polymorphisms in the gene for aromatase which is required for production of both estrone and 17β-estradiol have been associated more with metabolic cardiovascular risk factors including insulin sensitivity and diabetes than with vasomotor symptoms (Table 2) (Crandall et al., 2006; Lo et al., 2006; Sutton-Tyrrell et al., 2005). Interestingly, polymorphisms in estrogen receptors were not consistently associated with blood lipids in women participating in SWAN as was reported in women from the WHI (Herrington and Howard, 2003; Sowers et al., 2006). In the future, analysis of testosterone and sex hormone binding globulin may be useful in identifying subgroups of asymptomatic women who might receive cardiovascular benefit from menopausal estrogen treatment, perhaps reflecting effects of estrogens at the level of the vascular wall depending on local gonadal steroid metabolism (Karim et al., 2008).
VI. Estrogenic Effects in Pathophysiology
A. Inflammation
Inflammation is a stereotypic response of tissues to injury (Kracht and Saklatvala, 2002). Because many cardiovascular diseases including atherosclerosis are thought to have an inflammatory etiology (Libby, 2002), some discussion regarding estrogenic effects on inflammation is warranted.
Literature concerning the effect of estrogens on inflammatory responses seems contradictory, with both pro-inflammatory and immunosuppressive effects reported. In animal models, anti-inflammatory effects have been clearly reported, but, in humans, estrogens are thought to have pro-inflammatory effects in chronic autoimmune diseases. The situation is further complicated by findings that estrogen metabolites produced by first-pass hepatic metabolism may have pro-inflammatory effects as well, causing differences in response depending on the route of administration. Further complications in understanding this field and its impact on vascular disease include the number of different ways in which inflammation is defined; the variety of stimuli, acute or chronic, used to initiate tissue injury; and differences in endpoints used to define the inflammatory response. Detailed and comprehensive reviews of the general topic of estrogenic regulation of inflammatory processes address some of these points and provide excellent diagrams and tables of specific actions of estrogens on regulation of specific cytokines and leukocytes associated with innate and acquired immunity (Stork et al., 2004; Straub, 2007). However, for the purposes of this review, a few points are important to emphasize in order to facilitate future research into estrogenic regulation of inflammation associated with vascular disease.
Pathogens, specifically gram negative bacteria, initiate inflammation through binding of bacterial lipopolysaccharides to toll-like receptors on the surface of vascular cells. Transient or sustained release of cytokines such as tissue necrosis factor (TNF-alpha) or several of the family of interleukins (IL-1, IL-6, IL-17, etc) sustains the inflammatory response through intracellular signaling cascades. These signaling cascades result in both transcriptional and translational modification of receptors, chemokines and enzymes including nitric oxide synthases and matrix metalloproteinases (Stork et al., 2004; Straub, 2007). Inflammatory responses may have either positive or negative consequences, depending in part on the time frame of occurrence. For example, expression of adhesion molecules on the surface of damaged cells with subsequent release of cytokines increases blood flow to the damaged area, serving a protective effect. Recruited leukocytes phagocytize damaged cells and/or invading organisms. In terms of negative effects, however, cytokines released from these cells may also facilitate re-growth of damaged tissue and secretion of extracellular matrix that can, for example, form fibrous scarring characteristic of fibrous plaque. Indeed genetic polymorphisms in toll-like receptors may be protective against cardiovascular disease while rendering the individual more susceptible to infection (Arbour et al., 2000; de Kleinj and Pasterkamp, 2003; Kiechl et al., 2002; Miller et al., 2004b; Zwaal et al., 2005). Evaluation of toll-like receptor polymorphisms has not yet been conducted for women participating in hormone treatment trials.
Many of the intracellular signaling cascades affected by toll-like receptors and interleukin molecules are common to those stimulated by surface estrogen receptors (see Section III). Therefore, it might be expected that estrogenic treatments would modulate inflammatory responses to infectious pathogens. However, the relationship of subclinical infection to progression of cardiovascular disease has not been considered in clinical studies of hormone treatments, for example in relation to periodontal disease (Ford et al., 2007). However, the most consistent reports of estrogenic modulation of inflammation in vascular tissue of animals involve activation of cytokine pathways of inflammation. Estrogenic abrogation of inflammation initiated by TNFα is perhaps the most consistent of these effects and may be critical for linking infection to cardiovascular disease as TNFα increases transiently even with low levels of lipopolysaccharide challenge (Jayachandran et al., 2007). Interestingly, in rats estrogen has been shown to suppress vascular inflammatory responses to IL-1β and lipopolysaccharide treatments, and this response has also been shown to vary through the estrous cycle (Galea et al., 2002; Ospina et al., 2004; Sunday et al., 2006). An intriguing result is that this anti-inflammatory effect of estrogen is lost in older female animals (Miller et al., 2004a; Sunday et al., 2007).
Vascular sensitivity to infection-associated inflammation in relationship to integrity of specific estrogen receptors has not been studied directly and may provide insight into identifying individuals susceptible to infection and to cardiovascular disease with aging. For example, as discussed elsewhere in this article, in response to vascular injury, deficiencies in ERα lead to accelerated development of atherosclerosis in men (Shearman et al., 2003; Sudhir et al., 1997b), and estrogen treatments do not reduce the vascular response to injury following endothelial denudation in mice deficient in this receptor (Pare et al., 2002). ERα mediates estrogenic abrogation of some but not all cytokine-induced expression of cell adhesion molecules in endothelial cells (Caulin-Glaser et al., 1996; Chen et al., 1999; Cid et al., 1994). Alternatively, ERβ mediated the estrogenic abrogation of TNFα-induced inflammation in cultured smooth muscle cells (Xing et al., 2007).
In transfection experiments, ERβ modulates expression of ERα (Hall and McDonnell, 1999). Therefore, differential expression of estrogen receptors in either endothelial or smooth muscle cells and in blood elements interacting with the vascular wall may be critical in defining how various estrogenic products modulate an inflammatory response because various estrogenic products such as SERMS, CEE or estrogen metabolites such as estrone, estriol, estrone sulfate do not bind to estrogen receptors with equal affinity (Ensrud et al., 2006; Hsia et al., 2006b). Furthermore, as some inflammatory cytokines may regulate both expression and activity of steroid sulfatase and sulfotransferases, metabolism of estrogen at the level of the vascular wall may be critical in establishing pro- or anti-inflammatory outcomes (Hebbring et al., 2007; Nakamura et al., 2003).
An individual’s ability to metabolize estrogen may be critical in determining whether estrogenic treatments, oral or transdermal, are beneficial. Oral 17β-estradiol is metabolized in the liver and oral (CEE) which contains a variety of estrogen metabolites is also modified in the liver. Thus, the relationship among circulating 17β-estradiol, estrone, estrone sulfate, etc. will vary with an individual’s ability to metabolize the initial product (Hebbring et al., 2007). Effects of estrogen on all parameters of inflammation are dose-dependent (see (Straub, 2007) for review), and metabolism of 17β-estradiol as well as estrone sulfate can occur within vascular cells (Dubey et al., 2004; Nakamura et al., 2003). Metabolic products of 17β-estradiol have various biological activities. Therefore, effective dosing may vary depending upon which product has the greatest effect on a given parameter of the inflammatory process and an individual’s ability to metabolize the estrogen (Dubey et al., 2000). For example, in response to an inter-arterial challenge of amyloid-β in ovariectomized rats, adherence of leukocytes to both mesenteric arterioles and venules was reduced in rats treated with oral CEE compared to those treated with oral 17β-estradiol. Further, inhibition was dose-dependent, such that leukocyte adhesion was reduced with increases in the dose of oral 17β-estradiol. Therefore, interpretation of effects of estrogen on a this inflammatory response depend both on formulation and dose of the estrogenic product (Thomas et al., 2003). The effective dose of estrogen may also be dependent upon the cytokine milieu. For example, interleukin 1β may regulate expression of enzymes which affect local production of 17β-estradiol by inhibiting sulfatase and stimulating expression of estrogen sulfotransferase (Nakamura et al., 2003).
Oral estrogenic preparations are considered to be pro-inflammatory because hepatic metabolism of 17β-estradiol produces proinflammatory metabolites in higher concentrations than might be produced by more slowly absorbed transdermal products. (Brosnan et al., 2007; De Lignieres et al., 1986; Lacut et al., 2003; Seed et al., 2000; Strandberg et al., 2003; Vehkavaara et al., 2001). However, inflammation in clinical studies is defined by changes in circulating levels of cytokines, for example C-reactive protein, P-selectin, TNF-α, ICAM-1, VCAM-1 and fibrinogen. Such measurements do not identify the cell of origin of the cytokine/protein or take into account kinetics of their production, biological half-life or degradation. Furthermore, in many clinical trials, these measurements represent two time points (pre- and post- intervention) and are related to changes in risk factor profile rather than measurable physical changes in the vascular wall or disease progression (Miller et al., 2007b; Stork et al., 2004). In contrast, in studies using experimental animals, effects of estrogen on vascular inflammation evaluate structural changes in the vascular wall including infiltration of leukocytes or expression of adhesion molecules or secretion of enzymes, such as matrix metalloproteinases. Changes in these parameters are linked to a physiological consequence such as cell proliferation, migration or receptor expression showing that estrogenic treatments reduce infiltration of leukocytes to arteries following endothelium denudation and cytokine-induced gene transcription in smooth muscle (Chen et al., 1996; Oparil et al., 1999; Tolbert et al., 2001; Wang et al., 2005; White et al., 1998; Xing et al., 2007). Therefore, studies are needed to better define estrogenic effects on specific parameters of the inflammatory process associated with progression of vascular disease in humans. These studies will require longitudinal assessment of soluble factors as well as evaluation of vascular anatomy and immuno-competence.
Most studies comparing oral to transdermal preparations of estrogenic treatments on plasma markers of inflammation have evaluated transdermal preparations of 17β estradiol (Chen et al., 2001; Girdler et al., 2004; Seed et al., 2000; Sendag et al., 2002; Stevenson et al., 2004; Strandberg et al., 2003; Vehkavaara et al., 2001). Few studies have examined transdermal preparations of estrogen metabolites, for example, estriol (Mishra et al., 2006), or transdermal preparations of compounded formulations of estriol and estrone sulfate. These latter preparations have gained popularity as more natural, bio-dentical formulations, but data supporting their superiority over other estrogenic formulations are scant in regard to specific measures of vascular physiology.
In addition to modulation of production of inflammatory proteins produced by the liver or direct effect on the vascular wall, estrogen may also modulate inflammatory responses indirectly through the hypothalamic-pituitary-adrenal axis, including stimulating release of corticotropin-releasing hormone from the hypothalamus and corticosteroids from the adrenal glands. Activation of the hypothylamic-pituitary-adrenal axis associates hormonally mediated events to centrally mediated events and to the manifestation of depression, stress and inflammation (Kaplan et al., 1996; Kelly et al., 2005). In general, estrogen treatment suppresses the stress response to challenges such as intra-arterial injection of interleukin 1β, hemorrhage and hypoxia and to emotional challenges such as noise and psychosocial factors (Buller et al., 1999; Dayas et al., 2000; Kaplan et al., 1996; Smith et al., 1995) albeit through different neuronal pathways. Links between stress and estrogenic treatments have implications in understanding sex-based differences in susceptibility to infection, chronic inflammatory conditions and hypertension (Critchlow et al., 1963; Cutolo et al., 2002; Dayas et al., 2000; Fernander et al., 2004; Kaplan et al., 1996; Matthews et al., 1995) and should be considered as potential physiological risk factors for cardiovascular disease in women in addition to the usual psycho-social risk factors such as marital status, level of education, income, etc.
B. Atherosclerosis
Changes in vascular anatomy characterizing atherosclerotic lesions occur over decades. Although there is a large body of evidence that estrogen affects the vascular wall, the molecular mechanisms of vascular responsiveness to sex steroids during different stages of development of atherosclerosis are not clear. Because the response of cells in the non-atherogenic artery may not be the same as those in developing plaque, both the timing and nature of interventions to reduce the rate of these changes should matter when considering the question of whether estrogenic treatments provide protection against cardiovascular disease. This unifying hypothesis has emerged from numerous evaluations of data from preclinical, observational, epidemiological and prospective clinical trials (Clark, 2006; Clarkson, 2007; Hodis and Mach, 2007; Rossouw et al., 2007; Schnatz, 2006; Shapiro, 2006). For example, if estrogenic therapy was initiated within the first 10 years of menopause, the odds ratio for adverse cardiac events was reduced (Grodstein et al., 2006; Hsia et al., 2006b). This reduction was independent of the type of menopause, that is, natural or surgical (Grodstein et al., 2006; Hsia et al., 2006b; Mack et al., 2004; Manson et al., 2007; Rocca et al., 2006). However, the same consistent pattern was not observed for stroke risk (see Section VI.C.). These observations in humans suggest that arteries of different anatomical origin may have different susceptibilities to stimuli initiating and sustaining atherosclerotic and other pathological processes (Moreau et al., 2002).
1. Peripheral arterial disease (PAD)
Few studies have evaluated effects of estrogenic treatments on the incidence of PAD. In women with existing cardiovascular disease participating in the Heart and Estrogen/progestin Replacement Study (HERS), the combined hormone treatment did not reduce the incidence of PAD (Grady et al., 2002; Hsia et al., 2000). Likewise in older, but generally more healthy women participating in the WHI, estrogenic treatments also did not reduce the rate of PAD but the overall incidence was low (Hsia et al., 2006a; Hsia et al., 2004). However, in studies where femoral arterial diameter and anatomy were evaluated by ultrasound, the intimal to medial ratio was lower in treated versus untreated menopausal women (Moreau et al., 2002; Moreau et al., 2003; Naessen and Rodriguez-Macias, 2006). It remains to be determined whether or not timing of initiation of estrogenic treatments impacts development of PAD independent of other risk factors such as smoking, diabetes and hypertension (Hsia et al., 2000).
2. Carotid intimal medial thickness
In experimental animals, estrogenic treatments consistently reduced development of carotid intimal medial thickness following a mechanical injury or atherosclerotic diet (Chen et al., 1996; Foegh et al., 1995; Karas et al., 1999; Oparil et al., 1999). In humans, a consequence of carotid arterial atherosclerosis, incidence of ischemic stroke, was not reduced in women participating in the WHI (Rossouw et al., 2007; Writing, 2002). But no assessment of carotid anatomy was performed in these women. However, as with the femoral arteries, in studies in which the carotid arteries have been evaluated by ultrasound, a consistent reduction in carotid intimal medial thickness is observed in post-menopausal women using estrogenic treatments compared to those who do not (Deneke et al., 2000; Hodis et al., 2001; Karim et al., 2008; Mihmanli et al., 2002; Moreau et al., 2002; Sator et al., 1998; Takahashi et al., 2004). A direct testing of the timing hypothesis of estrogen intervention on progression of carotid intimal medial thickness is ongoing in the Early vs Late Intervention Trial with Estradiol (ELITE, NCT00114517). ELITE will compare the effect of oral 17β-estradiol (oral 1mg/day) on the rate of change of carotid intimal medial thickness in women less than six years after menopause to women who are more than 10 years past menopause. Effects of oral conjugated equine estrogen (0.425 mg/day) and transdermal 17β-estradiol (50 μg weekly patches) on both progression of carotid intimal medial thickness and coronary calcification are being evaluated in women who are within three years of menopause in the Kronos Early Estrogen Prevention Study (KEEPS; NCT00154180). Therefore, within the next five years, data will be available to allow evaluation of three different estrogenic formulations on the same outcome of disease progression, i.e. carotid intimal medial thickness, in an aged spectrum spanning two decades of menopause. These studies will provide valuable evidence regarding efficacy of products which may affect a risk for ischemic stroke (see section VI C. below).
3. Coronary arterial calcification
Coronary calcification, a predictor of future adverse cardiovascular events, can be present in early menopausal women who do not present with the usual cadre of risk factors for cardiovascular disease (Hecht et al., 2006; Hodis et al., 2001; Rumberger et al., 1994). Estrogenic treatments reduce coronary arterial calcification in post-menopausal women (Budoff et al., 2005; Mackey et al., 2005; Manson et al., 2007). And as discussed above (sectionV.C.3.), reduced calcification was observed with CEE treatment even in women who did not experience menopausal symptoms (Manson et al., 2007) (Table3). These data support a cardiovascular protective effect of CEE in women for whom estrogenic treatments would not be prescribed under the current practice guidelines. Mechanisms by which estrogen reduces calcification are multifactorial but most likely include modulation of cell differentiation (Abedin et al., 2004; Anderson et al., 2004; Fitzpatrick et al., 2003; Rzewuska-Lech et al., 2005), genetic variation related to bone matrix proteins and osteoblast/clast activation (Doherty et al., 2003), and perhaps susceptibility to infection by calcifying nanoparticles (Miller et al., 2004b). Much remains to be learned about the contribution of specific estrogen receptors in arterial calcific processes. However, ERβ is prominent in coronary arterial plaque, and polymorphisms in the gene for ERβ were associated with myocardial infarction in women (Christian et al., 2006; Rexrode et al., 2007). Intracellular processes mediated by this receptor that contribute to or limit calcification are not known.
4. Endothelial dysfunction and other modalities to assess cardiovascular risk in menopausal women
Modulation of quantity of intimal hyperplasia and arterial calcification reflect long-term effects of estrogen on components of the vascular wall. However, effects of estrogen on endothelial function are noted within three days of ovariectomy in rabbits and within months in humans (Bush et al., 1998; Gisclard et al., 1988; Lieberman et al., 1994). Since endothelial dysfunction may reflect the earliest stages of disease processes (Feletou and Vanhoutte, 2006; Hamburg et al., 2004), evaluation of endothelial function relative to menopausal age may provide another diagnostic modality to identify women who might receive cardiovascular benefit from estrogenic treatments. In a prospective study which stratified women by age since menopause, forearm vasodilatation, as an indication of endothelial function, increased following 3 months of oral estrogen treatment (estradiol valerate, 1 mg/day) in all women. However, the magnitude of the increase diminished with age past menopause (Vitale et al., 2008). In women who had used estrogenic treatments prior to the study, the effect of aging was diminished. These results confirm the hypothesis that endothelial function diminishes with age, that estrogen maintains endothelial function and that even temporary use of estrogen may delay detrimental impact of aging on endothelial function. However, longitudinal, prospective studies are needed to provide additional data regarding the rate of change of endothelial function with age and to determine the duration of “carryover’ effect of estrogen on endothelial function once treatment is stopped (Hynes and Duckles, 1987; Seals et al., 2006; Sherwood et al., 2007). This latter point is especially important given the current prescribing recommendation to use estrogen treatments for the shortest period of time to relieve menopausal symptoms.
Additional parameters, including endothelial function, are needed for cardiovascular risk stratification in early menopausal women as standard characterization of risk using parameters of hypertension, plasma lipids and smoking status (i.e. Framingham Risk Score) does not adequately predict risk in this group of women (Lakoski et al., 2007; Miller et al., 2007b; Shaw et al., 2006; Sherwood et al., 2007). Search for a set of blood biomarkers has not yielded a reliable indicator of early disease (Kullo et al., 2006; Kullo et al., 2003; Redberg et al., 2000). Because coronary calcification increases risk for future adverse events (Budoff et al., 2005; Desai et al., 2004; Raggi et al., 2003), identifying women at risk by inexpensive screening modalities, such as assessing arterial calcification using mammograms, may provide an additional, inexpensive way to stratify risk for women. However, additional studies are needed to confirm in newly menopausal women the relationship between breast and coronary calcification that has been found in older women (Kataoka et al., 2006; Maas et al., 2004; Rotter et al., 2008).
Another potential diagnostic tool to evaluate risk in early menopausal women might be analysis of blood borne microparticles (or microvesicles). Microparticles are formed during activation and apoptosis of activated cells. These circulating spheres of membrane range in size from 0.1–1μm and carry surface signature molecules of their cell of origin, varying with specific disease conditions including various cardiovascular diseases (Boulanger et al., 2006; Lynch and Ludlam, 2007). Few studies have evaluated microparticle populations in asymptomatic populations. Expression of phosphatidylserine on microparticles varied among early, asymptomatic menopausal women being screened for the KEEPS trial (Miller et al., 2008). The level of expression was not associated with the usual risk factors for cardiovascular disease such as plasma lipids, hsCRP, BMI, blood pressure or smoking status. However, in this group of women, the quantity of endothelium- and platelet-derived microparticles expressing phosphatidylserine were significantly and positively correlated with subclinical coronary disease defined by a coronary calcification score >50 Agatston Units (Jayachandran et al., 2008 (in review)). Therefore, analysis of populations of microparticles may provide insight into cell-cell interactions in early disease processes. With refinement and standardization of methods to detect and analyze populations of microparticles, this approach may help identify early disease processes in otherwise asymptomatic populations. Development of such new, easily accessible markers of the progression of vascular disease would aid in understanding the impact of a variety of therapies, including gonadal steroid hormones.
C. Stroke
Ischemic stroke is uncommon in women before menopause and increases substantially as women age, leading to the premise that women are protected early in life by reproductive hormones (Barrett-Connor and Bush, 1991; Wenger et al., 1993). However, findings of recent clinical trials have been surprising in not showing protective effects of hormonal therapy at reducing the incidence of stroke therapy. Careful analysis of data from the WHI demonstrates that daily administration of CEE alone in women without a uterus did not protect against ischemic stroke (Hendrix et al., 2006). In the youngest group of women (50–59 years) the cumulative hazard ratio for ischemic stroke was 1.09, while this was 1.72 in women aged 60 to 69. Thus, it is clear that treatment with CEE increased the risk of ischemic stroke in a group of generally healthy postmenopausal women in whom a decrease in coronary calcification was recorded. This study makes it clear that CEE, not the medroxyprogesterone given to women with a uterus, was responsible for the increased risk of stroke in the WHI as a whole (Rossouw et al., 2002). Nevertheless, it remains clear that sex and hormonal status are important factors in the pathophysiology of many diseases, including ischemic stroke. For example, a recent study of randomized low-dose aspirin for the primary prevention of cardiovascular disease in women demonstrated that aspirin lowered the risk of stroke without affecting the risk of myocardial infarction (Ridker et al., 2005). This result is significantly different from earlier findings in men, again raising the importance of the variable of sex in understanding pathophysiology of cerebrovascular disease.
In animal studies, that treatment with the natural estrogen, 17β-estradiol, protects against cardiovascular disease and neuronal damage of experimental ischemic stroke. For example, administration of 17β-estradiol consistently decreases lesion size in rodent ischemic stroke (Alkayed et al., 2000; McCullough and Hurn, 2003). A number of possible explanations for the discrepancy between recent clinical trials and animal studies have been offered. These include the possibility that the oral hormone replacement therapy regimen used in recent studies may not be the most advantageous for positive cardiovascular effects. In contrast to animal studies where 17β-estradiol was administered intraperitoneally or subcutaneously, in human trials, CEE was administered orally. Thus another possibility is that some of the various, little-studied estrogenic compounds in the CEE preparation were deleterious (Turgeon et al., 2004). Although animal studies have demonstrated untoward effects of medroxyprogesterone (Sunday et al., 2006), analysis of the CEE only arm of the WHI appears to rule out this explanation (Barrett-Connor and Stuenkel, 1999; Hendrix et al., 2006; Turgeon et al., 2004). However, it is particularly notable that, in the Heart and Estrogen/Progestin Replacement Study (HERS), only women with existing coronary disease were studied (Hulley et al., 1998), while in the Women’s Health Initiative study (WHI), the mean age of women at initial screening was 63 years, and over 65% of the women were older than 60 years of age (Rossouw et al., 2002). If 17β-estradiol is protective, but cannot reverse pre-existing vascular disease, then perhaps hormone replacement therapy has not been administered early enough in menopause in these studies to be effective (Naftolin et al., 2004).
What do we know about the actions of 17β-estradiol on cardiovascular function that might explain how this hormone could have powerful protective effects against cardiovascular disease and stroke but not be effective against existing disease? As described above, non-reproductive effects of estrogen on the cardiovascular system may have protective effects in stroke, including beneficial effects on lipid metabolism, increased vascular endothelial production of NO and prostacyclin, promotion of endothelial cell growth and angiogenesis and suppression of inflammatory responses. Furthermore, if individuals lack exposure to estrogen for a period of time, progression of atherosclerosis due to changes in expression of estrogen receptors, an unfavorable lipid profile and consequent endothelial dysfunction may not be reversed by subsequent estrogen administration. However, actions of estrogen on vascular mitochondrial function may provide additional explanations. Changes in mitochondrial function with age would not be reversible. Ischemic stroke, with a much greater incidence in older individuals, is an age-related disease, thus it makes sense to hypothesize that age-related mitochondrial changes contribute to the pathophysiology of ischemic stroke. If estrogen is protective, then treatment of women well past menopause who have not been continually exposed to estrogen would not be protected from stroke. In fact, it would be quite possible that, through other mechanisms, such as prothrombotic actions, estrogen might even increase stroke incidence, as was seen in clinical trials of CEE. Current understanding of the biological actions of estrogen on cerebral blood vessels and the cerebral microvesicles that support neuronal function is incomplete.
There is increasing appreciation that the health of neurons during ischemic stroke is dependent on local microvessels and supporting cells such as astrocytes (del Zoppo, 2006; Iadecola et al., 2006). Together, these elements comprise what is referred to as the “neurovascular unit” because of the close association and interaction among cerebrovascular cells, astrocytes and neurons. Perivascular neurons appear to communicate to blood vessels through astrocytic processes to adjust local blood flow (Hamel, 2006; Zonta et al., 2003). Astrocytes also provide metabolic support for neurons, utilizing energy substrates supplied by the microvessels (Pellerin and Magistretti, 1994). While stroke studies in animals have traditionally focused on neuronal survival mechanisms, it is becoming apparent that it is the “neurovascular unit” that must be protected from ischemic injury to improve stroke outcome (del Zoppo, 2006; Iadecola et al., 2006). This complexity is challenging for the researcher because each cell type within the unit has varying responses and coping strategies during the progression of stroke injury and recovery.
Three key cell types of the neurovascular unit, neurons, astrocytes and endothelial cells, are highly metabolic, requiring energy to maintain ion pumps and transporters critical to the proper functioning of the brain. With particular relevance to this review, cerebrovascular endothelial cells are highly metabolic compared with other vascular beds, containing more mitochondria than other types of endothelium (Nag, 2003). Cerebral endothelial cells have the unique function of maintaining the blood-brain barrier, a critical site of injury during ischemic stroke that leads to vasogenic edema. Furthermore, vascular dysfunction during stroke compromises local blood flow, contributing to the evolution of brain injury. The impact of ischemic stroke on the various components of the neurovascular unit differs in response and timing. For example, in models of transient ischemia/reperfusion, the peak of superoxide production, as measured by hydroethidine (HEt) oxidation, was seen in neurons after 1 h reperfusion but not until 4 h in endothelial cells (Kim et al., 2002). αB-crystallin, a small heat-shock protein (HSP), is induced transiently in neurons of the peri-infarct (penumbra) region at 4 h after ischemia/reperfusion but does not appear in astrocytes until 2–4 d later (Piao et al., 2005). Another heat-shock protein, HSP70, is expressed only in endothelial and glial cells, but not neurons in the ischemic core. In the penumbra, HSP70 is also expressed in metabolically stressed neurons (Kokubo et al., 2003; Sharp et al., 2000). Early expression of matrix metalloproteinase-9 (MMP-9) and VEGF after ischemia/reperfusion is associated with microvessels, causing degradation of the vascular matrix and blood-brain barrier leakage (Zhao et al., 2006). However, 7–14 days after stroke these factors in the peri-infarct zone are primarily involved in neurovascular remodeling and repair (Zhang et al., 2002; Zhao et al., 2006). More work is needed to understand the effect of estrogen on all components of the neurovascular unit, and the mechanisms by which estrogen may affect the progression of ischemic injury in order to develop useful therapeutic interventions (Bushnell et al., 2006).
D. Migraine
In the preceding section, the concept of the neurovascular unit was introduced. The neurovascular unit is also implicated in the etiology of migraine. Although migraines may be considered an “essentially benign condition” (Bousser, 2004; Bousser and Welch, 2005), they certainly have a negative impact on quality of life for the migraineurs. Migraines express as pain and are associated with vasodilatation of cerebral and meningeal arteries. They are classified as occurring with or without a visual aura, thus implicating different neuronal involvement between the two types of migraines (Bousser, 2004; Bousser and Welch, 2005; Brandes, 2006; MacGregor et al., 2006; Wessman et al., 2004). Indeed, individuals who experience aura can be biochemically differentiated from those who do not (Ferrari, 1992).
Migraines show a three-fold-greater prominence in women compared to men (Brandes, 2006; Martin and Behbehani, 2006). In some women, migraines may be associated with the menstrual period, ameliorated by pregnancy, diminished at menopause and may worsen with menopausal hormone treatment. These observations suggest that fluctuations in estrogen levels, especially a decrease, may be a precipitating factor in migraines without aura (Bousser, 2004; MacGregor et al., 2006; Wessman et al., 2004). However, differences in circulating levels of estrogen were not observed between women with and without menstrual migraine. Urinary excretion of estrone-3-glucuronide was more than 2-fold higher in women with migraine than those who did not experience migraine, suggesting the ability to metabolize estrogen may relate to development of migraine (MacGregor et al., 2006). In depth analyses related to estrogen metabolism among women who experience migraines, with or without aura, and women who do not, needs further investigation. In particular, production of catecholestrogens, perhaps influencing production and disposition of adrenergic neurotransmitters, could participate in neuronally-induced cerebral vasospasm (Ferrari, 1992; Martin and Behbehani, 2006).
Several genetic polymorphisms are associated with familial migraine including genetic variation in ERα (G594A polymorphism of exon 8) (Colson et al., 2006; Colson et al., 2004; Johnson et al., 2007). As discussed above, estrogen receptors are located within brain nuclei that innervate the cerebral vasculature as well as other nuclei regulating cardiovascular function (See section V.C; (Martin and Behbehani, 2006)). Thus, in addition to influencing adrenergic mechanisms, estrogen may also modulate central opioidergic tone, release of peptidergic transmitters from trigeminal nuclei and the GABAergic system, perhaps modulating NO (Bergerot et al., 2006; Brandes, 2006; Johnson et al., 2005; Martin and Behbehani, 2006; Puri et al., 2006). Since ERα stimulates NO production in vascular endothelium, there might also be direct modification of migraine occurrence through this pathway. Implicating NO in the etiology of migraine are observations that platelet production of NO was greater in women with menstrual migraine than in those without (Brandes, 2006). NO released from platelets could contribute to decreases in cerebral vascular tone in vivo or reflect changes in synthesis of NO that might occur locally within the cerebral vasculature. A polymorphism (Glu298Asp) in endothelial nitric oxide synthase (eNOS) results in decreased activity of the enzyme. This variant is associated with increased risk for cardiovascular and cerebrovascular disease. The homozygous variant was an independent risk factor for stroke in persons with migraine with aura (Borroni et al., 2006). About 80% of individuals participating in this study were female, reflecting the prominence of the condition in women, suggesting that this variant is prevalent among women with migraine (Borroni et al., 2006). The association of this genetic variation in eNOS with those of ERα in a larger population remains to be determined. If the genetic variant results in decreased activity of eNOS, these results are difficult to interpret within the context that increased production of NO may trigger migraine (Thomsen and Olesen, 2001). Some evidence implicates neuronally derived NO in the etiology of migraine, but no association of migraine with genetic variation of neuronal nitric oxide synthase was found (Bergerot et al., 2006; Borroni et al., 2006; Johnson et al., 2005). More information is needed regarding estrogenic modulation of all three isoforms of nitric oxide synthase in the cerebrovascular unit.
In addition to estrogenic modulation of neuronal transmission associated with pain and endothelial NO (Martin and Behbehani, 2006; Rossouw et al., 2002; Welch et al., 2006), estrogen may induce migraine through direct effects on vascular smooth muscle cells. For example, estrogen increased the efflux of magnesium from cultured cerebral smooth muscle cells (Li et al., 2001). Indeed, magnesium is an effective treatment for migraine in some individuals (Ferrari, 1992; Martin, 2007).
The relationship between migraine and stroke should be considered, especially as related to brain ischemia. In doing so, however, it is important to try to distinguish migraine as an underlying pathology increasing the risk for stroke as opposed to migraine as a symptom resulting from a stroke. Most evidence indicating that migraine may be a risk factor for stroke comes from studies of incidence of ischemic stroke in young women who experience migraine with aura. Although the risk for stroke overall is low, about 18 per 100,000 per year, the relative risk for stroke is 3.8–6.2 in women who have migraine with aura compared to those who experience migraine without aura (Bousser, 2004; Bousser and Welch, 2005). The relative risk for ischemic stroke increases in this group with oral contraception use and smoking. As the former may increase the risk for thrombosis and the latter is a known risk factor for cardiovascular disease, suggests that migraine with aura may reflect an underlying vascular pathology which is exacerbated by these environmental stressors (Bousser, 2004). Middle aged (average about 58 years) women participating in the population based study, Atherosclerosis Risk in Communities Study, who experienced migraine with aura were also at increased risk for ischemic stroke (Stang et al., 2005). This observation also points to an underlying pathology of the neurovascular unit contributing to migraine. In the WHI, incidence of stroke was greater even in the CEE-only arm of the study where those randomized to treatment had lower incidence of myocardial infarction and coronary calcification (Manson et al., 2007; Rossouw et al., 2002; Rossouw et al., 2007). Incidence of migraine in this cohort was not assessed. These observations point to the need to understand and differentiate factors contributing to stroke risk from those contributing to cardiac risk (Bousser and Welch, 2005; Bushnell et al., 2006).
There are several chronic alterations in small arterial anatomy and function which predispose an individual to ischemic stroke and migraine with aura which may not show sex-difference in frequency. One syndrome, mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS), is associated with mutations in mitochondrial DNA (Bousser and Welch, 2005). The relationship between these mitochondrial mutations and mitochondrial pathways modulated by estrogen as discussed in Section IV remains to be explored.
E. Thrombosis
Risk of venous thrombosis is a “black box” warning required by the United States Food and Drug Administration on labeling of estrogenic products. Although increased incidence of venous thrombosis has been reported in numerous clinical trials of estrogenic products in menopausal women (Barrett-Connor et al., 2002; Grodstein et al., 2001; Herrington et al., 1998; Scarabin et al., 1997; Vickers et al., 2007; Writing, 2002) little is known about what actually constitutes risk for an individual woman. Concentrations of soluble markers in the blood associated with either inflammation or proteins of the coagulation cascade including substances also associated with arterial disease such as C-reactive protein, fibrinogen, and homocysteine were higher in individuals who experienced a thrombotic event compared to those who did not (Eilertsen et al., 2005; Meijers et al., 2000; Pradhan et al., 2002; van Hylckama Vlieg and Rosendaal, 2003). While assays for these plasma/serum markers are clinically validated, no global assessment tool has been established to identify an “at risk” phenotypic profile for an individual woman contemplating use of estrogenic treatments.
An alternative approach to define a procoagulant phenotype is to use genetic analysis based on that obtained from individuals who have experienced an adverse event. Perhaps the most consistent risk for estrogenic treatment and venous thrombosis is Factor V Leiden. However, individuals who do not carry the mutation may be at risk for a thrombotic event while those who carry it may never experience an event (Heit, 2006; Herrington et al., 2002b; Miller et al., 2006; Price and Ridker, 1997; Simon et al., 2006). The formulation of the estrogenic treatment may also be critical for increasing risk even in individuals with this mutation, as incidence of venous thrombosis was less in individuals with Factor V Leiden using transdermal compared to oral estrogenic products (Scarabin et al., 2003; Straczek et al., 2005). As discussed in other sections of this review, the first pass metabolism of oral products in the liver may increase inflammatory cytokines such as hsCRP and other coagulation proteins increasing risk of thrombosis in susceptible individuals (Brosnan et al., 2007; Scarabin et al., 1997; Scarabin et al., 2003). Alternatively, detoxification of the catecholestrogens may impact production of prostacyclin which acts to inhibit platelet aggregation (Needleman and Parks, 1982). Direct comparison of oral CEE and transdermal 17β estradiol on soluble proteins and other cytokines related to coagulation and inflammation together with an estrogen metabolic profile will be measured in the KEEPS trial (Harman et al., 2005a).
Genetic variants in platelet surface receptors and estrogen receptors have been evaluated in regard to individual risk for arterial thrombotic events but without consistent findings (Alessio et al., 2007; Kjaergaard et al., 2007). No studies have evaluated an individual’s genotype for enzymes that metabolize estrogen and related that to thrombotic risk. Differences in copy number of cytosolic sulfotransferase reflect the ability to metabolize estrogen (Hebbring et al., 2007). However, how this differences translates to risk for disease, i.e. thrombosis, cancer, etc, remains to be determined. In the future it will be important to take a polygenomic approach to estimating thrombotic risk, including evaluation of estrogen receptors, enzymes metabolizing estrogen and receptors for environmental factors and exposure to inflammatory provoking pathogens (Arbour et al., 2000; Jayachandran et al., 2007; Kiechl et al., 2002; Mari et al., 2007; van Hylckama Vlieg and Rosendaal, 2003). Interaction of age, hormonal status and environmental factors should also be considered. For example, a dose of lipopolysaccahride which was not lethal to reproductively competent mice became lethal in reproductively senescent mice and to a greater extent if the mice lacked ERβ (Miller et al., 2006). Additional studies are needed to exploit new models for studying thrombosis in experimental animals (VanLangevelde et al., 2005).
Contrary to evidence that an early menopause increases risk for arterial cardiovascular disease (de Kleijn et al., 2002; Hu et al., 1999; Rocca et al., 2006), data from a hospital based case-controlled study suggest that the risk of venous thromboembolism decreases with early menopause (Simon et al., 2006). That is, the shorter the exposure to endogenous estrogen (an early menopause), the less the risk of a venous thrombotic event. The reason for these apparent contrary findings is unclear, underscoring the need for basic research into how hormones affect the venous wall. However, the impact of number of pregnancies and related issues of changes in vessel compliance resulting from connective tissue reorganization and physical challenges related to obstruction of venous return also need to be considered carefully (Simon et al., 2006).
As generation of a clot, arterial or venous, requires interaction of the blood with a biochemical or mechanical lesion in the vascular wall, it is also critical to understand how estrogenic treatments impact formed elements in the blood. Platelets are the formed element in the blood critical for the generation of thrombin. Both platelets and their precursors, megakaryocytes in the bone marrow, contain estrogen receptors (Bracamonte et al., 2002c; Jayachandran and Miller, 2003; Khetawat et al., 2000; van Kesteren et al., 1997; Yang et al., 2004). Thus, changes in hormonal status, i.e., at the transition to puberty, menopause and hormonal treatments, influence the phenotype of the circulating platelet pool (Jayachandran and Miller, 2002; Jayachandran et al., 2005b; Jayachandran et al., 2004). In general, in studies of estrogen treatment to large animals, platelet aggregation, and secretion decreased compared to ovariectomized animals. However, not all specific components of the platelet secretome were regulated the same by different formulations of oral estrogens. In particular, stimulated release of nitric oxide was greater in platelets from animals treated with 17β estradiol than in those treated with oral CEE or raloxifene (Jayachandran et al., 2005a). Other phenotypic changes including expression of adhesion molecules, phosphatidylserine and CD40, which allow the platelet to interact with leukocytes and endothelial cells of the vascular wall, were affected by hormonal status and implicated in progression of arterial disease (Cognasse et al., 2007; Henn et al., 1998; Jayachandran et al., 2005a; Jayachandran et al., 2005b; Prasad et al., 2003; Schonbeck et al., 2001; Wu and Li, 2006). However, expression of these adhesion molecules is also increased with infection, thus perhaps linking environmental stimuli to thrombotic susceptibility with estrogenic treatments.
As discussed above, activation and interaction of cells of the vascular wall with blood elements result in the formation of membrane-derived microparticles. Microparticles bind fibrinogen initiating platelet microaggregation (Holme et al., 1998) and also act as carriers between cells of biochemically active molecules including tissue factor (Losche et al., 2004; Morel et al., 2004) contributing to activation of the coagulation cascade. Microvesicles of endothelial origin are elevated in persons with venous thromboembolism (Chirinos et al., 2005). However, prospective studies are needed to determine if elevation of specific populations of microvesicles define a thrombotic phenotype prior to an event and if so, how that phenotype may be impacted by estrogenic treatment. Because circulating proteins and peptides turn over rapidly and their source usually cannot be identified, evaluating changes relative to hormonal therapy has not provided meaningful information for identifying an “at risk” thrombotic phenotype (Andersson et al., 2005; Healy et al., 2006; Miller et al., 2008 (in press); Pradhan et al., 2002; Ridker et al., 2003; Schonbeck et al., 2001). Therefore, evaluation of cellular origin of microparticles and their functional characteristics (i.e. thrombin generating capacity), may allow for a more consistent mechanism to define an at risk thrombotic phenotype in early menopausal women.
VII. Future Directions: Summary
Although areas needed for future research have been mentioned in each of the preceeding section, there are several points deserving particular attention.
A. Pharmacogenomics
Delivering the right drug, at the right dose to the right person at the right time is a treatment goal. Genetic testing to reach this goal is already realized in prescribing the selective estrogen receptor modulator, tamoxifen, as an adjuvant treatment for breast cancer (Andersson et al., 2005; Borges et al., 2006; Goetz et al., 2005). A picture emerging from genomic data published from several large scale trials evaluating efficacy of estrogenic treatments is that, in the future, a polygenomic or genome-wide approach will be needed to determine dosing and formulations to maximize benefit and reduce risk for women considering using these treatments. Such an approach may consider both receptors (pharmacodynamics) and pathways that metabolize the hormone (pharmacokinetics) (Weinshilboum and Wang, 2006). Thus, depending upon the ability of an individual to metabolize these chemicals, treatment with 17β-estradiol alone may not prove equally efficacious to conjugated equine estrogen, estriol or estrone. Therefore, experiments are needed to more accurately define an estrogen metabolome relative to menopausal symptoms and disease susceptibility.
In addition, research is scant regarding long-term systemic effects of oral or transdermal products compared to sublingual or vaginal products and compounded products. It will be desirable that an interdisciplinary approach be taken in evaluating women using the various formulations in order to increase the evidence base for prescribing these products. That means, for example, when studies are conducted to evaluate efficacy of products to treat menopausal symptoms such as hot flashes, other parameters of cardiovascular health should be evaluated simultaneously. A limiting factor for such interdisciplinary approaches, of course, is immediate cost of conducting the study. However, with careful design the up front costs of such studies may outweigh the expense of additional future trials. Furthermore, increased collaborations requiring sharing of data bases and distribution of banked samples to laboratories with specific expertise will be needed so that efforts are not duplicated but validated.
B. Variation of physiological/pathological impact of hormones across the lifespan
A unifying concept emerging from the various observational and clinical trials in humans and preclinical studies in animals regarding the vascular actions of estrogenic treatments is that timing matters (Mendelsohn and Karas, 2007). As discussed above, age may be an important factor in estrogenic effects on inflammatory responses, with a loss of estrogenic efficacy in older animals. Hormonal treatment given to animals or individuals with established cardiovascular disease may initiate a different set of physiological responses compared to responses in the absence of established disease. For example, estrogen may have a disadvantageous effect in vessels with developing or established plaque compared to an advantageous effect to decrease development of atherosclerotic lesions when administered earlier in the disease progression. Thus, the ability of estrogenic treatment to prevent or slow progression of vascular remodeling or plaque formation may be limited to a narrow window of opportunity. Another aspect of the importance of timing involves the effects of estrogen on mitochondrial function. Earlier in life, the effect of estrogen to reduce mitochondrial ROS production may delay accumulation of mtDNA mutations. However, once such mutations have accumulated, estrogen may be unable to reverse this effect. Thus, actions of estrogen on mitochondrial function may only be protective, but estrogen may lack the ability to reverse existing disease processes. Questions regarding regulation of hormonal receptors, their co-regulators and other factors affecting their transcription and translation as well as changes in down-stream signaling cascades prior to and following slow hormone withdrawal, as in natural menopause, or abrupt, as in surgical menopause, need to be addressed and may lead to novel markers of early disease processes as well as better identification of when to intervene with treatment for maximal benefit and minimal harm.
Acknowledgments
Authors work reported in this manuscript was supported by NIH grants HL-50775 to SPD and HL-78638 and HL-51736 to VMM, the Kronos Longevity Institute, and the Mayo Foundation. The authors thank Ms. Wendy Reidt for her expert assistance with the manuscript.
Financial Support: Authors work reported in this manuscript was supported by NIH grants HL-50775 to SPD and HL-78638 and HL-51736 to VMM, the Kronos Longevity Institute, and the Mayo Foundation.
Contributor Information
Virginia M. Miller, Professor, Surgery and Physiology, Mayo Clinic College of Medicine, Email: miller.virginia@mayo.edu, Phone: 507-284-2290, Fax: 507-266-2233.
Sue P. Duckles, Professor, Pharmacology, University of California, Irvine, School of Medicine, Email: spduckle@uci.edu, Phone: 949-824-4265, Fax: 949-824-4855.
References
- Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- Alessio AM, Hoehr NF, Siqueira LH, Ozelo MC, de Padua Mansur A, Annichino-Bizzacchi JM. Association between estrogen receptor alpha and beta gene polymorphisms and deep vein thrombosis. Thromb Res. 2007;120:639–645. doi: 10.1016/j.thromres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke; a journal of cerebral circulation. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Andersson T, Flockhart DA, Goldstein DB, Huang S-M, Kroetz DL, Milos PM, Ratain MJ, Thummel K. Drug-metabolizing enzymes: Evidence for clinical utility of pharmacogenomic tests. Clin Pharmacol Ther. 2005;78:559–581. doi: 10.1016/j.clpt.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- Arnal JF, Bayard F. Alteration in endothelial estrogen receptor expression: a potential key of vasculoprotection by estrogens? Circ Res. 2002;91:759–760. doi: 10.1161/01.res.0000041047.50799.e2. [DOI] [PubMed] [Google Scholar]
- Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006;8:368–376. doi: 10.1007/s11906-006-0080-1. [DOI] [PubMed] [Google Scholar]
- Ball P, Knuppen R. Formation, metabolism, and physiologic importance of catecholestrogens. Am J Obstet Gynecol. 1990;163:2163–2170. doi: 10.1016/0002-9378(90)90558-o. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Campbell DR, Weston AP, Banerjee DK. Biphasic estrogen response on bovine adrenal medulla capillary endothelial cell adhesion, proliferation and tube formation. Mol Cell Biochem. 1997;177:97–105. doi: 10.1023/a:1006888020596. [DOI] [PubMed] [Google Scholar]
- Barber D, Miller V. Endothelium-dependent vasoconstrictors. Endothelial Cell Research. 1998:167–185. [Google Scholar]
- Barber DA, Sieck GC, Fitzpatrick LA, Miller VM. Endothelin receptors are modulated in association with endogenous fluctuations in estrogen. Am J Physiol. 1996;271:H1999–H2006. doi: 10.1152/ajpheart.1996.271.5.H1999. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265:1861–1867. [PubMed] [Google Scholar]
- Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautaharju P, Harper KD. Raloxifene and cardiovascular events in osteoporotic postmenopausal women. Four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847–857. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Stuenkel C. Hormones and heart disease in women: Heart and Estrogen/Progestin Replacement Study in perspective. J Clin Endocrinol Metab. 1999;84:1848–1853. doi: 10.1210/jcem.84.6.5667. [DOI] [PubMed] [Google Scholar]
- Batty S, Chow EM, Kassam A, Der SD, Mogridge J. Inhibition of mitogen-activated protein kinase signalling by Bacillus anthracis lethal toxin causes destabilization of interleukin-8 mRNA. Cell Microbiol. 2006;8:130–138. doi: 10.1111/j.1462-5822.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- Bergerot A, Holland PR, Akerman S, Bartsch T, Ahn AH, MaassenVanDenBrink A, Reuter U, Tassorelli C, Schoenen J, Mitsikostas DD, van den Maagdenberg AM, Goadsby PJ. Animal models of migraine: looking at the component parts of a complex disorder. The European journal of neuroscience. 2006;24:1517–1534. doi: 10.1111/j.1460-9568.2006.05036.x. [DOI] [PubMed] [Google Scholar]
- Best PJM, Berger PB, Miller VM, Lerman A. The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. AnnInternMed. 1998;128:285–288. doi: 10.7326/0003-4819-128-4-199802150-00006. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther. 2005;313:1203–1208. doi: 10.1124/jpet.104.082867. [DOI] [PubMed] [Google Scholar]
- Bolego C, Vegeto E, Pinna C, Maggi A, Cignarella A. Selective agonists of estrogen receptor isoforms: new perspectives for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2192–2199. doi: 10.1161/01.ATV.0000242186.93243.25. [DOI] [PubMed] [Google Scholar]
- Borges S, Desta Z, Li L, Skaar T, Ward B, Nguyen A, Jin Y, Storniolo A, Nikoloff D, Wu L, Hillman G, Hayes D, Stearns V, Flockhart DA. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clinical Pharmacology & Therapeutics. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Borroni B, Rao R, Liberini P, Venturelli E, Cossandi M, Archetti S, Caimi L, Padovani A. Endothelial nitric oxide synthase (Glu298Asp) polymorphism is an independent risk factor for migraine with aura. Headache. 2006;46:1575–1579. doi: 10.1111/j.1526-4610.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- Bousser MG. Estrogens, migraine, and stroke. Stroke; a journal of cerebral circulation. 2004;35:2652–2656. doi: 10.1161/01.STR.0000143223.25843.36. [DOI] [PubMed] [Google Scholar]
- Bousser MG, Welch KM. Relation between migraine and stroke. Lancet neurology. 2005;4:533–542. doi: 10.1016/S1474-4422(05)70164-2. [DOI] [PubMed] [Google Scholar]
- Bracamonte MP, Jayachandran M, Rud KS, Miller VM. Acute effects of 17β-estradiol on femoral veins from adult, gonadally intact and ovariectomized female pigs. Am J Physiol:Heart Circ Physiol. 2002a;283:H2389–H2396. doi: 10.1152/ajpheart.00184.2002. [DOI] [PubMed] [Google Scholar]
- Bracamonte MP, Miller VM. Vascular effects of estrogens: arterial protection versus venous thrombotic risk. Trends Endocrinol Metab. 2001;12:204–209. doi: 10.1016/s1043-2760(01)00406-4. [DOI] [PubMed] [Google Scholar]
- Bracamonte MP, Rud KS, Miller VM. Mechanism of raloxifene-induced relaxation in femoral veins depends on ovarian hormonal status. J Cardiovasc Pharmacol. 2002b;39:704–713. doi: 10.1097/00005344-200205000-00011. [DOI] [PubMed] [Google Scholar]
- Bracamonte MP, Rud KS, Owen WG, Miller VM. Ovariectomy increases mitogens and platelet-induced proliferation of arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002c;283:H853–H860. doi: 10.1152/ajpheart.00201.2002. [DOI] [PubMed] [Google Scholar]
- Brandes JL. The influence of estrogen on migraine: a systematic review. Jama. 2006;295:1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- Brosnan JF, Sheppard BL, Norris LA. Haemostatic activation in post-menopausal women taking low-dose hormone therapy: less effect with transdermal administration? Thromb Haemost. 2007;97:558–565. [PubMed] [Google Scholar]
- Brosnihan KB, Moriguchi A, Nakamoto H, Dean RH, Ganten D, Ferrario CM. Estrogen augments the contribution of nitric oxide to blood pressure regulation in transgenic hypertensive rats expressing the mouse Ren -2 gene. Am J Hypertension. 1994;7:576–582. doi: 10.1093/ajh/7.7.576. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Chen GP-W, Hunter CJ, Takasu J, Agrawal N, Sorochinsky B, Mao S. Effects of hormone replacement on progression of coronary calcium as measured by electron beam tomography. J Women’s Health. 2005;14:410–417. doi: 10.1089/jwh.2005.14.410. [DOI] [PubMed] [Google Scholar]
- Buller KM, Smith DW, Day TA. NTS catecholamine cell recruitment by hemorrhage and hypoxia. Neuroreport. 1999;10:3853–3856. doi: 10.1097/00001756-199912160-00024. [DOI] [PubMed] [Google Scholar]
- Bulut D, Albrecht N, Imohl M, Gunesdogan B, Bulut-Streich N, Borgel J, Hanefeld C, Krieg M, Mugge A. Hormonal status modulates circulating endothelial progenitor cells. Clin Res Cardiol. 2007;96:258–263. doi: 10.1007/s00392-007-0494-z. [DOI] [PubMed] [Google Scholar]
- Bush DE, Jones CE, Bass KM, Walters GK, Bruza JM, Ouyang P. Estrogen replacement reverses endothelial dysfunction in postmenopausal women. American Journal of Medicine. 1998;104:552–558. doi: 10.1016/s0002-9343(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MSV, Stern BJ, Herrington D, Ford-Lynch G, Gorelick P, James A, Brown CM, Choi ES, Bray P, Newby LK, Goldstein LB, Simpkins J. Advancing the study of stroke in women. Summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke; a journal of cerebral circulation. 2006 doi: 10.1161/01.STR.0000236053.37695.15. in press. [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney international. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- Case J, Davison CA. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J Pharmacol Exp Ther. 1999;291:524–530. [PubMed] [Google Scholar]
- Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri EL, Stack DE, Devanesan PD, Todorovic R, Dwivedy I, Higginbotham S, Johansson SL, Patil KD, Gross ML, Gooden JK, Ramanathan R, Cerny RL, Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc Natl Acad Sci U S A. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos C, Ribes C, Amado JA, Perez J, Garcia Unzueta MT, de Berrazueta JR. Venous endothelial function in postmenopausal women who are receiving long-term estrogen and progestagen therapy. Fertil Steril. 2000;74:268–273. doi: 10.1016/s0015-0282(00)00627-0. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- Chen FP, Lee N, Soong YK, Huang KE. Comparison of transdermal and oral estrogen-progestin replacement therapy: effects on cardiovascular risk factors. Menopause. 2001;8:347–352. doi: 10.1097/00042192-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am J Physiol Endocrinol Metab. 2004;286:E1011–1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- Chen S-J, Li H, Durand J, Oparil S, Chen Y-F. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, Horstman LL, Soriano AO, Zambrano JP, Ahn YS. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. Journal of the American College of Cardiology. 2005;45:1467–1471. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- Christian R, Liu P, Harrington S, Ruan M, Miller VM, Fitzpatrick LA. Intimal Estrogen Receptor (ER) beta, But Not ERalpha Expression, Is Correlated with Coronary Calcification and Atherosclerosis in Pre-and Postmenopausal Women. The Journal of Endocrinology & Metabolism. 2006;91:2713–2720. doi: 10.1210/jc.2005-2672. [DOI] [PubMed] [Google Scholar]
- Ciardullo AV, Panico S, Bellati C, Rubba P, Rinaldi S, Iannuzzi A, Cioffi V, Iannuzzo G, Berrino F. High endogenous estradiol is associated with increased venous distensibility and clinical evidence of varicose veins in menopausal women. J Vasc Surg. 2000;32:544–549. doi: 10.1067/mva.2000.107768. [DOI] [PubMed] [Google Scholar]
- Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest. 1994;93:17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid MC, Schnaper HW, Kleinman HK. Estrogens and the vascular endothelium. Ann N Y Acad Sci. 2002;966:143–157. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- Clark J. A Critique of Women’s Health Initiative Studies (2002–2006) Perspective. 2006;4:1–10. doi: 10.1621/nrs.04023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–384. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- Cognasse F, Lafarge S, Chavarin P, Acquart S, Garraud O. Lipopolysaccharide induces sCD40L release through human platelets TLR4, but not TLR2 and TLR9. Intensive Care Med. 2007;33:382–384. doi: 10.1007/s00134-006-0488-8. [DOI] [PubMed] [Google Scholar]
- Colson NJ, Lea RA, Quinlan S, Griffiths LR. No role for estrogen receptor 1 gene intron 1 Pvu II and exon 4 C325G polymorphisms in migraine susceptibility. BMC medical genetics. 2006;7:12. doi: 10.1186/1471-2350-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson NJ, Lea RA, Quinlan S, MacMillan J, Griffiths LR. The estrogen receptor 1 G594A polymorphism is associated with migraine susceptibility in two independent case/control groups. Neurogenetics. 2004;5:129–133. doi: 10.1007/s10048-004-0181-4. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Gimbrone MA, Jr, McLaughlin MK, Halpern W, Alexander RW. Increased vascular catecholamine sensitivity and α-adrenergic receptor affinity in female and estrogen-treated male rats. Circ Res. 1982;50:805–811. doi: 10.1161/01.res.50.6.805. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine reviews. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- Crandall CJ, Crawford SL, Gold EB. Vasomotor symptom prevalence is associated with polymorphisms in sex steroid-metabolizing enzymes and receptors. Am J Med. 2006;119:S52–60. doi: 10.1016/j.amjmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:131–142. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacological reviews. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Zhang Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ Res. 1998;83:388–395. doi: 10.1161/01.res.83.4.388. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. Journal of neuroendocrinology. 2000;12:784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. American journal of epidemiology. 2002;155:339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- de Kleinj D, Pasterkamp G. Toll-like receptors in cardiovascular diseases. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/s0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]
- De Lignieres B, Basdevant A, Thomas G, Thalabard JC, Mercier-Bodard C, Conard J, Guyene TT, Mairon N, Corvol P, Guy-Grand B, et al. Biological effects of estradiol-17 beta in postmenopausal women: oral versus percutaneous administration. J Clin Endocrinol Metab. 1986;62:536–541. doi: 10.1210/jcem-62-3-536. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang H-H, Anderson RGW, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- Deneke T, Grewe PH, Ruppert S, Balzer K, Muller KM. Atherosclerotic carotid arteries--calcification and radio-morphological findings. Z Kardiol. 2000;89(Suppl 2):36–48. doi: 10.1007/s003920070098. [DOI] [PubMed] [Google Scholar]
- Desai MY, Nasir K, Braunstein JB, Rumberger JA, Post WS, Budoff MJ, Blumenthal RS. Underlying risk factors incrementally add to the standard risk estimate in detecting subclinical atherosclerosis in low- and intermediate-risk middle-aged asymptomatic individuals. American heart journal. 2004;148:871–877. doi: 10.1016/j.ahj.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Doherty TM, Asotra K, Fitzpatrick LA, Qiao J-H, Wilkin DJ, Dentrano RC, Dunstan CR, Shah PK, Rajavashisth TB. Calcification in atherosclerosis: Bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci. 2003;100:11201–11206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G, Cruz MN, Poston L, Gustafsson JA, Kublickiene K. Functional characterization and sex differences in small mesenteric arteries of the estrogen receptor-beta knockout mouse. Am J Physiol Regul Integr Comp Physiol. 2008;294:R112–120. doi: 10.1152/ajpregu.00421.2007. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ. Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and mitogen-activated protein kinase activity. Arterioscler Thromb Vasc Biol. 2000;20:964–972. doi: 10.1161/01.atv.20.4.964. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37:640–644. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharmacol Exp Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: multiplicity of action. Clinical and experimental pharmacology & physiology. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN, Stirone C, Procaccio V. Estrogen and mitochondria: a new paradigm for vascular protection? Mol Interv. 2006;6:26–35. doi: 10.1124/mi.6.1.6. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development (Cambridge, England) 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annual review of physiology. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Eilertsen AL, Hoibraaten E, Os I, Andersen TO, Sandvik L, Sandset PM. The effects of oral and transdermal hormone replacement therapy on C-reactive protein levels and other inflammatory markers in women with high risk of thrombosis. Maturitas. 2005;52:111–118. doi: 10.1016/j.maturitas.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Ensrud K, Genazzani AR, Geiger MJ, McNabb M, Dowsett SA, Cox DA, Barrett-Connor E. Effect of raloxifene on cardiovascular adverse events in postmenopausal women with osteoporosis. Am J Cardiol. 2006;97:520–527. doi: 10.1016/j.amjcard.2005.09.083. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Felmeden DC, Lip GY. Hormone replacement therapy and hypertension. Blood Press. 2000;9:246–249. doi: 10.1080/080370500448614. [DOI] [PubMed] [Google Scholar]
- Fernander AF, Duran RE, Saab PG, Schneiderman N. John Henry Active Coping, education, and blood pressure among urban blacks. J Natl Med Assoc. 2004;96:246–255. [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD. Biochemistry of migraine. Pathologie-biologie. 1992;40:287–292. [PubMed] [Google Scholar]
- Ferrero V, Ribichini F, Matullo G, Guarrera S, Carturan S, Vado A, Vassanelli C, Piazza A, Uslenghi E, Wijns W. Estrogen receptor-α polymorphisms and angiographic outcome after coronary artery stenting. Arterioscler Thromb Vasc Biol. 2003;23:2223–2228. doi: 10.1161/01.ATV.0000101181.81022.BF. [DOI] [PubMed] [Google Scholar]
- Fieber CB, Eldridge J, Taha TA, Obeid LM, Muise-Helmericks RC. Modulation of total Akt kinase by increased expression of a single isoform: requirement of the sphingosine-1-phosphate receptor, Edg3/S1P3, for the VEGF-dependent expression of Akt3 in primary endothelial cells. Exp Cell Res. 2006;312:1164–1173. doi: 10.1016/j.yexcr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Fish JE, Matouk CC, Yeboah E, Bevan SC, Khan M, Patil K, Ohh M, Marsden PA. Hypoxia-inducible expression of a natural cis-antisense transcript inhibits endothelial nitric-oxide synthase. J Biol Chem. 2007;282:15652–15666. doi: 10.1074/jbc.M608318200. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA, Turner RT, Ritman ER. Endochondral bone formation in the heart: A possible mechanism of coronary calcification. Endocrinology. 2003;144:2214–2219. doi: 10.1210/en.2002-0170. [DOI] [PubMed] [Google Scholar]
- Foegh M, Rego A, Lou H, Katz N, Ramwell P. Gender effects on graft myointimal hyperplasia. TransplantProc. 1995;27:2070–2072. [PubMed] [Google Scholar]
- Ford PJ, Yamazaki K, Seymour GJ. Cardiovascular and oral disease interactions: what is the evidence? Primary Dental Care. 2007;14:59–66. doi: 10.1308/135576107780556806. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10:197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- Galea E, Santizo R, Feinstein DL, Adamsom P, Greenwood J, Koenig HM, Pelligrino DA. Estrogen inhibits NF kappa B-dependent inflammation in brain endothelium without interfering with I kappa B degradation. Neuroreport. 2002;13:1469–1472. doi: 10.1097/00001756-200208070-00024. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension. 1999;33:323–328. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- Garnier M, Mesange F, Dupont M, Gas N, Zanibellato C, Bayard F, Faye J. Ligands of the antiestrogen binding site block endothelial cell proliferation reversibly. Exper Cell Res. 1993;205:191–194. doi: 10.1006/excr.1993.1075. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Gonadal hormones affect diameter of male rat cerebral arteries through endothelium-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2000;279:H610–618. doi: 10.1152/ajpheart.2000.279.2.H610. [DOI] [PubMed] [Google Scholar]
- Geary GG, McNeill AM, Ospina JA, Krause DN, Korach KS, Duckles SP. Selected contribution: cerebrovascular nos and cyclooxygenase are unaffected by estrogen in mice lacking estrogen receptor-alpha. J Appl Physiol. 2001;91:2391–2399. doi: 10.1152/jappl.2001.91.5.2391. discussion 2389–2390. [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, De Paola V, Calabro A, Becherini L, Martini G, Nuti R. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGE review. American journal of epidemiology. 2005;161:307–320. doi: 10.1093/aje/kwi055. [DOI] [PubMed] [Google Scholar]
- Gibson LL, Hahner L, Osborne-Lawrence S, German Z, Wu KK, Chambliss KL, Shaul PW. Molecular basis of estrogen-induced cyclooxygenase type 1 upregulation in endothelial cells. Circ Res. 2005;96:518–525. doi: 10.1161/01.RES.0000158967.96231.88. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Hinderliter AL, Wells EC, Sherwood A, Grewen KM, Light KC. Transdermal versus oral estrogen therapy in postmenopausal smokers: hemodynamic and endothelial effects. Obstet Gynecol. 2004;103:169–180. doi: 10.1097/01.AOG.0000103998.48122.0b. [DOI] [PubMed] [Google Scholar]
- Gisclard V, Miller VM, Vanhoutte PM. Effect of 17β-estradiol on endothelium-dependent responses in the rabbit. JPharmExpTher. 1988;244:19–22. [PubMed] [Google Scholar]
- Goetz M, Rae J, Suman V, Safgren S, Ames M, Visscher D, Reynolds C, Couch F, Lingle W, Flockhart D, Desta Z, Perez E, Ingle J. Pharmacogenetics of Tamoxifen Biotransformation Is Associated With Clinical Outcomes of Efficacy and Hot Flashes. Journal of Clinical Oncology. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont PJ. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. American heart journal. 2003;146:S5–12. doi: 10.1016/j.ahj.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Ansar S, Duckles SP, Krause DN. Androgenic/estrogenic balance in the male rat cerebral circulation: metabolic enzymes and sex steroid receptors. J Cereb Blood Flow Metab. 2007;27:1841–1852. doi: 10.1038/sj.jcbfm.9600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology. 2007;148:218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) Jama. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Annals of Internal Medicine. 2001;135:1–8. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: The role of time since menopause and age at hormone initiation. J Women’s Health. 2006;15:35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- Hagen K, Stovner LJ, Skorpen F, Pettersen E, Zwart JA. The impact of the catechol-O-methyltransferase Val158Met polymorphism on survival in the general population--the HUNT study. BMC medical genetics. 2007;8:34. doi: 10.1186/1471-2350-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114:2261–2270. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- Hamburg N, Charbonneau F, Gerhard-Herman M, Ganz P, Creager MA. Comparison of Endothelial Function in Young Men and Women With a Family History of Premature Coronary Artery Disease. American Journal of Cardiology. 2004;94:783–785. doi: 10.1016/j.amjcard.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hamlet MA, Rorie DK, Tyce GM. Effects of estradiol on release and disposition of norepinephrine from nerve endings. Am J Physiol. 1980;239:H450–H456. doi: 10.1152/ajpheart.1980.239.4.H450. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocrine reviews. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005a;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Harman SM, Naftolin F, Brinton EA, Judelson DR. Is the Estrogen Controversy Over? Deconstructing the Women’s Health Initiative Study: A Critical Evaluation of the Evidence. Ann N Y Acad Sci. 2005b;1052:43–56. doi: 10.1196/annals.1347.004. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Molecular and cellular endocrinology. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR. A G protein-coupled receptor for estrogen: the end of the search? Mol Interv. 2005;5:158–161. doi: 10.1124/mi.5.3.5. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Maeda S, Iemitsu M, Otsuki T, Sugawara J, Tanabe T, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Sex differences in the relationship between estrogen receptor alpha gene polymorphisms and arterial stiffness in older humans. Am J Hypertens. 2007;20:650–656. doi: 10.1016/j.amjhyper.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys Res Commun. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- Healy AM, Pickard MD, Pradhan AD, Wang Y, chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker P, Simon DI. Platelet Expression Profiling and Clinical Validation of Myeloid-Related Protein-14 as a Novel Determinant of Cardiovascular Events. Circulation. 2006;113:2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- Hebbring SJ, Adjei AA, Baer JL, Jenkins GD, Zhang J, Cunningham JM, Schaid DJ, Weinshilboum RM, Thibodeau SN. Human SULT1A1 gene: copy number differences and functional implications. Human molecular genetics. 2007;16:463–470. doi: 10.1093/hmg/ddl468. [DOI] [PubMed] [Google Scholar]
- Hecht HS, Budoff MJ, Berman DS, Ehrlich J, Rumberger JA. Coronary artery calcium scanning: Clinical paradigms for cardiac risk assessment and treatment. American heart journal. 2006;151:1139–1146. doi: 10.1016/j.ahj.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Heit JA. Epidemiology of venous thromboembolism. In: Colman RW, editor. Hemostasis and thrombosis: basic principles and clinical practice. Lippincott: Williams & Wilkins; 2006. pp. 1227–1233. [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiological reviews. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Simonian SX, Thanky NR, Bicknell RJ. Oestrogen modulation of noradrenaline neurotransmission. Novartis Found Symp. 2000;230:74–85. doi: 10.1002/0470870818.ch7. discussion 85–93. [DOI] [PubMed] [Google Scholar]
- Herrington D, Howard H. ER-alpha variants and the cardiovascular effects of hormone replacement therapy. Pharmacogenetics. 2003;4:269–277. doi: 10.1517/phgs.4.3.269.22686. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Fong J, Sempos CT, Black DM, Schrott HG, Rautaharju P, Bachorik PS, Blumenthal R, Khan S, Wenger NK. Comparison of the heart and estrogen/progestin replacement study (HERS) cohort with women with coronary disease from the National Health and Nutrition Examination Survey III (NHANES III) American heart journal. 1998;136:115–124. doi: 10.1016/s0002-8703(98)70191-7. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Howard TD, Hawkins GA, Reboussin DM, Xu J, Zheng SL, Brosnihan KB, Meyers DA, Bleecker ER. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002a;346:967–974. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- Herrington DM, Vittinghoff E, Howard TD, Major DA, Owen J, Reboussin DM, Bowden D, Vittner V, Simon JA, Grady D, Hulley SB. Factor V Leiden, hormone replacement therapy, and risk of venous thromboembolic events in women with coronary disease. Arterioscler Thromb Vasc Biol. 2002b;22:1012–1017. doi: 10.1161/01.atv.0000018301.91721.94. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Ghraf R. Effects of short-term exposure to catecholestrogens on catecholamine turnover in the preoptic-hypothalamic brain of ovariectomized rats. Brain Res. 1982;240:295–301. doi: 10.1016/0006-8993(82)90224-4. [DOI] [PubMed] [Google Scholar]
- Hirata S, Shoda T, Kato J, Hoshi K. Isoform/variant mRNAs for sex steroid hormone receptors in humans. Trends Endocrinol Metab. 2003;14:124–129. doi: 10.1016/s1043-2760(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2005;70:382–387. doi: 10.1016/j.steroids.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hodis H, Mach W. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14:1–14. doi: 10.1097/gme.0b013e31802e8508. [DOI] [PubMed] [Google Scholar]
- Hodis H, Mack W, Lobo R, Shoupe D, Sevanian A, Mahrer P, Selzer R, Liu C-r, Liu C-i, Azen S. Estrogen in the Prevention of Atherosclerosis. A Randomized, Double-Blind, Placebo-Controlled Trial. Annals of Internal Medicine. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme PA, Solum NO, Brosstad F, Pedersen T, Kveine M. Microvesicles bind soluble fibrinogen, adhere to immobilized fibrinogen and coaggregate with platelets. Thromb Haemost. 1998;79:389–394. [PubMed] [Google Scholar]
- Hong L, Colpan A, Peptan IA, Daw J, George A, Evans CA. 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007;13:1197–1203. doi: 10.1089/ten.2006.0317. [DOI] [PubMed] [Google Scholar]
- Horner S, Pasternak G, Hehlmann R. A statistically significant sex difference in the number of colony-forming cells from human peripheral blood. Ann Hematol. 1997;74:259–263. doi: 10.1007/s002770050296. [DOI] [PubMed] [Google Scholar]
- Hsia J, Criqui MH, Herrington DM, Manson JE, Wu L, Heckbert SR, Allison M, McDermott MM, Robinson J, Masaki K. Conjugated equine estrogens and peripheral arterial disease risk: the Women’s Health Initiative. American heart journal. 2006a;152:170–176. doi: 10.1016/j.ahj.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hsia J, Criqui MH, Rodabough RJ, Langer RD, Resnick HE, Phillips LS, Allison M, Bonds DE, Masaki K, Caralis P, Kotchen JM. Estrogen plus progestin and the risk of peripheral arterial disease: the Women’s Health Initiative. Circulation. 2004;109:620–626. doi: 10.1161/01.CIR.0000115309.63979.92. [DOI] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R. Conjugated equine estrogens and coronary heart disease. Archives of internal medicine. 2006b;166:357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- Hsia J, Simon JA, Lin F, Applegate WB, Vogt MT, Hunninghake D, Carr M. Peripheral arterial disease in randomized trial of estrogen with progestin in women with coronary heart disease: the Heart and Estrogen/Progestin Replacement Study. Circulation. 2000;102:2228–2232. doi: 10.1161/01.cir.102.18.2228. [DOI] [PubMed] [Google Scholar]
- Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Archives of internal medicine. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Koller A, Kaley G. Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol. 1998;275:R1571–1577. doi: 10.1152/ajpregu.1998.275.5.R1571. [DOI] [PubMed] [Google Scholar]
- Huang A, Wu Y, Sun D, Koller A, Kaley G. Effect of estrogen on flow-induced dilation in NO deficiency: role of prostaglandins and EDHF. J Appl Physiol. 2001;91:2561–2566. doi: 10.1152/jappl.2001.91.6.2561. [DOI] [PubMed] [Google Scholar]
- Huber M, Poulin R. Post-translational cooperativity of ornithine decarboxylase induction by estrogens and peptide growth factors in human breast cancer cells. Molecular and cellular endocrinology. 1996;117:211–218. doi: 10.1016/0303-7207(95)03749-7. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Hynes MR, Duckles SP. Effect of increasing age on the endothelium-mediated relaxation of rat blood vessels in vitro. JPharmExpTher. 1987;241:387–392. [PubMed] [Google Scholar]
- Iadecola C, Goldman SS, Harder DR, Heistad DD, Katusic ZS, Moskowitz MA, Simard JM, Sloan MA, Traystman RJ, Velletri PA. Recommendations of the National Heart, Lung, and Blood Institute working group on cerebrovascular biology and disease. Stroke; a journal of cerebral circulation. 2006;37:1578–1581. doi: 10.1161/01.STR.0000221297.57305.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res. 2002;91:814–820. doi: 10.1161/01.res.0000038304.62046.4c. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Hano T, Nishio I. Estrogen reduces angiotensin II-induced acceleration of senescence in endothelial progenitor cells. Hypertens Res. 2005a;28:263–271. doi: 10.1291/hypres.28.263. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Hano T, Nishio I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J Hypertens. 2005b;23:1699–1706. doi: 10.1097/01.hjh.0000176788.12376.20. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Kobayashi K, Hano T, Nishio I. Effect of estrogen on differentiation and senescence in endothelial progenitor cells derived from bone marrow in spontaneously hypertensive rats. Hypertens Res. 2005c;28:763–772. doi: 10.1291/hypres.28.763. [DOI] [PubMed] [Google Scholar]
- Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005;72:1290–1296. doi: 10.1095/biolreprod.105.040014. [DOI] [PubMed] [Google Scholar]
- Jacob J, Sebastian KS, Devassy S, Priyadarsini L, Farook MF, Shameem A, Mathew D, Sreeja S, Thampan RV. Membrane estrogen receptors: genomic actions and post transcriptional regulation. Molecular and cellular endocrinology. 2006;246:34–41. doi: 10.1016/j.mce.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Brunn GJ, Karnicki KRSM, Owen WG, Miller VM. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: Implications for thrombotic risk. Appl Physiol. 2007;102:429–433. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Karnicki K, Miller RS, Owen WG, Korach KS, Miller VM. Platelet characteristics change with aging: Role of estrogen receptorβ. J Gerontol: Biol Sci. 2005a;60:815–819. doi: 10.1093/gerona/60.7.815. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Litwiller RD, Owen WG, Heit JA, Behrenbeck T, Mulvagh SL, Araoz PA, Budoff M, Harman SM, Miller VM. Phenotype of Blood Borne Microparticles as a Marker of Premature Coronary Calcification in Recently menopausal women. Am J Physiol. 2008 doi: 10.1152/ajpheart.00193.2008. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M, Miller VM. Ovariectomy upregulates expression of estrogen receptors, NOS, and HSPs in porcine platelets. Am J Physiol:Heart Circ Physiol. 2002;283:H220–H226. doi: 10.1152/ajpheart.00950.2001. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Miller VM. Human platelets contain estrogen receptorα, caveolin-1 and estrogen receptor associated proteins. Platelets. 2003;14:75–81. doi: 10.1080/0953710031000080562. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Mukherjee R, Steinkamp T, LaBreche P, Bracamonte MP, Okano H, Owen WG, Miller VM. Differential effects of 17β-estradiol, conjugated equine estrogen and raloxifene on mRNA expression, aggregation and secretion in platelets. Am J Physiol: Heart Circ Physiol. 2005b;288:H2355–H2362. doi: 10.1152/ajpheart.01108.2004. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Okano H, Chatrath R, Owen WG, McConnell JP, Miller VM. Sex-specific changes in platelet aggregation and secretion with sexual maturity in pigs. J Appl Physiol. 2004;97:1445–1452. doi: 10.1152/japplphysiol.01074.2003. [DOI] [PubMed] [Google Scholar]
- Ji Y, Moon I, Zlatkovic J, Salavaggione OE, Thomae BA, Eckloff BW, Wieben ED, Schaid DJ, Weinshilboum RM. Human hydroxysteroid sulfotransferase SULT2B1 pharmacogenomics: gene sequence variation and functional genomics. J Pharmacol Exp Ther. 2007;322:529–540. doi: 10.1124/jpet.107.122895. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Fernandez F, Colson NJ, Griffiths LR. A pharmacogenomic evaluation of migraine therapy. Expert opinion on pharmacotherapy. 2007;8:1821–1835. doi: 10.1517/14656566.8.12.1821. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Lea RA, Colson NJ, Macmillan JC, Griffiths LR. A population genomics overview of the neuronal nitric oxide synthase (nNOS) gene and its relationship to migraine susceptibility. Cellular and molecular biology (Noisy-le-Grand, France) 2005;51:285–292. [PubMed] [Google Scholar]
- Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: A review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17:55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Juan SH, Chen JJ, Chen CH, Lin H, Cheng CF, Liu JC, Hsieh MH, Chen YL, Chao HH, Chen TH, Chan P, Cheng TH. 17beta-estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1254–1261. doi: 10.1152/ajpheart.00723.2003. [DOI] [PubMed] [Google Scholar]
- Jun SS, Chen Z, Pace MC, Shaul PW. Estrogen upregulates cyclooxygenase-1 gene expression in ovine fetal pulmonary artery endothelium. J Clin Invest. 1998;102:176–183. doi: 10.1172/JCI2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Karas RH, Gauer EA, Bieber HE, Baur WE, Mendelsohn ME. Growth factor activation of the estrogen receptor in vascular cells occurs via a mitogen-activated protein kinase-independent pathway. J Clin Invest. 1998;101:2851–2861. doi: 10.1172/JCI1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas RH, Hodgin JB, Kwoun M, Krege JH, Aronovitz M, Mackey W, Gustafsson JA, Korach KS, Smithies O, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor beta-deficient female mice. Proc Natl Acad Sci USA. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93:131–138. doi: 10.1210/jc.2007-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M, Warren R, Luben R, Camus J, Denton E, Sala E, Day N, Khaw KT. How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography? AJR Am J Roentgenol. 2006;187:73–80. doi: 10.2214/AJR.05.0365. [DOI] [PubMed] [Google Scholar]
- Kawagoe J, Ohmichi M, Takahashi T, Ohshima C, Mabuchi S, Takahashi K, Igarashi H, Mori-Abe A, Saitoh M, Du B, Ohta T, Kimura A, Kyo S, Inoue M, Kurachi H. Raloxifene inhibits estrogen-induced up-regulation of telomerase activity in a human breast cancer cell line. J Biol Chem. 2003;278:43363–43372. doi: 10.1074/jbc.M304363200. [DOI] [PubMed] [Google Scholar]
- Keay J, Bridgham JT, Thornton JW. The Octopus vulgaris estrogen receptor is a constitutive transcriptional activator: evolutionary and functional implications. Endocrinology. 2006;147:3861–3869. doi: 10.1210/en.2006-0363. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen signaling in the hypothalamus. Vitam Horm. 2005;71:123–145. doi: 10.1016/S0083-6729(05)71005-0. [DOI] [PubMed] [Google Scholar]
- Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor β and androgen receptor (AR): Testosterone regulates AR expression. Blood. 2000;95:2289–2296. [PubMed] [Google Scholar]
- Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- Kikuchi N, Urabe M, Iwasa K, Okubo T, Tsuchiya H, Hosoda T, Tatsumi H, Honjo H. Atheroprotective effect of estriol and estrone sulfate on human vascular smooth muscle cells. The Journal of steroid biochemistry and molecular biology. 2000;72:71–78. doi: 10.1016/s0960-0760(99)00149-1. [DOI] [PubMed] [Google Scholar]
- Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke; a journal of cerebral circulation. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Kim HK, Rao VP, Park YS, Miller VM, Tazelaar HD, McGregor CG. Pulmonary arterial reactivity during induced infection of single lung allografts. Eur J Cardiothorac Surg. 2007;31:475–481. doi: 10.1016/j.ejcts.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- Kjaergaard A, Ellervik C, Tybjaerg-Hansen A, Axelsson C, Gronholdt M-L, Grande P, Jensen G, Nordestgaard BG. Estrogen receptor α polymorphism and risk of cardiovascular disease, cancer, and hip fracture. Circulation. 2007;115:861–871. doi: 10.1161/CIRCULATIONAHA.106.615567. [DOI] [PubMed] [Google Scholar]
- Klauber N, Parangi S, Flynn E, Hamel E, D’Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997;57:81–86. [PubMed] [Google Scholar]
- Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Forstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension. 1998;31:582–588. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Nelson MA. ATP-sensitive K+ currents in cerebral arterial smooth muscle: pharmacological and hormonal modulation. Am J Physiol. 1995;269:H1634–H1640. doi: 10.1152/ajpheart.1995.269.5.H1634. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am J Physiol. 1999;276:H961–969. doi: 10.1152/ajpheart.1999.276.3.H961. [DOI] [PubMed] [Google Scholar]
- Kokubo Y, Liu J, Rajdev S, Kayama T, Sharp FR, Weinstein PR. Differential cerebral protein synthesis and heat shock protein 70 expression in the core and penumbra of rat brain after transient focal ischemia. Neurosurgery. 2003;53:186–190. doi: 10.1227/01.neu.0000069023.01440.d6. discussion 190–181. [DOI] [PubMed] [Google Scholar]
- Kracht M, Saklatvala J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 2002;20:91–106. doi: 10.1006/cyto.2002.0895. [DOI] [PubMed] [Google Scholar]
- Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation. 1997;95:1768–1772. doi: 10.1161/01.cir.95.7.1768. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullo IJ, Li G, Bielak LF, Bailey KR, Sheedy PF, II, Peyser PA, Turner ST, Kardia SLR. Association of plasma homocysteine with coronary artery calcification in different categories of coronary heart disease risk. Mayo Clin Proc. 2006;81:177–182. doi: 10.4065/81.2.177. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, McConnell JP, Bailey KR, Kardia SL, Bielak LF, Peyser PA, Sheedy PF, 2nd, Boerwinkle E, Turner ST. Relation of C-reactive protein and fibrinogen to coronary artery calcium in subjects with systemic hypertension. Am J Cardiol. 2003;92:56–58. doi: 10.1016/s0002-9149(03)00466-1. [DOI] [PubMed] [Google Scholar]
- Lacut K, Oger E, Le Gal G, Blouch M-T, Abgrall J-F, Kerlan V, Scarabin P-V, Mottier D Investigators atS. Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C-reactive protein. Thromb Haemost. 2003;90:124–131. [PubMed] [Google Scholar]
- Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary Artery Calcium Scores and Risk for Cardiovascular Events in Women Classified as “Low Risk” Based on Framingham Risk Score: The Multi-Ethnic Study of Atherosclerosis (MESA) Archives of internal medicine. 2007;167:2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93:1127–1133. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
- Lewandowski S, Kalita K, Kaczmarek L. Estrogen receptor beta. Potential functional significance of a variety of mRNA isoforms. FEBS letters. 2002;524:1–5. doi: 10.1016/s0014-5793(02)03015-6. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Avsar M, LaBreche P, Bracamonte MP, Jayachandran M, Miller VM. Treatment with raloxifene and 17beta-estradiol differentially modulates nitic oxide and prostanoids in venous endothelium and platelets of ovariectomized pigs. J Cardiovasc Pharmacol. 2006;48:231–238. doi: 10.1097/01.fjc.0000247800.34991.a1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Bracamonte MP, Rud KS, Miller VM. Genome and Hormones: Gender Differences in Physiology Selected Contribution: Effects of sex and ovariectomy on responses to platelets in porcine femoral veins. J Appl Physiol. 2001;91:2823–2830. doi: 10.1152/jappl.2001.91.6.2823. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lowell RC, Cambria RA, Roche PC, Gloviczki P, Miller VM. Production of endothelium-derived factors from sodded expanded polytetrafluoroethylene grafts. J Vasc Surg. 1997;1:187–197. doi: 10.1016/s0741-5214(97)70337-9. [DOI] [PubMed] [Google Scholar]
- Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, Sanjay A, Collinge M, Baron R, Sessa WC, Bender JR. Variant estrogen receptor-c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proc Natl Acad Sci U S A. 2007;104:16468–16473. doi: 10.1073/pnas.0704315104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zheng T, Altura BM, Altura BT. Sex steroid hormones exert biphasic effects on cytosolic magnesium ions in cerebral vascular smooth muscle cells: possible relationships to migraine frequency in premenstrual syndromes and stroke incidence. Brain research bulletin. 2001;54:83–89. doi: 10.1016/s0361-9230(00)00428-7. [DOI] [PubMed] [Google Scholar]
- Li X, Geary GG, Gonzales RJ, Krause DN, Duckles SP. Effect of estrogen on cerebrovascular prostaglandins is amplified in mice with dysfunctional NOS. Am J Physiol Heart Circ Physiol. 2004;287:H588–594. doi: 10.1152/ajpheart.01176.2003. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent flow-mediated vasodilation in postmenopausal women. AnnInternMed. 1994;121:936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- Lippert C, Seeger H, Mueck AO, Lippert TH. The effects of A-ring and D-ring metabolites of estradiol on the proliferation of vascular endothelial cells. Life Sci. 2000;67:1653–1658. doi: 10.1016/s0024-3205(00)00747-5. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol. 2003;285:H2188–2193. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- Lo JC, Zhao X, Scuteri A, Brockwell S, Sowers MR. The association of genetic polymorphisms in sex hormone biosynthesis and action with insulin sensitivity and diabetes mellitus in women at midlife. Am J Med. 2006;119:S69–78. doi: 10.1016/j.amjmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Loose-Mitchell DS, Stancel GM. Estrogens and Progestins. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2001. pp. 1597–1634. [Google Scholar]
- Losche W, Scholz T, Temmler U, Oberle V, Claus RA. Platelet-derived microvesicles tranfer tissue factor to monocytes but not to neutrophils. Platelets. 2004;15:109–115. doi: 10.1080/09537100310001649885. [DOI] [PubMed] [Google Scholar]
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A. 2004;101:17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda, Md. 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- Maas AH, van der Schouw YT, Mali WP, van der Graaf Y. Prevalence and determinants of breast arterial calcium in women at high risk of cardiovascular disease. Am J Cardiol. 2004;94:655–659. doi: 10.1016/j.amjcard.2004.05.036. [DOI] [PubMed] [Google Scholar]
- MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67:2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Slater CC, Xiang M, Shoupe D, Lobo RA, Hodis HN. Elevated subclinical atherosclerosis associated with oophorectomy is related to time since menopause rather than type of menopause. Fertility & Sterility. 2004;82:391–397. doi: 10.1016/j.fertnstert.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Mackey RH, Kuller LH, Sutton-Tyrrell K, Evans RW, Holubkov R, Matthews KA. Hormone therapy, lipoprotein subclasses, and coronary calcification: the Healthy Women Study. Archives of internal medicine. 2005;165:510–515. doi: 10.1001/archinte.165.5.510. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- Manson J, Allison M, Rossouw JE, Carr J, Langer R, Hsia J, Kuller L, Cochrane B, Hunt J, Ludlam S, Pettinger M, Gass M, Margolis K, Nathan L, Ockene J, Prentice R, Robbins JR, Stefanick M. Estrogen Therapy and Coronary-Artery Calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146–5151. doi: 10.4049/jimmunol.175.8.5146. [DOI] [PubMed] [Google Scholar]
- Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2007 doi: 10.1016/j.exger.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Martin V. Targeted treatment strategies for menstrual migraine. The Journal of family practice. 2007;56:13–22. [PubMed] [Google Scholar]
- Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis--part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, Itoh R, Iwaguro H, Eguchi M, Iwami Y, Tanaka R, Nakagawa Y, Sugimoto A, Ninomiya S, Hayashi S, Kato S, Asahara T. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101:598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Caggiula AR, McAllister CG, Berga SL, Owens JF, Flory JD, Miller AL. Sympathetic reactivity to acute stress and immune response in women. Psychosom Med. 1995;57:564–571. doi: 10.1097/00006842-199511000-00009. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Flory JD, Owens JF, Harris KF, Berga SL. Influence of estrogen replacement therapy on cardiovascular responses to stress of healthy postmenopausal women. Psychophysiology. 2001;38:391–398. [PubMed] [Google Scholar]
- Matthews KA, Owens JF, Salomon K, Harris KF, Berga SL. Influence of hormone therapy on the cardiovascular responses to stress of postmenopausal women. Biol Psychol. 2005;69:39–56. doi: 10.1016/j.biopsycho.2004.11.004. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Kim N, Duckles SP, Krause DN, Kontos HA. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke; a journal of cerebral circulation. 1999;30:2186–2190. doi: 10.1161/01.str.30.10.2186. [DOI] [PubMed] [Google Scholar]
- Meijers JCM, Tekelenburg WLH, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Rosano GM. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol. 2000;85:787–789. A789. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- Mestas J, Crampton SP, Hori T, Hughes CC. Endothelial cell co-stimulation through OX40 augments and prolongs T cell cytokine synthesis by stabilization of cytokine mRNA. Int Immunol. 2005;17:737–747. doi: 10.1093/intimm/dxh255. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. Journal of medicinal chemistry. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mihmanli V, Mihmanli I, Atakir K, Kantarci F, Aydin T, Sengun Y, Uysal O. Carotid intima-media thickness in surgical menopause: women who received HRT versus who did not. Maturitas. 2002;42:37–43. doi: 10.1016/s0378-5122(02)00028-2. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke; a journal of cerebral circulation. 2007a;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004a;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- Miller VM, Jayachandran M, Hashimoto K, Heit JA, Owen WG. Estrogen, Inflammation and Platelet Phenotype. Gender Medicine. 2008;5:S91–S102. doi: 10.1016/j.genm.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Miller VM, Jayachandran M, Heit JA, Owen WG. Estrogen therapy and thrombotic risk. Pharmacology & Therapeutics. 2006;111:792–807. doi: 10.1016/j.pharmthera.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Miller VM, Li L, Sieck GC. Endothelium-dependent effects of estrogen on vasomotor tone: Consequences of non-genomic actions. Vasc Pharmacol. 2002;38:109–113. doi: 10.1016/s0306-3623(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Miller VM, Mulvagh SL. Sex steroids and endothelial function: translating basic science to clinical practice. Trends Pharmacol Sci. 2007;28:263–270. doi: 10.1016/j.tips.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Miller VM, Redfield MM, McConnell JP. Use of BNP and CRP as Biomarkers in Assessing Cardiovascular disease: Diagnosis Versus Risk. Current Vascular Pharmacology. 2007b;5:15–25. doi: 10.2174/157016107779317251. [DOI] [PubMed] [Google Scholar]
- Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, Yagubyan M, Rodgers JC, Cockerill FR, III, Folk RL, Kumar V, Farell-Baril G, Lieske JC. Evidence of nanobacterial-like structures in human calcified arteries and cardiac valves. Am J Physiol: Heart Circ Physiol. 2004b;287:H1115–H1124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- Mishra RG, Stanczyk FZ, Burry KA, Oparil S, Katzenellenbogen BS, Nealen ML, Katzenellenbogen JA, Hermsmeyer RK. Metabolite ligands of estrogen receptor-beta reduce primate coronary hyperreactivity. Am J Physiol Heart Circ Physiol. 2006;290:H295–303. doi: 10.1152/ajpheart.00468.2005. [DOI] [PubMed] [Google Scholar]
- Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol. 2007;102:890–895. doi: 10.1152/japplphysiol.00877.2006. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, Dinenno FA, Blackett SD, Hoetzer GL, Desouza CA, Tanaka H. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol. 2002;283:H1409–1417. doi: 10.1152/ajpheart.00035.2002. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Tanaka H, Jones PP, Gates PE, Seals DR. Basal leg blood flow in healthy women is related to age and hormone replacement therapy status. J Physiol. 2003;547:309–316. doi: 10.1113/jphysiol.2002.032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–164. doi: 10.1097/01.moh.0000131441.10020.87. [DOI] [PubMed] [Google Scholar]
- Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- Nabholtz JM, Gligorov J. Cardiovascular safety profiles of aromatase inhibitors : a comparative review. Drug Saf. 2006;29:785–801. doi: 10.2165/00002018-200629090-00003. [DOI] [PubMed] [Google Scholar]
- Naessen T, Rodriguez-Macias K. Menopausal estrogen therapy counteracts normal aging effects on intima thickness, media thickness and intima/media ratio in carotid and femoral arteries. An investigation using noninvasive high-frequency ultrasound. Atherosclerosis. 2006;189:387–392. doi: 10.1016/j.atherosclerosis.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Taylor HS, Karas R, Brinton E, Newman I, Clarkson TB, Mendelsohn M, Lobo RA, Judelson DR, Nachtigall LE, Heward CB, Hecht H, Jaff MR, Harman SM. The Women’s Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition. Fertil Steril. 2004;81:1498–1501. doi: 10.1016/j.fertnstert.2004.02.095. [DOI] [PubMed] [Google Scholar]
- Nag S. The Blood Brain Barrier. Humana Press; Totowa, New Jersey: 2003. [Google Scholar]
- Nair GV, Herrington DM. The ERA trial: Findings and implications for the future. Climacteric. 2000;3:227–232. doi: 10.1080/13697130008500132. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Miki Y, Suzuki T, Nakata T, Darnel AD, Moriya T, Tazawa C, Saito H, Ishibashi T, Takahashi S, Yamada S, Sasano H. Steroid sulfatase and estrogen sulfotransferase in the atherosclerotic human aorta. Am J Pathol. 2003;163:1329–1339. doi: 10.1016/S0002-9440(10)63492-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman SW, Parks WM. Catechol estrogens and thrombosis: differential effect of 2-hydroxyestradiol and estradiol on prostacyclin release. Contraception. 1982;26:317–320. doi: 10.1016/0010-7824(82)90079-8. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baumer AT, Grohe C, Kahlert S, Strehlow K, Rosenkranz S, Stablein A, Beckers F, Smits JFM, Daemen MJAP, Vetter H, Bohm M. Estrogen modulates AT 1 receptor gene expression in vitro and in vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiological reviews. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Okano H, Jayachandran M, Yoshikawa A, Miller VM. Differential effects of chronic treatment with estrogen receptor ligands on regulation of nitric oxide synthase in porcine aortic endothelial cells. J Cardiovasc Pharmacol. 2006;47:621–628. doi: 10.1097/01.fjc.0000211749.24196.98. [DOI] [PubMed] [Google Scholar]
- Oparil S, Chen SJ, Chen YF, Durand JN, Allen L, Thompson JA. Estrogen attenuates the adventitial contribution to neointima formation in injured rat carotid arteries. Cardiovasc Res. 1999;44:608–614. doi: 10.1016/s0008-6363(99)00240-0. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1beta-mediated induction of COX-2 pathway in rat cerebral blood vessels. Am J Physiol Heart Circ Physiol. 2004;286:H2010–2019. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Duckles SP, Krause DN. 17beta-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am J Physiol Heart Circ Physiol. 2003;285:H241–250. doi: 10.1152/ajpheart.00018.2003. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Krause DN, Duckles SP. 17beta-estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke; a journal of cerebral circulation. 2002;33:600–605. doi: 10.1161/hs0202.102732. [DOI] [PubMed] [Google Scholar]
- Owens JF, Stoney CM, Matthews KA. Menopausal status influences ambulatory blood pressure levels and blood pressure changes during mental stress. Circulation. 1993;88:2794–2802. doi: 10.1161/01.cir.88.6.2794. [DOI] [PubMed] [Google Scholar]
- Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- Paden CM, McEwen BS, Fishman J, Snyder L, DeGroff V. Competition by estrogens for catecholamine receptor binding in vitro. J Neurochem. 1982;39:512–520. doi: 10.1111/j.1471-4159.1982.tb03974.x. [DOI] [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- Parvizi N, Wuttke W. Catecholestrogens affect catecholamine turnover rates in the anterior part of the mediobasal hypothalamus and medial preoptic area in the male and female castrated rat. Neuroendocrinology. 1983;36:21–26. doi: 10.1159/000123523. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of Functional Estrogen Receptors at the Plasma Membrane. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao CS, Kim SW, Kim JB, Lee JK. Co-induction of alphaB-crystallin and MAPKAPK-2 in astrocytes in the penumbra after transient focal cerebral ischemia. Exp Brain Res. 2005;163:421–429. doi: 10.1007/s00221-004-2197-2. [DOI] [PubMed] [Google Scholar]
- Post WA, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa J-P. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43:985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease. Prospective analysis from the Women’s Health Initiative Observational Study. JAMA. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Togaibayeva AA, Kannan MS, Miller VM, Fitzpatrick LA, Sieck GC. Estrogen increases [Ca2+] efflux from female porcine coronary arterial smooth muscle. Am J Physiol (Heart Circ Physiol) 1999;45:H926–H934. doi: 10.1152/ajpheart.1999.276.3.H926. [DOI] [PubMed] [Google Scholar]
- Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Current Opinion in Hematology. 2003;10:356–361. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: A clinical perspective. AnnInternMed. 1997;127:895–903. doi: 10.7326/0003-4819-127-10-199711150-00007. [DOI] [PubMed] [Google Scholar]
- Pritchard KI, Abramson BL. Cardiovascular health and aromatase inhibitors. Drugs. 2006;66:1727–1740. doi: 10.2165/00003495-200666130-00005. [DOI] [PubMed] [Google Scholar]
- Puri V, Puri S, Svojanovsky SR, Mathur S, Macgregor RR, Klein RM, Welch KM, Berman NE. Effects of oestrogen on trigeminal ganglia in culture: implications for hormonal effects on migraine. Cephalalgia. 2006;26:33–42. doi: 10.1111/j.1468-2982.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Pharmacology of ATP-sensitive K+ currents in smooth muscle cells from rabbit mesenteric artery. 1995;269:C1112–C1118. doi: 10.1152/ajpcell.1995.269.5.C1112. [DOI] [PubMed] [Google Scholar]
- Quraishi A, Losordo DW. Ischemic tissue repair by autologous bone marrow-derived stem cells: scientific basis and preclinical data. Handb Exp Pharmacol. 2007:167–179. doi: 10.1007/978-3-540-68976-8_7. [DOI] [PubMed] [Google Scholar]
- Raggi P, Cooil B, Shaw LJ, Aboulhson J, Takasu J, Budoff M, Callister TQ. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am J Cardiol. 2003;92:827–829. doi: 10.1016/s0002-9149(03)00892-0. [DOI] [PubMed] [Google Scholar]
- Rauschemberger MB, Selles J, Massheimer V. The direct action of estrone on vascular tissue involves genomic and non-genomic actions. Life Sci. 2008;82:115–123. doi: 10.1016/j.lfs.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Redberg RF, Rifai N, Gee L, Ridker P. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: Implications for coronary artery disease screening. Journal of the American College of Cardiology. 2000;36:39–43. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocrine reviews. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Ridker PM, Hegener HH, Buring JE, Manson JE, Zee RY. Polymorphisms and haplotypes of the estrogen receptor-beta gene (ESR2) and cardiovascular disease in men and women. Clinical chemistry. 2007;53:1749–1756. doi: 10.1373/clinchem.2007.091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events. An 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Rocca W, Grossardt B, Andrade M, Malkasian G, Melton LI. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- Rochira V, Balestrieri A, Madeo B, Spaggiari A, Carani C. Congenital estrogen deficiency in men: a new syndrome with different phenotypes; clinical and therapeutic implications in men. Molecular and cellular endocrinology. 2002;193:19–28. doi: 10.1016/s0303-7207(02)00092-8. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Hinney A, Ziegler A, Hermann H, Fichter M, Mayer H, Siegfried W, Young JK, Remschmidt H, Hebebrand J. Systematic mutation screening of the estrogen receptor beta gene in probands of different weight extremes: identification of several genetic variants. J Clin Endocrinol Metab. 1998;83:4524–4527. doi: 10.1210/jcem.83.12.5471. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Rotter MA, Schnatz PF, Currier AA, O’Sullivan DM. Breast arterial calcifications (BACs) found on screening mammography and their association with cardiovascular disease menopause. 2008;15 doi: 10.1097/gme.0b013e3181405d0a. in press. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Johns A, Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Rumberger JA, Schwartz RS, Sheedy PF, III, Edwards WD, Fitzpatrick LA. Coronary calcification and pathologic stenosis: an ROC analysis to predict atherosclerotic severity and the influence of gender using ultrafast computed tomography. Am J Cardiol. 1994;74:1169–1173. doi: 10.1016/0002-9149(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Rzewuska-Lech E, Jayachandran M, Fitzpatrick LA, Miller VM. Differential effects of 17β-estradiol and raloxifene on VSMC phenotype and expression of osteoblast-associated proteins. Am J Physiol Endocrinol Metab. 2005;289:E105–E112. doi: 10.1152/ajpendo.00366-02004. First published February 115, 2005. [DOI] [PubMed] [Google Scholar]
- Saab PG, Matthews KA, Stoney CM, McDonald RH. Premenopausal and postmenopausal women differ in their cardiovascular and neuroendocrine responses to behavioral stressors. Psychophysiology. 1989;26:270–280. doi: 10.1111/j.1469-8986.1989.tb01917.x. [DOI] [PubMed] [Google Scholar]
- Saceda M, Lippman ME, Lindsey RK, Puente M, Martin MB. Role of an estrogen receptor-dependent mechanism in the regulation of estrogen receptor mRNA in MCF-7 cells. Mol Endocrinol. 1989;3:1782–1787. doi: 10.1210/mend-3-11-1782. [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ. Role of oestrogen in the central regulation of autonomic function. Clinical and experimental pharmacology & physiology. 2007;34:827–832. doi: 10.1111/j.1440-1681.2007.04663.x. [DOI] [PubMed] [Google Scholar]
- Salpeter S, Walsh J, Greyber E, EE S. Coronary heart disease events associated with hormone therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2006;21:363–366. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter S, Walsh J, Greyber E, Ormiston T, Salpeter E. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J Gen Intern Med. 2004;19:791–804. doi: 10.1111/j.1525-1497.2004.30281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sator MO, Joura EA, Gruber DM, Wieser F, Jirecek S, Tschugguel W, Huber JC. The effect of hormone replacement therapy on carotid arteries: measurement with a high frequency ultrasound system. Maturitas. 1998;30:63–68. doi: 10.1016/s0378-5122(98)00036-x. [DOI] [PubMed] [Google Scholar]
- Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women: A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071–3078. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. The Lancet. 2003;362:428–432. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- Schnatz PF. Hormonal therapy: does it increase or decrease cardiovascular risk? Obstet Gynecol Surv. 2006;61:673–681. doi: 10.1097/01.ogx.0000238674.98471.bb. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104:2266–2268. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, van Leeuwen JP, de Jong FH, Zillikens MC, Hofman A, Pols HA, Uitterlinden AG. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. Jama. 2004;291:2969–2977. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Experimental Gerontology. 2006;41:501–507. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Seed M, Sands RH, McLaren M, Kirk G, Darko D. The effect of hormone replacement therapy and route of administration on selected cardiovascular risk factors in post-menopausal women. Fam Pract. 2000;17:497–507. doi: 10.1093/fampra/17.6.497. [DOI] [PubMed] [Google Scholar]
- Sendag F, Karadadas N, Ozsener S, Bilgin O. Effects of sequential combined transdermal and oral hormone replacement therapies on serum lipid and lipoproteins in postmenopausal women. Arch Gynecol Obstet. 2002;266:38–43. doi: 10.1007/pl00007497. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Risk of cardiovascular disease in relation to the use of combined postmenopausal hormone therapy: detection bias and resolution of discrepant findings in two Women’s Health Initiative studies. Climacteric. 2006;9:416–420. doi: 10.1080/13697130601012061. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Vittner V, Kelsey SF, Olson M, Delia Johnson B, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. Journal of the American College of Cardiology. 2006;47:4S–20S. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Shearman AM. Oestrogen receptor genetics: a needle that cuts through many haystacks? European heart journal. 2006;27:1519–1520. doi: 10.1093/eurheartj/ehl065. [DOI] [PubMed] [Google Scholar]
- Shearman AM, Cooper JA, Kotwinski PJ, Miller GJ, Humphries SE, Ardlie KG, Jordan B, Irenze K, Lunetta KL, Schuit SC, Uitterlinden AG, Pols HA, Demissie S, Cupples LA, Mendelsohn ME, Levy D, Housman DE. Estrogen receptor alpha gene variation is associated with risk of myocardial infarction in more than seven thousand men from five cohorts. Circ Res. 2006;98:590–592. doi: 10.1161/01.RES.0000210578.62102.a6. [DOI] [PubMed] [Google Scholar]
- Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D. Association between estrogen receptor alpha gene variation and cardiovascular disease. Jama. 2003;290:2263–2270. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, Shaul PW. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. American journal of respiratory cell and molecular biology. 2002;26:610–616. doi: 10.1165/ajrcmb.26.5.4528. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- Simon T, Beau Yon De Jonage-Canonico M, Oger E, Wahl D, Conard J, Meyer G, Emmerich J, Barrellier M-T, Guiraud A, Scarabin PY. Indicators of lifetime endogenous estrogen exposure and risk of venous thromboembolism. Journal of Thrombosis and Haemostasis. 2006;4:71–76. doi: 10.1111/j.1538-7836.2005.01693.x. [DOI] [PubMed] [Google Scholar]
- Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci. 1995;15:7979–7988. doi: 10.1523/JNEUROSCI.15-12-07979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Sokabe T, Yamamoto K, Ohura N, Nakatsuka H, Qin K, Obi S, Kamiya A, Ando J. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H2027–2034. doi: 10.1152/ajpheart.00260.2004. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Symons JP, Jannausch ML, Chu J, Kardia SR. Sex steroid hormone polymorphisms, high-density lipoprotein cholesterol, and apolipoprotein A-1 from the Study of Women’s Health Across the Nation (SWAN) Am J Med. 2006;119:S61–68. doi: 10.1016/j.amjmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Srivastava RA, Tang J, Baumann D, Schonfeld G. Hormonal and nutritional stimuli modulate apolipoprotein B mRNA editing in mouse liver. Biochem Biophys Res Commun. 1992;188:135–141. doi: 10.1016/0006-291x(92)92360-a. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stang PE, Carson AP, Rose KM, Mo J, Ephross SA, Shahar E, Szklo M. Headache, cerebrovascular symptoms, and stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2005;64:1573–1577. doi: 10.1212/01.WNL.0000158326.31368.04. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. Journal of medicinal chemistry. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Steimer T. Steroid Hormone Metabolism 2003 [Google Scholar]
- Stevenson JC, Oladipo A, Manassiev N, Whitehead MI, Guilford S, Proudler AJ. Randomized trial of effect of transdermal continuous combined hormone replacement therapy on cardiovascular risk markers. Br J Haematol. 2004;124:802–808. doi: 10.1111/j.1365-2141.2004.04846.x. [DOI] [PubMed] [Google Scholar]
- Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol. 2005a;67:105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- Stirone C, Chu Y, Sunday L, Duckles SP, Krause DN. 17 Beta-estradiol increases endothelial nitric oxide synthase mRNA copy number in cerebral blood vessels: quantification by real-time polymerase chain reaction. Eur J Pharmacol. 2003a;478:35–38. doi: 10.1016/j.ejphar.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003b;284:E184–192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005b;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Stork S, van der Schouw YT, Grobbee DE, Bots ML. Estrogen, inflammation and cardiovascular risk in women: a critical appraisal. Trends Endocrinol Metab. 2004;15:66–72. doi: 10.1016/j.tem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Straczek C, Oger E, de Jonage-Canonico BY, Plu-Bureau G, Conard J, Meyer G, Alhenc-Gelas M, Levesque H, Trillot N, Barrellier M-T, Wahl D, Emmerich J, Scarabin P-V. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women. Impact of the route of estrogen administration. Circulation. 2005;112:3495–3500. doi: 10.1161/CIRCULATIONAHA.105.565556. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Ylikorkala O, Tikkanen MJ. Differing effects of oral and transdermal hormone replacement therapy on cardiovascular risk factors in healthy postmenopausal women. Am J Cardiol. 2003;92:212–214. doi: 10.1016/s0002-9149(03)00542-3. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocrine reviews. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003a;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003b;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- Stringer B, Waddington R, Houghton A, Stone M, Russell G, Foster G. Serum from postmenopausal women directs differentiation of human clonal osteoprogenitor cells from an osteoblastic toward an adipocytic phenotype. Calcif Tissue Int. 2007;80:233–243. doi: 10.1007/s00223-007-9016-2. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation. 1997a;96:3774–3777. doi: 10.1161/01.cir.96.10.3774. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet. 1997b;349:1146–1147. doi: 10.1016/S0140-6736(05)63022-X. [DOI] [PubMed] [Google Scholar]
- Sumi D, Hayashi T, Jayachandran M, Iguchi A. Estrogen prevents destabilization of endothelial nitric oxide synthase mRNA induced by tumor necrosis factor α through estrogen receptor mediated system. Life Sci. 2001;69:1651–1660. doi: 10.1016/s0024-3205(01)01251-6. [DOI] [PubMed] [Google Scholar]
- Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol. 2007;292:H2333–2340. doi: 10.1152/ajpheart.01057.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday L, Tran MM, Krause DN, Duckles SP. Estrogen and progestagens differentially modulate vascular proinflammatory factors. Am J Physiol Endocrinol Metab. 2006;291:E261–267. doi: 10.1152/ajpendo.00550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torrens JI. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanaka E, Murakami M, Mori-Abe A, Kawagoe J, Takata K, Ohmichi M, Kurachi H. Long-term hormone replacement therapy delays the age related progression of carotid intima-media thickness in healthy postmenopausal women. Maturitas. 2004;49:170–177. doi: 10.1016/j.maturitas.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Tempfer CB, Riener EK, Hefler LA, Huber JC, Muendlein A. DNA microarray-based analysis of single nucleotide polymorphisms may be useful for assessing the risks and benefits of hormone therapy. Fertil Steril. 2004;82:132–137. doi: 10.1016/j.fertnstert.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Thampan RV. The nuclear binding of estradiol stimulates ribonucleoprotein transport in the rat uterus. J Biol Chem. 1985;260:5420–5426. [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond WD, Howard V, Runsfeld J, Manolio TA, Zhi-Jie Z, Flegal K, O’Donnell CP, Kittner S, Lloyd-Jones D, Goff D, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger VL, Sidney S, Srorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf PH. Heart Disease and Stroke Statistics - 2006 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thomas TN, Rhodin JA, Clark L, Garces A, Bryant M. A comparison of the anti-inflammatory activities of conjugated estrogens and 17-β estradiol. Inflamm Res. 2003;52:452–460. doi: 10.1007/s00011-003-1198-0. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Olesen J. Nitric oxide in primary headaches. Current opinion in neurology. 2001;14:315–321. doi: 10.1097/00019052-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Tian YM, Mole DR, Ratcliffe PJ, Gleadle JM. Characterization of different isoforms of the HIF prolyl hydroxylase PHD1 generated by alternative initiation. Biochem J. 2006;397:179–186. doi: 10.1042/BJ20051996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert T, Thompson A, Bouchard P, Oparil S. Estrogen-induced vasoprotection is independent of inducible nitric oxide synthase expression. Evidence from the mouse caarotid artery ligation model. Circulation. 2001;104:2740–2745. doi: 10.1161/hc4701.099581. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Totary-Jain H, Naveh-Many T, Riahi Y, Kaiser N, Eckel J, Sasson S. Calreticulin destabilizes glucose transporter-1 mRNA in vascular endothelial and smooth muscle cells under high-glucose conditions. Circ Res. 2005;97:1001–1008. doi: 10.1161/01.RES.0000189260.46084.e5. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Maldonado A, Damian-Matsumura P, Timossi C. Endocrine regulation of gonadotropin glycosylation. Arch Med Res. 2001;32:520–532. doi: 10.1016/s0188-4409(01)00319-8. [DOI] [PubMed] [Google Scholar]
- Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- van Hylckama Vlieg A, Rosendaal FR. High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thromb Haemost. 2003;1:2677–2678. doi: 10.1111/j.1538-7836.2003.0543b.x. [DOI] [PubMed] [Google Scholar]
- van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol. 1997;47:337–342. doi: 10.1046/j.1365-2265.1997.2601068.x. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- VanLangevelde LA, Anchill SE, Wrobleski SK, Linn MJ, Wakefield TW, Myers DD., Jr Gender differences in deep venous thrombosis in a rat model: A preliminary study. Comp Med. 2005;55:55–60. [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocrine reviews. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Vehkavaara S, Silveira A, Hakala-Ala-Pietila T, Virkamaki A, Hovatta O, Hamsten A, Taskinen MR, Yki-Jarvinen H. Effects of oral and transdermal estrogen replacement therapy on markers of coagulation, fibrinolysis, inflammation and serum lipids and lipoproteins in postmenopausal women. Thromb Haemost. 2001;85:619–625. [PubMed] [Google Scholar]
- Verdier-Sevrain S, Bonte F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Experimental dermatology. 2006;15:83–94. doi: 10.1111/j.1600-0625.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- Vickers M, Martin JF, Meade T. The Women’s international study of long-duration oestrogen after menopause (WISDOM): a randomised controlled trial. BMC Women’s Health. 2007;7:1–17. doi: 10.1186/1472-6874-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vin F, Allaert FA, Levardon M. Influence of estrogens and progesterone on the venous system of the lower limbs in women. The Journal of dermatologic surgery and oncology. 1992;18:888–892. doi: 10.1111/j.1524-4725.1992.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Vitale C, Mercuro G, Cerquetani E, Marazzi G, Patrizi R, Volterrani M, Fini M, Collins P, Rosano GM. Time Since Menopause Influences the Acute and Chronic Effect of Estrogens on Endothelial Function. Arterioscler Thromb Vasc Biol. 2008;28 doi: 10.1161/ATVBAHA.107.158634. in press. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging and cancer: A dawn for evolutionary medicine. Annual Review of Genetics. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Oparil S, Chen Y-F, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:2094–2099. doi: 10.1161/01.ATV.0000179602.85797.3f. [DOI] [PubMed] [Google Scholar]
- Wehling M. Specific, nongenomic actions of steroid hormones. Annual review of physiology. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM. Pharmacogenomics: catechol O-methyltransferase to thiopurine S-methyltransferase. Cell Mol Neurobiol. 2006;26:539–561. doi: 10.1007/s10571-006-9095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum RM, Wang L. Pharmacogenetics and Pharmacogenomics: Development, Science, and Translation. Annu Rev Genomics Hum Genet. 2006;7:223–245. doi: 10.1146/annurev.genom.6.080604.162315. [DOI] [PubMed] [Google Scholar]
- Welch KM, Brandes JL, Berman NE. Mismatch in how oestrogen modulates molecular and neuronal function may explain menstrual migraine. Neurol Sci. 2006;27(Suppl 2):S190–192. doi: 10.1007/s10072-006-0599-6. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. N Engl J Med. 1993;329:247–256. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- Wessman M, Kaunisto MA, Kallela M, Palotie A. The molecular genetics of migraine. Annals of medicine. 2004;36:462–473. doi: 10.1080/07853890410018060. [DOI] [PubMed] [Google Scholar]
- White CR, Shelton J, Chen S-J, Darley-Usmar V, Allen L, Nabors C, Sanders PW, Chen Y-F, Oparil S. Estrogen restores endothelial cell function in an experimental model of vascular injury. Circulation. 1998;96:1624–1630. doi: 10.1161/01.cir.96.5.1624. [DOI] [PubMed] [Google Scholar]
- White RE, Darkow DJ, Falvo Lang JL. Estrogen relaxes coronary arteries by opening BK+Ca++ channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension. 2003;42:991–996. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- Williams JK, Adams MR, Herrington DM, Clarkson TB. Short-term administration of estrogen and vascular responses of atherosclerotic coronary arteries. Journal of the American College of Cardiology. 1992;20:452–457. doi: 10.1016/0735-1097(92)90116-5. [DOI] [PubMed] [Google Scholar]
- Worda C, Sator MO, Schneeberger C, Jantschev T, Ferlitsch K, Huber JC. Influence of the catechol-O-methyltransferase (COMT) codon 158 polymorphism on estrogen levels in women. Hum Reprod. 2003;18:262–266. doi: 10.1093/humrep/deg059. [DOI] [PubMed] [Google Scholar]
- Writing WGftWsHII. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Wu H, Sun L, Zhang Y, Chen Y, Shi B, Li R, Wang Y, Liang J, Fan D, Wu G, Wang D, Li S, Shang Y. Coordinated regulation of AIB1 transcriptional activity by sumoylation and phosphorylation. J Biol Chem. 2006;281:21848–21856. doi: 10.1074/jbc.M603772200. [DOI] [PubMed] [Google Scholar]
- Wu M, Li YG. The expression of CD40-CD40L and activities of matrix metalloproteinases in atherosclerotic rats. Mol Cell Biochem. 2006;282:141–146. doi: 10.1007/s11010-006-1741-8. [DOI] [PubMed] [Google Scholar]
- Wu Z, Maric C, Roesch DM, Zheng W, Verbalis JG, Sandberg K. Estrogen regulates adrenal angiotensin AT1 receptors by modulating AT1 receptor translation. Endocrinology. 2003;144:3251–3261. doi: 10.1210/en.2003-0015. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Carlson SH. Effects of hormone replacement therapy on the sympathetic nervous system and blood pressure. Curr Hypertens Rep. 2003;5:241–246. doi: 10.1007/s11906-003-0027-8. [DOI] [PubMed] [Google Scholar]
- Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am J Physiol Heart Circ Physiol. 2007;292:H2607–2612. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- Yager JD, Chen JQ. Mitochondrial estrogen receptors--new insights into specific functions. Trends Endocrinol Metab. 2007;18:89–91. doi: 10.1016/j.tem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying AK, Hassanain HH, Roos CM, Smiraglia DJ, Issa JJ, Michler RE, Caligiuri M, Plass C, Goldschmidt-Clermont PJ. Methylation of the estrogen receptor-alpha gene promoter is selectively increased in proliferating human aortic smooth muscle cells. Cardiovasc Res. 2000;46:172–179. doi: 10.1016/s0008-6363(00)00004-3. [DOI] [PubMed] [Google Scholar]
- Zacharia LC, Gogos JA, Karayiorgou M, Jackson EK, Gillespie DG, Barchiesi F, Dubey RK. Methoxyestradiols mediate the antimitogenic effects of 17beta-estradiol: direct evidence from catechol-O-methyltransferase-knockout mice. Circulation. 2003;108:2974–2978. doi: 10.1161/01.CIR.0000106900.66354.30. [DOI] [PubMed] [Google Scholar]
- Zacharia LC, Jackson EK, Gillespie DG, Dubey RK. Catecholamines abrogate antimitogenic effects of 2-hydroxyestradiol on human aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:1745–1750. doi: 10.1161/hq1001.097064. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Li SH, Liu SM, Szmitko PE, He XQ, Fedak PW, Verma S. C-Reactive protein upregulates receptor for advanced glycation end products expression in human endothelial cells. Hypertension. 2006;48:504–511. doi: 10.1161/01.HYP.0000234904.43861.f7. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zhu BT. Catechol-O-Methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3:321–349. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson J-A, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]