Abstract
Numerous antagonists of tumor necrosis factor alpha (TNFα) have been developed to attenuate inflammation and accompanying pain in many disease processes. Soluble TNF receptor type II (sTNFRII) is one such antagonist that sequesters TNFα away from target receptors and attenuates its activity. Systemic delivery of soluble TNF receptors or other antagonists may have deleterious side effects associated with immune suppression, so that strategies for locally targeted drug delivery are of interest. Elastin-like polypeptides (ELPs) are biopolymers capable of in situ drug depot formation through thermally-driven supramolecular complexes at physiological temperatures. A recombinant fusion protein between ELP and sTNFRII was designed and evaluated for retention of bivalent functionality. Thermal sensitivity was observed by formation of supramolecular submicron-sized particles at 32°C, with gradual resolubilization from the depot observed at physiological temperatures. In vitro refolding of the sTNFRII domain was required and the purified product exhibited an equilibrium dissociation constant for interacting with TNFα that was seven-fold higher than free sTNFRII. Furthermore, anti-TNF activity was observed in inhibiting TNFα-mediated cytotoxicity in the murine L929 fibrosarcoma assay. Potential advantages of this ELP-sTNFRII fusion protein as an anti-TNFa drug depot include facility of injection, in situ depot formation, low endotoxin content, and functionality against TNFα.
Keywords: fusion protein, tumor necrosis factor alpha, soluble tumor necrosis factor receptor, drug depot, elastin-like polypeptide
Introduction
Tumor necrosis factor alpha (TNFα) is a pro-inflammatory cytokine that has been implicated as a key mediator of inflammation in multiple systemic and local disease processes including rheumatoid arthritis[1–3], inflammatory bowel disease[4, 5], sepsis[6–8], and radiculopathy[9–11]. TNFα acts through two transmembrane receptors, TNFRI and TNFRII, that dissociate the cytotoxic and proinflammatory effects in target cells.[12] Soluble TNF receptors (sTNFRI and sTNFRII) are the extracellular domains of these membrane receptors that are liberated from the transmembrane domain by proteolytic cleavage. Upon removal, these truncated receptors become soluble proteins and retain the appropriate tertiary structure to act as decoy receptors sequestering TNFα away from the cell surface receptors.[13, 14] Cleavage products of the Type I and Type II TNF receptors (sTNFRI and sTNFRII respectively) have 27.5% sequence identity[15] and have TNFα equilibrium dissociation constants of 1.2 nM and 0.35 nM.[16]
Both TNFα-binding antibodies (infliximab and adalimumab) and molecules based on sTNFRI (pegsuncercept) or sTNFRII (etanercept) are used to sequester the cytokine away from target cell surface receptors in inflammatory disease.[13, 17, 18] These therapeutic high molecular weight conjugates shift clearance from renal to hepatic providing for longevity in the systemic circulation.[13, 19] Such agents are delivered subcutaneously or intravenously causing systemic toxicity in patients due to immunosuppression.[18] While systemic diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis demand parenteral treatment, local inflammation in monoarticular osteoarthritis or disc-herniation radiculopathy exhibit local upregulation of TNFα-activity and may benefit from local treatment.[20] A delivery system that sustains sTNFRII activity in a targeted compartment may increase therapeutic efficacy and minimize serum exposure and associated side effects. Soluble TNF receptors are complex proteins with tertiary structure of extensive disulfide bridging[21] requiring expression in E. coli to include in vitro oxidation-reduction refolding.[22] Purification of such agents, alone or conjugated to a carrier, has involved affinity purification with a TNFα-functionalized column.[14, 17, 23]
Thermally-responsive elastin-like polypeptides (ELPs) have been evaluated as drug carriers to diarthrodial joints,[24] dorsal root ganglia,[25] and solid tumors.[26] ELPs consist of pentapeptide repeats of a Val-Pro-Gly-Xaa-Gly sequence with structural homology to mammalian elastin (Xaa is a guest residue other than proline).[27] Aqueous solutions of these polymers exhibit inverse phase transition behavior: ELPs are soluble monomers below a characteristic transition temperature (Tt), but upon heating the solution above their Tt, they undergo a reversible hydrophobic-association into micron-sized, supramolecular complexes. This property can be exploited to form drug depots in situ by delivering ELPs in solution at room temperature that spontaneously associate upon delivery to a local cavity at body temperature. Indeed, prior studies have demonstrated that an ELP designed to undergo thermal phase transition upon intra-articular injection exhibited a 25-fold increase in its intra-articular half-life compared to a soluble, non-transitioning ELP.[24] In other work on local delivery, a depot-forming ELP delivered to the dorsal root ganglion exhibited a seven-fold increase in local half-life compared to a soluble ELP of comparable molecular weight.[25] Furthermore, both studies revealed that systemic exposure to the depot-forming ELP was substantially decreased by the phase-transitioning property of the polymer. It remains unclear if this longevity will provide for greater or sustained activity of conjugated therapeutics delivered locally in a disease model. Local targeting after systemic delivery has also been accomplished by intravenous delivery of soluble ELPs with a Tt of 40°C followed by application of local hyperthermia to trigger the phase transition and permit ELP phase separation and accumulation within a tumor.[28, 29] These results support that the inverse phase transition behavior of an ELP conjugated to a drug may provide a facile means of generating an in situ forming depot, with slow release increasing drug longevity in the targeted compartment and reducing serum exposure to the attached therapeutic. Following intravenous administration, ELPs are cleared with a terminal half-life of 8.4 hours.[30] The reported biodistribution studies relate only to the ELP carrier, and it remains uncertain if therapeutic fusion proteins would exhibit those desired benefits of sustained release and attenuated serum exposure to potent immunosuppressive agents. Drugs conjugated with ELPs gain properties of thermally-induced phase transition and also maintain in vitro bioactivity. This has been shown for chemically-conjugated chemotherapeutics such as doxorubicin,[26] recombinant oligopeptide fusions with cell penetrating peptides[31] and a c-myc oncogene inhibitor,[32] and recombinant protein fusions with interleukin-1 receptor antagonist[33] and other proteins[34, 35]. Surfaces coated with an ELP fused to the RGD or fibronectin CS5 cell binding sequence also retain an ability to support in vitro endothelial cell adhesion and spreading.[36] Other applications of ELP, including entrapment of small molecules such as dexamethasone,[37] have also been investigated and are elsewhere reviewed.[38]
The primary objective of this study was to create a fusion protein between an ELP and sTNFRII that would retain the ELP inverse phase transition behavior and sTNFRII domain bioactivity. This study is the first step towards realizing the long-term objective exploring the feasibility of attenuating local inflammation from TNFα hyperactivity in joint, nerve, and intervertebral disc spaces via local delivery and sustained release of the immunomodulator therapeutic. An ELP-sTNFRII gene was designed and the fusion protein was expressed in Escherichia coli. Results are presented for the phase transition and resolubilization behavior of the fusion protein, as well as binding affinity of ELP-sTNFRII to immobilized TNFα and in vitro anti-TNF bioactivity. The results indicate that ELP-sTNFRII retains functionality of both domains, establishing the potential of this therapeutic as an injectable local immunomodulatory protein.
Materials and Methods
Fusion Protein Synthesis
The gene encoding human sTNFRII was inserted into a pUC57 cloning vector (GenScript, Piscataway, NJ) with the coding sequence flanked by unique XbaI and HindIII restriction sites, with the inclusion of an SfiI restriction site at the 3’ end. This plasmid was linearized with SfiI and treated with Calf Intestinal Phosphatase (New England Biolabs, Ipswich, MA). A cassette for the ELP gene encoding (VPGVG)60 was removed from a pUC19 cloning vector (generously provided by Dr. Chilkoti, Duke University) by double digestion using PflmI and BglI, followed by electrophoretic separation and agarose gel extraction. The ELP cassette was then ligated into the linearized pUC57 vector. The fusion gene cassette was removed by double digestion using XbaI and HindIII, followed by electrophoretic separation and agarose gel extraction. Separately, a pET25b(+) expression vector was modified by double digestion with XbaI and HindIII and ligation with annealed custom-designed non-phosphorylated oligonucleotides (Integrated DNA Technologies, Coralville, IA) to move the XbaI site and downstream to the Shine-Delgarno variant ribosomal-binding site. This modified pET25b(+) vector was double digested with XbaI and HindIII, treated with CIP, agarose gel purified, and then ligated with the fusion gene cassette to yield the target fusion gene (ELP-sTNFRII) in an expression vector. The BL21trxB(DE3) expression strain of E. coli was transformed with the ligation mixture using heat shock poration.
One liter of TB-Dry (MoBio, Carlsbad, CA) media with 100 µg/mL of ampicillin (Sigma-Aldrich, St. Louis, MO) was inoculated with the expression strain and grown using a hyperexpression protocol.[39] Cells were harvested by centrifugation (3200 g, 15 minutes) and resuspended in 35 mL PBS. They were lysed by sonication at 4°C and centrifuged (11500 g, 15 minutes, 4°C) to eliminate cell debris. Nucleic acids were precipitated using polyethyleneimine (0.5% w/v) and removed by centrifugation (11500 g, 15 minutes, 4°C). Fusion protein was purified by four rounds of inverse transition cycling (ITC) as described previously[40] under reducing conditions with 0.15% β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) for samples to be refolded, or non-reducing conditions for samples to be directly evaluated. SDS-PAGE was performed at 80 mV with ReadyGel™ 4–20% gradient gels (Bio-Rad, Hercules, CA) and stained with SimplyBlue™ (Invitrogen, Carlsbad, CA) to confirm fusion protein purity. Concentrations were determined spectrophotometrically using calculated extinction coefficients.[41]
Thermal Characterization
The phase-transitioning behavior of the ELP-sTNFRII protein (MW = 44.3 kDa) was characterized in order to confirm retention of thermal transitioning behavior. Dynamic light scattering (DLS) at a 90° scattering angle (DynaPro LSR with Peltier temperature control; Wyatt Technology Corp., Santa Barbara, CA) was used to evaluate the temperature-dependence of the hydrodynamic radius of the soluble ELP fusion protein and its supramolecular complexes for a 25 µM solution in PBS filtered through a 20 nm Whatman Anodisc filter. This low protein concentration on the order of 0.1% w/v helps control the solution viscosity to allow accurate estimates of particle size from the Stokes-Einstein equation, while also helping protect against the complicating feature of multiple scattering events. A broader range of fusion protein concentrations (500 nM to 100 µM) in both PBS and cell culture media was evaluated for the inverse phase transition using turbidity analysis, optical density at 350 nm (OD350), measured over the 15–60°C range (Cary 300 UV-vis spectrophotometer with multicell thermoelectric temperature controller; Varian, Walnut Creek, CA) with the transition temperature defined as the temperature at which the OD350 is increased by 5% of the maximal observed change.
Fusion Protein Resolubilization
Fusion protein resolubilization was tested to evaluate the kinetics of monomer resolubilization from the multiparticle depots at clinically-relevant concentrations. Current etanercept dosing ranges from systemic delivery for rheumatoid arthritis or ankylosing spondylitis of 25 mg subcutaneously per injection,[42] to local delivery in clinical trials for radiculopathy of 2–6 mg per injection.[43] Solutions of ELP or ELP-sTNFRII at 25 mg/mL concentration were thermally-triggered to undergo the inverse phase transition in a volume of 1.5 mL at the bottom of 15 mL conical tubes by heating to 37°C followed by warm centrifugation. Protein resolubilization was analyzed in triplicate. Aliquots (7–10 µL) were periodically taken to measure concentration by UV-VIS spectrophotometry at 280 nm until the aqueous phase concentration reached steady-state. The entire supernatant was withdrawn and replaced with an equivalent volume of 37°C PBS. The dilute supernatant, free of soluble protein, would drive depot dissociation. Aqueous phase aliquots were periodically withdrawn and supernatant concentrations measured to monitor protein release. Data were transformed into the mass fraction of soluble protein, and fit by non-linear regression to a first-order kinetic model:
| (Equation 1) |
where x(t) is the mass fraction of soluble form ELP as a function of time, xss is the steady-state mass fraction of soluble form ELP, and τ is the characteristic time constant. Goodness of fit was evaluated by the coefficient of determination, r2. Student’s t-test was used to assess differences in xss and τ between the fusion protein and the free ELP at a significance level of 0.05.
In Vitro Fusion Protein Refolding
Recombinant protein expression was not anticipated to yield a uniform population of folded proteins with functional conformation. This assumption was based upon previous studies expressing sTNFRI and sTNFRII as free proteins[14], or sTNFRI as a fusion with maltose binding protein[22], and motivated development of a protocol to refold soluble ELP-sTNFRII after the initial purification step. Refolding of ELP-sTNFRII began with complete protein denaturation at 2.5 mg/mL using 8M urea and 0.15% β-mercaptoethanol at 65°C for 10 minutes. The protein was then diluted 1:50 to a final concentration of 50 µg/mL into refolding buffer that contained 0.1 M Na2CO3 with a variety of tested conditions. Refolding variables included duration and temperature of refolding, buffer pH, ratios of oxidized and reduced glutathione constituents of the oxidation-reduction buffer, presence of co-solutes that were added to promote refolding, and the application of an enzymatic catalyst, protein disulfide isomerase. Refolding effectiveness was evaluated using a fluorescence immunoassay with specific defunctionalizing anti-sTNFRII antibody (Abcam, Cambridge, MA) as the primary detection antibody and AlexaFluor488 anti-IgG (Invitrogen, Carlsbad, CA) as the secondary reporter antibody.
Fusion Protein Precipitation
For further characterization, immunoprecipitation was used to capture ELP-sTNFRII with appropriate conformation using defunctionalizing monoclonal anti-sTNFRII antibodies (Abcam, Cambridge, MA). Briefly, using the Seize Primary Immunoprecipitation Kit (Pierce, Rockford, IL), 100 µg of antibody was immobilized onto agarose functionalized with activated aldehyde groups. Five hundred microliters of a 50 µM solution of refolded fusion protein was incubated with this gel at 4°C for 24 hours, after which the agarose was washed with 1M saline, and elution was performed under acidic conditions (pH 2.8). SDS-PAGE followed by silver staining was used to confirm fusion protein capture. To confirm the immunoprecipitation results, a separate ligand-affinity precipitation experiment was performed with immobilization of 20 µg of TNFα, and again 24 hours incubation with 500 µL of a 50 M solution of refolded fusion protein at 4°C. The column was eluted under acidic conditions and SDS-PAGE with SimplyBlue™ staining was performed to confirm the presence of ELP-sTNFRII isolated by TNFα affinity.
TNFα Binding Affinity of ELP-sTNFRII
The binding kinetics between ELP-sTNFRII and immobilized TNFα were evaluated by surface plasmon resonance (SPR) using a BIACore X instrument (BIACore AB, Uppsala, Sweden). TNF (100 µL at a concentration of 500 nM in PBS) was immobilized onto a self-assembled monolayer (SAM) of 16-mercaptohexadecanoic acid on gold-coated glass slides using activated ester chemistry. First, eighty microliters of an equal volume mixture of 0.2 M N-hydroxysulfosuccinimide and 0.4 M 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide was added to activate the terminal carboxylic acid groups of the SAM, after which the cytokine was added at a flow rate of 5 µL/min. Seventy-five microliters of 1M ethanolamine was used to deactivate residual carboxylic acid groups, after which the surface was washed with PBS. Binding kinetics were measured at 20°C, below the protein transition temperature, by monitoring the surface’s refractive index changes upon injection of 100 µL aliquots of seven test solutions in PBS at 25 µL/min flow rate: ELP-sTNFRII purified by ITC (non-reducing conditions), unfolded ELP-sTNFRII, refolded ELP-sTNFRII, immunoprecipitated ELP-sTNFRII, TNFα-purified ELP-sTNFRII, commercial sTNFRII (Abcam, Cambridge, MA), or ELP alone. Dissociation kinetics were measured by changing the flow to PBS. Data was fit by non-linear least-squares regression to a model of bimolecular interaction (BIAevaluation Software, BIACore, AB). Resulting kon, koff and KD values were tested by one-factor ANOVA with post-hoc Dunn’s test for differences among fusion protein, commercial sTNFRII, and free ELP (α = 0.05 significance level).
Protein Endotoxin Content
Three samples of ELP or ELP-sTNFRII obtained from three independent cultures were purified by three rounds of ITC and serially-diluted from a stock concentration of 25 µM in endotoxin-free water. Briefly, endotoxin content of these samples was evaluated using the Limulus Amebocyte Lysate Complete 20 kit (Cambrex, Charles City, IA) in which serial dilutions of a sample are tested to determine the presence of a critical endotoxin concentration at which coagulation occurs. These results were compared against an endotoxin control to estimate the amount of endotoxin in the purified ELP and ELP-sTNFRII preparations.
Anti-TNFα Activity of Fusion Protein using Murine L929 Fibrosarcoma Cells
Murine fibrosarcoma L929 cells (ATCC, Rockville, MD) were grown in 75 cm2 tissue culture flasks containing DMEM medium with 4.5 g/L glucose (Invitrogen, Carlsbad, CA), 10% horse serum, 50 U/mL penicillin, and 50 µg/mL streptomycin (Sigma-Aldrich, St. Louis, MO). Cells were incubated for 24 hours in a 96-well plate at density of 10,000 cells/well in 70 µL of culture medium. Cells were sensitized to cytotoxicity using Actinomycin D (1 µg/mL, Sigma-Aldrich, St.Louis, MO) for one hour, after which 0.25 ng/mL TNFα was added.[44, 45] Treated cells were coincubated with ELP-sTNFRII or commercial sTNFRII to investigate dose-dependent cytoprotection. The assay plate was incubated for a further 24 hours after which cell survival was quantified by the CellTiter Glo luminescence assay (Promega, Madison, WI). Treatment effects were normalized against the observed survival of cells without TNFα. The dose-response relationship was fit by non-linear regression to a logistic curve to derive IC50 data:
| (Equation 2) |
where SBaseline is the fraction of cell survival without TNF, Smax is the maximal observed cytotoxicity on incubation with TNFα, and S is the concentration-dependent fractional survival by the given antagonist. The Hill slope, k, and the antagonist inhibitory concentration, IC50, are fit parameters. Goodness of fit was evaluated by the coefficient of determination, r2. Student’s t-test evaluated differences amongst the antagonist IC50 values at a significance level of 0.05. Further, to characterize the activity of material resolubilized from the depot, the ELP-sTNFRII supernatant fraction from the resolubilization experiment (n = 4 trials) was evaluated (n = 8 replicates) in the murine L929 bioassay and compared against fusion protein that had not been aggregated. Single-factor ANOVA with post-hoc Dunn’s test (α = 0.05 significance level) evaluated cytoprotection differences between depot-resolubilized and non-aggregated protein.
Results
Fusion Protein Synthesis
The fusion ELP-sTNFRII gene was successfully assembled and subcloned into the modified pET25b(+) expression vector. The fusion protein was expressed in the BL21trxB(DE3) strain of E. coli, followed by ITC purification under reducing conditions. SDS-PAGE with SimplyBlue™ stain revealed a single band with the appropriate molecular weight of 44 kDa after four rounds of ITC. Protein yield after ITC purification was 35–50 mg per liter of E. coli culture.
Thermal Characterization
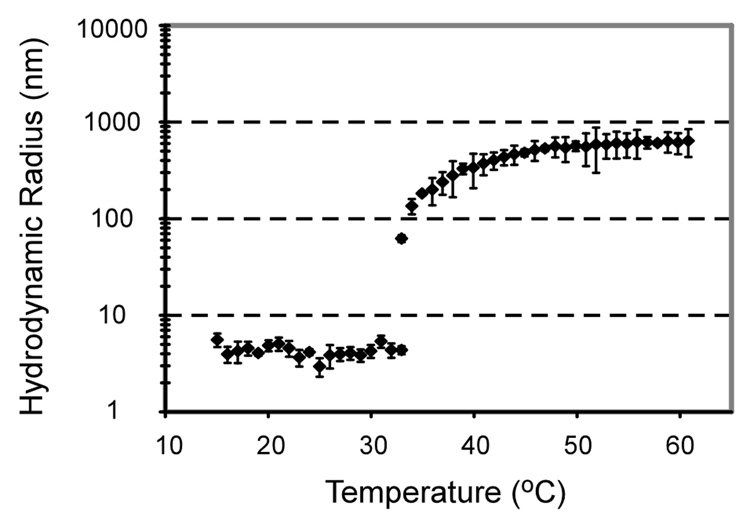
The ELP-sTNFRII fusion protein exhibited thermally-responsive behavior in the DLS experiment as shown by a temperature-dependent increase in hydrodynamic radius from monomers to larger nanoparticles (Figure 1). The particle sizes were consistently less than 10 nm for temperatures below Tt consistent with the presence of soluble protein monomers. Upon reaching a transition temperature of approximately 32°C, the proteins formed supramolecular complexes and the particle size increased dramatically, growing to the sub-micron order. Results of the turbidity analysis demonstrate temperature-dependent increases in OD350 for fusion protein concentrations ranging from 500 nM to 100 µM, with the transition temperature observed to be subphysiological for all concentrations in both PBS and cell culture media (data not shown).
Figure 1.
Dynamic light scattering data (mean ± standard deviation) for ELP-sTNFRII at 25 µM shows a transition from monomers to larger, micron-sized complexes at subphysiological temperatures.
Fusion Protein Resolubilization
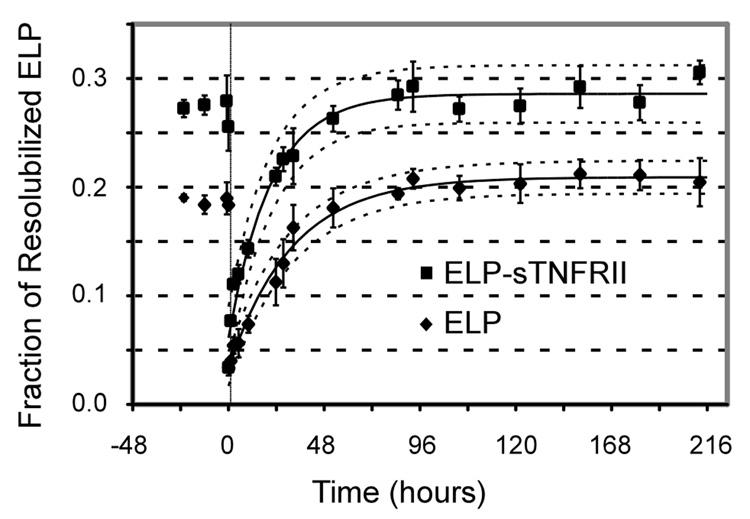
The time-dependent release of ELP-sTNFRII fusion proteins and the non-fusion ELP controls from the coacervate phase into fresh PBS supernatant is shown in Figure 2. Steady-state mass fraction of non-fusion ELP (xss) was determined to be 20.9 ± 0.3% with a time constant of 31 ± 3 h. The ELP-sTNFRII fusion protein resolubilized to a greater extent (28.6 ± 0.6%, p < 0.01) and with a shorter time constant (21 ± 3 h, p < 0.01) compared to non-fusion ELP. This observation likely results from the hydrophilic sTNFRII domain within the ELP-sTNFRII fusion protein that would promote favorable thermodynamics of solvation during the resolubilization process.
Figure 2.
ELP (♦) and ELP-sTNFRII (■) proteins resolubilize into warm PBS supernatant reaching a steady-state thermodynamic equilibrium. Data were fit to an increasing monoexponential (Equation 1) with coefficients of determination, r2, exceeding 0.98 for both datasets. The extent of protein release into the supernatant was greater for the fusion protein than for the free ELP (p < 0.05). Furthermore, ELP-sTNFRII resolubilizes more rapidly than the non-fusion ELP (p < 0.05).
In Vitro Fusion Protein Refolding
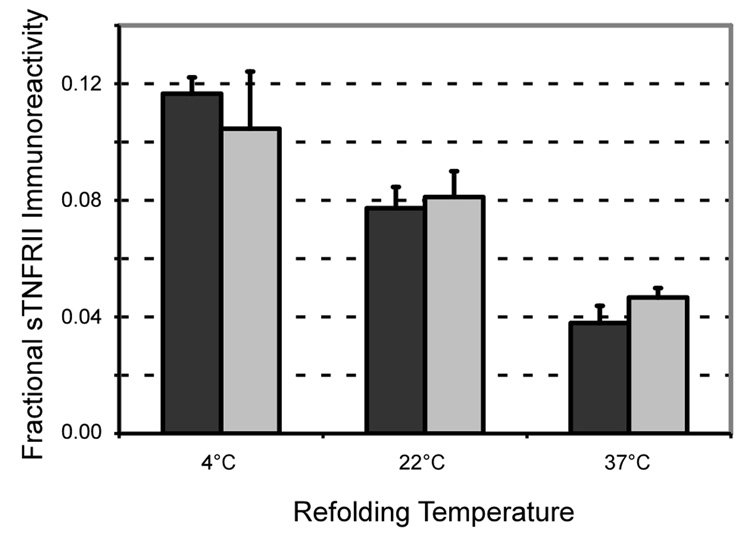
The optimal temperature for fusion protein refolding was found to be 4°C (Figure 3) with duration benefits observed up to 120 hours (data not shown). The nature of the oxidation-reduction buffer was also important, with the depicted experiments conducted under ideal refolding conditions involving a 4:1 ratio of reduced to oxidized glutathione. Other attempts to enhance refolding such as serially diluting the unfold mixture to the target concentration, including adjuvants such as polyethylene glycol, arginine, or proline, or changing concentrations of the oxidation-reduction buffer components did not prove to be of benefit (data not shown). Using the described conditions, we observed 25 ± 3% immunoreactivity in the refolded ELP-sTNFRII compared to an equimolar quantity of commercial sTNFRII.
Figure 3.
The temperature at which ELP-sTNFRII refolding was performed impacted upon yield, with the optimal temperature observed to be 4°C (ANOVA, p < 0.05). No difference was noted in the absence (black) or presence (grey) of protein disulfide isomerase (ANOVA, p = 0.86).
Fusion Protein Precipitation
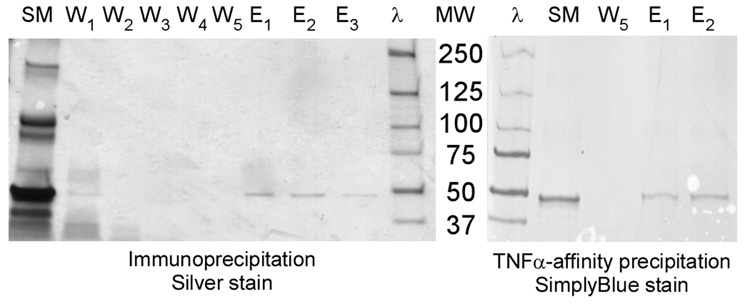
Immunoprecipitation successfully isolated a protein of 44 kDa molecular weight assessed by SDS-PAGE using silver staining (Figure 4). There was evidence of contaminant proteins in low concentrations in the ITC purified material as evidenced by weak but multiple bands present in the first washes of the immunoprecipitation column. These proteins were not seen on the ITC purification SDS-PAGE stained by SimplyBlue™; and, as expected, the immunoprecipitation effectively eliminated this contamination in five wash steps. Similar precipitation of a single 44 kDa molecular weight protein was observed by the TNFα-affinity purification (Figure 4).
Figure 4.
SDS-PAGE of successful ELP-sTNFRII immunoprecipitation (left) and TNFα-affinity precipitation (right) yielding a 44 kDa protein. Depicted for the immunoprecipitation are starting material (SM), washing steps (W1–5), elutions (E1–3), and molecular weight ladder (λ). Silver staining reveals several contaminant proteins in the starting material that are effectively eliminated during the immunoprecipitation. Depicted for the TNFα-affinity precipitation are the molecular weight ladder (λ), starting material (SM), the fifth final wash step, and the first two eluted fractions. The lower sensitivity SimplyBlue™ staining does not reveal the same contaminant proteins as evident from silver staining.
TNFα Binding Affinity of ELP-sTNFRII
After ITC purification alone, fusion protein binding to TNFα (Table 1) showed the equilibrium dissociation constant (KD) to be 153-fold greater than that of commercial sTNFRII in the SPR analysis (Dunn’s test, α = 0.05). The observed KD for the commercial sTNFRII protein of 0.47 ± 0.12 nM compares favorably with literature reports of the full length TNFRII protein KD of 0.35 nM.[16] Following in vitro refolding, KD was improved to 35-fold greater than sTNFRII. Immunoprecipitation further enhanced molecular affinity for the TNFα target with an observed KD of 3.5 ± 0.3 nM, only 7-fold higher than the commercial antagonist. Ligand affinity chromatography against TNFα further enhanced this purification with a KD of 2.1 ± 0.9 nM.
Table 1.
Surface Plasmon Resonance Data for Antagonist Binding to Immobilized TNFα
| Antagonist | kon (104 M−1 s−1) | koff (10−4 s−1) | KD (nM) | KD/KD,sTNFRII |
|---|---|---|---|---|
| sTNFRII | 4.5 ± 0.5 | 0.22 ± 0.08 | 0.47 ± 0.12 | 1 |
| ELP-sTNFRII (ITC purified) | 1.4 ± 0.6 | 10 ± 6* | 73 ± 12* | 153 |
| ELP-sTNFRII (Unfolded) | 0.12 ± 0.08 | 5 ± 2* | 470 ± 180* | 989 |
| ELP-sTNFRII (refold mixture) | 0.7 ± 0.4 | 1.1 ± 0.4* | 17 ± 8* | 35 |
| ELP-sTNFRII (immunoprecipitated) | 4.4 ± 1.8 | 1.6 ± 0.7* | 3.5 ± 0.3* | 7 |
| ELP-sTNFRII (TNFα-affinity purified) | 4.6 ± 1.8 | 0.9 ± 0.2* | 2.1 ± 0.9* | 4.5 |
| ELP | 0.013 ± 0.018# | 42 ± 39 | 7400 ± 400# | 150000 |
kon – association rate constant
koff – dissociation rate constant
KD – equilibrium dissociation constant
values for ELP-sTNFRII are significantly different from values for sTNFRII at p < 0.05
values for ELP are significantly different from values for both sTNFRII and ELP-sTNFRII at p < 0.05
The dissociation rate constant for the receptor-ligand complex (koff) was faster for purified ELP-sTNFRII, although no difference was observed between the association rate constants (kon) among all antagonist preparations (Dunn’s test, α = 0.05). The equivalency of association for commercial sTNFRII and the immunoprecipitated ELP-sTNFRII fusion protein suggests that the ELP tag does not significantly hinder association with immobilized TNFα. However, the faster observed dissociation and higher equilibrium dissociation constant compared to the commercial antagonist may reflect a destabilizing steric effect or conformational change in the fusion protein following ligand binding. A similar pattern of similar kon and faster koff was observed for material purified by TNFα-affinity precipitation, although the more favorable koff confirms the greater purity conferred by TNFα-mediated purification than antibody-mediated precipitation. Affinity for the immobilized TNFα cannot be attributed to the ELP domain, with non-fusion ELP having a KD in this study that was 150000-fold greater than the commercial antagonist.
Protein Safety
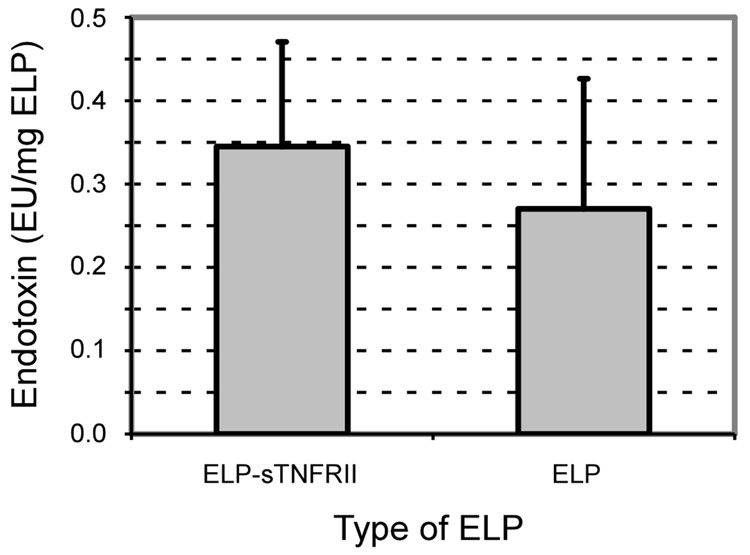
Non-fusion ELP and ELP-sTNFRII had endotoxin contents of 0.27 ± 0.16 EU/mg and 0.35 ± 0.13 EU/mg respectively (Figure 5). These values and are slightly higher than reported values of less than 0.2 EU/mg ELP following five rounds of ITC purification.[46] These remain exceptionally low results when compared with other techniques of endotoxin removal such as polymyxin B or histidine chromatographic affinity purification with reported levels of 250 EU/mg for creatine kinase, myoglobin, and cardiac troponin I.[47] However, validity of this claim can only be fully assessed after determining the clinically-necessary doses.
Figure 5.
Thermal purification of both ELP and ELP-sTNFRII by inverse thermal cycling effectively removes endotoxin from these recombinant proteins. The endotoxin content of ELP-sTNFRII is 0.35 ± 0.13 EU/mg and that of non-fusion ELP is 0.27 ± 0.16 EU/mg.
Anti-TNFα Activity of Fusion Protein using Murine L929 Fibrosarcoma Cells
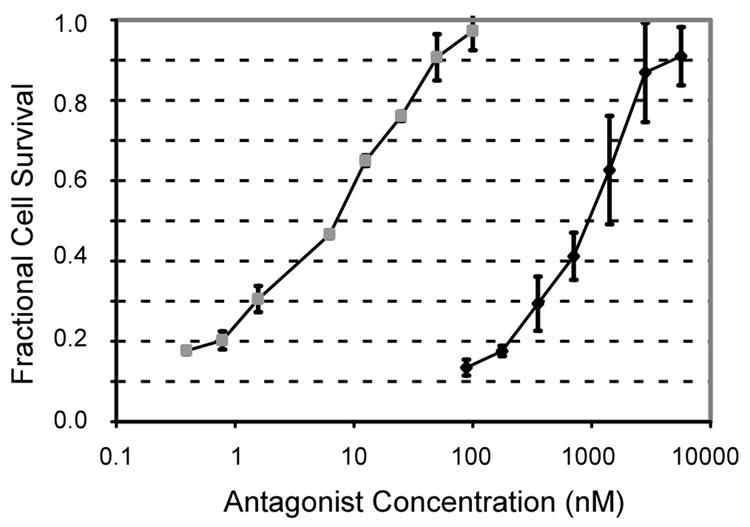
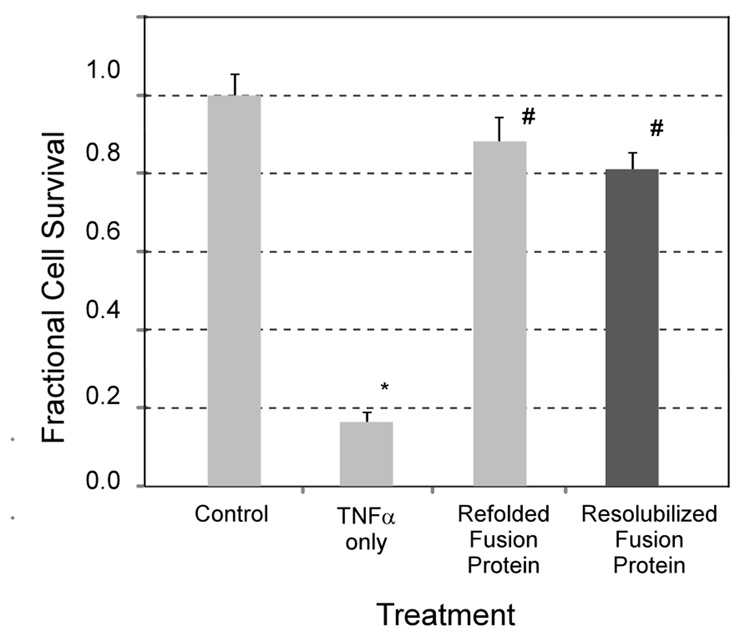
Figure 6 shows bioactivity for sTNFRII and ELP-sTNFRII against TNFα-mediated L929 cell cytotoxicity. The commercial antagonist had an IC50 of 12 ± 4 nM, and the fusion protein was less active with an IC50 of 1200 ± 500 nM (p < 0.01). This observation may reflect association of ELP-sTNFRII at 37°C into supramolecular particles from which only the soluble fraction of monomers are bioactive. Alternately, the ELP tag may destabilize the fusion protein interaction with TNFα, as evidenced by the higher KD from the SPR experiments, thereby requiring a higher dose to treat TNFα activity. Further, the supernatant fraction of ELP-sTNFRII from the resolubilization experiment has in vitro bioactivity equivalent to protein that was not processed as such (Figure 7), suggesting the lower potency is due to lower activity of the monomer.
Figure 6.
In vitro bioactivity of ELP-sTNFRII (♦) against TNFα-mediated L929 cytotoxicity has an IC50 of 1200 ± 500 nM. This is significantly reduced (p < 0.01) compared with commercial sTNFRII ( ) with an IC50 of 12 ± 4 nM. Coefficients of determination, r2, for fitting to a dose-response curve (Equation 2) ranged from 0.94 to 0.99.
) with an IC50 of 12 ± 4 nM. Coefficients of determination, r2, for fitting to a dose-response curve (Equation 2) ranged from 0.94 to 0.99.
Figure 7.
The supernatant ELP-sTNFRII fractions obtained from the fusion protein resolubilization experiment demonstrate in vitro bioactivity (#, p < 0.01) against TNFα-mediated L929 cytotoxicity (0.25 ng/mL TNFα, *, p < 0.01). The extent of cytoprotection was equivalent to that of ELP-sTNFRII that was not put through the depot-formation and resolubilization experiment.
Discussion
A fusion protein of ELP and sTNFRII was designed towards the goal of developing an in situ forming depot for localized anti-TNF therapy. This approach maintained both phase transition behavior as well as activity for the drug domain, consistent with results for other ELP-drug conjugates.[32, 33] Advantages of this system include acceptable expression levels of chimeric proteins with high drug-to-carrier ratios compared with chemical conjugation.[48–50] Purification of genetically engineered ELPs generates proteins of high purity and low endotoxin levels that have been demonstrated to be non-immunogenic, non-pyrogenic, and biocompatible, with endogenous amino acid degradation products.[51] It is unclear if ELP conjugates with small molecule drugs or fusions with bioactive protein domains will be equally biocompatible. Such investigation is important for this technology to be applied in an animal disease model. Genetic engineering also affords optimization of ELP sequences for desired behavior by precise control of stereochemistry, amino acid sequence, chain length, and transition temperature.[48]
Preservation of the thermally-triggered phase transition and resolubilization behaviors for the fusion protein is prerequisite for this drug delivery concept. A novel protein was expressed with sTNFRII fused to the C-terminus of a thermally-responsive ELP domain. The fusion protein retained ELP domain thermal sensitivity, with increased solution turbidity and hydrodynamic radii upon heating above 32° (Figure 1). Functionality of this domain was further evaluated by studying fusion protein resolubilization from an in vitro depot. After formation of a stable depot, monomer or small particle release was driven by disruption of thermodynamic equilibrium by replacing supernatant with warm PBS (Figure 2). The resulting association and resolubilization of monomers in dilute supernatant was assumed to occur by a first-order process, although multiparticle interactions in the supernatant are possible. More extensive resolubilization was observed for ELP-sTNFRII than ELP alone, likely due to the intrinsic sTNFRII domain solubility that cannot partake in the hydrophobic association. While the results for steady-state concentrations suggest that 70% of the available ELP is retained in multimeric particle form, ELPs are cleared from the perineural space with a half-life of 39 hours[25] and the joint space with a half-life of 3.4 days,[24] demonstrating that the retained ELP fraction will be removed in vivo. Previous work investigating incubation of tritium-labeled ELPs in rat serum demonstrates degradation at 0.33% per day to low molecular weight fragments.[25] Together, these findings suggest that serum and in vivo application do not interfere with the delivery mechanism but may result in resolubilization rates that are incompletely represented by in vitro results.
The sTNFRII protein is intricately folded with 24 cysteine residues in 12 disulfide bonds.[21] Appropriate conformation is required for TNFα ligand interaction,[16] and previous authors describing TNFα receptor expression in E. coli used in vitro refolding with chromatographic isolation of appropriate foldamers.[14, 22] Conventional refolding adjuvants including arginine, proline, and polyethylene glycol did not enhance yield, results consistent with work by Merli and coworkers[22] in their expression of sTNFRI. The optimal refolding temperature of 4°C (Figure 3) may reflect the transition at higher temperatures promoting molecular proximity and intermolecular disulfide bond formation as a competing process. The optimally-refolded material demonstrated 25 ± 3% immunoreactivity of the commercial sTNFRII molecule, in line with the 21% reported in the aforementioned study. This suggests that the ELP fusion tag is not deleterious to refolding, but certainly the ELP phase transition behavior may affect fusion domain folding during the initial protein expression in E. coli. We eliminated such effects by beginning the refolding process with fully denatured protein. The use of glutathione during refolding may contribute to the fraction of non-immunoreactive material. Further, misfolded material with non-native disulfide bridges would not be expected to achieve immunoreactivity. Consequently, the purity benefits of immunoprecipitation or TNFα-affinity precipitation are required for application to in vivo animal or clinical settings. Chromatographic purification of this mixture yielded a protein with heightened TNFα affinity in receptor-ligand binding studies.
The ELP-sTNFRII molecule also retained functionality for the sTNFRII domain. First, fusion protein binding to immobilized TNFα was observed with kon values comparable to commercially available sTNFRII (Table 1), a finding that suggests that accessibility of ELP-sTNFRII to immobilized TNFα is not impaired by the ELP domain. However, the higher dissociation rate (koff) observed for the fusion protein compared to the commercial antagonist may reflect a conformational change in the fusion protein following ligand binding. It is known that the hydrophilicity of the conjugated domain in ELP-based fusion proteins impacts on the molecule’s transitioning characteristics.[35] It is possible that shielding of the hydrophilic TNFα-binding site on the sTNFRII domain could induce a change in ELP conformation destabilizing the receptor-ligand interaction and leading to a slight increase in dissociation rate. Potential methods to decrease this steric effect could include changing the amino acid sequence or size of the ELP domain to still exhibit thermal sensitivity while permitting access to the therapeutic domain, or by introducing a linker peptide that does not participate in the hydrophobic association process.
In vitro bioactivity was demonstrated by the fusion protein antagonizing TNFα-mediated cytotoxicity of murine L929 fibrosarcoma cells, although requiring higher doses than the commercial antagonist (Figure 6). The higher IC50 probably reflects that ELP-sTNFRII expectedly transitions into supramolecular complexes and only the soluble fraction of monomers exhibits bioactivity. Proteolytic, lymphatic, and vascular clearance of this fraction will drive further resolubilization and sustain the presence of the therapeutic agent. It is unlikely that the ELP domain contributes anticytokine activity, and no specific interaction was observed for non-fusion ELP to immobilized TNFα in the SPR experiments Further, Mecham and co-workers[52] have shown that a variety of hydrophobic sequences, including the repeat sequence VPGVG used here, do not interact with cell-surface elastin receptors. The higher fusion protein KD and IC50 compared against commercial sTNFRII is a trend for other fusion proteins.[33, 53] The necessary KD or IC50 required for this protein to achieve efficacy in the drug delivery concept proposed here depends also on other features of the in vivo system, such as clearance time and mechanism from the targeted space, total amount of protein delivered, and resolubilization kinetics. Caution is important in directly comparing against the commercial sTNFRII because the gene sequence and technique for synthesis and purification are quite different. The nature of these differences and how they impact direct comparison is not well understood.
Previous studies have demonstrated the feasibility of local intra-articular[24] and perineural[25] delivery of depot-forming ELPs, with the findings that this behavior prolonged target compartment residence time. Furthermore, both studies also demonstrated that peak serum exposure of the depot-forming carrier was less than for a similar molecular weight, soluble polypentapeptide sequence. There was negligible protein accumulation in the peripheral organs. Indeed, the balance of competing digestion and distribution will ultimately govern the necessary dose to treat local inflammatory disease.
In summary, this study describes the expression of a recombinant fusion protein between thermally-responsive ELP and the sTNFRII protein therapeutic of complex tertiary structure. Conjugation of the thermally-responsive ELP tag with sTNFRII is expected to yield high doses upon local injection, to provide sustained release, and to lower systemic exposure to reduce systemic side-effects.[25] While Urry and co-workers[51] have described biocompatibility and non-immunogenicity for ELPs, the immunogenicity of the chimeric fusion protein remains unknown and would require in vivo testing which was beyond the scope of the present study. Further, it is unclear how the reticuloendothelial system will respond to such multimeric particles formed by hydrophobic association. Nonspecific uptake of the complex or receptor-mediated uptake of the fusion protein may accelerate clearance, or the hydrophobic core surrounded by a hydrophilic corona in a micellar structure could inhibit clearance. Such effects are likely to be protein specific and will also require in vivo testing. Nevertheless, we show the feasibility of expressing a recombinant fusion protein between thermally-responsive ELP with a complex bioactive protein. These in vitro experiments are requisite to demonstrate bidomain functionality for the fusion protein prior to undertaking evaluation of the anti-TNFα therapeutic in a specific body site. Lower drug domain bioactivity for the fusion protein likely results from suboptimal protein folding and steric hinderance of receptor-ligand association, and may be tolerable in therapeutic application if sustained release from the drug depot prolongs the presence of therapeutic doses. Future studies will involve determining if slowed release of therapeutic fusion proteins will effectively treat local inflammation in animal models of cytokine-mediated disease.
Acknowledgements
This work was funded by NIH R01EB002263 (LAS), NIH R21AR052745 (LAS), NIH R01GM061232 (AC), and a Pratt-Gardner Predoctoral Research Fellowship (MFS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dayer JM. The saga of the discovery of IL-1 and TNF and their specific inhibitors in the pathogenesis and treatment of rheumatoid arthritis. Joint Bone Spine. 2002;69(2):123–132. doi: 10.1016/s1297-319x(02)00363-9. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nature medicine. 2003;9(10):1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 3.Maini RN, Elliott M, Brennan FM, Williams RO, Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. Apmis. 1997;105(4):257–263. doi: 10.1111/j.1699-0463.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 4.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory bowel diseases. 2006;12 Suppl 1:S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 5.Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Buschenfelde KH, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. European journal of immunology. 1997;27(7):1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 7.Remick DG, Kunkel RG, Larrick JW, Kunkel SL. Acute in vivo effects of human recombinant tumor necrosis factor. Laboratory investigation; a journal of technical methods and pathology. 1987;56(6):583–590. [PubMed] [Google Scholar]
- 8.Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 9.Igarashi T, Kikuchi S, Shubayev V, Myers RR. Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25(23):2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21(2):218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30(1):44–53. doi: 10.1097/01.brs.0000149186.63457.20. discussion 54. [DOI] [PubMed] [Google Scholar]
- 12.Barbara JA, Smith WB, Gamble JR, Van Ostade X, Vandenabeele P, Tavernier J, Fiers W, Vadas MA, Lopez AF. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. Embo J. 1994;13(4):843–850. doi: 10.1002/j.1460-2075.1994.tb06327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelmann H, Novick D, Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990;265(3):1531–1536. [PubMed] [Google Scholar]
- 14.Hale KK, Smith CG, Baker SL, Vanderslice RW, Squires CH, Gleason TM, Tucker KK, Kohno T, Russell DA. Multifunctional regulation of the biological effects of TNF-alpha by the soluble type I and type II TNF receptors. Cytokine. 1995;7(1):26–38. doi: 10.1006/cyto.1995.1004. [DOI] [PubMed] [Google Scholar]
- 15.Fu ZQ, Harrison RW, Reed C, Wu J, Xue YN, Chen MJ, Weber IT. Model complexes of tumor necrosis factor-alpha with receptors R1 and R2. Protein Eng. 1995;8(12):1233–1241. doi: 10.1093/protein/8.12.1233. [DOI] [PubMed] [Google Scholar]
- 16.Chen PC, DuBois GC, Chen MJ. Mapping the domain(s) critical for the binding of human tumor necrosis factor-alpha to its two receptors. J Biol Chem. 1995;270(6):2874–2878. doi: 10.1074/jbc.270.6.2874. [DOI] [PubMed] [Google Scholar]
- 17.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J Exp Med. 1991;174(6):1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheinfeld N. A comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumab. J Dermatolog Treat. 2004;15(5):280–294. doi: 10.1080/09546630410017275. [DOI] [PubMed] [Google Scholar]
- 19.Solorzano CC, Kaibara A, Hess PJ, Edwards PD, Ksontini R, Abouhamze A, McDaniel S, Frazier J, Trujillo D, Kieft G, Seely J, Kohno T, Cosenza ME, Clare-Salzler M, MacKay SL, Martin SW, Moldawer LL, Edwards CK., 3rd Pharmacokinetics, immunogenicity, and efficacy of dimeric TNFR binding proteins in healthy and bacteremic baboon. J Appl Physiol. 1998;84(4):1119–1130. doi: 10.1152/jappl.1998.84.4.1119. [DOI] [PubMed] [Google Scholar]
- 20.Brisby H, Olmarker K, Larsson K, Nutu M, Rydevik B. Proinflammatory cytokines in cerebrospinal fluid and serum in patients with disc herniation and sciatica. Eur Spine J. 2002;11(1):62–66. doi: 10.1007/s005860100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc Res Tech. 2000;50(3):184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Merli S, Corti A, Cassani G. Production of soluble tumor necrosis factor receptor type I in Escherichia coli: optimization of the refolding yields by a microtiter dilution assay. Anal Biochem. 1995;230(1):85–91. doi: 10.1006/abio.1995.1441. [DOI] [PubMed] [Google Scholar]
- 23.Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, Wong GH, Gatanaga T, Granger GA, Lentz R, Raab H, et al. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- 24.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115(2):175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Shamji MF, Whitlatch L, Friedman AH, Richardson WJ, Chilkoti A, Setton LA. An injectable and in situ-gelling biopolymer for sustained drug release following perineural administration. Spine. 2008;33(7) doi: 10.1097/BRS.0b013e3181695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91(1–2):31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 27.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. Journal of Physical Chemistry B. 1997;101(51):11007–11028. [Google Scholar]
- 28.Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia. Cancer Res. 2001;61(4):1548–1554. [PubMed] [Google Scholar]
- 29.Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. J Control Release. 2001;74(1–3):213–224. doi: 10.1016/s0168-3659(01)00319-4. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Dreher MR, Chow DC, Zalutsky MR, Chilkoti A. Tracking the in vivo fate of recombinant polypeptides by isotopic labeling. J Control Release. 2006;114(2):184–192. doi: 10.1016/j.jconrel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Massodi I, Bidwell GL, 3rd, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release. 2005;108(2–3):396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Bidwell GL, 3rd, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4(7):1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 33.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56(11):3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 34.Trabbic-Carlson K, Liu L, Kim B, Chilkoti A. Expression and purification of recombinant proteins from Escherichia coli: Comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 2004;13(12):3274–3284. doi: 10.1110/ps.04931604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabbic-Carlson K, Meyer DE, Liu L, Piervincenzi R, Nath N, LaBean T, Chilkoti A. Effect of protein fusion on the transition temperature of an environmentally responsive elastin-like polypeptide: a role for surface hydrophobicity? Protein Eng Des Sel. 2004;17(1):57–66. doi: 10.1093/protein/gzh006. [DOI] [PubMed] [Google Scholar]
- 36.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5(2):497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 37.Herrero-Vanrell R, Rincon AC, Alonso M, Reboto V, Molina-Martinez IT, Rodriguez-Cabello JC. Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. J Control Release. 2005;102(1):113–122. doi: 10.1016/j.jconrel.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Simnick AJ, Lim DW, Chow DC, Chilkoti A. Biomedical and Biotechnological Applications of Elastin-Like Polypeptides. Journal of Macromolecular Science, Part C: Polymer Reviews. 2007;47:121–154. [Google Scholar]
- 39.Daniell H, Guda C, McPherson DT, Zhang X, Xu J, Urry DW. Hyperexpression of a synthetic protein-based polymer gene. Methods Mol Biol. 1997;63:359–371. doi: 10.1385/0-89603-481-X:359. [DOI] [PubMed] [Google Scholar]
- 40.McPherson DT, Xu J, Urry DW. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr Purif. 1996;7(1):51–57. doi: 10.1006/prep.1996.0008. [DOI] [PubMed] [Google Scholar]
- 41.Gill S, von Hippel P. Calculation of protein extinction coefficients from amino acid sequence data. Analytical Biochemistry. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- 42.Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63(9):1120–1123. doi: 10.1136/ard.2003.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johns Hopkins University, Efficacy of Epidural Etanercept in the Treatment of Sciatica. Bethesda (MD): National Library of Medicine (US); ClinicalTrials.gov [Internet] 2006 present. Available from http://www.clinicaltrials.gov/show/NCT00364572.
- 44.Meager A, Leung H, Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- 45.Tomkins P, Cooper K, Webber D, Bowen G. The L929 cell bioassay for murine tumour necrosis factor is not influenced by other murine cytokines. J Immunol Methods. 1992;151(1–2):313–315. doi: 10.1016/0022-1759(92)90133-e. [DOI] [PubMed] [Google Scholar]
- 46.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11(11–12):1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Tobias R, McClure S, Styba G, Shi Q, Jackowski G. Removal of endotoxin from recombinant protein preparations. Clin Biochem. 1997;30(6):455–463. doi: 10.1016/s0009-9120(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 48.Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Deliv Rev. 2002;54(8):1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 49.Chow DC, Dreher MR, Trabbic-Carlson K, Chilkoti A. Ultra-high expression of a thermally responsive recombinant fusion protein in E. coli. Biotechnol Prog. 2006;22(3):638–646. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Cabello J, Reguera J, Girotti A, Alonso M, Testera A. Developing functionality in elastin-like polymers by increasing their molecular complexity: the power of the genetic engineering approach. Progress in Polymer Science. 2005;30:1119–1145. [Google Scholar]
- 51.Urry DW, Parker TM, Reid MC, Gowda DC. Biocompatibility of the bioelastic materials, Poly(GVGVP) and its gamma-irradiation cross-linked matrix: summary of generic biological test results. Journal of Bioactive and Compatible Polymers (USA) 1991;6(3):263–282. [Google Scholar]
- 52.Mecham RP, Hinek A, Griffin GL, Senior RM, Liotta LA. The elastin receptor shows structural and functional similarities to the 67-kDa tumor cell laminin receptor. J Biol Chem. 1989;264(28):16652–16657. [PubMed] [Google Scholar]
- 53.Kim DH, Smith JT, Chilkoti A, Reichert WM. The effect of covalently immobilized rhIL-1ra-ELP fusion protein on the inflammatory profile of LPS-stimulated human monocytes. Biomaterials. 2007;28(23):3369–3377. doi: 10.1016/j.biomaterials.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]