Abstract
Objectives
Intravascular thrombosis remains a major barrier to successful pig-to-primate xenotransplantation. However, the precise factors initiating thrombosis are unknown. In this study, we investigated the contribution of recipient platelets and monocytes.
Methods
Primary pig aortic endothelial cells (PAEC) were incubated with combinations of fresh or heat-inactivated (HI) human plasma, platelets, or monocytes, following which they were separated and analysed individually by flow cytometry for tissue factor (TF) expression and for their ability to clot recalcified normal or FVII-deficient plasma.
Results
Procoagulant porcine TF was induced on PAEC only by fresh human plasma, not HI plasma, platelets or monocytes. In contrast, procoagulant human TF was induced on platelets and monocytes after incubation with PAEC, irrespective or whether plasma was present on not. In addition, human platelets caused the shedding of procoagulant TF-expressing aggregates from PAEC.
Conclusions
This work defines a cell-based in vitro assay system to address complex interactions between PAEC, human platelets and monocytes. The induction of procoagulant TF on PAEC by fresh human plasma was most likely dependent on xenoreactive natural antibody and complement present in fresh human plasma. In contrast, the shedding of procoagulant platelet-PAEC aggregates, induced by human platelets, and the induction of procoagulant TF on human platelets and monocytes by PAEC, occurred independently of these factors. These results suggest that different mechanisms may contribute to the initiation of thrombosis after xenotransplantation, some of which may not be influenced by further manipulation of the immune response against pig xenografts.
Keywords: Coagulation, Monocytes, Platelets, Tissue factor, Xenotransplantation
INTRODUCTION
Xenotransplantation promises an unlimited supply of organs for clinical use. Pigs are thought to be the most suitable source of xenografts (1, 2). However, the antibody-mediated immunologic barrier between primates and pigs hinders the success of xenotransplantation. Several strategies have been developed to overcome hyperacute rejection and prolong graft survival (2). Nonetheless, acute humoral xenograft rejection (AHXR) ensues and leads to intravascular thrombosis.
For example, transplanting hearts from α1,3-galactosyltransferese knock-out pigs (3) into baboons prolonged median survival to 78 days, but eventually all grafts succumbed to ischemic necrosis from thrombotic microangiopathy (TM) (4, 5). Nevertheless, the pathology in these grafts was different from typical AHXR and revealed microvascular thrombosis in arterioles, capillaries, and venules, with only rare interstitial mononuclear cells. Whether these changes resulted from low-grade humoral rejection or non-immunologic factors, such as coagulation dysregulation, remains uncertain.
Tissue factor (TF) binds factor VII/factor VIIa (FVII/VIIa), and the complex TF-FVIIa activates FX and FIX to initiate coagulation (6, 7). Endothelial cells (EC) and monocytes constitute the main origins of TF, as shown in inflammation and sepsis models (8, 9). Microparticles shed from EC, or monocytes, are the main source of circulating TF, and transfer TF to platelets (10, 11). Recently, platelets have been shown to be capable of synthesizing and expressing functional TF (12).
The importance of TF as the initiator of thrombosis after xenotransplantation has not been formally studied. In vivo studies demonstrated that expression of TF was up-regulated in necrotic xenografts (13, 14). The expression of TF on PAEC was up-regulated by activated platelets or complement by xenogeneic antibodies (15, 16). These studies suggested TF as an initiator of xenograft thrombosis.
The importance of other proteins, such as the fibrinogen-like protein-2 (fgl-2), remains to be demonstrated. Grafts from fgl-2-defiicient mice are largely resistant to thrombosis when transplanted into rats, but, in the same model, overexpressing human tissue factor pathway inhibitor within the transplanted heart can completely inhibit intragraft thrombosis, suggesting that TF might be the primary initiator (17, 18)
However, the origins of TF and the interaction between porcine aortic endothelial cells (PAEC), human monocytes and platelets are not fully understood. In this study, we developed an in vitro model to attempt to elucidate the interactions between PAEC and human monocytes and platelets in terms of expression of TF, and we attempted to demonstrate that TM is initiated by TF.
MATERIALS AND METHODS
In vitro model system
PAEC or HAEC adherent to a culture flask were pre-incubated for 8h with fresh or heatinactivated (HI) human plasma (HP) (5%), human platelets (5×107/ml), monocytes (5×105/ml), or combinations of all three. Five percent (5%) HP was selected because this concentration resulted in near-saturation of IgG and IgM binding to PAEC by flowcytometry, and caused <10% complement-dependent cytotoxicity (CDC) (data not shown). HP, human platelets and monocytes were isolated from blood type A volunteer donors to minimize the effect of ABO-incompatibility. After coculture, human monocytes or platelets were collected from supernatants, and PAEC were harvested by prewarmed 0.5% trypsin (Gibco, Paisley, UK) at 37°C for flow cytometry and recalcified clotting assay analysis, respectively.
Cell culture
PAEC were isolated from fresh aortae and were maintained in 2% gelatin-coated tissue culture flasks in RPMI 1640 (Gibco) supplemented with 10% fetal calf serum (Globepharm, Surrey, UK), penicillin (50 units/ml) streptomycin (50µg/ml) and L-glutamine (2mM) at 37°C in 5% CO2. For all experiments, PAEC and HAEC of less than 6 passages were used. HAEC (as an allograft control) and a human breast cancer cell line (ZR-75-1; as a positive allograft control for the expression of human TF) were cultured in EBM-2 medium ( Lonza, Walkersville, MD) under the same conditions (19).
Preparation of human platelets
Blood type A platelet-rich HP was obtained from blood by centrifugationat 80g for 10min, followed by dilution at 1:20 with 1% ammoniumoxalate and 2.5mM Gly-Pro-Arg-Pro peptide (Sigma, St Louis, MO). Samples were placed in a counting chamber in a moist Petri dish, and the platelets in 1mm2 counted (= N). The number of platelets per liter of blood equaled 2N × 109. Platelet phenotypewas confirmed by flow cytometric analysis with an anti-CD41 monoclonal antibody (Serotec, Oxford, UK).
Human plasma and monocyte preparations
Human blood was drawn from blood type A volunteers with heparin. HP was collected and HI-HP was prepared after heating at 56°C for 30min. Peripheral blood mononuclear cells (PBMC) were prepared by Ficoll-Hypaque density gradient (AppliChem GmbH, Darmstadt, Germany). Monocytes were positively selected from PBMC by using anti-human CD14 magnetic beads (Miltenyi Biotec, Auburn, CA).
Flow cytometry analysis
PAEC, HAEC, human monocytes and platelets were harvested and washed with PBS/1% bovine serum albumin (BSA) before incubation with primary antibody or control IgG antibody, and incubated on ice for 30min. After washing x3 with PBS/1%BSA, the cells were then incubated on ice with suitable FITC-conjugated IgG for a further 30min, before three more washes and analysis on an EPICS XL flow cytometer (Coulter, Luton, UK). The following antibodies were used; monoclonal mouse anti-pig CD106 (clone 10.2C7, a generous gift from Professor D.O. Haskard, Imperial College London), anti-human CD41 (Serotec), antiporcine CD31 (Antigenix, Huntington Station, NY), and polyclonal sheep-anti human TF (Affinity Biologicals, Ancaster, ON, Canada). Mouse IgG1 and IgG2b (Pharmingen, San Diego, CA) and sheep IgG (Affinity Biologicals) were used as isotype controls. Goat anti-mouse and donkey anti-sheep conjugated antibodies (Sigma) were used for second-layer staining.
Human plasma recalcification assay
The appropriate number of PAEC (1× 105), human monocytes (1× 105), and platelets (1× 107) were suspended in 50µl Tris-buffered saline and mixed with 100µl of normal human plasma (Sigma) in glass tubes (Corning, Corning, NY). Ten (10) µl of 250mM CaCl2 in Tris-buffered saline combined with 90µl phospholipids (Diagnostic Reagents, Oxford, UK) were added, and the tube incubated at 37°C in a water bath; the time for a fibrin clot to form was determined in triplicate, during which time the tubes were continuously agitated by tilting. In these assays, TF-dependent thrombin generation requires the participation of FVIIa, and therefore FVII-deficient plasma (Diagnostic Reagents) was used in a separate assay to determine whether clotting was TF-dependent. In other assays, cells or platelets were treated with an anti-human TF antibody (1mg/ml) for 30min at 4°C before determining clotting times.
Apoptosis detection assay
PAEC were assessed for early apoptosis by evaluating phosphatidylserine exposure on the cell surface using annexin V-FITC (BioVision, Mountain View, CA). Late-stage apoptosis associated with compromised membrane permeability was measured by propidium iodide (PI) staining by flow cytometry. The results were basedon the percentage of total gated 104 cells.
Immunofluorescence
Platelets were isolated after incubation with PAEC, and double-stained with anti-TF and anti-CD106 antibodies, followed by FITC-conjugated donkey anti-sheep and TRITC-conjugated goat anti-mouse antibodies (Sigma). The slides were examined under an immunofluorescence microscope (Axiovert S100 TV; Zeiss, Welwyn Garden City, UK). Images were analyzed using the MetaMorph system (Universal Imaging, Downingtown, PA).
Statistical analysis
Data are presented as mean ± SEM. Significance of the difference between two groups was determined by paired Student's t-test. Values of P<0.05 were considered significant.
RESULTS
The characteristics of sheep anti-human TF antibody
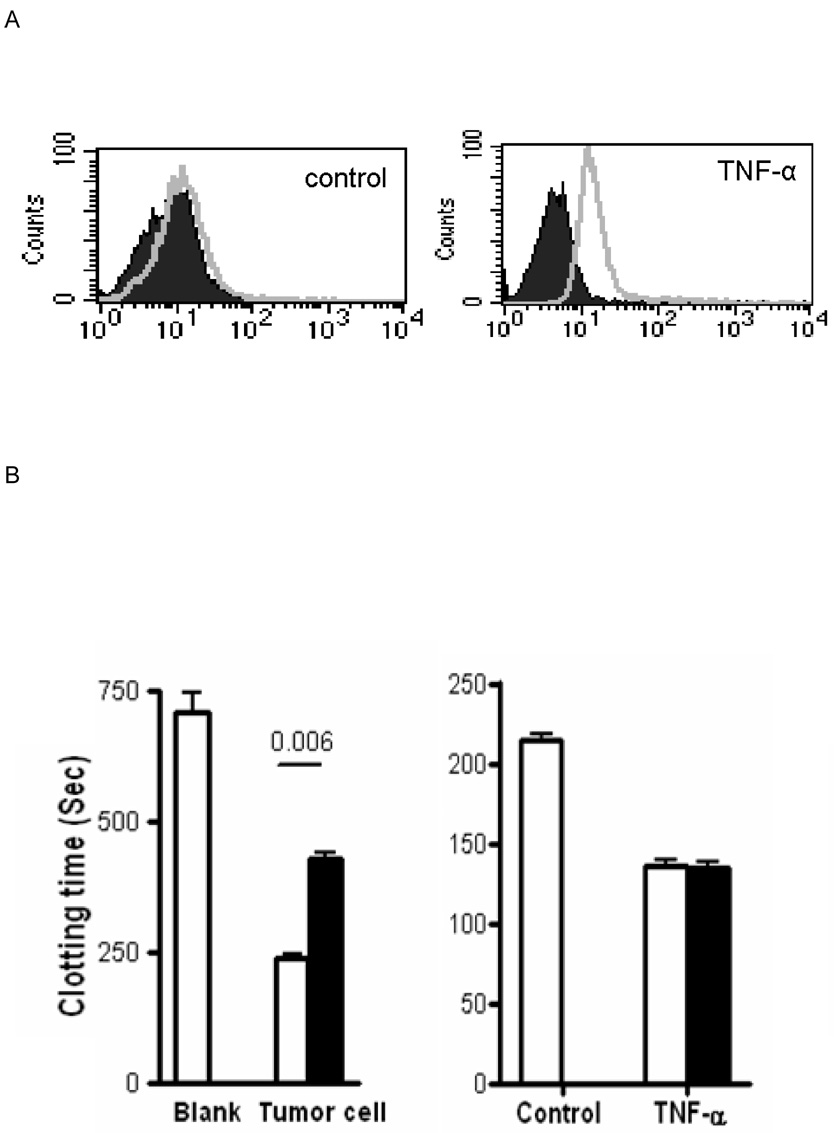
Up-regulation of cell surface TF expression was detected on PAEC after activation by TNF-α by FACS analysis using the sheep anti-human TF antibody (Figure 1A). These cells promoted shorter clotting times of recalcified normal plasma compared to control unactivated PAEC (Figure 1B), which was TF-dependent since the shortening of clotting time disappeared in FVII-deficient plasma (Figure 1C). This antibody (1mg/ml) was able to block the activity of human TF on a TF-positive control human breast cancer cell line, but failed to inhibit porcine TF-initiated clotting since successful blocking of porcine TF needed a much higher concentration of the antibody (10mg/ml) (Figure 1B). Therefore, this antibody was used to differentiate the origin of TF, either from human or pig.
Figure 1. Characterisation of the sheep anti-human TF antibody.
(A) PAEC stimulated by TNF-α (10ng/ml) for 8h to induce porcine TF activity measured by flow cytometery using the sheep anti-human TF antibody (open file) or an isotype control (closed file).
(B) Clotting times of recalcified normal human plasma. Left panel; results without exposure to cells (control, blank) or after exposure to a human TF-positive tumor cell line. Right panel; results following no activation (control) or TNFα activation of PAEC. (Open bars - no additional sheep anti-human TF antibody. Closed bars - sheep anti-human TF antibody incubated with cells for 30min prior to the clotting assay.)
(C) Recalcified coagulation assay using factor VII-deficient plasma.
Procoagulant activity of PAEC
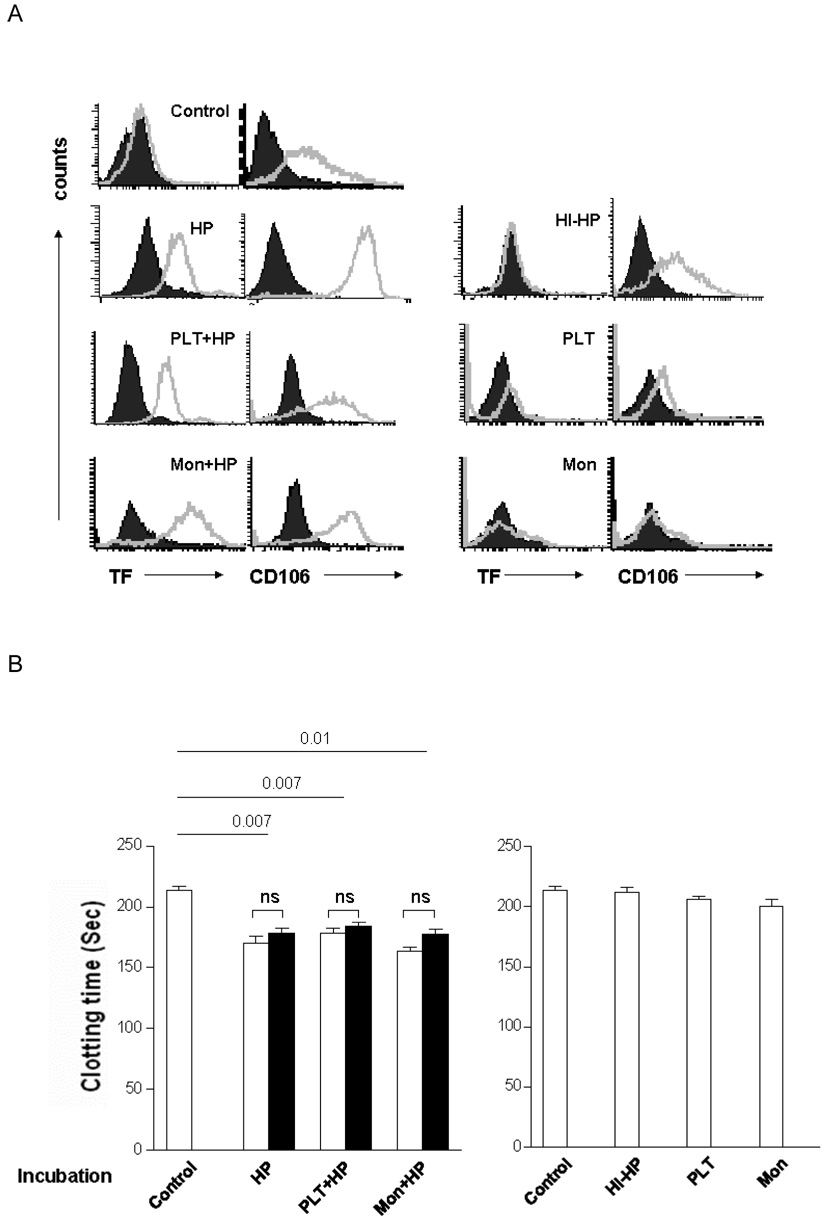
After incubation with HP, human platelets, human monocytes, or combinations of all three, PAEC were analysed by flow cytometry for expression of TF and VCAM-1 (CD106; used as a control for activation). Untreated PAEC were TF-negative and VCAM-1-positive. HI-HP caused no induction of TF or VCAM-1. In contrast, prior incubation with HP induced TF and VCAM-1 expression (Figure 2A). Platelets or monocytes did not induce TF expression and, when each was included in the incubation step with HP, they had no additional impact on TF or VCAM-1 expression (Figure 2A). The observed induction of TF on the surface of PAEC was mirrored by increased TF activity as evidenced by a reduction in clotting time in the plasma recalcification assay (Figure 2B), but not in FVII-deficient plasma (Figure 2C). The failure of the anti-human TF antibody to inhibit clotting activity demonstrated that this activity was related to porcine TF and not to human TF derived from the plasma (Figure 2B).
Figure 2. PAEC express TF as determined by flow cytometry and demonstrate porcine TF activity in the recalcified clotting assay.
PAEC were co-incubated with various stimuli for 8h, and studied by flow cytometery and the recalcified coagulation assay performed using both normal and factor VII-deficient plasma. Addition of sheep anti-human TF antibody was performed separately to determine the origin of the TF.
(A) Results of flow cytometry of TF and CD106 expression (open files) on PAEC (closed files: isotype control).
(B) Recalcified coagulation assay using normal human plasma with (open bars) or without (closed bars) incubation with sheep anti-human TF antibody after PAEC were pre-incubated in the presence (left) or absence (right) of fresh human plasma.
(C) Recalcified coagulation assay using factor VII-deficient human plasma after PAEC were pre-incubated in the presence (left) or absence (right) of fresh human plasma.
(ns = no significant difference, HP = human plasma, HI-HP = heated-inactivated human plasma, Mon = monocytes, PLT = platelets).
Pro-coagulant activity of human monocytes and platelets
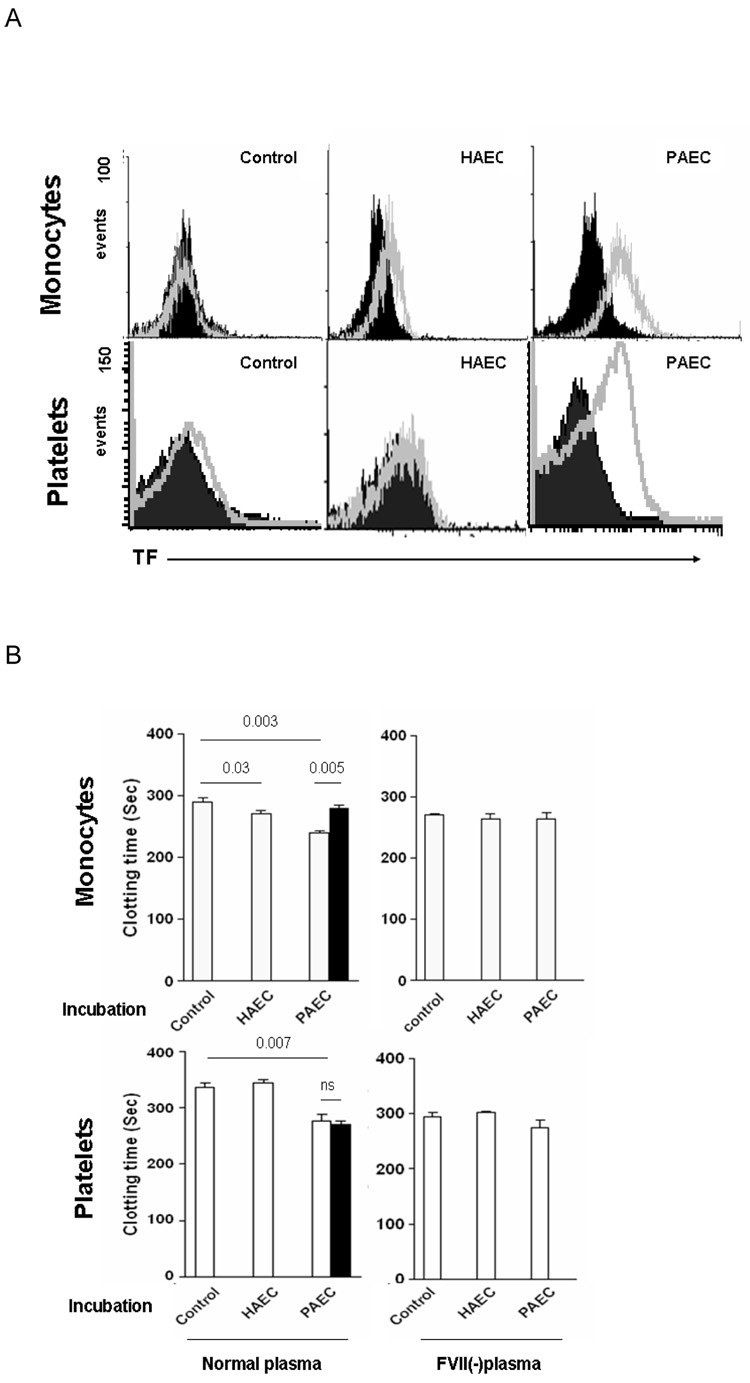
To determine the effect on monocytes or platelets of 8h incubation with PAEC and HP, these cells were isolated and analyzed by flow cytometry. TF was not detected on untreated monocytes and platelets. Low levels of TF were induced on monocytes after co-incubation with HAEC+HP, but not on platelets (Figure 3A). However, TF was significantly induced on both monocytes and platelets after co-incubation with PAEC+HP (Figure 3A). In clotting assays with recalcified normal plasma, clotting times induced by monocytes and platelets after co-incubation with PAEC+HP were shortened compared to those associated with medium or after pre-incubation with HAEC+HP (Figure 3B). Reduction in clotting time was not seen when FVII-deficient plasma was used (Figure 3B), demonstrating that this shortening was TF-dependent. The anti-human TF antibody inhibited the clotting, consistent with expression of human TF by the monocytes and platelets. Incubation with PAEC in the presence of HP therefore induced pro-coagulant human TF expression on both monocytes and platelets.
Figure 3. In the presence of human monocytes and platelets express human TF (after co-incubation with PAEC) as determined by flow cytometry, and demonstrate human TF activity in the recalcified clotting assay.
Human monocytes and platelets were harvested from supernatants after co-incubation with HAEC or PAEC in the presence of 5% human plasma for 8h.
(A) Results of flow cytometry of TF expression (open) on monocytes and platelets (closed files: isotype control).
(B) Clotting times of recalcified normal (left) and factor VII-deficient (FVII(-)) (right) human plasma in the presence of resting (control) or pre-incubated (with HAEC+HP or PAEC+HP) human monocytes or platelets. Coagulation assays were also performed following prior incubation of the monocytes or platelets with sheep anti-human TF antibody (closed bars).
To determine the contribution of HP to the induction of TF, these experiments were repeated without HP in the incubation step, so that monocytes and platelets were incubated with PAEC alone. Under these conditions, TF was still induced on both the monocytes and platelets (Figure 4A). In clotting assays, the results using monocytes were identical to before, indicating that procoagulant human TF was induced on the monocytes by incubation with PAEC (Figure 4B). However, the changes in clotting times induced by platelets were different. Although the results from FVII-deficient plasma confirmed that shortened clotting times were TF-dependent, the anti-human TF antibody failed to inhibit TF activity (Fig 4B), indicating that procoagulant porcine TF was associated with platelets following incubation with PAEC.
Figure 4. In the absence of human monocytes express human TF activity (after coincubation with PAEC), but only platelets confer porcine TF activity.
Human monocytes and platelets were harvested from supernatants after co-incubation with PAEC in the absence of 5% human plasma for 8h.
(A) Results of flow cytometry of TF expression on monocytes and platelets (open files) (closed files: isotype control).
(B) Clotting times of recalcified normal (left) and factor VII-deficient (FVII(-)) (right) human plasma in the presence of resting (control) or pre-incubated (with HAEC or PAEC) human monocytes or platelets. Coagulation assays were also performed following prior incubation of the monocytes or platelets with the sheep anti-human TF antibody (closed bars). (ns = no significant difference).
Human platelets induce PAEC apoptosis and shedding of TF-positive platelet-PAEC aggregates
To investigate these results further, we examined the interaction between human platelets and PAEC. After incubation with human platelets, the PAEC underwent significant apoptosis, as assessed by flow cytometry using annexin V and PI (Figure 5A), which was partially inhibited by co-incubation with HP. In contrast, incubation with human monocytes provoked only a minimal increase in apoptosis of the PAEC (Figure 5A). Platelets were isolated after incubation with PAEC in the absence of HP, and stained with CD106 (red) and TF (green), and examined using immunocytochemical techniques (Figure 5B). Compared to control platelets, which were incubated in medium alone, platelets incubated with PAEC became TF-positive, but CD106-negative, indicating that the encounter with PAEC had activated them. However, this study clearly revealed the presence of larger, irregular particles that were double-positive for both markers. These were assumed to be fragments of apoptotic PAEC that expressed porcine TF. The presence of significant quantities of these platelet- PAEC aggregates among the purified platelets explained the clotting time results (shown in Figure 4B), suggesting the presence of porcine TF.
Figure 5. Human platelets induce PAEC apoptosis.
(A) PAEC were isolated after co-incubation with various stimuli for 8h. Apoptosis was measured by flow cytometry, double-staining with annexin V-FTIC and propidium iodide-PE defined apoptotic cells. The percentage of cells double-positive for TF and annexin V or single-positive for annexin V are indicated.
(B) Immunocytochemical analysis of platelets following co-incubation with or without PAEC for 8h. Cells were double-stained with the sheep anti-human-TF antibody (FITC, green) and anti-porcine CD106 antibody (TRITC, red), then examined by fluorescence microscopy. TF-positive and CD106-negative cells are indicated by arrows. The larger, irregular particles double-positive for both markers are indicated by arrowheads; these were assumed to be fragments of apoptotic PAEC that expressed porcine TF (platelet-PAEC aggregates).
DISCUSSION
The expression of TF has been increasingly recognized during hyperacute rejection or AHXR (15, 20) and linked to the activation of PAEC. The resulting procoagulation stimuli have been hypothesised to play an important role in early xenograft failure. Most previous studies have focused on EC activation by xenoreactive antibody and complement as the trigger to thrombotic graft failure. However, two other factors are important to consider.
First, molecular incompatibilities of the coagulation-anticoagulation system between pig and primate may also contribute to the development of a consumptive coagulopathy. For example, porcine von Willibrand factor (vWF) aggravates the thrombotic process, and, conversely, porcine TF pathway inhibitor and thrombomodulin are unable to down-regulate the propagation of thrombosis (21, 22). Second, thrombosis can be initiated by blood-borne TF, expressed by monocytes or platelets or existing as circulating microparticles, all of which may have prothrombotic activity and substantially contribute to the formation of thrombosis (23, 24).
Our study has attempted to address the relevance of this blood-borne TF as a potential mechanism that may contribute to the thrombosis seen in AHXR and TM. The present study confirmed that PAEC express TF after incubation with HP, and that this effect is lost after heat inactivation of the HP, compatible with it being a complement-dependent phenomenon, similar to the findings of other groups (15). We have assumed this is due to the presence of xenoreactive natural antibodies, so it could be labeled as a procoagulant effect that is humoral immune response-dependent.
Our results also clearly show that resting platelets and monocytes are unable to up-regulate TF expression of PAEC in vitro, consistent with other studies that have shown that only activated platelets can activate PAEC to express TF (16, 25). In an allogeneic setting, one study has shown that activated, but not resting, monocytes were able to activate HAEC, although others reported that resting monocytes could also up-regulate HAEC expression of TF (26, 27).
In systemic inflammatory processes, monocytes and platelets are activated and express TF. It has been previously reported that monocytes can be activated by allogeneic or xenogeneic EC (28). Our results also showed monocytes were weakly activated by HAEC, but much more strongly by PAEC. However, there are no reports of platelets expressing TF in AHXR. In our study, resting PAEC activated monocytes and platelets to express TF, without the involvement of HP. This is clearly independent of the action of xenoreactive natural antibodies or complement, so could be labeled as being humoral immune response-independent. The situation appears complex for platelets, but mainly because, in the absence of HP, PAEC undergo significant apoptosis that results in porcine TF-expressing platelet-PAEC aggregates, which then contribute to the enhanced clotting seen in recalcified HP. Nevertheless, our staining experiments showed that platelets were activated in these circumstances to express human TF, so we can state with some confidence that PAEC induce TF expression on platelets independent of xenoreactive natural antibodies and complement, and therefore independent of a humoral immune response.
These results suggest that recipient monocytes and platelets may make a significant contribution to the thrombotic complications associated with porcine organ xenotransplantation, and that overcoming this contribution will not be achieved by further manipulation of the humoral immune response against the xenograft An important question is whether the apoptosis seen in PAEC after incubation with human platelets (or, to a lesser extent, monocytes) and resulting shedding of TF-positive platelet-PAEC aggregates that we observed is relevant. Although AHXR is mainly associated with EC activation and fibrin deposition along the wall of blood vessels, rather than apoptosis in the endothelium (29), in one study using human decay-accelerating factor (hDAF) transgenic kidney grafts, numerous TUNEL-positive cells were detected with the destruction of the glomerular network, concomitant with the development of TM (13). Our study showed both human platelets and monocytes, but not HP alone, can induce apoptosis of porcine cells (by detection of annexin V expression). The disruption of integrity of the EC and exposure of phosphatidylserine change the EC to become prothrombotic (30), so that any degree of apoptosis of luminal EC in vivo might contribute to thrombosis.
Unlike humans, only A or H blood group antigen is expressed on pig epithelial cells and neither antigen is present on vascular EC (31). Only blood types A and O exist in pigs. In our experiments, the blood type of the pig from which cells were obtained was not determined (32). Therefore, blood type A HP, platelets and monocytes were used as the recipient blood microenvironment to minimize the side effects of ABO-incompatibility.
In summary, we have defined a novel in vitro model in which we can examine independently the contribution to thrombosis/TM made by PAEC, human platelets and monocytes. We have demonstrated that each may contribute to the expression of procoagulant TF after pig-to-primate xenotransplantation. The model is expected to be useful to investigate the mechanism of TM after transplantation of organs from α1,3-galactosyltransferase geneknockout pigs and to provide insights into the type of graft manipulation that could modify the process.
ACKNOWLEDGMENTS
Work in our laboratories is supported in part by NIH grant A1068642 (AD, DKCC)
Abbreviations
- AHXR
acute humoral xenograft rejection
- EC
endothelial cells
- FVII/VIIa
factor VII/factor VIIa
- HAEC
human aortic endothelial cells
- HI
heated-inactivated
- HP
human plasma
- PAEC
porcine aortic endothelial cells
- PBMC
peripheral blood mononuclear cells
- TF
tissue factor
- TM
thrombotic micoangiopathy
REFERENCES
- 1.Cooper DK, Gollackne B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DK, Dorling A, Pierson RN, 3rd, et al. Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84(1):1. doi: 10.1097/01.tp.0000260427.75804.f2. [DOI] [PubMed] [Google Scholar]
- 3.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Tseng YL, Kuwaki K, Dor FJ, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80(10):1493. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 6.Morrissey JH. Tissue factor: an enzyme cofactor and a true receptor. Thromb Haemost. 2001;86(1):66. [PubMed] [Google Scholar]
- 7.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24(6):1015. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 8.Warr TA, Rao LV, Rapaport SI. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75(7):1481. [PubMed] [Google Scholar]
- 9.Keller TT, Mairuhu AT, de Kruif MD, et al. Infections and endothelial cells. Cardiovasc Res. 2003;60(1):40. doi: 10.1016/s0008-6363(03)00354-7. [DOI] [PubMed] [Google Scholar]
- 10.Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci USA. 1999;96(5):2311. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monroe DM, Key NS. The tissue factor-factor VIIa complex: procoagulant activity regulation, and multitasking. J Thromb Haemost. 2007;5(6):1097. doi: 10.1111/j.1538-7836.2007.02435.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwertz H, Tolley ND, Foulks JM, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenicity of human platelets. J Exp Med. 2006;203(11):2433. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu A, Yamada K, Yamamoto S, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005;16(9):2732. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 14.Nagayasu T, Saadi S, Holzknecht RA, Plummer TB, Platt JL. Expression of tissue factor mRNA in cardiac xenografts: clues to the pathogenesis of acute vascular rejection. Transplantation. 2000;69(4):475. doi: 10.1097/00007890-200002270-00003. [DOI] [PubMed] [Google Scholar]
- 15.Gollackner B, Goh SK, Qawi I, et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation. 2004;77(11):1735. doi: 10.1097/01.tp.0000131167.21930.b8. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Arnaud F, Tadaki DK, Burkly LC, Harlan DM, Kirk AD. Human platelets activate porcine endothelial cells through a CD154-dependent pathway. Transplantation. 2001;72(11):1858. doi: 10.1097/00007890-200112150-00029. [DOI] [PubMed] [Google Scholar]
- 17.Mendicino M, Liu M, Ghanekar A, et al. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation. 2005;112(2):248. doi: 10.1161/CIRCULATIONAHA.105.534271. [DOI] [PubMed] [Google Scholar]
- 18.Chen D, Weber M, McVey JH, et al. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004;4(12):1958. doi: 10.1111/j.1600-6143.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Pinto M, Carvajal A, et al. Progesterone increases tissue factor gene expression, procoagulant activity, and invasion in the breast cancer cell line ZR-75-1. J Clin Endocrinol Metab. 2005;90(2):1181. doi: 10.1210/jc.2004-0857. [DOI] [PubMed] [Google Scholar]
- 20.Nagayasu T, Saadi S, Holzknecht RA, Plummer TB, Platt JL. Induction of tissue factor mRNA in acute vascular rejection: localization by in situ reverse transcriptase polymerase chain reaction. Transplant Proc. 2000;32(5):970. doi: 10.1016/s0041-1345(00)01066-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen D. Microcoagulation process after xenotransplantation. Curr Opin Organ Transplant. 2005;10(3):240. [Google Scholar]
- 22.Schulte am Esch J, 2nd, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men--impact on xenografting. Ann Transplant. 2001;6(3):12. [PubMed] [Google Scholar]
- 23.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9(4):458. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 25.Bustos M, Saadi S, Platt JL. Platelet-mediated activation of endothelial cells: implications for the pathogenesis of transplant rejection. Transplantation. 2001;72(3):509. doi: 10.1097/00007890-200108150-00025. [DOI] [PubMed] [Google Scholar]
- 26.Napoleone E, Di Santo A, Lorenzet R. Monocytes upregulate endothelial cell expression of tissue factor: a role for cell-cell contact and cross-talk. Blood. 1997;89(2):541. [PubMed] [Google Scholar]
- 27.Fan ST, Edgington TS. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J Immunol. 1993;150(7):2972. [PubMed] [Google Scholar]
- 28.Kopp CW, Robson SC, Siegel JB, et al. Regulation of monocyte tissue factor activity by allogeneic and xenogeneic endothelial cells. Thromb Haemost. 1998;79(3):529. [PubMed] [Google Scholar]
- 29.Holzknecht ZE, Kuypers KL, Plummer TB, et al. Apoptosis and cellular activation in the pathogenesis of acute vascular rejection. Circ Res. 2002;91(12):1135. doi: 10.1161/01.res.0000046236.20251.fa. [DOI] [PubMed] [Google Scholar]
- 30.Bombelic T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89(7):2429. [PubMed] [Google Scholar]
- 31.Oriol R, Ye Y, Koren E, Cooper DK. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 1993;56(6):1433. doi: 10.1097/00007890-199312000-00031. [DOI] [PubMed] [Google Scholar]
- 32.Busch J, Specht S, Ezzelarab M, Cooper DK. Buccal mucosal cell immunohistochemistry: a simple method of determining the ABH phenotype of baboons, monkeys, and pigs. Xenotransplantation. 2006;13(1):63. doi: 10.1111/j.1399-3089.2005.00255.x. [DOI] [PubMed] [Google Scholar]