Abstract
Slow local skin heating (LH) causes vasodilator responses, some of which are dependent on sympathetic nerve function. It is not known, however, how the rate of LH affects either the sympathetic or the nonadrenergic components of the responses to LH and whether the adrenergic effects of LH depend on tonic sympathetic activity or whether LH stimulates transmitter release. In part 1, cutaneous vascular conductance (CVC) responses to slow and fast LH (+0.1° and +2°C/min) from 34° to 40°C were compared both at control sites and at sites pretreated with bretylium tosylate (BT; blocks transmitter release from adrenergic terminals). We confirmed, as previously found, the axon reflex (AR) response to slow LH to be blocked by BT (P < 0.05). Pretreatment with BT reduced the AR only with fast LH. BT inhibited the peak vasodilation achieved with both rates of LH (P < 0.05). Longer-term LH was associated with a slow fall in CVC, the classical “die away” phenomenon, at untreated sites (P < 0.05) but not at BT-pretreated sites. Thus the LH-stimulated AR is only partially dependent on intact sympathetic function, and the “die away” phenomenon is dependent on such function. In part 2, we tested whether the conditions in part 1 (whole body and local skin temperatures of 34°C) completely suppressed sympathetic nerve activity. The infusion of BT by microdialysis did not change the CVC (P > 0.05), suggesting the absence of tonic activity in those conditions and therefore that the adrenergic components of the responses in part 1 are via the stimulation of the transmitter release by LH.
Keywords: bretylium, norepinephrine release, local control of blood flow, axon reflex, human
a major function of the cutaneous circulation is the maintenance of a stable body temperature. Under conditions of heat stress, skin blood flow can increase for more than 6 l/min (23). In contrast, with an exposure to extreme cold, skin blood flow can fall to levels that are almost negligible (16). This range of skin blood flow can also be achieved by direct local cooling and heating of the skin. Recently, the mechanisms for the effects of local cooling and heating of the skin have attracted renewed interest (8, 11, 12, 27).
A recent intriguing finding is that for a complete cutaneous vasodilation in response to slow local heating, functional vasoconstrictor nerves are required (11, 12). A presynaptic blockade of vasoconstrictor nerves with bretylium tosylate (BT) abolished the axon reflex and greatly reduced the peak vasodilator response to local skin heating, neither of which was restored by the infusion of norepinephrine (NE) during vasoconstrictor nerve blockade (12). By performing separate postsynaptic blockade of α- and β-adrenergic receptors and of Y1 receptors, we (11) found evidence of roles for both NE and the cotransmitter neuropeptide Y (NPY).
In the work by Houghton et al. (12) and Hodges et al. (11), it was found that an inhibition of nitric oxide synthase (NOS) and thus the production of nitric oxide also abolished the axon reflex portion of the response to local skin heating and caused a large attenuation of the peak vasodilator response. However, previous work by Kellogg et al. (19) and Minson et al. (20), in which rapid local heating was used, did not find any effect of NOS antagonism on the magnitude of the axon reflex, although they both reported an attenuation of the peak vasodilator response. This raised the possibility that the axon reflex and the involvement of NE and NPY in the cutaneous vasodilator response to local heating may be dependent on the rate of temperature change. To that end, we performed part 1 of this investigation, in which we compared the cutaneous vascular responses to slow and rapid local heating with and without vasoconstrictor nerve blockade.
The involvement of NE and NPY in the cutaneous vasodilator response to local skin heating begs the question as to whether the increasing local temperature stimulates the release of these neurotransmitters or whether it is dependent on an extant, tonic release. In vitro, local cooling reduces the release, synthesis, and reuptake of NE (3, 5, 13, 28, 29). Could local heating actually be stimulating the release of NE and/or NPY? To answer that question, it was important that tonic vasoconstrictor nerve activity be negligible, so we performed part 2 of this investigation, in which we measured the cutaneous vascular response to the infusion of BT via microdialysis at a whole body skin temperature (Tsk) of 34°C, the conditions used in part 1. This allowed us to evaluate the effects of the acute blockade of transmitter release from vasoconstrictor nerves on cutaneous blood flow and thereby determine whether interrupting vasoconstrictor nerve function has an influence on resting cutaneous blood flow under conditions of a whole body Tsk of 34°C, generally felt to be thermoneutral with little or no adrenergic tone (16). The importance of this portion of the study is that, if tonic vasoconstrictor nerve activity is negligible with that background Tsk, then any contribution by adrenergic function to the responses to local heating (at that Tsk) would most likely be because the local heating stimulated transmitter release.
METHODS
Subjects
All studies were approved by the local Institutional Review Board. All subjects were fully informed of the methods and risks before written consent was obtained. Seven subjects participated in both parts (5 men and 2 women). All were moderately active, healthy nonsmokers, and not taking any medications, and all refrained from alcoholic and caffeinated beverages for at least 12 h before the study. The menstrual status of the female participants was recorded; nevertheless, their responses did not differ perceptively from those of the men, and their results were combined. All experiments were performed in the morning and conducted during the fall months.
Instrumentation and Measurements
All measurements were performed with the subjects resting in the supine posture. Skin blood flow was measured from the ventral aspect of the forearm by laser-Doppler flowmetry (MoorLAB, Moor Instruments, Axminster, UK) and expressed as laser-Doppler flow (LDF) (15, 21). LDF measures are limited to the skin and are not affected by underlying skeletal muscle blood flow (24). Local temperature control was achieved with custom-built Peltier cooling/heating metal probe holders (8, 17, 31): these controlled surface temperature over an area of 6.3 cm2 with the exception of a small aperture (0.28 cm2) in the center of the holder to enable the placement of the laser-Doppler probe. A thermocouple between the skin surface and the probe holder enabled a local skin temperature assessment and feedback control. Local skin temperature can be precisely maintained within 0.05°C with this device. Blood pressure was recorded noninvasively and continuously by the Penaz method (22) from the middle finger of the experimental arm (Finapres, Ohmeda, Madison, WI). Mean arterial pressure was obtained from the electrical integration of the continuous blood pressure signal. Cutaneous vascular conductance (CVC), in arbitrary units, was calculated as the ratio of LDF to mean arterial pressure. Whole body Tsk was recorded as the weighted mean from six thermocouples placed on the body surface and controlled by the use of a water-perfused suit (26). The suit covered the entire body surface apart from the head, hands, feet, and forearm used for the blood flow measurements. All variables were collected at 1-s intervals and stored as 20-s averages for off-line analysis.
In part 2, subjects had two microdialysis probes placed intradermally on the ventral aspect of the forearm as previously described (4, 19). These probes consisted of 1 cm of microdialysis tubing (regenerated cellulose, inner diameter 200 μm, 18 kDa nominal molecular mass cut off) attached at each end to polyimide tubing. Before implantation, the area of skin was temporarily anesthetized by the application of an ice pack for 5 min. A 25-gauge needle was introduced aseptically for ∼2.5 cm into the dermis before exiting. The microdialysis probe and the connecting tubing were introduced into the skin via the lumen of the needle, which was then removed, leaving the probe in place. Both probes were placed in this manner. To allow for the effects of the insertion trauma to subside, we waited 1.5 h before beginning any protocols (1, 6). The different probes were placed 3–5 cm apart.
Drugs
A blockade of the transmitter release from vasoconstrictor nerves was achieved by the use of BT (Schweizerhall, South Plainfield, NJ). In part 1, BT was administered via iontophoresis at a concentration of 10 mM at 250 μA for 10 min to a 0.64-cm2 area of skin (18). In part 2, BT was administered via microdialysis at a concentration of 10 mM for 60 min at a rate of 4 μl/min (12, 25, 30). An administration of BT causes a localized blockade of neurotransmitter release from the cutaneous adrenergic vasoconstrictor nerves (7, 18).
Protocols
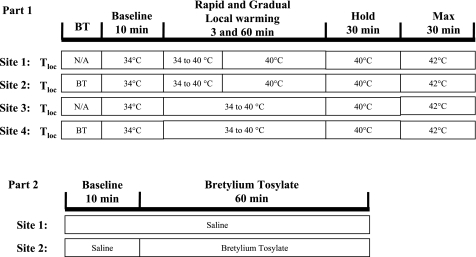
Part 1 was designed to test whether the abolition of the axon reflex and the attenuation of the peak vasodilator response under the condition of vasoconstrictor nerve blockade were dependent on the rate of heating. To this end, four sites on the ventral aspect of the forearm were completed with LDF probes and heater/cooler Peltier holders as described in Instrumentation and Measurements. Whole body Tsk was maintained at 34°C throughout. The treatments were as follows: sites 1 and 3, untreated; and sites 2 and 4, pretreated with BT via iontophoresis (Fig. 1, part 1). Sites 1 and 2 were subjected to rapid local heating, +2°C/min, and sites 3 and 4 to slow local heating, +0.1°C/min. This enabled the measurement of responses to both rates of heating with and without a blockade of vasoconstrictor nerve function. The protocol began with the local temperature held at 34°C for 10 min (baseline measures). Local temperature was then increased as previously described and indicated in Figure 1. At the site undergoing rapid heating, the increase in temperature to 40°C took 3 min, and the local temperature was maintained there until 100 min (Fig. 1). Temperature at sites 3 and 4, undergoing slow local heating, reached 40°C after 60 min. That temperature was held for 30 min (experimental time point 100), and all sites were then heated further to 42°C to obtain maximal CVC (CVCmax) values for each site (14).
Fig. 1.
Protocols. Part 1 (top) was designed to test for a role of the rate of local heating. Using 4 skin sites, 2 pretreated with bretylium tosylate (BT) via iontophoresis (sites 2 and 4) and 2 untreated (sites 1 and 3) are shown. Local heating raised local temperature (Tloc) from 34° to 40°C rapidly (+2°C/min; sites 1 and 2) or slowly (+0.1°C/min; sites 3 and 4). After local heating was completed, Tloc was maintained at 40°C until experiment time 100 min. Tloc was then increased to 42°C for 30 min to obtain maximal skin blood flow. In part 2 (bottom), we sought to determine whether, at whole body skin temperature of 34°C, there was any tonic cutaneous vasoconstrictor nerve activity. At 2 skin sites, microdialysis fibers were perfused with saline for 10 min. The infusion at site 2 was changed to BT (10 mM) for a further 60 min. An increase in cutaneous vascular conductance (CVC) in response to the infusion of BT would indicate the existence of tonic vasoconstrictor activity. Max, maximum; N/A, not applicable.
Part 2 was performed to confirm whether the whole body Tsk of 34°C, as used in part 1, is sufficiently high to remove tonic vasoconstrictor nerve activity. Two ventral forearm skin sites were instrumented with microdialysis fibers, LDF probes, and heater/cooler Peltier holders. Whole body Tsk was maintained at 34°C throughout. The protocol began with both sites perfused with saline. Site 1 acted as an untreated control. Following 10 min of baseline measurements, site 2 was infused, via microdialysis, with BT for 60 min (Fig. 1, part 2). This allowed us to evaluate the effects of acute vasoconstrictor nerve blockade on cutaneous blood flow and thereby determine whether the interrupting vasoconstrictor nerves have an influence on the resting cutaneous blood flow under conditions of a whole body Tsk of 34°C. The importance of this portion of the study is that, if tonic vasoconstrictor nerve activity is negligible with that background Tsk, then any contribution by adrenergic function to the responses to local heating (at that Tsk) would most likely be because the local heating stimulated transmitter release.
Statistical Analysis
Data are presented as means ± SE and analyzed with statistical software (OpenStat, v. 16.9). The analysis was performed by paired statistics or, when appropriate, repeated-measures ANOVA. A power analysis indicated that, for parts 1 and 2, a minimum of six subjects in each study would be required for a P < 0.05 with 95% power (nQuery Advisor v. 3). In part 1, we evaluated the response to the two rates of local heating for both the axon reflex and the peak vasodilation achieved during the local heating protocol. When CVC is expressed as a percentage of CVCmax, the axon reflexes were typically at least 10 to 15% of CVCmax and thus easily identifiable and distinguishable from the movement or other artifacts. The data for the net vasodilator response were expressed as a percentage of the CVCmax achieved at that site as produced by a local skin temperature of 42°C (14). In part 2, data were expressed as percentages of baseline to allow an analysis of the small changes in CVC (8, 17, 31). The levels of CVC at the sites infused with BT after 60 min were compared with the preinfusion baseline at the same sites and to the levels at the untreated sites at the same time.
RESULTS
There were no significant changes in heart rate or blood pressure in either protocol.
Part 1
Axon reflex.
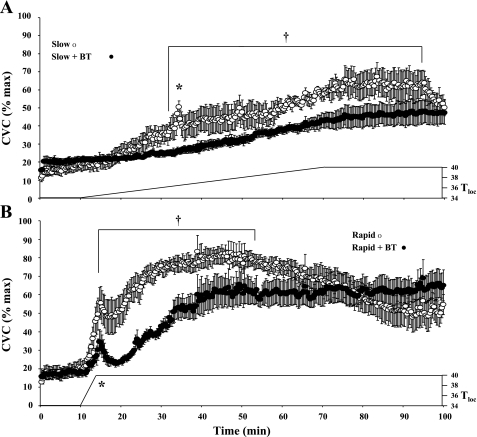
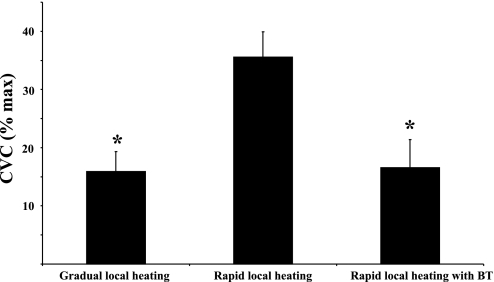
Slow local heating consistently produced an axon reflex at untreated control sites (Δ15.92 ± 3.43 CVC %max) but failed to produce an axon reflex vasodilator response at sites treated with BT (Fig. 2A). However, rapid local heating produced a large axon reflex both at the untreated sites (Δ35.54 ± 4.36 CVC %max) and under the conditions of vasoconstrictor nerve blockade (Δ16.27 ± 4.81 CVC %max, Fig. 2B). The magnitude of the axon reflex was nevertheless significantly (P < 0.05) reduced under conditions of vasoconstrictor nerve blockade (Fig. 3).
Fig. 2.
Results from part 1. Data averaged from all 7 subjects. CVC responses to slow local heating (A) and rapid local heating (B) from both control sites and sites pretreated with BT are shown. Slow local heating causes a marked axon reflex (*) and a pronounced peak vasodilator response. Under conditions of adrenergic blockade with BT, the axon reflex was abolished and a lower peak vasodilator response achieved. Rapid local heating caused a pronounced axon reflex and a greater peak vasodilator response at the untreated site. Pretreatment with BT reduced (P < 0.05) but did not abolish the axon reflex to rapid heating. BT also causes an attenuated peak vasodilator response to local heating. The untreated sites showed a decline in CVC following rapid heating. This “die away” phenomenon was abolished by pretreatment with BT. †P < 0.05, CVC at these points is significantly higher at the untreated sites compared with that at the BT treated sites for that heating protocol.
Fig. 3.
Summary data from all 7 subjects of the changes in CVC, expressed as a percentage of CVCmax during the axon reflex from part 1. There were no axon reflexes observed at BT-treated sites with slow local heating. At the slowly heated and rapidly heated sites pretreated with BT, axon reflexes were significantly (P < 0.05) less than at the untreated, rapidly heated sites. *P < 0.05, significantly lower than at the untreated sites during rapid local heating.
Peak vasodilator responses.
As local heating continued, CVC increased, but the peak vasodilator response was attenuated by the pretreatment with BT with both rapid (untreated 80.68 ± 4.88 vs. BT 63.81 ± 7.03 CVC %max; P < 0.05; Fig. 2) and slow local heating (untreated 60.03 ± 7.77 vs. BT 47.28 ± 6.54 CVC %max; P < 0.05; Fig. 2). The peak vasodilator response was significantly (P < 0.05) greater at the sites previously exposed to rapid local heating (peak occurred from 40 to 50 min, Fig. 2B) than at the sites exposed to slow local heating (peak occurred from 80 to 90 min, Fig. 2A).
Die away.
With longer-term local heating, CVC declined at the untreated sites following rapid local heating, dropping from a peak CVC of 80.68 ± 4.88 to 50.95 ± 7.66%max despite continued local heating (starts ∼55 min; P < 0.05; Fig. 2B). This “die away” phenomenon was absent at the skin sites without functional vasoconstrictor nerves. Although there was a slight tendency for the “die away” phenomenon to occur at untreated sites following slow local heating, this did not achieve statistical significance. There was no such tendency at BT-treated sites following either heating rate.
Part 2
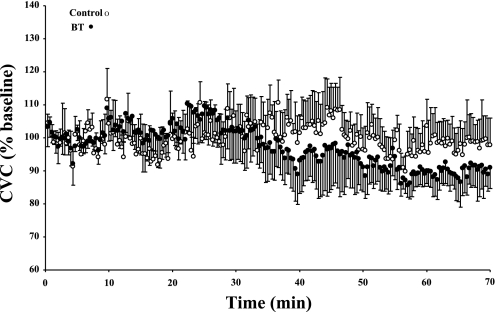
Infusion of BT via microdialysis at a whole body Tsk of 34°C did not cause any significant (P > 0.05) changes in CVC compared with the original baseline of that site (91.99 ± 7.20 CVC %baseline) or to CVC at the untreated control site (98.45 ± 7.64 CVC %baseline; Fig. 4). There was a small, statistically insignificant, downward trend in CVC, which is in the direction opposite to any effects of vasoconstrictor nerve blockade.
Fig. 4.
Data from all 7 subjects, displaying the responses in CVC, expressed as a percentage of baseline, at untreated sites and during the infusion of BT via microdialysis in part 2. There was neither a significant (P > 0.05) change in CVC during the infusion of BT compared with the original baseline nor a significant (P > 0.05) difference at the end of the BT infusion phase between the control and BT-treated sites.
DISCUSSION
Our findings show that sympathetic vasoconstrictor nerve function is important in several elements of the cutaneous vasodilator responses to local skin heating. We found that the axon reflex seen early in local heating requires intact sympathetic function for its full expression, as does the peak vasodilator response while heating is maintained. When local heating is prolonged, the “die away” phenomenon is dependent on an intact vasoconstrictor system. Finally, our results indicate that local heating provides a stimulus for an increased release of transmitter from sympathetic nerve endings.
In part 1, we found that rapid local heating or BT-treated skin partially restored the axon reflex that was eliminated with slow local heating by BT treatment. This suggests that the abolition of the axon reflex under conditions of vasoconstrictor nerve blockade (11, 12) is dependent on the rate of heating and, therefore, that the axon reflex response to local skin heating is not strictly dependent on intact vasoconstrictor nerve function. The attenuation of the peak vasodilator response seen with slow local heating under conditions of vasoconstrictor nerve blockade (11, 12) is still present with rapid local heating, but the vasodilator response was greater with the faster heating protocol. Thus functional vasoconstrictor nerves play roles in both the axon reflex and the peak vasodilator response to local heating in human skin, in agreement with earlier findings (11, 12). Additionally, in part 1, we found that the “die away” phenomenon, first described by Barcroft and Edholm (2) in response to prolonged submaximal skin heating, is abolished by presynaptic vasoconstrictor nerve blockade. This suggests the involvement of the vasoconstrictor nerve system in that classical phenomenon. Whether this is through a depletion of neurotransmitter(s), receptor desensitization, or some other mechanism is not clear.
These data, however, did not distinguish whether this interaction between local heating and vasoconstrictor nerve function was dependent on tonic release of transmitter(s) or whether such release was, in fact, stimulated by local heating. In part 2, we found that the infusion of BT at a whole body Tsk of 34°C (the level used in both parts 1 and 2) does not elicit a change in CVC, which suggests that, at a whole body Tsk of 34°C, the basal cutaneous vasoconstrictor nerve tone is absent. These data, therefore, suggest that these effects of vasoconstrictor nerves on the response to local heating do not depend on their tonic activity and that the release of NE and/or NPY is increased in response to local heating. Although it might be argued that direct recordings of skin sympathetic activity would answer this question, it is technically very difficult to make recordings of such activity unambiguously from forearm skin. We felt that the functional measurement of CVC in part 2 would answer the question arising about changes in CVC observed in part 1.
Houghton et al. (12) were the first to report the involvement of vasoconstrictor nerves in the cutaneous vasodilator response to local heating in humans. They found that the presynaptic blockade of vasoconstrictor nerves abolished the axon reflex and greatly attenuated the peak vasodilator response. The authors tried an interesting approach of infusing a low concentration of NE via microdialysis, under conditions of presynaptic vasoconstrictor nerve blockade, in an attempt to restore the axon reflex and the peak vasodilator response. The fact that this attempt failed suggested the possible involvement of other transmitters. Hodges et al. (11) tested whether the cotransmitter NPY was involved. Through separate and combined postsynaptic blockade of adrenergic and Y1 receptors, Hodges et al. (11) established that both NE and NPY were required for a complete axon reflex and peak vasodilator response to local skin heating, which might explain why the infusion of only NE by Houghton et al. (12) was unsuccessful in restoring the axon reflex. Both Houghton et al. (12) and Hodges et al. (11) reported that, with slow local heating, NOS inhibition, in most instances, also abolished the axon reflex. Previous work by Kellogg et al. (19) and Minson et al. (20) using rapid local heating did not show NOS inhibition to abolish the axon reflex. The different results between these studies suggested a role for the rate of change in temperature. Indeed, Yamazaki et al. (31) noted an important role for the rate of change of temperature in the cutaneous response to local cooling. Therefore, we were interested in determining whether there was a role for the rate of local heating in the axon reflex and in the peak vasodilator response. We found that rapid local heating partially restored the axon reflex in the presence of the blockade of vasoconstrictor nerve function. It is of interest that a role for the rate of local heating in cutaneous vasodilation extends beyond the period of temperature change. As shown in Fig. 2, the peak vasodilation following rapid local heating (40–50 min) is greater than that following slow heating (80–90 min), both in control and following BT pretreatment. It is not clear from the present studies how the rate of heating has this stimulatory effect, although many neural processes are rate dependent. It is likely, however, that the axon reflex and its stimulation by the rate of change of temperature have a protective role by quickly raising blood flow in response to noxious heat (e.g., a hot surface) before the more slowly developing NOS mechanism.
Does local heating stimulate the release of NE, or are its effects dependent on an existing release following from tonic levels of sympathetic activity? In vitro work shows that NE release, synthesis, and reuptake are reduced with local cooling (3, 5, 13, 28, 29). Thus there is a possibility that local heating might stimulate the release of NE (and NPY). In part 2, we sought to determine whether at a whole body Tsk of 34°C (the level of Tsk used in part 1), there was any tonic vasoconstrictor nerve activity. This Tsk is generally viewed as the upper end of thermoneutral conditions and therefore associated with little, if any, tonic vasoconstrictor nerve activity (16). The infusion of BT via microdialysis caused no significant changes in CVC. This, therefore, suggests that the NE-dependent effects of local heating in part 1 are due to a stimulation of that release by the increased local temperature. What is not clear from these data is whether conditions in which there is a tonically elevated sympathetic nerve activity, as with reduced whole body Tsk, would have shown an additional NE-dependent vasodilator effect of local heating.
Barcroft and Edholm (2) observed an interesting phenomenon in which prolonged submaximal local heating for more than an hour led to a decrease in flow, which they termed the “die away.” Other than a speculation as to the basis of this phenomenon, nothing further about this phenomenon was investigated then or since. Although not an initial aim of the present study, in part 1 we observed this phenomenon following rapid submaximal local heating. Importantly, it did not occur at the skin sites treated with BT. This observation shows the “die away” phenomenon to be dependent on intact vasoconstrictor nerve function. Could the “die away” be due to some prolonged stimulatory effect on the adrenergic system? For example, it could be due to a depletion of neurotransmitter(s) or a desensitization of the receptors. In contrast, are the vasoconstrictor effects of NE revealed during prolonged local heating? This phenomenon requires further investigation; nevertheless, these observations show the “die away” phenomenon to be of an adrenergic origin.
Experimental Considerations
It is possible that BT was not only acting to block transmitter release from the vasoconstrictor nerves but may have also been having an anesthetic-like effect. The attenuation of the axon reflex during the fast heating protocol produces a CVC response pattern that is similar to work produced by Minson et al. (20) in which topical local anesthesia was used. Although this possibility cannot be dismissed entirely, we feel this to be unlikely. First, multiple studies with BT show it not to measurably affect vasodilator nerve function (18). Second, either the combination of yohimbine and propranolol or the NPY receptor antagonist BIBP3226 delays the axon reflex response to skin heating, and the combination of all three antagonists abolishes it (11). This reflects exactly the effect of presynaptic vasoconstrictor nerve blockade by BT through the antagonism of the relevant postsynaptic receptors. The data gathered from the two female subjects did not differ perceptively from those of the male subjects, but it is within reason that their results might have varied according to menstrual status. This would most probably be a quantitative difference rather than any difference in control mechanisms. Finally, we did not test the adequacy of vasoconstrictor blockade by BT with whole body cooling in part 2, other than in preliminary studies. We used the same concentration of BT and twice the microdialysis flow rate as used by others in previous studies (12, 24, 30). In those studies, vasoconstrictor system blockade was uniformly successful as challenged by whole body cooling, so we feel confident that, with our use of twice the delivery rate, such was also the case in the present investigation.
In summary, we found that the abolition of the axon reflex under conditions of vasoconstrictor nerve blockade during slow local heating to be partially reversed if the rate of local heating is increased. Because the axon reflex is not fully restored and the attenuation of the peak vasodilator response also remains, the suggestion is reinforced that functional vasoconstrictor nerves are required for a complete vasodilator response to local skin heating. We also provide evidence to support the notion that local heating actually promotes the release of cutaneous vasoconstrictor nerve neurotransmitters, further supporting their role in the cutaneous vasodilator response to local heating. We also found evidence to suggest that the “die away” phenomenon observed during prolonged submaximal heating is vasoconstrictor nerve based, but whether this is due to transmitter depletion, the receptor desensitization or some other mechanism remains unclear.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grant RO1-HL-059166.
Acknowledgments
We thank the volunteers for their time and enthusiastic participation. G. J. Hodges is now at The University of Western Ontario (ghodges@uwo.ca).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol 102: 807–811, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol 102: 5–20, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boles PJ, Verbeuren TJ, Vanhoutte PM. Moderate cooling depresses the accumulation of newly synthesized catecholamines in isolated canine saphenous veins. Experientia 41: 1374–1377, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Crandall CG, Etzel RA, Johnson JM. Evidence of functional β-adrenoceptors in the cutaneous vasculature. Am J Physiol Heart Circ Physiol 273: H1038–H1043, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Flavahan NA The role of vascular α2 adrenoceptors as cutaneous thermosensors. News Physiol Sci 6: 251–255, 1991. [Google Scholar]
- 6.Groth L, Serup J. Cutaneous microdialysis in man: effects of needle insertion trauma and anaesthesia on skin perfusion, erythema and skin thickness. Acta Derm Venereol 78: 5–9, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Haeusler G, Haefely W, Huerlimann A. On the mechanism of the adrenergic nerve blocking action of bretylium. Naunyn Schmiedebergs Arch Pharmacol 265: 260–277, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol 574: 849–857, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges GJ, Traeger JA 3rd, Tang T, Kosiba WA, Zhao K, Johnson JM. Role of sensory nerves in the cutaneous vasoconstrictor response to local cooling in humans. Am J Physiol Heart Circ Physiol 293: H784–H789, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Hodges GJ, Kosiba WA, Zhao K, Alvarez GE, Johnson JM. The role of baseline in the cutaneous vasoconstrictor responses during combined local and whole body cooling in humans. Am J Physiol Heart Circ Physiol 293: H3187–H3192, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol 105: 233–240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens WJ, Vanhoutte PM. Instantaneous changes of α-adrenoceptor affinity caused by moderate cooling in canine cutaneous veins. Am J Physiol Heart Circ Physiol 234: H330–H337, 1978. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JM The cutaneous circulation. In: Laser-Doppler Blood Flowmetry, edited by Shepherd AP and Öberg PA. New York: Springer, 1990, p. 121–139.
- 16.Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology. Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect 4, vol. I, p. 215–243. [Google Scholar]
- 17.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288: H1573–H1579, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg DL, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Öberg PA Laser-Doppler flowmetry. Crit Rev Biomed Eng 18: 125–163, 1990. [PubMed] [Google Scholar]
- 22.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Rowell LB Cardiovascular aspects of human thermoregulation. Circ Res 52: 367–379, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Saumet JL, Kellogg DL Jr, Taylor WF, Johnson JM. Cutaneous laser-Doppler flowmetry: influence of underlying muscle blood flow. J Appl Physiol 65: 478–481, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Simmons GH, Minson CT, Cracowski JL, Halliwill JR. Systemic hypoxia causes cutaneous vasodilation in healthy humans. J Appl Physiol 103: 608–615, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol 292: H1700–H1705, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Vanhoutte PM Physical factors of regulation. In: Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol. Soc., 1980, sect. 2, vol. II, chap. 16, p. 443–474. [Google Scholar]
- 29.Vanhoutte PM, Verbeuren TJ. Depression by local cooling of 3H-norepinephrine release by nerve stimulation in cutaneous veins. Blood Vessels 13: 92–99, 1976. [DOI] [PubMed] [Google Scholar]
- 30.Wilson TE, Monahan KD, Short DS, Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol 287: R1230–R1234, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J Appl Physiol 100: 42–50, 2006. [DOI] [PubMed] [Google Scholar]