Abstract
Flow-mediated dilatation (FMD) has become a commonly applied approach for the assessment of vascular function and health in humans. Recent studies emphasize the importance of normalizing the magnitude of FMD to its apparent eliciting stimulus, the postdeflation arterial shear. However, the relationship between shear stress and the magnitude of FMD may differ between groups. The aim of this study was to examine the relationship between the brachial FMD and four different indexes of postdeflation shear rate (SR) in healthy children (n = 51, 10 ± 1 yr) and young (n = 57, 27 ± 6 yr) and older (n = 27, 58 ± 4 yr) adults. SR was calculated from deflation (time 0) until 9 s (peak), 30 s (0–30), 60 s (0–60), or until the time-to-peak diameter in each individual (0-ttp). Edge detection and wall tracking of high resolution B-mode arterial ultrasound images were used to calculate the conduit artery diameter. In young adults, the brachial artery FMD demonstrated a significant correlation with the area under the SR curve (SRAUC) 0–30 s (r2 = 0.12, P = 0.009), 0–60 s (r2 = 0.14, P = 0.005), and 0-ttp (r2 = 0.14, P = 0.005) but not for the peak SRAUC 0–9 s (r2 = 0.04, P = 0.12). In children and older adults, the magnitude of the brachial artery FMD did not correlate with any of the four SRAUC stimuli. These findings suggest that in young subjects, postdeflation SRAUC correlates moderately with the magnitude of the FMD response. However, the relationship between FMD and postdeflation shear appears to be age dependent, with less evidence for an association in younger and older subjects. Therefore, we support presenting SRAUC stimuli but not normalizing FMD responses for the SRAUC when using this technique.
Keywords: eliciting stimulus, Doppler, blood flow, methodology
flow-mediated dilatation (FMD) describes the vasodilatation of a conduit artery following an increase in shear stress, typically induced by 5 min ischemia provoked by a limb cuff placed distal to the recording probe (2). With the assumption that the appropriate methodology is used (12, 39), the FMD response is largely nitric oxide mediated (14, 20) and provides information about the endothelial function (40). Since impaired endothelial vasodilator function is considered an early and integral manifestation of vascular disease, which predicts cardiovascular events (11, 16, 18, 21, 26), the FMD approach has become a popular and frequently applied technique in clinical and physiological research.
In a series of experiments, Pyke et al. (28–30) explored the relationship between FMD and its apparent eliciting stimulus. They concluded that the area under the shear rate (SR) curve following cuff deflation (SRAUC), rather than peak SR, is the critical determinant of the FMD response. As a result of recommendations arising from these studies, including the suggestion that the appropriate strategy for normalization of the FMD response is to relate FMD to the total SRAUC from cuff deflation to the time of peak diameter measurement (29), recent studies have begun to correct the magnitude of the peak diameter for the eliciting shear (1, 5, 15, 17, 24, 42). However, the suggestion that SRAUC be used to normalize FMD is based on repeated-measure studies of relatively small samples (n = 8–24) of healthy, young adults (28, 29, 33), and this relationship may conceivably differ with age and cardiovascular risk. Furthermore, confusion persists regarding the appropriate time frame across which SRAUC should be calculated, with different studies using different predetermined time windows (3, 8, 15, 22, 25, 38) (e.g., 0–30 or 0–60 s) or the time-to-peak diameter (1). The aim of this study was to examine the relationship between FMD and four different SRAUC stimuli that have been used in the literature in a cross section of children and young and older adults. Our aim was to identify the best method to normalize FMD for differences in its eliciting SR stimulus. We hypothesized that correcting the FMD for the SRAUC from cuff deflation to the time of peak diameter, but not peak SR or a SRAUC calculated within a predetermined time window, will abolish individual differences in the magnitude of the FMD response within and between the age groups.
METHODS
Subjects.
Healthy volunteers (n = 135) were recruited from the community and were stratified based on age into three different groups: children (n = 51, 9–10 yr), young adults (n = 57, 20–41 yr), and older adults (n = 27, 50–66 yr) (Table 1). All subjects were nonsmokers and normotensive (<140/90 mmHg), and none had any history of diabetes, insulin resistance, or cardiovascular disease. None of the subjects used any medications known to interfere with the cardiovascular system. The Ethics Committee of the Liverpool John Moores University approved the study protocols, which adhered to the Declaration of Helsinki. Informed consent from subjects and informed consent of parents/guardians of children were obtained before their participation in the study.
Table 1.
Subject characteristics of the participants divided into a group of children and young and older adults
| Children | Young Adults | Older Adults | ANOVA | |
|---|---|---|---|---|
| n | 51 | 57 | 27 | |
| Age, yr | 10±1* | 27±6* | 58±4* | <0.001 |
| Sex, male/female | 20/31 | 48/9 | 15/12 | |
| Systolic blood pressure, mmHg | 106±8* | 116±10 | 121±16 | <0.001 |
| Diastolic blood pressure, mmHg | 63±4* | 67±7* | 71±10* | <0.001 |
| Mean blood pressure, mmHg | 78±5* | 81±11* | 87±11* | <0.001 |
| Height, cm | 142±7* | 177±8* | 168±9* | <0.001 |
| Weight, kg | 39.7±9.1* | 76.5±11.3 | 75.1±17.6 | <0.001 |
| BMI, kg/m2 | 19.5±3.2* | 24.3±2.8* | 26.2±4.6* | <0.001 |
Values are means ± SD; n, number of participants.
P < 0.05, post hoc significant from all other groups.
Experimental design.
After the participants reported to the laboratory, the brachial artery FMD response was measured after a resting period of at least 20 min. Testing was performed between 9:00 am and 4:00 pm. Previous studies found, despite an attenuated FMD immediately after waking, that the FMD does not appear to be influenced across the time frame adopted in our study, when testing is performed under well-controlled conditions (23, 34). Accordingly, all measures were performed under standardized conditions in a quiet, temperature-controlled room and after at least 6 h of fast and at least 8 h of abstinence from caffeine or alcohol. No subject performed strenuous physical activity for at least 24 h before testing, since this can result in a transient change in FMD (4).
Brachial artery FMD.
Patients rested supine with the right arm extended and immobilized with foam, supported at an angle of ∼80° from the torso. Heart rate and mean arterial pressure were determined from an automated sphygmomanometer (GE Pro 300V2, Dinamap, Tampa, FL) on the contralateral arm. For the assessment of the FMD response, a rapid inflation/deflation pneumatic cuff was positioned on the imaged arm distal to the olecranon process to provide a stimulus to forearm ischemia. A 7.5- (Aspen, Acuson; Mountain View, CA) or 10-MHz (T3000, Terason, Aloka, UK) multifrequency linear array probe attached to a high-resolution ultrasound machine was used to image the brachial artery in the distal third of the upper arm. Ultrasound parameters were set to optimize longitudinal B-mode images of the lumen/arterial wall interface. A continuous Doppler velocity assessment was obtained simultaneously, and data were collected using the lowest possible insonation angle (always <60°), which did not vary during each study (27). After a resting period of at least 20 min, 1 min of baseline recording of the brachial artery diameter and velocity was performed. Subsequently, the occlusion cuff was inflated to >200 mmHg for 5 min. The brachial artery diameter and velocity recordings were restarted at least 30 s before cuff deflation and continued for at least 3 min after deflation. Peak artery diameter and flow, and the time to reach this peak after cuff deflation, were recorded.
Brachial artery diameter and blood flow.
Posttest analysis of the brachial artery diameter was performed using custom-designed edge-detection and wall-tracking software, which is independent of investigator bias (41). Briefly, the echo-Doppler signal was real-time encoded and stored as a digital file when using the Terason ultrasound machine. When using the Aspen machine, the video signal was taken directly from the ultrasound machine and, using an IMAQ-PCI-1407 card, was encoded and stored as a digital DICOM file on the PC. In both situations, subsequent software analysis of the data was performed at 30 Hz using an icon-based graphical programming language and toolkit (LabView 6.02, National Instruments, Austin, TX).
The initial phase of image analysis involved the identification of regions of interest (ROIs) on the first frame of every individual study. These ROIs allowed for an automated calibration of diameters on the B-mode image and velocities on the Doppler strip. A ROI was drawn around the optimal area of the B-mode image, and within this ROI, a pixel-density algorithm automatically identified the angle-corrected near and far-wall e-lines for every pixel column within the ROI. The algorithm begins by dividing the ROI into an upper half, containing the near-wall lumen-intima interface, and a lower half containing the far-wall interfaces. The near-wall intimal edge is identified by a Rake routine that scans from the bottom to the top of the upper half of the ROI. The position of the edge is established by determining the point where the pixel intensity changes most rapidly. Typical B-mode ROIs, therefore, contained ∼200–300 diameter measures per frame, the average of which was calculated and stored. This process occurred at 30 frames/s. A final ROI was drawn around the Doppler waveform and automatically detected the peak of the envelope of this waveform 30 times/s for off-line analysis. The mean diameter measure derived from within the B-mode ROI (above) was synchronized with the velocity measure derived from the Doppler ROI at 30 Hz. Ultimately, from this synchronized diameter and velocity data, blood flow (the product of cross-sectional area and Doppler velocity) and SR (4 times velocity divided by diameter) were calculated at 30 Hz. All data were written to the file and retrieved for analysis in a custom-designed analysis package. We have shown that the reproducibility of diameter measurements using this semiautomated software is significantly better than manual methods, reduces observer error significantly, and possesses an intraobserver coefficient of variation of 6.7% (41).
Data analysis.
Baseline diameter and blood flow were determined during the 1 min before cuff inflation. Peak diameter following cuff deflation was automatically detected according to an algorithm that identified the maximum bracket of data subsequent to the performance of a moving window smoothing function. This smoothing routine calculates the median value from 100 consecutive frames (∼3 s) before the window shifts to the next bracket of data that shares 20% overlap with the preceding bracket (see Ref. 1 for more details). The maximum value of all the calculated median values is then automatically detected and chosen to represent the peak of the diameter curve. FMD was calculated as the percent rise of this peak diameter from the preceding baseline diameter.
From the simultaneously acquired blood flow velocity estimates and diameter measures, SR was calculated at 30 Hz. The postdeflation SR data were exported to a spreadsheet, and the SRAUC was calculated based on the Riemann sum technique. To examine the relationship between diameter dilation (FMD%) and the eliciting SR, we then calculated the most frequently applied methods for SR correction used in the literature: peak SR (AUC from 0–9 s) (3, 29) and the SRAUC from cuff deflation up to the arbitrary time point of 30 (15) and 60 s (8) and from deflation to the time of peak diameter for each individual (1).
Statistics.
Statistical analyses were performed using SPSS 14.0 (SPSS, Chicago, Illinois) software. All data are reported as means (SD), and the statistical significance was assumed at P ≤ 0.05. Pearson's correlation coefficient was used to examine the correlation between the different correction methods for the eliciting shear stimulus and the FMD for the whole group, but also for the different age groups. These correlations were calculated for the FMD presented as a relative (FMD%) as well as the absolute change in millimeter from baseline (FMDmm) (35). A linear regression model was used to examine the impact of various factors [SRAUC stimuli, diameter, blood pressure, and body mass index (BMI)] upon the magnitude of the FMD%. In addition, an ANOVA procedure was used to examine differences in baseline characteristics between the three groups. Post hoc analysis was used to identify the groups that differed significantly.
RESULTS
Subject characteristics.
Age, height, BMI, and diastolic blood pressure differed significantly between all three groups (Table 1). Systolic arterial blood pressure and weight were significantly lower in children, compared with young and older adults, whereas older men had a higher systolic blood pressure than children and young adults (Table 1).
Brachial artery FMD% versus SR stimuli.
Baseline and peak brachial artery diameter was significantly lower in children compared with young and older adults (Table 2). The FMD, when expressed as the relative change from baseline, was significantly higher in children than in young and older adults. The time-to-peak diameter did not significantly differ between groups (Table 2).
Table 2.
Brachial artery characteristics at baseline and during the FMD response of participants divided into a group of children and young and older adults
| Children | Young Adults | Older Adults | ANOVA | |
|---|---|---|---|---|
| n | 51 | 57 | 27 | |
| Baseline diameter, mm | 2.6±0.3* | 4.1±0.6 | 4.1±0.9 | <0.001 |
| Peak diameter, mm | 2.9±0.4* | 4.4±0.7 | 4.4±1.0 | <0.001 |
| Change from baseline, mm | 0.3±0.1 | 0.3±0.1 | 0.2±0.1† | 0.047 |
| Change from baseline, FMD% | 10.7±4.9* | 7.5±3.1* | 6.0±2.9* | <0.001 |
| Time-to-peak dilation, s | 71±32 | 71±37 | 86±32 | 0.14 |
| Peak SRAUC, s−1, ×103 | 5.0±2.1* | 4.3±2.2 | 3.2±2.2 | <0.001 |
| SRAUC 0–30 s, s−1, ×103 | 18.6±5.4* | 11.4±5.9 | 11.9±5.9 | <0.001 |
| SRAUC 0–60 s, s−1, ×103 | 31.3±8.8* | 17.0±8.8 | 17.5±7.9 | <0.001 |
| SRAUC 0-ttp, s−1, ×103 | 35.6±16.7* | 17.5±8.8 | 20.4±9.5 | <0.001 |
| FMD/SRAUC 0-ttp, s−1, ×10−4 | 4.3±2.4 | 5.3±3.1 | 3.4±1.9† | 0.045 |
Values are means ± SD; n, number of participants. Four different methods for postdeflation shear rate area-under-the-curve (SRAUC) calculation were presented: peak shear rate and shear rate 0–30 s, 0–60 s, and 0 up to time-to-peak (0-ttp) diameter. FMD%, flow-mediated dilatation, presented as %change from baseline diameter.
P < 0.05, post hoc significant from all other groups.
P < 0.05, post hoc significant between young and older adults.
The SRAUC differed significantly between the four methods (peak, 0–30 s, 0–60 s, and 0-ttp) for children and young and older adults (ANOVA, all P < 0.001), with the largest SRAUC stimulus when calculating SR until the time-to-peak diameter (Table 2).
Correlations across and within different age groups.
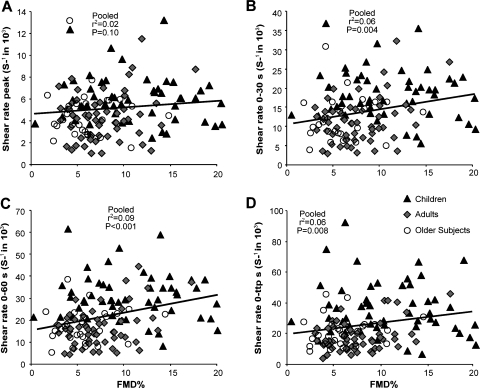
The correlation between the magnitude of the FMD% response and the four different SRAUC stimuli for the pooled data set (n = 135) revealed no significant correlations for the peak SRAUC (Fig. 1). In contrast, the FMD% correlated significantly with the SRAUC stimulus when calculated to 30 s, 60 s, and until the individual time of peak diameter (Fig. 1). The magnitude of the FMDmm response and the four different SRAUC stimuli for the pooled data set revealed no correlations.
Fig. 1.
Correlations between brachial artery flow-mediated dilatation, presented as %change from baseline diameter (FMD%) and 4 different shear rate stimuli. Peak shear rate (A), 0–30-s shear rate (B), 0–60-s shear rate (C), and 0 time-to-peak diameter (0-ttp) shear rate (D) are shown in the pooled data set of children (n = 51, ▴), young adults (n = 57,  ), and older adults (n = 27, ○).
), and older adults (n = 27, ○).
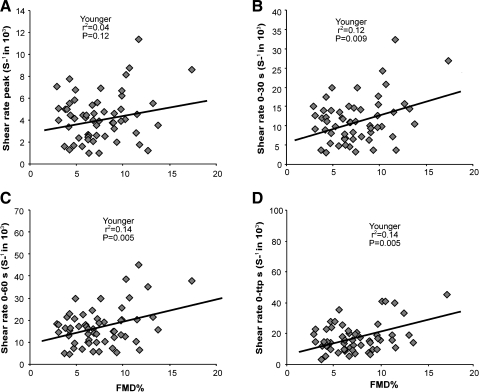
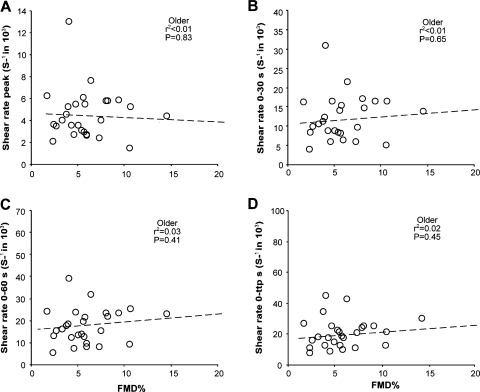
In parallel with the pooled data, we found no correlation between the brachial artery FMD% and the peak SRAUC stimulus in the individual groups of children and young and older adults (Figs. 2, 3, and 4). Younger adults showed a significant correlation between the FMD% and the SRAUC stimulus until 30 s, 60 s, and the time of peak diameter (Fig. 3). In marked contrast, children and older adults reported no significant correlation between the FMD% and the SRAUC stimuli (Figs. 2 and 4, respectively). The FMDmm response and the four different SRAUC stimuli did not correlate in children or young or older adults.
Fig. 2.
Correlations between brachial artery FMD% and 4 different shear rate stimuli. Peak shear rate (A), 0–30-s shear rate (B), 0–60-s shear rate (C), and 0-ttp shear rate (D) are shown in children (n = 51, ▴).
Fig. 3.
Correlations between brachial artery FMD% and 4 different shear rate stimuli. Peak shear rate (A), 0–30-s shear rate (B), 0–60-s shear rate (C), and 0-ttp shear rate (D) are shown in young adults (n = 57,  ).
).
Fig. 4.
Correlations between brachial artery FMD% and 4 different shear rate stimuli. Peak shear rate (A), 0–30-s shear rate (B), 0–60-s shear rate (C), and 0-ttp shear rate (D) are shown in older adults (n = 27, ○).
Although FMD% correlated significantly with age (r = −0.39, r2 = 0.15, P = 0.004), BMI (r = −0.36, r2 = 0.13, P < 0.001), mean arterial blood pressure (r = −0.17, r2 = 0.03, P = 0.03) and SRAUC stimuli (Fig. 1), a stepwise linear regression model performed to examine the contribution of these various parameters to the FMD% in the pooled data set (n = 135) identified baseline diameter as the strongest predictor for the magnitude of the FMD% (r = −0.53, r2 = 0.28, P < 0.001). The other variables listed above did not demonstrate a significant β-coefficient and could not be added to the regression model.
DISCUSSION
In recent years, several studies have provided support for the concept that the FMD response should be normalized for the magnitude of its eliciting postdeflation shear stress stimulus to make valid comparisons between subjects (17, 29, 32). While this principle is based on studies adopting a “within” subjects approach in small samples of young subjects (28, 29, 33), we examined the relationship between artery dilation and four commonly adopted methods to correct for the eliciting shear stimulus between subjects and between groups (children and young and older adults). We found a significant correlation between the FMD% response and the SRAUC using a predetermined window (0–30 and 0–60 s) or between deflation and the individual time-to-peak diameter, but not for the peak SR. This finding confirms the above studies, which were performed in similarly young and healthy subjects, indicating that the SRAUC, rather than the peak SR, determines the magnitude of the FMD (29). However, relatively weak correlations were found in this group of 57 subjects, with the SRAUC (0–60 s) explaining only ∼14% of the FMD% response. More importantly, the correlation between FMD and the eliciting SRAUC stimulus appears to be group specific. Since young adults showed modest significant correlations between FMD and SRAUC to 30 s, 60 s, or the time-to-peak diameter, no correlations were reported between any of these measures of SR and FMD in either children or older subjects. The correlation between the brachial artery FMD and the SR stimulus, therefore, appears to be age dependent. These observations indicate that the SRAUC normalization approaches explain only a relatively small portion of the magnitude of the FMD response and that any relationships present are dependent on the groups studied. This finding has important implications for future studies using the FMD, where a consideration is given to normalization procedures for comparisons between individuals or groups.
In an attempt to improve the validity of FMD testing and methodology, Pyke and Tschakovsky performed an elegant series of experiments to gain better insight into the nature of the eliciting shear stress stimulus for the FMD response. They found that the SRAUC stimulus, rather than the peak shear, is the critical determinant of the FMD response (29). Our data in a large group of young, healthy adults reinforce these findings since we demonstrated a significant correlation between FMD% and the SRAUC using a predetermined time window (0–30 or 0–60 s) or up to the peak diameter, but not using the peak SRAUC. The correlations found between FMD% and the three distinct methods to calculate the SRAUC stimulus did not importantly differ. This indicates that extending the time window for the calculation of the SRAUC after 30 s postdeflation does not significantly improve the correlation between the magnitude of the FMD and the eliciting SR stimulus in our dataset.
A recent influential study suggested, based on of the finding that the SRAUC stimulus explained 56% of the variation in the FMD response, that the FMD should be corrected for this SRAUC stimulus (29). In our study, however, the SRAUC stimulus predicts only 9% of the magnitude of the FMD%, and other methods were even more modestly predictive (e.g., other SRAUC stimuli and FMDmm). A possible explanation for these contrasting findings is that we examined a heterogeneous age group (9–66 yr), whereas most previous studies examined young subjects. A subgroup analysis on our data demonstrated enhanced correlations between the magnitude of the FMD response and the SRAUC stimuli in healthy, young subjects (Fig. 2). However, our correlations are still markedly lower than reported previously (r = 0.74; see Ref. 29). A crucial difference between both studies is that we used a between-subject design in 57 subjects, whereas Pyke and Tschakovsky performed a repeated-measures design of six repeated measurements within 10 subjects. The latter approach is likely to overestimate the correlations typical of between-subject comparisons. We suggest that, if the FMD response is primarily, or even largely, dependent on the postdeflation eliciting shear evident between subjects, then it might be expected that the variance in FMD responses between subjects would be more than ∼15% dependent on measured indexes of shear.
To explore which variables, other than SRAUC stimuli, might contribute to the magnitude of the FMD response, we performed a stepwise linear regression analysis. Interestingly, this analysis revealed that the baseline diameter provided the most powerful prediction of the FMD% response (r = 0.53, r2 = 0.28, P < 0.001). This finding reinforces previous studies that identified artery diameter as a strong predictor for the FMD (13, 17, 28, 29, 33). Some studies have attributed this relationship to the impact of diameter on postdeflation shear. On the basis of a modest relationship between SRAUC and FMD, the impact of baseline diameter is unlikely to be fully explained through its impact on the SRAUC stimulus alone (35). Possibly, inherent differences in the wall architecture of different sized arteries may partly explain the relationship between size and functional responsiveness, as originally proposed by Folkow et al. (9, 10). Indeed, in a recent study, we observed similar relationships between endothelium-independent vasodilation and baseline artery size as those present between artery size and FMD, suggesting an important role for the structural aspects of the vessel wall determining the magnitude of FMD (35).
To our knowledge, this is the first study to examine the correlation between indexes of postdeflation shear (SRAUC) and FMD in populations in whom, a priori, one might expect different levels of endothelial function. Indeed, we observed significant differences between FMD and corrected FMD between the groups we studied. However, we found no correlation between the FMD and any of the SRAUC stimuli in either children or older subjects, which indicates that the relationship between FMD and the eliciting shear stimulus differs between groups of differing age. This raises the possibility that many typically compared groups, such as those with and without cardiovascular disease or risk factors, may also have a different relationship between the FMD response and the SRAUC stimulus. More importantly, the different relationships between FMD and SRAUC stimuli between groups indicate that FMD normalization for the SRAUC stimulus could lead to a misleading reassurance that the normalization of artery function has been conferred for all subjects to a similar degree. Although postdeflation shear is clearly an important stimulus for artery dilation during the FMD (17, 28, 29, 32), other factors likely contribute to the magnitude of this response. We propose that FMD normalizing for SR, a procedure involving multiplication of error in the process of dividing of a ratio (i.e., FMD) by yet another measure (SRAUC), may lead to less precision in vascular function studies than simply providing a comparison between subjects or groups of SRAUC. We therefore suggest that future studies present SRAUC stimulus data, but we question the validity of normalization of FMD measures for SRAUC. Since the dependence of FMD on SRAUC is modest and the relationship between FMD and SR as the eliciting dilator stimulus differs between groups, a normalization of FMD may even lead to misleading results when comparing groups.
We can only speculate as to the reasons for differences between age groups in the strength of the relationship between FMD% and SRAUC stimuli. One possible reason relates to age-related differences in vascular wall characteristics, for example elasticity (19), wall thickness (6), and baseline diameter (7). These characteristics of the vasculature are suggested to influence the responsiveness of arteries to physiological stimuli (9, 10) and may, therefore, contribute to differences in the relationship between FMD and SRAUC stimuli in different age groups. Another reason relates to age-dependent differences in the sensitivity of the vasodilator [e.g., nitric oxide (31)] and vasoconstrictor pathways [e.g., angiotensin-II and endothelin-1 (37)]. Age-dependent differences in response to vasoactive substances likely contribute to the differences in the relationship between FMD and SRAUC stimuli. Future studies should further elucidate the potential mechanisms that explain the differences in the strength of the relationship between FMD% and SRAUC stimuli in age groups but also focus on other groups that differ regarding this relationship (e.g., sex difference).
In conclusion, our data support previous studies that suggested that the SRAUC stimulus, rather than the peak SR postdeflation, correlates with the magnitude of the FMD%, whereas we found no differences in the correlations between FMD and three typically used methods for SRAUC stimulus calculation. However, in contrast to the current, widely held view, we found the eliciting SRAUC stimulus explaining only 10–15% of the FMD response. This reinforces the suggestion that, besides individual differences in the SRAUC stimulus, other shear-independent factors contribute to individual differences in the magnitude of FMD responses. Moreover, the correlation between the SRAUC stimulus and the FMD response differs between age groups, with no association between SRAUC and FMD evident in either children or older adults. While we strongly support measurement and presentation of the eliciting SRAUC stimulus in future studies comparing FMD between groups, our results suggest that further studies will be required before the practice of normalizing FMD for differences in the SRAUC is adopted. This procedure introduces error and may even lead to a misinterpretation of data, especially when analyzing FMD responses between groups that have a different relationship between the FMD and SRAUC stimulus.
GRANTS
This study was supported by The Netherlands Organization for Scientific Research NWO-Grant 82507010 (to D. H. J. Thijssen) and British Heart Foundation Grant FS/05/117/19971 (to M. A. van Bemmel).
Acknowledgments
We thank Chris Reed for assistance with the development of edge detection and wall tracking software.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Chironi G, Craiem D, Miranda-Lacet J, Levenson J, Simon A. Impact of shear stimulus, risk factor burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. J Hypertens 26: 508–515, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Dawson EA, Whyte GP, Black MA, Jones H, Hopkins ND, Oxborough D, Gaze D, Shave RE, Wilson M, George KP, Green DJ. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 105: 1562–1568, 2008. [DOI] [PubMed] [Google Scholar]
- 5.de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol 287: H374–H380, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol 278: H1205–H1210, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 290: H1446–H1453, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Folkow B The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med Suppl 4: 3s–22s, 1978. [DOI] [PubMed] [Google Scholar]
- 10.Folkow B, Grimby G, Thulesius O. Adaptive structural changes in the vascular walls in hypertension and their relation to the control of peripheral resistance. Acta Physiol Scand 44: 255–272, 1955. [DOI] [PubMed] [Google Scholar]
- 11.Gocke N, Keaney JF, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105: 1567–1572, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Green D Point: Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1233–1234, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Herrington DM, Fan L, Drum M, Riley WA, Pusser BE, Crouse JR, Burke GL, McBurnie MA, Morgan TM, Espeland MA. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk 8: 319–328, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuvin JT, Patel RR, Sliney KA, Pandian NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a non-invasive indicator of coronary artery disease. J Am Coll Cardiol 38: 1843–1849, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, and Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension 44: 134–139, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40: 505–510, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation 104: 1627–1632, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner G, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 86: 207–210, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 109: 2507–2510, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol 291: H3043–H3049, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Parker BA, Smithmyer SL, Jarvis SS, Ridout SJ, Pawelczyk JA, Proctor DN. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol 292: H1148–H1156, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Perticone F, Ceravolo R, Puji A, Ventura G, Iacopino S, Scozzafva A, Ferraro A, Chello M, Mastroroberto P, Verdechhia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Potter K, Reed CJ, Green DJ, Hankey GJ, Arnolda LF. Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound 6: 6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol 105: 1323–1332, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol 38: 1859–1865, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Silber HA, Ouyang P, Bluemke DA, Gupta SN, Foo TK, Lima JA. Why is flow-mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase-contrast magnetic resonance imaging. Am J Physiol Heart Circ Physiol 288: H822–H828, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Ter Avest E, Holewijn S, Stalenhoef AF, de Graaf J. Variation in non-invasive measurements of vascular function in healthy volunteers during daytime. Clin Sci (Lond) 108: 425–431, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans impact of arterial size. Am J Physiol Heart Circ Physiol 295: H1927–H1934, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol 292: H2874–H2880, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006. [DOI] [PubMed] [Google Scholar]