Abstract
Sphingosine-1-phosphate (S1P) regulates various molecular and cellular events in cultured endothelial cells, such as cytoskeletal restructuring, cell-extracellular matrix interactions, and intercellular junction interactions. We utilized the venular leakage model of the cremaster muscle vascular bed in Sprague-Dawley rats to investigate the role of S1P signaling in regulation of microvascular permeability. S1P signaling is mediated by the S1P family of G protein-coupled receptors (S1P1-5 receptors). S1P1 and S1P2 receptors, which transduce stimulatory and inhibitory signaling, respectively, are expressed in the endothelium of the cremaster muscle vasculature. S1P administration alone via the carotid artery was unable to protect against histamine-induced venular leakage of the cremaster muscle vascular bed in Sprague-Dawley rats. However, activation of S1P1-mediated signaling by SEW2871 and FTY720, two agonists of S1P1, significantly inhibited histamine-induced microvascular leakage. Treatment with VPC 23019 to antagonize S1P1-regulated signaling greatly potentiated histamine-induced venular leakage. After inhibition of S1P2 signaling by JTE-013, a specific antagonist of S1P2, S1P was able to protect microvascular permeability in vivo. Moreover, endothelial tight junctions and barrier function were regulated by S1P1- and S1P2-mediated signaling in a concerted manner in cultured endothelial cells. These data suggest that the balance between S1P1 and S1P2 signaling regulates the homeostasis of microvascular permeability in the peripheral circulation and, thus, may affect total peripheral vascular resistance.
Keywords: spingosine-1-phosphate receptor subtypes, vascular integrity, signal transduction
spingosine-1-phosphate (S1P), a serum-borne bioactive lipid mediator, regulates an array of biological activities in various cell types (13, 14, 28, 42). Most, if not all, S1P-regulated functions are mediated by the S1P family of G protein-coupled receptors (1, 20, 48). Five members of the S1P receptor family have been identified: S1P1, S1P2, S1P3, S1P4, and S1P5, previously known as endothelial differentiation gene (EDG)-1, -5, -3, -6, and -8, respectively (6). It was demonstrated that S1P receptor subtypes couple to different Gα polypeptides to regulate specific signaling pathways (2, 16, 46a). S1P receptor subtypes are expressed in distinct combinations in different cell types to produce an appropriate biological effect. For example, S1P1 and S1P3 are expressed in cultured endothelial cells (ECs) (18). The signaling pathways regulated by the S1P1 and S1P3 receptors in ECs are required for chemotaxis, adherens junction assembly, morphogenesis, and angiogenic response in vitro and in vivo (18–20). However, the functional outcomes resulting from the concerted effects of the distinct S1P receptor signaling pathways are unknown in a physiological environment.
In contrast to S1P1-stimulated chemotaxis, S1P2-mediated signaling was shown to negatively regulate cell migration (12, 38, 43). For example, embryonic fibroblasts isolated from S1P2-null mouse exhibited enhanced chemotaxis toward S1P, serum, and platelet-derived growth factor; this enhancement was reversed by reintroduction of S1P2 receptors (12). Recently, the mechanisms for S1P2-regulated inhibition of chemotaxis have been identified in several laboratories. It was shown that the inhibition of migration by S1P2 was mediated by Gα12/13-dependent Rac inactivation (43). In addition, Rho-dependent phosphatase and tensin homolog deleted on chromosome Ten (PTEN) activation was demonstrated to account for the S1P2-mediated inhibitory effect (38). These data indicate that S1P is able to control two opposing biological activities via the activation of specific S1P receptor signaling pathways: S1P1 stimulates chemotaxis, and S1P2 inhibits it. Thus the physiological responses of S1P may be an orchestrated manifestation between the signaling cascades activated by the different S1P receptor subtypes.
The development of pharmacological agonists/antagonists has significantly advanced our understanding of specific signaling and function regulated by distinct S1P receptor subtypes. For example, FTY720, a potent agonist of S1P1, S1P3, S1P4, and S1P5 receptors (4, 36, 45), is shown to downregulate S1P1 receptors on T and B lymphocytes and results in defective egress of these cells from spleen, lymph nodes, and Peyer's patch (24). Similar immune-suppressive activity was observed after treatment with SEW2871, a selective S1P1 receptor agonist that is not active for the S1P2-5 receptors (39). In addition, VPC 23019, a competitive antagonist of S1P1 and S1P3 receptors (8), has been used to examine the role of S1P1 in S1P-induced contraction and nitric oxide generation in isolated cerebral arteries (35). Furthermore, the role of S1P2-mediated signaling in inhibiting migration and contraction of vascular smooth muscle cells has been elucidated by using JTE-013, a selective S1P2 receptor antagonist (29, 30).
Cultured ECs abundantly express the S1P1 receptor subtype (18). In vitro analyses showed that S1P-mediated signaling pathways via S1P1 receptors regulate cytoskeletal structures (18), integrin activation (31, 46), and assembly of adherens (18, 20) and tight junctions (TJs) (17) in cultured ECs. Together, these in vitro lines of evidence imply that S1P may function as a novel modulator in regulation of vascular permeability in vivo. In agreement with these findings, we recently showed that S1P-mediated signaling pathways involving the S1P1 receptor stimulated TJ formation and, thus, enhanced transendothelial electrical resistance (TEER) in vitro (17). In the present study, we utilized the venular leakage model in the cremaster muscle vasculature of Sprague-Dawley (SD) rats to examine the molecular basis of S1P-regulated vascular permeability in vivo. We showed that S1P/S1P1 signaling protected against microvascular permeability in vivo. Importantly, evidence presented in the present study suggests that the homeostasis of peripheral microvascular permeability is regulated by the balance between S1P1- and S1P2-mediated signaling pathways. This study is the first to demonstrate how the functional balance between S1P1 and S1P2 signaling regulates the physiology of the vasculature.
MATERIALS AND METHODS
Reagents.
S1P (Biomol, Plymouth Meeting, PA) was dissolved in methanol (0.5 mg/ ml). SEW2871, JTE-013, and FTY720 (Cayman Chemical, Ann Arbor, MI) and VPC 23019 (Avanti, Alabaster, AL) were solubilized in ethanol (20 mg/ml) and divided into aliquots, which were vacuumed dried and stored at −20°C. When needed, the aliquots were resuspended in 4% fatty acid-free BSA (Sigma, St. Louis, MO) by sonication to make a stock solution of 1 mg/ml. Polyclonal rabbit anti-S1P1 and anti-S1P2 were kindly provided by Dr. Suzanne Mandala (Merck Research Laboratories). These antibodies have been shown to specifically immunoreact with the S1P1 and S1P2 receptor subtypes (5, 41). Purified mouse anti-rat platelet EC adhesion molecule (PECAM)/CD31 was purchased from Chemicon (Temecula, CA). Goat anti-S1P1 and anti-S1P3 and mouse anti-α-actin were obtained from Santa Cruz Biotechnology. Alexa 488-, Alexa 594-, and Alexa 647-conjugated secondary antibodies were purchased from Molecular Probes (Carlsbad, CA). Other reagents, unless specified, were purchased from Sigma.
Microvascular permeability assay.
In accordance of National Institutes of Health guidelines for animal research, all animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Male SD rats (200–250 g body wt; Harlan, Indianapolis, IN) were anesthetized with pentobarbital sodium (50 mg/kg ip), and a tracheal cannula was inserted to maintain a patent airway. Animals were placed on a heating pad to maintain body temperature at 37 ± 1°C. Mean arterial blood pressure (MABP) and heart rate (HR) were continuously monitored through a polyethylene catheter (PE-50) inserted into the carotid artery and connected to a transducer and a blood pressure analyzer (Micro-Med, Nicholasville, KY).
The microvascular permeability assay in cremaster muscle vascular bed was performed essentially as we described previously (22). Briefly, the skin of the right half of the scrotum was opened and the cremaster muscle was incised longitudinally, with care taken to keep the principal nerves and blood vessels to the muscle intact. The cremaster muscle was spread with sutures over the bottom of a tissue bath that contained 60 ml of modified Krebs bathing solution (in mM: 113 NaCl, 25.5 NaHCO3, 11.6 dextrose, 4.7 KCl, 2.6 CaCl2, 1.2 MgSO4, and 1.2 KH3PO4). The pH of the bath was maintained by a constant supply of CO2 gas, and the temperature was maintained at 35°C by a heating coil. The rat and the cremaster muscle preparation were positioned on a modified stage of a microscope (model MM-11, Nikon, Tokyo, Japan) so that the cremaster muscle, which is ∼200–250 μm thick, could be observed by transmitted light or epi-illumination (22, 23).
After the surgical preparation, there was a 60-min equilibration period. At 30 min of the equilibration period, stock solutions of the agonists/antagonists in BSA were injected via the carotid artery cannula. In addition, the agonists/antagonists were locally added to the tissue bath at the same concentrations at this time. When JTE-013 or VPC 23019 was injected, S1P was injected via the cannula 10 min later. Before each experiment, autofluorescence of the observed area was recorded over a standard range of camera gains (22, 23). FITC tagged to BSA (300 μg/ml) was injected intra-arterially (0.2 ml/100 g body wt) and allowed to circulate for ∼10 min. Cremaster muscle circulation was surveyed to ensure that there was no spontaneous leakage in the observed area, which would indicate compromised vascular integrity.
Venules were identified by observation of the topology of the cremaster muscle circulation and blood flow direction. A rectangular area of interest (AOI), ∼2,000 μm2 in the interstitium adjacent to a venular wall, was assessed for venular leakage. In each rat, three different venular segments were chosen for observation. There was no spontaneous leakage or other visible vessels in the chosen AOIs. Microvascular leakage was measured in animals injected with S1P, SEW2871, JTE-013 with or without S1P, VPC 23019 with or without S1P, and FTY720. Animals injected with vehicle (4% fatty acid-free BSA) alone were used as a control group. Subsequently, leakage was induced by sequential addition of 10−8–10−4 M histamine to the tissue bath containing surgically prepared cremaster muscle. Each concentration of histamine was additively administered for 10 min to the surgically exposed cremaster muscle vascular bed. Microvascular leakage was assessed and quantitated by the presence of FITC-albumin in the extravenular space at 0, 5, and 10 min after histamine addition. The AOIs for three venular segments in the cremaster vascular bed were observed for 10 min after each histamine application. Histamine-induced microvascular leakage for each animal was determined as an average of data obtained from the measurements of the fluorescence intensity in AOIs in each of the three venular segments. At least three animals were used for each group of agonist/antagonist treatment.
The histamine-induced microvascular leakage, shown by the presence of FITC-albumin in the interstitium, was imaged and quantitated as described previously (22). An epi-illumination system, consisting of a mercury arc lamp and a Ploem system with appropriate filters, was used to excite intravascular FITC. The AOI was exposed to blue light (∼490 nm) for 10–15 s with a light power density of 2 J/cm2. The microscope images were acquired by a light-sensitive silicon-intensified tube camera (model C2400, Hamamatsu) and image acquisition system (Marvel G450-eTV, Matrox Graphics). The camera output voltage was standardized with a 50 ng/ml fluorescein diacetate standard (Eastman Kodak, Rochester, NY) for each experiment.
Immunofluorescent analysis.
Rat cremaster muscle tissues were surgically excised and immediately fixed in 4% formaldehyde for 2 h. After they were soaked in 0.3 M sucrose-PBS for 16 h, tissues were sectioned with a cryostat. Sections (8 μm) were stained with PECAM/CD31, α-actin, or S1P receptor subtype-specific antibodies followed by Alexa 488-, Alexa 594-, or Alexa 647-conjugated secondary antibodies. Confocal images were obtained using a confocal microscope (FluoView FV1000, Olympus).
RESULTS
S1P administration via carotid artery was unable to protect from histamine-induced microvascular leakage.
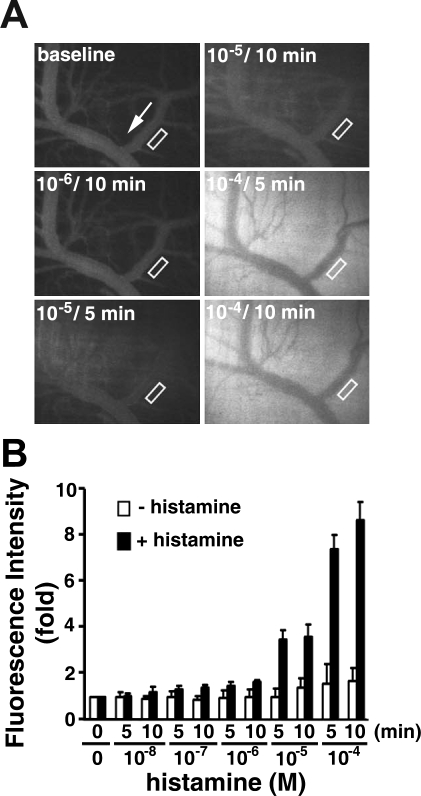
We studied microvascular leakage in venules of the cremaster muscle in SD rats to determine the effect of S1P on regulation of vascular integrity in vivo. Histamine, a biogenic amine known to dilate blood vessels and make vessel walls abnormally permeable, was used to induce microvascular leakage. Sequential addition of 10−8–10−4 M histamine resulted in a dose-dependent increase of FITC-albumin in the interstitium adjacent to venules, indicating microvascular leakage (Fig. 1). Leakage of FITC-albumin was most pronounced at higher doses of histamine. There was a 3.54 ± 0.43 and 7.56 ± 0.59 fold (n = 11) increase in fluorescence intensity in the extravenular space after 5 min of treatment with 10−5 and 10−4 M histamine, respectively. In contrast, without histamine treatment, there was only a 1.01 ± 0.36 and 1.57 ± 0.89 fold (n = 2) increase of fluorescence intensity in the interstitium after 5 min (Fig. 1B). Because of the significant difference in microvascular leakage induced by higher doses of histamine, to test the effects of S1P, SEW2871, JTE-013, VPC 23019, or FTY720, we used only high concentrations (10−5 and 10−4 M) of histamine to induce microvascular leakage.
Fig. 1.
Histamine induces macromolecular leakage in cremaster venular microvessels of Sprague-Dawley (SD) rats. A: images recorded before (baseline) and 5 and 10 min after sequential application of increasing concentrations of histamine. White arrow indicates flow direction in veins. B: quantitation of changes in fluorescence intensity with (n = 11) or without (n = 2) histamine in extravascular space. Venular microvascular leakage was assessed by measurement of fluorescence intensity in area of interest (AOI; i.e., areas enclosed in rectangles in A). Values are means ± SE.
Injection of vehicle (4% fatty acid-free BSA) did not alter MABP and HR (103 ± 5 mmHg and 380 ± 10 beats/min, n = 3) in the control group compared with values before injection (104 ± 3 mmHg and 361 ± 11 beats/min). Injection of S1P, SEW2871, and JTE-013 + S1P did not have an effect on MABP or HR: 104 ± 9 mmHg and 364 ± 5 beats/min (n = 5), 101 ± 8 mmHg and 376 ± 16 beats/min (n = 3), and 112 ± 18 mmHg and 368 ± 19 beats/min (n = 3), respectively. These values were similar to MABP and HR in respective controls for S1P, SEW2871, and JTE-013 + S1P groups that were injected with vehicle only: 100 ± 4 mmHg and 366 ± 14 beats/min (n = 3), 104 ± 14 mmHg and 355 ± 17 beats/min (n = 3), and 113 ± 8 mmHg and 340 ± 11 beats/min (n = 2), respectively.
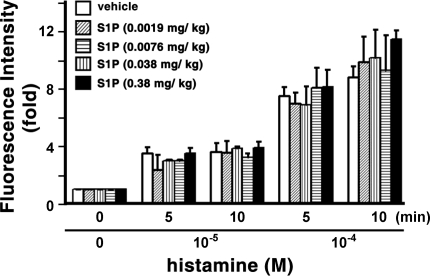
Subsequently, we examined the effects of S1P on histamine-induced venular leakage in the cremaster muscle microvascular beds of SD rats. Animals were arterially injected with 0.0019–0.38 mg/kg body wt S1P; then the surgically exposed cremaster muscle vascular beds were treated with histamine. There was no statistical difference in histamine-induced microvascular leakage between the S1P- and vehicle-injected groups (Fig. 2; also see supplemental Fig. 1 in the online version of this article). Although leakage was greater in the group injected with 0.38 mg/kg body wt S1P than in the control group at 10 min after 10−4 M histamine treatment (Fig. 2), this difference is unlikely to be of physiological significance, since leakage of FITC-albumin into the extravenular space was maximal in vehicle- and S1P-injected rats at this concentration of histamine (see supplemental Fig. 1).
Fig. 2.
Arterial injection of spingosine-1-phosphate (S1P) is unable to diminish histamine-induced macromolecular leakage of cremaster venular microvessels. Changes in fluorescence intensity were measured after injection of vehicle (n = 11) or S1P (n = 3–5 for each dose). Microvascular leakage was assessed by measurement of fluorescence intensity of FITC-albumin in extravascular space. Values are means ± SE.
S1P1 and S1P2 receptor subtypes are expressed in the vascular endothelium of the cremaster muscle.
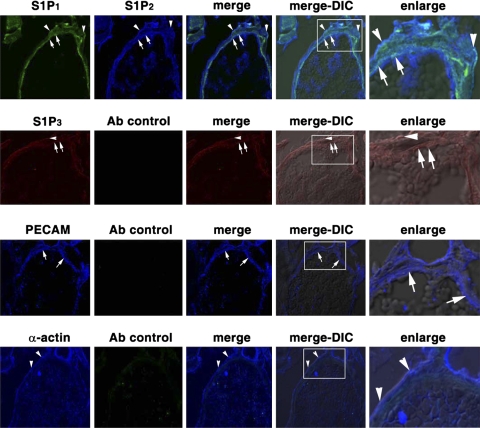
We next used immunostaining to determine the expression of S1P receptor subtypes in the cremaster muscle vascular bed. The endothelium and smooth muscle cells of the cremaster muscle vasculature were identified by immunostaining with PECAM and α-actin antibodies (Fig. 3; see supplemental Fig. 2), respectively. S1P1 and S1P2 receptors were clearly detected in the endothelium and smooth muscle cells of the cremaster muscle vessels (Fig. 3). In contrast, S1P3 receptors were weakly detected in the vasculature of the cremaster muscle vessels (Fig. 3). The immunostaining assay is specific, because no signals were detected when immunostaining was performed in the absence of primary antibodies (Fig. 3; see supplemental Fig. 2). Moreover, S1P1 and S1P2 receptors are present in endothelium and smooth muscle cells of the cremaster muscle arterial and venular vessels in SD rats (see supplemental Fig. 2B).
Fig. 3.
Expression of S1P1 and S1P2 in the venule of the cremaster muscle circulatory bed. Cremaster specimens were surgically removed from SD rats. After sections were fixed with 4% paraformaldehyde and embedded in OCT compound, frozen 8-μm-thick sections were immunostained with primary antibodies and then with Alexa 488 (green)-, Alexa 594 (red)-, or Alexa 647 (blue)-conjugated secondary antibodies and analyzed by a confocal microscope. Top to bottom: goat anti-S1P1 and rabbit anti-S1P2 primary antibodies and Alexa 488-donkey anti-goat and Alexa 647-donkey anti-rabbit secondary antibodies; specimens analyzed with Alexa 488 and Alexa 647 filters (row 1); goat anti-S1P3 primary antibody and Alexa 594-donkey anti-goat secondary antibody; specimens analyzed with Alexa 594 and Alexa 647 filters (row 2); mouse anti-rat platelet endothelial cell adhesion molecule (PECAM) primary antibody and Alexa 647-donkey anti-mouse and Alexa 488-donkey anti-goat secondary antibodies; specimens analyzed with Alexa 488 and Alexa 647 filters (row 3); mouse anti-α-actin primary antibody and Alexa 647-donkey anti-mouse secondary antibody; specimens analyzed with Alexa 488 and Alexa 647 filters (row 4). Endothelium (arrows) and smooth muscle cells (arrowheads) were identified by staining with PECAM (row 3) and α-actin (row 4) antibodies, respectively. S1P1 and S1P2 receptors are expressed in endothelium (arrows) and smooth muscle cells (arrowheads) of cremaster muscle venules (row 1), and S1P3 receptors were weakly detected in the venules of cremaster muscle vasculature (row 2). Enlarge, enlarged images of boxed areas in merge-differential interference contrast (DIC) image; merge-DIC, fluorescence merged images overlapped with DIC images.
S1P1-mediated signaling in histamine-induced microvascular leakage.
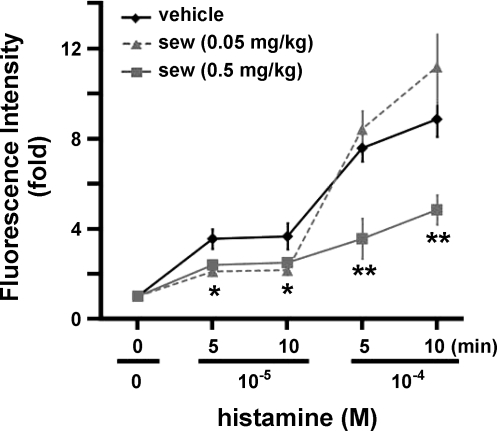
SEW2871 is a selective S1P1 receptor agonist that is not active on the S1P2–5 receptors (39). Thus we injected SEW2871 via the carotid artery to specifically activate the S1P1-mediated signaling pathway and examine the role of S1P1-mediated signaling in regulation of microvascular integrity of the peripheral vasculature in vivo. Injection of 0.05 mg/kg body wt SEW2871 significantly diminished the leakage of FITC-albumin into the extravenular space in rats treated with 10−5 M histamine (Fig. 4). There was little increase in microvascular leakage after application of 10−5 M histamine in animals injected with 0.05 mg/kg body wt SEW2871 (2.10 ± 0.20 and 2.15 ± 0.21 fold increase at 5 and 10 min, respectively, n = 3) compared with the significantly higher values in control (vehicle-injected) animals (3.54 ± 0.43 and 3.65 ± 0.58 fold increase at 5 and 10 min, respectively, n = 11, P < 0.01, SEW2871 vs. control vehicle at the same time points). At 10−4 M, histamine induced little difference in leakage between SEW2871-treated (8.40 ± 0.81 and 11.16 ± 1.09 fold increase at 5 and 10 min, respectively, n = 3) and control (7.56 ± 0.59 and 8.84 ± 0.77 fold increase at 5 and 10 min, respectively, n = 11) rats. Although the data indicate greater leakage at 10−4 M histamine in rats treated with 0.05 mg/kg body wt SEW2871, this is not physiologically important, inasmuch as FITC-albumin had saturated the interstitium at this dose (see supplemental Fig. 3).
Fig. 4.
SEW2871 (sew) inhibits histamine-induced venular leakage. Changes in fluorescence intensity were measured after injection with vehicle (n = 11), 0.05 mg/kg body wt SEW2871 (n = 3), or 0.5 mg/kg body wt SEW2871 (n = 3). Values are means ± SE. *P < 0.01 (t-test), SEW2871 at both doses vs. vehicle at respective time points. **P < 0.01 (t-test), 0.5 mg/kg body wt SEW2871 vs. vehicle at respective time point.
Strikingly, injection of a higher dose of SEW2871 (0.5 mg/kg body wt) inhibited venular leakage at 10−5 and 10−4 M histamine (Fig. 4). In rats treated with this dose of SEW2871, there was scarcely an increase in microvascular leakage at 10−5 M histamine (2.39 ± 0.14 and 2.49 ± 0.18 fold increase at 5 and 10 min, respectively, n = 3). These values were significantly less than in respective control (vehicle-injected) animals (P < 0.01, SEW2871 vs. vehicle at the same time points; Fig. 4). Furthermore, 10−4 M histamine induced maximal microvascular leakage in rats injected with vehicle (see supplemental Fig. 3). However, 0.5 mg/kg body wt SEW2871 dramatically inhibited the microvascular leakage induced by 10−4 M histamine (see supplemental Fig. 3). Fluorescence intensity increased 7.56 ± 0.59 and 8.84 ± 0.77 fold in vehicle-injected rats at 5 and 10 min, respectively, after 10−4 M histamine but increased only 3.55 ± 0.89 and 4.83 ± 0.66 fold in rats injected with 0.5 mg/kg body wt SEW2871 at 5 and 10 min, respectively (n = 3, P < 0.01, SEW2871 vs. vehicle at the same time points).
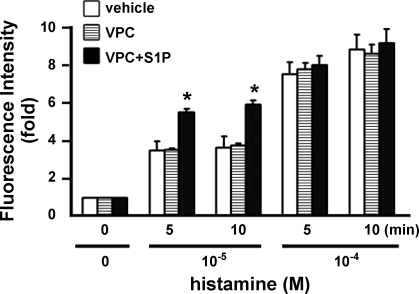
It has been shown that treatment with VPC 23019 antagonizes S1P1- and S1P3-mediated signaling cascades (8, 35). Therefore, the role of S1P1-regulated signaling in control of vascular permeability was further examined by injection of SD rats with VPC 23019 or VPC 23019 + S1P. There is no statistical difference among vehicle, VPC 23091, and VPC 23019 + S1P in vascular leakage induced by 10−4 M histamine (Fig. 5; see supplemental Fig. 4). However, VPC 23019 + S1P significantly enhanced vessel leakage induced by 10−5 M histamine (P < 0.01; Fig. 5, see supplemental Fig. 4). These data strongly suggest that S1P1-mediated signaling plays a critical role in regulation of vessel integrity in cremaster muscle vascular beds of SD rats.
Fig. 5.
Coinjection of VPC 23019 (VPC) and S1P enhances venular leakage. Changes in fluorescence intensity were measured after injection with 0.05 mg/kg body wt VPC 23019 (n = 6) or 0.05 mg/kg body wt VPC 23019 + 0.5 mg/kg body wt S1P (n = 5) before and after application of histamine. VPC 23019 + S1P significantly increases venular leakage at 10−5 M histamine. *P < 0.01 (t-test), VPC 23019 + S1P vs. VPC 23019 or vehicle at respective time points.
S1P2-mediated signaling in histamine-induced microvascular leakage.
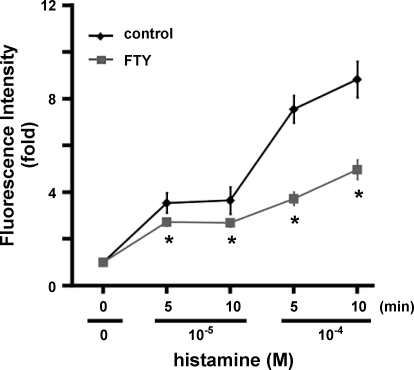
Two pharmacological reagents were utilized to examine the role of S1P2 in regulation of vascular permeability in cremaster muscle vasculature. FTY720 is phosphorylated by sphingosine kinase, which then functions as a potent agonist of S1P1, S1P3, S1P4, and S1P5 receptors (4, 36, 45). However, FTY720 has no effect on activation of signaling cascades transduced by S1P2 receptors (4, 24). In the present study, similar to administration of SEW2871 (Fig. 4), treatment with FTY720 significantly inhibited histamine-induced venular leakage (P < 0.01, FTY720 vs. vehicle at the same time points; Fig. 6, see supplemental Fig. 5). This result strongly argues that the S1P2-regulated events are attributable to the inability of S1P to prevent vascular leakage (Fig. 2).
Fig. 6.
FTY720 (FTY) protects against leakage of venular microvessels induced by histamine. Changes in fluorescence intensity were measured after injection with vehicle (n = 11) or 1 mg/kg body wt FTY720 (n = 3). *P < 0.01 (t-test), FTY720 vs. control at respective time points.
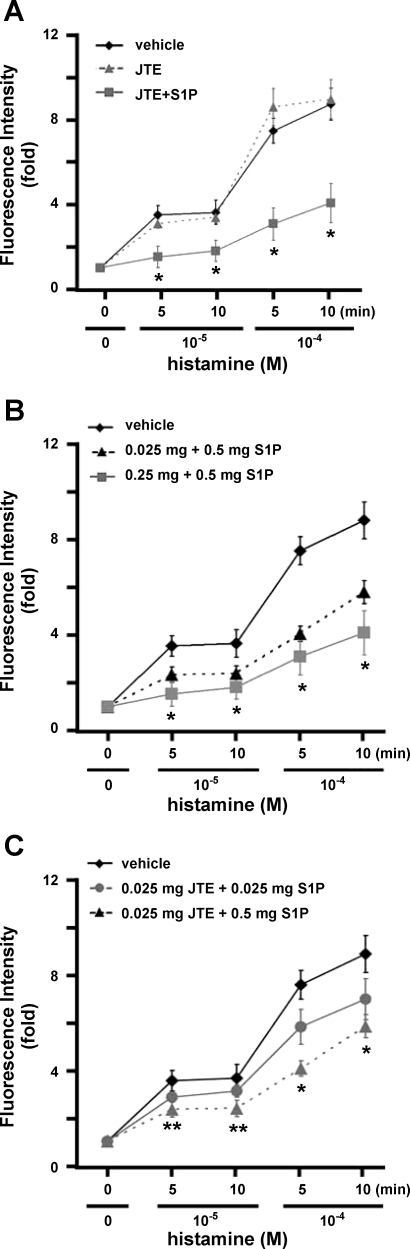
We next investigated whether S1P was able to protect against microvascular leakage in vivo if S1P2-mediated signaling was inhibited by pretreatment with JTE-013, a highly selective antagonist of the S1P2 receptor (29, 50). Administration of JTE-013 + S1P significantly facilitated the leakage inhibition effects of S1P at 10−5 and 10−4 M histamine (P < 0.01, JTE-013 + S1P vs. vehicle at the same time points; Fig. 7A). Similar to SEW2871 administration (Fig. 4), there was little vascular leakage in the presence of 10−5 M histamine when rats were treated with JTE-013 + S1P (1.52 ± 0.52 and 1.81 ± 0.51 fold increase at 5 and 10 min, respectively, n = 3), whereas there was obvious leakage in the absence of JTE-013 + S1P (Fig. 7A). Furthermore, maximal microvascular leakage induced by 10−4 M histamine was significantly diminished in rats that were treated with JTE-013 + S1P (Fig. 7A, see supplemental Fig. 6A). The fluorescence intensities of the leaked albumin in rats treated with 10−4 M histamine for 5 and 10 min in the presence of JTE-013 + S1P were increased 3.12 ± 0.77 and 4.18 ± 0.94 fold, respectively (n = 3). Treatment with JTE-013 alone did not prevent histamine-induced leakage (Fig. 7A, see supplemental Fig. 6A), indicating that protection of microvascular leakage by JTE-013 + S1P is attributable to S1P. Moreover, pretreatment with JTE-013 dose-dependently enhanced S1P effects on inhibition of histamine-induced microvascular leakage (Fig. 7B, see supplemental Fig. 6B). Also, S1P, in a dose-dependent manner, diminished vessel leakage in the presence of JTE-013 (Fig. 7C, see supplemental Fig. 6B). Together, these results suggest that inhibition of S1P2 signaling greatly facilitates the S1P1-mediated enhancement of microvascular endothelial integrity.
Fig. 7.
S1P inhibits histamine-induced venular leakage in the presence of JTE-013 (JTE). A: JTE-013 + S1P inhibits venular leakage. SD rats were injected with vehicle (n = 11), 0.25 mg/kg body wt JTE-013 (n = 3), or 0.25 mg/kg body wt JTE-013 + 0.5 mg/kg body wt S1P (n = 3). Values are means ± SE. *P < 0.01 (t-test), JTE-013 + S1P vs. vehicle and JTE-013 at respective time points. B: JTE-013 dose-dependently facilitates the inhibitory effect of S1P. SD rats were injected with vehicle, 0.025 mg/kg body wt JTE-013 + 0.5 mg/kg body wt S1P (n = 3), or 0.25 mg/kg body wt JTE-013 + 0.5 mg/kg body wt S1P (n = 3). *P < 0.01, both doses of JTE-013 + S1P vs. vehicle at respective time point. C: S1P dose-dependently inhibits venular leakage in the presence of JTE-013. Rats were injected with vehicle, 0.025 mg/kg body wt JTE-013 + 0.025 mg/kg body wt S1P, or 0.025 mg/kg body wt JTE-013 + 0.5 mg/kg body wt S1P (n = 3 for each dose of S1P). *P < 0.01, JTE-013 + both doses of S1P vs. vehicle at 10−4 M histamine. **P < 0.01, JTE-013 + 0.5 mg/kg body wt of S1P vs. vehicle at 10−5 M histamine.
S1P1 and S1P2 concertedly regulate integrity of EC function in vitro.
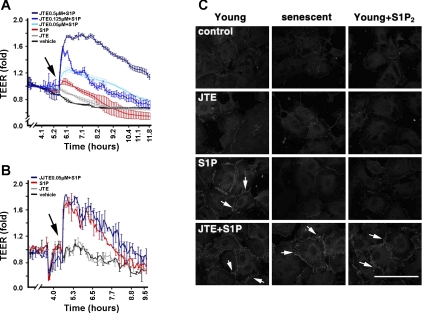
Recently, we showed that early-passaged ECs in vitro abundantly express S1P1 receptors and barely express S1P2 receptors. In contrast, S1P2 receptors are markedly increased in senescent ECs (9). The upregulation of S1P2 receptors was shown to mediate the impaired wound-healing and angiogenic responses in senescent ECs (9). Thus cultured young and senescent ECs were used as an in vitro model to examine whether endothelial S1P1- and S1P2-mediated signaling cascades are able to concertedly regulate vascular barrier function in vivo. A minimal rise of TEER, an in vitro measurement of endothelial barrier function (9, 17), in senescent ECs after S1P treatment is shown in Fig. 8A. Pretreatment with JTE-013 dose-dependently increased the S1P-stimulated increase of TEER in senescent ECs (Fig. 8A). Neither vehicle nor JTE-013 alone had an effect on the increase of TEER in senescent ECs. In sharp contrast, S1P stimulated a maximal increase of TEER in young ECs (Fig. 8B), which barely express S1P2 receptors (9, 18). Pretreatment with JTE-013 had no effect on the S1P-stimulated increase of TEER in young ECs. These data indicate that the S1P-induced increase of TEER is controlled by the expression profile of S1P1 and S1P2 receptor subtypes in cultured ECs.
Fig. 8.
JTE-013 restores the effect of S1P on regulation of barrier integrity of senescent endothelial cells (ECs). A: S1P stimulates increase of transendothelial electrical resistance (TEER) in JTE-013-pretreated senescent ECs. Cultured senescent ECs [cumulative population-doubling level (CPDL) ∼60], abundantly expressing S1P1 and S1P2 receptors (9), were washed and pretreated with different doses of JTE-013 for 15 min. After addition of S1P (0.5 μM, arrow), TEER rises were measured in a real-time manner (9, 17). B: JTE-013 does not augment S1P-stimulated TEER rise in young ECs. Human umbilical vein ECs at early passage (CPDL ∼15), abundantly expressing S1P1 only (9, 18), were pretreated with JTE (0.5 μM) for 15 min and then stimulated with or without S1P (0.5 μM). S1P stimulates maximal TEER rise and JTE-013 pretreatment does not further increase S1P-induced TEER rise in young ECs. JTE-013 or S1P alone in A and B were 0.5 μM each. Values are means ± SE of duplicate determinations of a representative experiment, which was repeated 3 times with similar results. C: JTE-013 pretreatment restores S1P-stimulated tight junction (TJ) formation in S1P2-expressing ECs. Cells were washed, pretreated with JTE-013 (0.5 μM) for 15 min, and then stimulated with S1P (0.5 μM) for 15 min. Cells were fixed and then immunostained with anti-zonula occludens-1, as described previously (17). Left: young ECs; middle: senescent ECs; right: young ECs expressing S1P2 receptor by transduction with adenoviral particles carrying S1P2 receptors (9). S1P stimulates TJ formation in young ECs (arrows), whereas S1P is unable to stimulate TJ formation in senescent and young ECs expressing S1P2 receptors. S1P is able to stimulate TJ formation in senescent and young ECs expressing S1P2 after pretreatment with JTE-013 (arrows). Scale bar, 27 μm.
We showed that S1P stimulates the formation of endothelial TJs (17). Knockdown of zonula occludens-1 (ZO-1), a TJ-associated polypeptide, significantly abrogated the S1P-induced increase of TEER (17), indicating that endothelial TJs play an essential role in the S1P-induced increase of TEER. Thus we next investigated whether endothelial S1P1 and S1P2 receptors, in combination, regulate the formation of TJs. S1P was able to stimulate TJ formation in young ECs (Fig. 8C), but not in senescent ECs or in young ECs ectopically expressing S1P2 receptors (Fig. 8C). The inability of senescent and young ECs expressing S1P2 receptors to form TJs was reversed by pretreatment with JTE-013 (Fig. 8C). Young ECs transduced with control adenoviral particles carrying β-galactosidase (9, 17, 19) behave identically to young ECs without adenoviral transduction (data not shown). Also, treatment with vehicle or JTE-013 alone had no effect on TJ formation in young, senescent, and S1P2-expressing young ECs. Together, these results suggest that endothelial TJs and barrier integrity are regulated by S1P1- and S1P2-mediated signaling in a coordinated manner.
DISCUSSION
Cultured human umbilical vein ECs abundantly express S1P1 receptors (18, 19). Using cultured human umbilical vein ECs as an in vitro model system, we and others showed that S1P signaling via the plasma membrane S1P1 receptors regulates various endothelial functions (18, 19). For example, treatment of the cultured ECs with S1P resulted in dramatic cytoskeletal rearrangement, including formation of stress fibers and cortical actins (18). Moreover, S1P stimulated the formation of vascular endothelial cadherin-based adherens junctions, which are critical in regulation of S1P-mediated morphogenesis of ECs (18). Recently, we showed that S1P enhances the cell-extracellular matrix interaction by activating endothelial αvβ3-integrin via the S1P1/Gi/Rho family of GTPases signaling pathway (46). Furthermore, it was shown that S1P stimulation results in formation of two distinct ZO-1 complexes that regulate the endothelial TJ formation and chemotactic response in ECs (17). The ability of S1P to control cytoskeletal architecture, integrin-extracellular matrix interactions, and intercellular interactions in vitro suggests that S1P/S1P1-mediated signaling pathways play a critical role in regulating the barrier function of the endothelium in vivo. In vitro studies showed that S1P treatment resulted in an increase in endothelial electrical resistance (10), a measurement of endothelial barrier integrity, and a decrease in albumin permeability across cultured EC monolayers (25). Moreover, it was shown that S1P-mediated cytoskeletal rearrangement, particularly the Rac and p21-associated kinase-dependent cortical actin assembly, plays an important role in regulating endothelial barrier integrity in vitro (10, 25).
Subsequently, we used the electrical cell-substrate impedance-sensing assay (Applied Biophysics) to investigate the mechanisms of S1P-regulated barrier function in vascular ECs (17). A robust increase of TEER was observed immediately after S1P treatment. This observation supports the notion that S1P is able to enhance endothelial barrier function in vitro. It should be noted that the S1P-induced barrier integrity was sustained for >6–10 h after S1P treatment and, thus, was not a transient event. S1P-enhanced TEER was markedly abrogated in S1P1-knockdown ECs (small interference S1P1), but not in control cells stably expressing small interfering RNA for luciferase (siLuc). This indicates that S1P-enhanced TEER is primarily controlled by the S1P1 receptor. This conclusion is supported by the observation that S1P-enhanced endothelial barrier integrity was abrogated in the presence of pertussis toxin, inasmuch as S1P1 signaling is dependent on the Gi heterotrimeric G protein. In addition, treatment with Ly-294002 (an inhibitor of phosphoinositide 3-kinase) or adenoviral particles carrying dominant-negative Akt or Rac constructs significantly inhibited the S1P-enhanced TEER. Importantly, we demonstrated that S1P significantly stimulates TJ formation in cultured ECs (17). Knockdown of ZO-1, a critical polypeptide associated with TJ plaque, markedly abrogated the S1P-induced increase of TEER in ECs (17). Together, these results indicate that the S1P-enhanced barrier function of vascular ECs is controlled by endothelial TJs via the S1P1-mediated Gi/phosphoinositide 3-kinase/Akt/Rac signaling pathway.
It was shown that S1P treatment reduces the endotoxin-induced extravasation of albumin in lung tissue (32). Also, platelet-activating factor-induced hydraulic conductivity in rat mesenteric venules was markedly inhibited by S1P addition (27). These studies suggest that S1P plays a critical role in regulating vascular integrity in vivo. Although the effect of S1P on inhibition of platelet-activating factor-induced permeability was shown to be pertussis toxin sensitive, the receptor subtype(s) involved in this inhibitory effect remains unidentified. Intriguingly, in the present study, we observed that S1P was unable to protect histamine-induced venular leakage in the cremaster muscle microvessels of SD rats (Fig. 2, see supplemental Fig. 1). The molecular basis of this discrepancy is unknown. It is possible that different tissues express distinct S1P receptor subtypes and, thus, exhibit contrasting responsiveness in S1P-regulated vascular permeability. In agreement, Sanchez and colleagues (37) showed that S1P2-mediated signaling regulates H2O2-induced vascular permeability in a rat perfused lung model, indicating that the S1P effect on regulation of functional integrity may be tissue specific.
Recently, Sanna and colleagues (40) showed that in vivo administration of SEW2871 markedly inhibited VEGF-induced vessel leakage, whereas administration of S1P1 antagonist greatly stimulated capillary leakage in lung tissue. On the basis of this observation, they proposed the “S1P-S1P1” rheostat model for control of capillary permeability under basal physiological conditions (33, 40). Similarly, we showed that treatment with S1P1 agonists significantly protected cremaster muscle venules from histamine-induced vessel leakage (Figs. 4 and 6). However, treatment with S1P1 antagonist alone did not markedly alter the basal (without histamine addition; see supplemental Fig. 4) or histamine-induced venular leakage of cremaster muscle vasculature (Fig. 5, see supplemental Fig. 4) compared with treatment with vehicle. In contrast, treatment with S1P after inhibition of S1P1 signaling markedly enhanced the histamine-induced venular leakage (Fig. 5, see supplemental Fig. 4). Moreover, treatment with JTE-013 alone to block S1P2 signaling had no effect on venular leakage (Fig. 7A), whereas JTE-013 + S1P significantly inhibited histamine-induced leakage in a dose-dependent manner (Fig. 7, B and C). Together, these data suggest that the balance of S1P1 and S1P2 signaling may play a more critical role than the S1P-S1P1 rheostat model in regulation of venular leakage of cremaster muscle vasculature.
Strikingly, a recent study showed that S1P administration via the airways, but not via the vasculature, induces lung leakage (11). Moreover, it was shown that S1P3 receptors are expressed in type I and II alveolar epithelial cells. The S1P/S1P3-mediated signaling regulates epithelial integrity and acts additively with TNF in compromising respiratory barrier function (11). The molecular basis of this S1P3-mediated barrier breakdown in alveolar epithelial cells is completely unknown. Nevertheless, the results of the present study, together with mounting evidence (10, 17, 25, 27, 32, 37) for the role of S1P in EC layer integrity, suggest that vascular barrier integrity is concertedly regulated by S1P1- and S1P2-mediated signaling cascades, whereas S1P3-mediated signaling pathways are important in controlling epithelial barrier function.
In the present study, we employed histamine-induced microvascular leakage of the cremaster muscle vascular bed in SD rats to investigate the underlying mechanisms of S1P-regulated microvascular permeability in vivo. Injection of agonists and topical application of histamine did not substantially alter MABP or HR in these SD rats. This finding suggests that any quantitative change in microvascular leakage should have been the result of the specific biological effect of agonist/antagonist, rather than a generic response to stress, surgical procedure, or systemic changes. However, our finding that there are no changes in MABP and HR is not definitive. There might be a minor alteration; however, the number of experiments (n = 3–5) and time for observation (1–1.5 h) might not be enough to derive statistical power of analysis for statistical difference.
In contrast to previous in vivo studies (27, 32), we found that arterial injection of S1P (0.0019–0.38 mg/kg body wt) was unable to protect against microvascular leakage of cremaster muscle venules induced by histamine application (Fig. 2). However, specific activation of S1P/S1P1-mediated signaling by SEW2871 significantly diminished histamine-induced macromolecular leakage of cremaster muscle venules (Fig. 4). A similar effect on inhibition of venular leakage was observed when we used FTY720 (Fig. 6), which is phosphorylated after in vitro and in vivo administration and is shown to activate S1P1 receptors (36, 45). Importantly, S1P treatment significantly abrogated histamine-induced venular leakage when rats were pretreated with JTE-013, whereas JTE-013 alone had no effect on venular leakage (Fig. 7). Thus it is unlikely that the inability of S1P to protect against histamine-induced venular leakage resulted from an inability of S1P to reach the endothelium of cremaster muscle microvascular bed or to degrade in the circulation. Instead, these data suggest that arterially injected S1P is able to reach the endothelium of the cremaster muscle vasculature, which expresses S1P1 and S1P2 receptors (Fig. 3, see supplemental Fig. 2) and that S1P2 signaling antagonizes the S1P1-regulated endothelial barrier function in the cremaster muscle circulatory bed.
Recently, it was shown that S1P/S1P2 signaling inhibited Rac activation and cell migration in a Chinese hamster ovary cell line ectopically expressing S1P2 receptors (43). This S1P2-mediated inhibition was subsequently demonstrated to be physiologically relevant in several cell lines, such as mouse embryonic fibroblasts, mast cells, glioblastoma, and vascular smooth muscle cells (12, 21, 34, 38, 44, 47). Moreover, we recently observed that S1P1 and S1P2 receptors were expressed in the endothelium of mouse aorta (unpublished observation). Together, these data suggest that S1P1-mediated enhancement of vascular integrity may be counteracted by S1P2 signaling in cremaster muscle vasculature, which results in the inability of S1P to inhibit histamine-induced venular leakage. Indeed, S1P2 receptors were found to be expressed in cremaster muscle vasculature (Fig. 3, see supplemental Fig. 2). Thus we examined the effect of S1P on vascular permeability after JTE-013-induced inhibition of S1P2 signaling. JTE-013 is a potent, selective S1P2 antagonist that specifically binds to the human and rat S1P2 receptor with IC50 of 17 and 22 nM, respectively (15, 29, 30), and reverses the inhibitory effects of S1P2 signaling on cell migration of vascular ECs and smooth muscle cells (3, 7, 15, 29, 30). Importantly, S1P administration was able to significantly prevent histamine-induced microvascular leakage following functional blockage of the S1P2 receptor by JTE-013 treatment (Fig. 7). In contrast, S1P greatly increased histamine-induced venular leakage when S1P1-mediated signaling was antagonized by treatment with VPC 23019 (Fig. 5). In addition, it is possible that neural stimulation or effects on other local cell types (e.g., macrophages, pericytes, and vascular smooth muscle) may also contribute to the pharmacological effects of agonists/antagonists that are observed in the present study. We recently showed that S1P1 and S1P2 receptors are expressed in cultured senescent ECs (9). Importantly, the effects of pharmacological agonists/antagonists can be recaptured in S1P1- and S1P2-expressing senescent ECs. For example, blockage of S1P2 signaling by JTE-013 significantly enhanced the effects of S1P on the increase of TEER, an in vitro measurement of endothelial integrity, as well as the formation of TJs in senescent ECs. This finding suggests that the regulation concertedly mediated by S1P1 and S1P2 receptors in the endothelium is critical, if not essential, for the biological responses that are demonstrated in the present study by use of the pharmacological agonists/antagonists in the whole animal model.
In summary, the data presented in the present study suggest that the homeostasis of vascular permeability under normal physiological conditions is regulated by a delicate balance between S1P1 and S1P2 signaling. Moreover, knowledge derived from this study may have therapeutic use in the future.
GRANTS
This work is supported by National Institutes of Health Grants R01 HL-071071 (M.-J. Lee), R01 HL-080394 (D. Lominadze), and R01 CA-111987 (B. W. Wattenberg) and American Heart Association Grant-in-Aid 0755245B (M.-J. Lee). D. L. Siow is a recipient of American Heart Association Predoctoral Fellowship 0815428D.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.An S, Goetzl EJ, Lee H. Signaling mechanisms and molecular characteristics of G protein-coupled receptors for lysophosphatidic acid and sphingosine 1-phosphate. J Cell Biochem Suppl 30–31: 147–157, 1998. [PubMed] [Google Scholar]
- 2.Ancellin N, Hla T. Differential pharmacological properties and signal transduction of sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J Biol Chem 274: 18997–19002, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. J Biol Chem 278: 32841–32851, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest 114: 1082–10899, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev 54: 265–269, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Damirin A, Tomura H, Komachi M, Tobo M, Sato K, Mogi C, Nochi H, Tamoto K, Okajima F. Sphingosine 1-phosphate receptors mediate the lipid-induced cAMP accumulation through cyclooxygenase-2/prostaglandin I2 pathway in human coronary artery smooth muscle cells. Mol Pharmacol 67: 1177–1185, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Spingosine-1-phosphate analogs as receptor antagonists. J Biol Chem 280: 9833–9841, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, Mathis SP, Sanchez T, Wang E, Kontos CD, Lin CY, Hla T, Haribabu B, Lee MJ. Up-regulating sphingosine-1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem 283: 30363–30375, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia JGN, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamburg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA 102: 9270–9275, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol 10: 4237–4249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol 58: 201–207, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi Y, Yatomi Y. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim Pol 45: 299–309, 1998. [PubMed] [Google Scholar]
- 15.Ikeda H, Satoh H, Yanase M, Inoue Y, Tomiya T, Arai M, Tejima K, Nagashima K, Maekawa H, Yahagi N, Yatomi Y, Sakurada S, Takuwa Y, Ogata I, Kimura S, Fujiwara K. Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology 124: 459–469, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Im DS, Heise CE, Ancellin N, O'Dowd BF, Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J Biol Chem 275: 14281–14286, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, Wang E, Lee MJ. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem 281: 29190–29200, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99: 301–312, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell 8: 693–704, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res 65: 3788–3795, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebravascular leakage in mice. Am J Physiol Heart Circ Physiol 290: H1206–H1213, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lominadze D, Joshua IG, Schuschke DA. In vivo platelet thrombus formation in microvessels of spontaneously hypertensive rats. Am J Hypertens 10: 1140–1146, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360, 2004. [DOI] [PubMed] [Google Scholar]
- 25.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 92: 1075–1085, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Minnear FL, Zhu L, He P. Sphingosine 1-phosphate prevents platelet-activating factor-induced increase in hydraulic conductivity in rat mesenteric venules: pertussis toxin sensitive. Am J Physiol Heart Circ Physiol 289: H840–H844, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Moolenaar WH Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res 253: 230–238, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res 58: 170–177, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 299: 483–487, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of αvβ3- and β1-containing integrins. J Biol Chem 276: 11830–11837, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JGN. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol 28: 102–107, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res 90: 325–332, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol 153: 140–147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem 278: 47281–47290, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312–1318, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez T, Thangada S, Wu MT, Kontos CD, Wu D, Wu H, Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci USA 102: 4312–4317, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanna G, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol 2: 434–441, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117: 2506–2516, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiegel S Sphingosine 1-phosphate: a prototype of a new class of second messengers. J Leukoc Biol 65: 341–344, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol 23: 1534–1545, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamama K, Tomura H, Sato K, Malchinkhuu E, Damirin A, Kimura T, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein inhibits migration of vascular smooth muscle cells through its sphingosine 1-phosphate component. Atherosclerosis 178: 19–23, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Vora KA, Nichols E, Porter G, Cui Y, Keohane CA, Hajdu R, Hale J, Neway W, Zaller D, Mandala S. Sphingosine 1-phosphate receptor agonist FTY720-phosphate causes marginal zone B cell displacement. J Leukoc Biol 78: 471–480, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Lee JF, Lin CY, Lee MJ. Rho GTPases mediated integrin αvβ3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem Cell Biol 129: 579–588, 2008. [DOI] [PubMed] [Google Scholar]
- 46a.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gi, Gq, and G12 families of heterotrimeric G proteins. J Biol Chem 274: 27351–27358, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Yokoo E, Yatomi Y, Takafuta T, Osada M, Okamoto Y, Ozaki Y. Sphingosine 1-phosphate inhibits migration of RBL-2H3 cells via S1P2: cross-talk between platelets and mast cells. J Biochem (Tokyo) 135: 673–681, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signaling through the G-protein-coupled receptor Edg-1. Biochem J 330: 605–609, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.