Abstract
Previously, we demonstrated that ischemia induces mitochondrial damage and dysfunction that persist throughout reperfusion and impact negatively on postischemic functional recovery and cellular viability. We hypothesized that viable respiration-competent mitochondria, isolated from tissue unaffected by ischemia and then injected into the ischemic zone just before reperfusion, would enhance postischemic functional recovery and limit infarct size. New Zealand White rabbits (n = 52) were subjected to 30 min of equilibrium and 30 min of regional ischemia (RI) induced by snaring the left anterior descending coronary artery. At 29 min of RI, the RI zone was injected with vehicle (sham control and RI vehicle) or vehicle containing mitochondria (7.7 × 106 ± 1.5 × 106/ml) isolated from donor rabbit left ventricular tissue (RI-Mito). The snare was released at 30 min of RI, and the hearts were reperfused for 120 min. Our results show that left ventricular peak developed pressure and systolic shortening in RI-Mito hearts were significantly enhanced (P < 0.05 vs. RI-vehicle) to 75% and 83% of equilibrium value, respectively, at 120 min of reperfusion compared with 57% and 62%, respectively, in RI-vehicle hearts. Creatine kinase-MB, cardiac troponin I, and infarct size relative to area at risk were significantly decreased in RI-Mito compared with RI-vehicle hearts (P < 0.05). Confocal microscopy showed that injected mitochondria were present and viable after 120 min of reperfusion and were distributed from the epicardium to the subendocardium. These results demonstrate that viable respiration-competent mitochondria, isolated from tissue unaffected by ischemia and then injected into the ischemic zone just before reperfusion, significantly enhance postischemic functional recovery and cellular viability.
Keywords: ischemia, infarct, apoptosis
previously, we and others demonstrated that myocardial ischemia initiates a succession of events resulting in alterations of mitochondrial volume, mitochondrial structure, mitochondrial oxidative phosphorylation, mitochondrial oxygen consumption, and mitochondrial calcium accumulation that extend into reperfusion to severely compromise myocardial postischemic functional recovery and significantly increase infarct size (IS) (11, 15, 30).
We and others attempted to modulate the effects of ischemia on mitochondrial structure and function by pharmaceutical and/or exogenous substrate intervention alone or in combination with procedural techniques to provide cardioprotection (2, 11, 15, 16, 30, 36). These protocols, for the most part, provided only limited cardioprotection, and mitochondrial damage and dysfunction remained after myocardial ischemia and continued to impact negatively on postischemic functional recovery and cellular viability. These data suggest that the current paradigm needs to be reviewed and that alternative approaches need to be explored to allow for preservation of myocardial function and cellular viability after ischemia and reperfusion.
In previous studies, a variety of cell types have been injected directly into the myocardium or seeded onto biodegradable scaffolds to enhance myocardial function and induce myocardial regeneration in the infarcted zone after an ischemic event (3, 7, 20, 25). The efficacy of these interventions is limited. Only minimal survival of the injected cells has been demonstrated, and only limited prevention of postischemic myocardial infarction progression and modest functional improvements have been reported (3, 7, 20, 25). One major disadvantage of these cellular interventions is the time, i.e., days to weeks, needed for cell isolation, growth, and expansion before use.
An alternative therapeutic intervention to cell transplantation and current cardioprotective protocols would be the replacement of mitochondria damaged during ischemia. We hypothesized that transplantation of mitochondria isolated from remote tissue unaffected by ischemia into the ischemic zone just before reperfusion would provide an alternative to cellular transplantation, allowing for a relatively rapid intervention to enhance postischemic functional recovery and cellular viability during reperfusion.
In the present study, we show that viable, respiration-competent mitochondria isolated from remote tissue unaffected by ischemia and then directly injected into the ischemic zone of myocardial tissue during early reperfusion significantly enhance regional and global postischemic functional recovery and significantly decrease IS in a model of surgical ischemia and reperfusion.
METHODS
Animal model.
New Zealand White rabbits (Millbrook Farm, Amherst, MA) were housed individually and provided with laboratory chow and water ad libitum. All experiments were approved by the Harvard Medical Area Standing Committee on Animals and conformed to the National Institutes of Health (NIH) guidelines regulating the care and use of laboratory animals (NIH Publication No. 5377-3, 1996). All research was performed in accordance with the American Physiological Society's “Guiding Principles in the Care and Use of Animals.”
Mitochondrial isolation.
Left ventricular (LV) mitochondria were isolated from donor hearts as previously described and used immediately for injection or stored at −20°C overnight (13, 28). Mitochondrial protein content, oxygen consumption, respiratory control index (RCI), and mitochondrial DNA and RNA were determined as previously described (10, 13, 28). Mitochondrial complex I, II, III, IV, and V activities were determined separately as previously described (1, 42).
Mitochondrial number.
Viable mitochondrial number was determined by labeling an aliquot (10 μl) of isolated mitochondria with MitoTracker Orange CMTMRos (5 μmol/l; Invitrogen, Carlsbad, CA). Aliquots of labeled mitochondria were spotted onto slides and counted using a spinning disk confocal microscope with a 63× C-apochromat objective (1.2 W Korr/0.17 NA, Zeiss). Mitochondria were counterstained with the mitochondria-specific dye MitoFluor Green (Invitrogen). Appropriate wavelengths were chosen for measurement of autofluorescence and background fluorescence with use of unstained cells and tissue. Briefly, 1 μl of labeled mitochondria was placed on a microscope slide and covered. Mitochondrial number was determined at low (×10) magnification covering the full specimen area using MetaMorph Imaging Analysis software.
Langendorff perfusion.
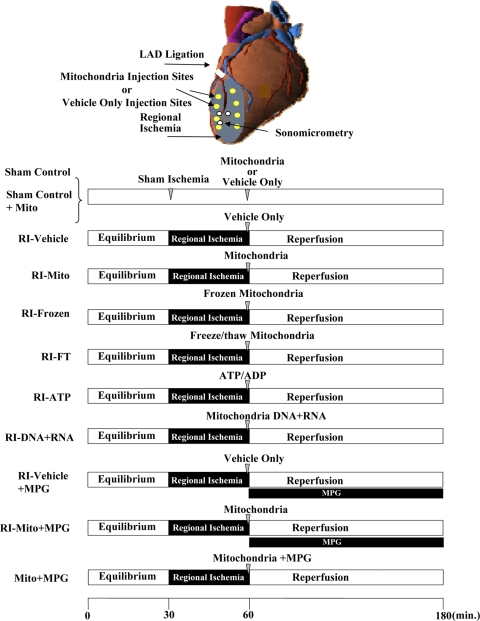
Langendorff retrograde perfusion was performed as described by Feinberg et al. (5). All rabbits were anesthetized with pentobarbital sodium (Nembutal, 100 mg/kg) and heparin (200 U/kg) intravenously through the marginal ear vein. The heart was excised and placed in a 4°C bath of Krebs-Ringer solution equilibrated with 95% O2-5% CO2 (pH 7.4 at 37°C), where spontaneous beating ceased within a few seconds. Krebs-Ringer solution contained (in mmol/l) 100 NaCl, 4.7 KCl, 1.1 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 1.7 CaCl2, 11.5 glucose, 4.9 pyruvic acid, and 5.4 fumaric acid. Langendorff retrograde perfusion was performed as previously described (4, 18). Briefly, a latex balloon containing a catheter-tipped transducer (Millar Instruments, Houston, TX) was inserted into the LV. The volume of the water-filled balloon was determined at a constant physiological end-diastolic pressure of 5–10 mmHg using a calibrated microsyringe during equilibrium, and this balloon volume was maintained for the duration of the experiment. The aorta was cannulated with a metal cannula, and the heart was subjected to Langendorff retrograde perfusion at a constant pressure of 75 cmH2O at 37°C. Hearts were paced through the right atrium at 180 ± 3 beats/min throughout the experiment using a stimulator (model 5330, Medtronic, Minneapolis, MN). Regional myocardial function was assessed by sonomicrometry (Sonometrics, London, ON, Canada) (18). Sonomicrometry was performed using three digital piezoelectric ultrasonic probes (1 mm), with each probe serving as both transmitter and receiver (Fig. 1 ). The probes were implanted in the subepicardial layer ∼3–4 mm apart in the regional ischemic (RI) zone: two probes were placed on the major axis of the heart, and one probe was placed parallel to the minor axis of the heart and secured to the epicardium with Z suture using polypropylene stitches. The probes were left in place until the end of the experiment (18, 34). Digital data were inspected for correct identification of end-diastolic and end-systolic points with use of postprocessing software (SonoView, Sonometrics). Measurements were made over at least three cardiac cycles in normal sinus rhythm and then averaged. Regional myocardial function was assessed as segment systolic shortening (SS), as previously described (34). Global function was determined using a PO-NE-MAH digital data acquisition system with an Acquire Plus processor board and LV pressure analysis software and an ECG/Biotach (Gould, Valley View, OH) (18).
Fig. 1.
Top: Langendorff perfusion. White circles show positions of digital piezoelectric ultrasonic probes. Bottom: experimental protocol. Rabbit hearts were subjected to Langendorff perfusion consisting of 30 min of equilibrium, 30 min of regional ischemia [RI; achieved by occlusion of the left anterior descending coronary artery (LAD)], and 120 min of reperfusion. After 29 min of sham or RI, sham control and RI-vehicle hearts were injected with vehicle, sham control + Mito and RI-Mito hearts with vehicle containing isolated mitochondria (7.7 × 106 ± 1.5 × 106/ml), RI-frozen hearts with isolated mitochondria (7.7 × 106 ± 1.5 × 106/ml) that had been frozen overnight at −20°C, RI-FT with mitochondrial components from mitochondria (7.7 × 106 ± 1.5 × 106/ml) that had been frozen and then thawed, RI-ATP hearts with vehicle containing 1 mM ATP + 1 mM ADP, RI-DNA + RNA hearts with vehicle containing mitochondrial DNA + RNA isolated from 7.7 × 106 ± 1.5 × 106/ml mitochondria, and Mito + MPG hearts with isolated mitochondria (7.7 × 106 ± 1.5 × 106/ml) containing the reactive oxygen species scavenger N-(2 mecaptopropionyl)glycine (MPG, 300 μmol/l). At 30 min of RI, RI-vehicle + MPG and RI-Mito + MPG hearts were reperfused for 120 min with MPG (300 μmol/l) in Krebs-Ringer solution for 120 min.
Experimental protocol.
The experimental protocol is shown in Fig. 1. Hearts from female New Zealand White rabbits (>32 mo old, 5–6 kg body wt, n = 32) were subjected to 30 min of equilibrium, 30 min of RI, and 120 min of reperfusion. Sham control hearts were subjected to sham RI by passage of a prolene suture around the left anterior descending coronary artery (LAD) with a cutting needle (34). No snare was tied in the sham control group. RI of the LAD was achieved by passage of both ends of the suture through a small vinyl tube to form a snare, which was then pulled tight and fixed with a mosquito clamp (34). After 29 min of RI, we used a 1-ml insulin syringe with a 28-gauge needle to administer eight, 0.1-ml injections of vehicle alone [respiration buffer A supplemented with 5 mmol/l glutamate, 5 mmol/l malate, and 8 mmol/l succinate for sham-control (n = 6) and RI-vehicle (n = 7)] or vehicle containing mitochondria [7.7 × 106 ± 1.5 × 106/ml; for sham control-Mito (n = 3) and RI-Mito (n = 7)] into the RI area.
Separate groups of hearts (n = 3 each) were injected, separately, with isolated mitochondria (7.7 × 106 ± 1.5 × 106/ml) that had been frozen overnight at −20°C, mitochondrial components that had been frozen and then thawed (1, 40), mitochondrial DNA + RNA (10), or vehicle containing 1 mM ATP and 1 mM ADP (ultra-pure, Sigma, St. Louis, MO) into the RI zone.
At 30 min of RI, the snare was released and the hearts were reperfused for 120 min.
To determine the role of reactive oxygen species (ROS), separate groups of hearts (n = 3 each) were perfused as described above with the addition of the ROS scavenger N-(2-mercaptopropionyl)glycine (MPG, 300 μmol/l; Sigma) to the Krebs-Ringer solution during reperfusion (4, 14).
Markers of ischemic injury.
Creatine kinase-MB (CK-MB) and cardiac troponin I (cTnI) were determined in aliquots (1.5 ml) collected from coronary sinus effluents at 30 min of equilibrium and at 30 min of ischemia. Samples were frozen immediately in liquid nitrogen and stored at −80°C. CK-MB was estimated using the CK-MB enzyme immunoassay kit (Biocheck, Foster City, CA), and cTnI was determined using the rabbit cTnI ELISA (Life Diagnostics, West Chester, PA) according to the manufacturers' directions and using manufacturers' standards.
Area at risk and IS determination.
After the end of reperfusion, the LAD was religated, and the area at risk (AAR) was delineated by injection of monastryl blue pigment into the aorta (18). The heart was rapidly removed, and samples from three predesignated sites in the AAR (0.25–0.3 g) were removed and either quick frozen in liquid nitrogen and then used for biochemical analysis or immediately used for wet-to-dry weight ratio (18). The heart was then sliced across the long axis of the LV, from apex to base, into 1-cm-thick transverse sections and traced onto a clear acetate sheet over a glass plate under room light. The sliced hearts were incubated in 1% triphenyltetrazolium chloride (Sigma) in phosphate buffer (pH 7.4) at 38°C for 20 min and fixed for 24 h in 10% neutral buffered formalin solution (18). A copy of the stained heart slices was traced onto a clear acetate sheet over a glass plate under room light. The AAR in the LV and the IS were measured using planimetry. The volumes of the infarcted zone and the AAR were calculated by multiplying the planimetered areas by the slice thickness. The ratio of AAR to LV weight was calculated. IS was expressed as a percentage of AAR for each heart (IS/AAR) (18).
Wet-to-dry weight ratios.
Wet-to-dry weight ratios in the AAR were determined after 120 min of reperfusion, as previously described (18).
ATP determination.
ATP was determined in frozen tissue sections from the AAR by fluorometric analysis according to the method described by Lowry and Passonneau (22).
Thiobarbituric acid-reactive substances.
Thiobarbituric acid-reactive substances (TBARS) were determined in frozen tissue sections from the AAR according to the method described by Ohkawa et al. (21).
Biodistribution of injected mitochondria.
A separate group of hearts was injected with vehicle labeled with MitoTracker Orange CMTMRos (sham control and RI-vehicle, n = 4 each) or vehicle containing mitochondria labeled with MitoTracker Orange CMTMRos (RI-Mito, n = 4). The same experimental protocol described in Fig. 1 was used; however, after 120 min of reperfusion, the hearts were perfusion fixed with 4% paraformaldehyde in PBS (pH 8.0) under pressure (33). Transmyocardial samples were dissected from the RI zone, embedded, sectioned (5–7 μm thick) completely, and then mounted on glass slides (∼90–100 slides).
Slides were used for routine histological staining (hematoxylin-eosin or Masson's trichrome) or immunohistochemically stained with an antibody or mitochondrial marker. Anti-α-actinin-2 polyclonal antibody (1:500 dilution) was detected with a highly cross-absorbed goat anti-rabbit Alexa Fluor 488 secondary antibody (Invitrogen; 1:250 dilution). 4′,6-Diamidino-2-phenylindole (Invitrogen) was diluted in the secondary antibody solution according to the manufacturer's directions. MitoFluor Green was used at a 20 nmol/l final concentration.
After the slides were mounted in fluorescent mounting medium (Dako), they were visualized on a multipoint spinning-disk confocal system (Atto, BD Biosciences, Rockville, MD) attached to a Zeiss Axiovert 200M microscope (33). The confocal system and microscope were each illuminated with an X-Cite 120 mercury-halide light source, and images were acquired using a CoolSNAP HQ camera (Photometrics, Huntington Beach, CA) or a MicroMAX 1300 YHS charge-coupled device camera (Princeton Instruments, Acton, MA) controlled with MetaMorph 6.2 software (Molecular Devices, Downingtown, PA). Image processing was accomplished with MetaMorph 6.2 and Photoshop CS (Adobe, San Jose, CA) software.
TdT-mediated dUTP nick-end labeling (TUNEL) was performed using the ApopTag fluorescein in situ apoptosis detection kit and positive control slides (Millipore, Billerica, MA), as previously described (18). Total cytoplasmic proteins were isolated as previously described (18). Caspase-3-like activity was determined in total cytoplasmic proteins using the caspase-3 colorimetric analysis kit, standards, and inhibitors according to the manufacturer's instructions (Chemicon International, Temecula, CA), as previously described (18).
Statistical analysis.
Statistical analysis was performed using SAS (version 6.12) software (SAS Institute, Cary, NC). Values are means ± SE. Statistical significance was assessed using repeated-measures ANOVA, with group as a between-subjects factor and time as a within-subjects factor. Tukey's honestly significant difference test was used for comparisons between sham control and other groups to adjust for the multiplicity of tests. A one-way ANOVA was used for AAR, IS, markers of ischemic injury, and TUNEL. Statistical significance was claimed at P < 0.05.
RESULTS
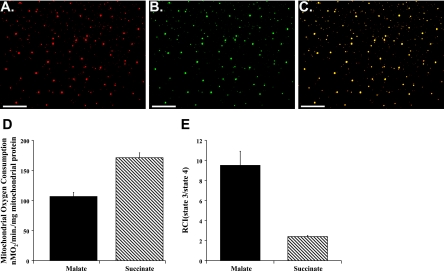
Mitochondria were isolated in ∼90 min (15, 30). MitoTracker Orange CMTMRos labeling demonstrated that isolated mitochondria maintained membrane potential and were viable (Fig. 2A). Mitochondrial identity was confirmed by counterstaining with mitochondria-specific MitoFluor Green (Fig. 2, B and C). The mitochondria appeared intact and were of the correct size and morphology.
Fig. 2.
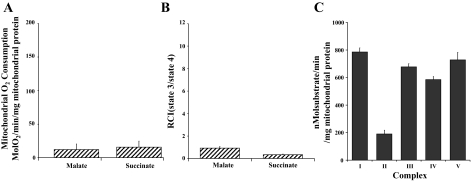
Isolated mitochondrial viability and oxygen consumption. A–C: confocal-microscopic images of isolated mitochondria. A: isolated mitochondria labeled with MitoTracker Orange CMTMRos, which labels mitochondria maintaining membrane potential. B: mitochondria counterstained with mitochondria-specific MitoFluor Green. C: overlay of A and B. Original magnification ×400. D and E: state 3 (active) oxygen consumption (ADP-stimulated respiration) and respiratory control index (RCI; state 3/state 4) for malate-induced (complex I) and succinate-induced (complex II) energized mitochondria. Values are means ± SE of 6 analyses.
To ensure that isolated mitochondria were respiration competent, an aliquot was used to determine mitochondrial oxygen consumption (15, 30). Our results (Fig. 2, D and E) demonstrate that mitochondrial oxygen consumption and RCI for malate-induced complex I and succinate-induced complex II were similar to earlier results reported by our laboratory and demonstrated that isolated mitochondria were respiration competent (15, 30).
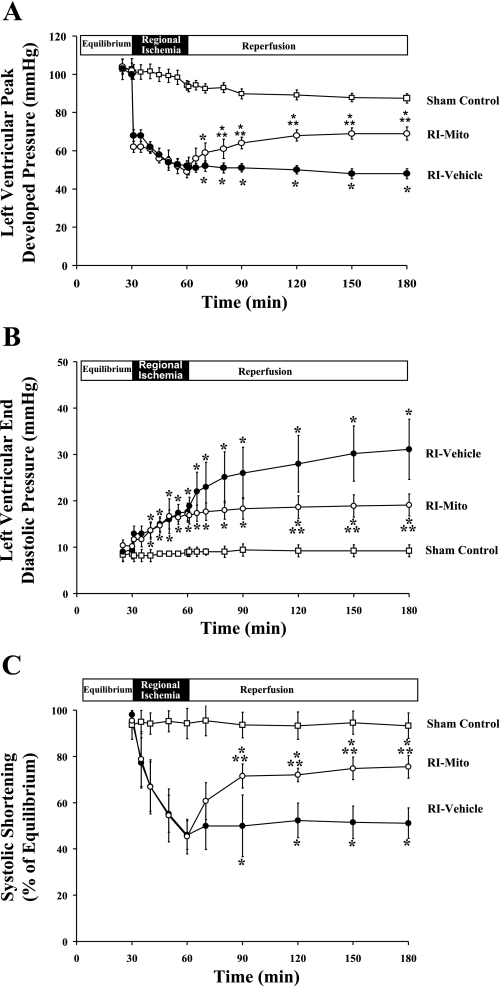
Global myocardial function was determined by LV peak developed pressure (LVPDP, mmHg) and LV end-diastolic pressure (LVEDP, mmHg). Regional function in the RI zone was determined as SS (percentage of equilibrium value). LVPDP in sham control and sham control + Mito hearts during equilibrium was 103 ± 5.4 and 101 ± 4.7 mmHg, respectively, and LVEDP was 8.6 ± 1.4 and 9.3 ± 0.7 mmHg, respectively. These values did not differ significantly among the three groups during equilibrium (Fig. 3). Moreover, there was no significant difference in these values between RI-vehicle and RI-Mito groups during RI and before injection of mitochondria. During ischemia, LVPDP was significantly decreased, LVEDP was significantly increased, and SS was significantly decreased in RI-vehicle and RI-Mito hearts relative to sham control hearts (P < 0.05).
Fig. 3.
Global and regional myocardial function during equilibrium, RI, and reperfusion: left ventricular (LV) peak developed pressure (LVPDP; A), LV end-diastolic pressure (B), and systolic shortening (SS; C) during equilibrium, RI, and reperfusion in sham control, RI-vehicle, and RI-Mito hearts. Values are means ± SE. *P < 0.05 vs. sham control. **P < 0.05 vs. RI-vehicle.
After 120 min of reperfusion, LVPDP, LVEDP, and SS were significantly decreased during reperfusion in RI-Mito and RI-vehicle compared with sham control hearts (P < 0.05; Fig. 3). Significant improvement in function was observed in RI-Mito hearts compared with RI-vehicle hearts. LVPDP was increased, LVEDP was decreased, and SS in the ischemic zone was increased (P < 0.05; Fig. 3). No significant difference was observed in LVPDP, LVEDP, or SS between sham control and sham control + Mito hearts throughout reperfusion.
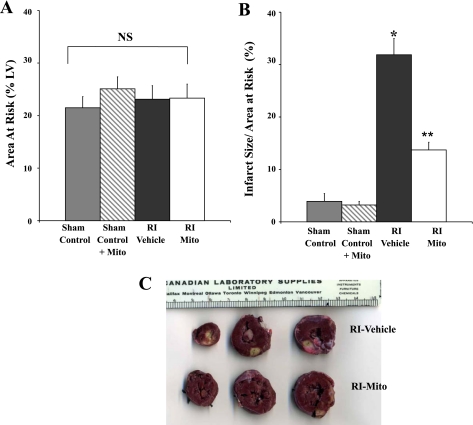
No significant difference was observed in AAR between sham control, sham control + Mito, RI-vehicle, and RI-Mito hearts (Fig. 4A). No significant difference was observed in IS/AAR after 30 min of RI and 120 min of reperfusion between sham control (3.9 ± 1.5%) and sham control + Mito (3.2 ± 0.7%) hearts. IS was significantly increased in RI-Mito hearts to 13.7 ± 3.6% (P < 0.05 vs. sham control) and in RI-vehicle hearts to 31.9 ± 7.8% (P < 0.05 vs. sham control and RI-Mito; Fig. 4B). IS was significantly decreased in RI-Mito hearts compared with RI-vehicle hearts (P < 0.05; Fig. 4B). Examination of tissue sections showed that infarct in RI-vehicle hearts was large and transmural whereas infarct in RI-Mito hearts was predominantly small, focal, and epicardial.
Fig. 4.
Myocardial injury after 30 min of RI and 120 min of reperfusion: myocardial area at risk (AAR; A) and infarct size (IS) relative to AAR (IS/AAR, B) in sham control, sham control + Mito, RI-vehicle, and RI-Mito hearts after 30 min of RI and 120 min of reperfusion. Sham control hearts were subjected to sham RI only. There was no significant difference in IS between sham control and sham control + Mito. *P < 0.05 vs. sham control and sham control + Mito. **P < 0.05 vs. RI-vehicle. C: representative photograph of rabbit heart slices from RI-vehicle and RI-Mito hearts stained with 1% triphenyltetrazolium chloride. AAR is outlined by white line. Myocardial infarct (yellow) was significantly increased in RI-vehicle compared with RI-Mito hearts. Scale is shown in cm.
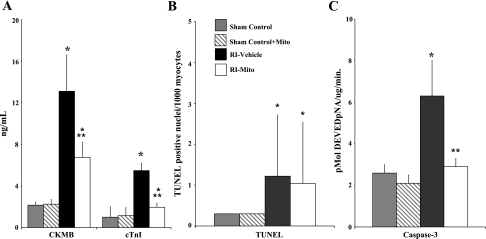
No significant differences were observed in CK-MB release, TUNEL-positive nuclei, or caspase-3 activity between sham control and sham control + Mito hearts (Fig. 5). CK-MB and cTnI were significantly increased in RI-Mito (P < 0.05 vs. sham control) and RI-Vehicle (P < 0.05 vs. sham control) hearts. CK-MB and cTnI were significantly decreased in RI-Mito hearts compared with RI-vehicle hearts (P < 0.05; Fig. 5, A and B). There was no significant difference (P = 0.65) in TUNEL-positive nuclei between RI-vehicle and RI-Mito hearts. Caspase-3-like activity was significantly increased in RI-vehicle hearts (P < 0.05), but there was no significant difference (P = 0.6149) in caspase-3-like activity in RI-Mito hearts compared with sham control and sham control + Mito hearts (Fig. 5C).
Fig. 5.
Markers of myocardial injury after 30 min of RI and 120 min of reperfusion. A: creatine kinase (CK-MB) and rabbit cardiac troponin I (cTnI) in myocardial effluents after 30 min of RI in sham control, sham control + Mito, RI-vehicle, and RI-Mito hearts. B and C: TdT-mediated dUTP nick-end-label (TUNEL)-positive nuclei per 1,000 myocytes and caspase-3-like activity after 30 min of RI and 120 min of reperfusion. pNA, peptide nucleic acid. Values are means ± SE. There were no significant differences in CK-MB, cTnI, TUNEL, or caspase-3 activity between sham control and sham control + Mito. *P < 0.05 vs. sham control and sham control + Mito. **P < 0.05 vs. RI-vehicle.
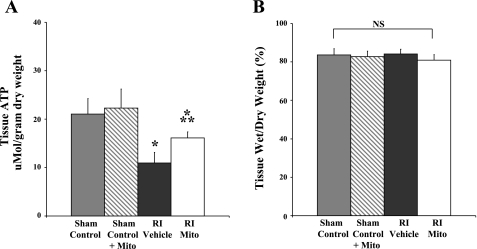
ATP content in the AAR was significantly increased (P < 0.05) in RI-Mito hearts to 16.14 ± 0.5 μmol/g dry wt compared with 10.95 ± 1.0 μmol/g dry wt in RI-vehicle hearts. Tissue ATP content in RI-Mito and RI-vehicle hearts was significantly decreased (P < 0.05) compared with 21.04 ± 1.4 μmol/g dry wt in sham control and 22.33 ± 3.9 μmol/g dry wt in sham control + Mito hearts (Fig. 6A).
Fig. 6.
Tissue ATP content (A) and tissue wet-to-dry weight ratio (B) in AAR after 30 min of RI and 120 min of reperfusion. There was no significant difference in tissue ATP content in AAR between sham control and sham control + Mito. *P < 0.05 vs. sham control and sham control + Mito. **P < 0.05 vs. RI-vehicle. NS, no statistical significance between or within groups.
Myocardial wet-to-dry weight ratio in the AAR was 83.6 ± 1.6% in sham control, 81.6 ± 0.7% in sham control + Mito, 83.7 ± 0.7% in RI-vehicle, and 81.3 ± 0.8% in RI-Mito hearts. There was no significant difference between sham control, sham control + Mito, RI-vehicle, and RI-Mito hearts (P = 0.13; Fig. 6B).
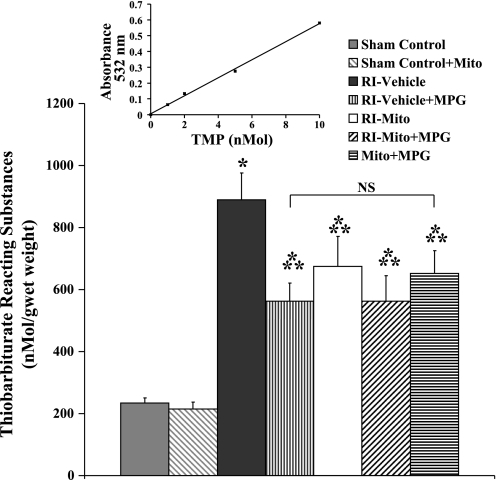
TBARS (nmol/g wet wt) were significantly decreased (P < 0.05) to 674 ± 97 in RI-Mito hearts compared with 890 ± 86 in RI-vehicle hearts (Fig. 7). TBARS in RI-Mito and RI-vehicle hearts were significantly increased (P < 0.05) compared with 235 ± 16 nmol/g wet wt in sham control hearts (Fig. 7).
Fig. 7.
Thiobarbituric acid-reactive substances (TBARS) in AAR after 30 min of RI and 120 min reperfusion. Inset: representative standard curve. TMP, 1,1,3,3-tetramethoxypropane. There was no significant difference in TBARS in AAR between sham control and sham control + Mito. *P < 0.05 vs. sham control and sham control + Mito. **P < 0.05 vs. RI-vehicle.
Addition of the ROS scavenger MPG (300 μmol/l) to Krebs-Ringer solution during reperfusion significantly decreased (P < 0.001) TBARS in RI-Mito + MPG (556 ± 58 nmol/g wet wt) and RI-vehicle + MPG (563 ± 82 nmol/g wet wt) hearts (Fig. 7). There was no significant difference in TBARS between RI-Mito + MPG and RI-vehicle + MPG hearts (P = 0.85). MPG had no effect on postischemic functional recovery (LVPDP, LVEDP, and SS) compared with RI-vehicle and RI-Mito hearts. LVPDP at the end of 120 min of reperfusion was 68.4 ± 4.2 mmHg in Mito + MPG, 71.6 ± 5.8 mmHg in RI-Mito + MPG, and 51.7 ± 8.1 mmHg in RI-vehicle + MPG hearts. LVEDP was 18.3 ± 2.4 mmHg in Mito + MPG, 18.5 ± 2.1 in RI-Mito + MPG, and 28.1 ± 5.3 in RI-vehicle + MPG hearts.
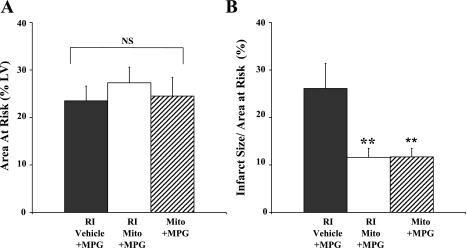
There was no significant difference in AAR between Mito + MPG, RI-Mito + MPG, and RI-vehicle + MPG hearts (P = 0.74; Fig. 8A). There was no significant difference in IS between Mito + MPG, RI-Mito + MPG, or RI-Mito hearts (P = 0.51) or between RI-vehicle + MPG and RI-vehicle hearts (P = 0.27; Figs. 4B and 8B).
Fig. 8.
Myocardial injury after 30 min of RI and 120 min of reperfusion: AAR (A) and IS/AAR (B). **P < 0.05 vs. RI-vehicle + MPG.
Mitochondria labeled with MitoTracker Orange CMTMRos in RI-Mito hearts were viable and evident after 120 min of reperfusion (Fig. 9). The labeled mitochondria were visible at the site of the injection and >2–3 mm from the site of the injection. The labeled mitochondria were distributed from the epicardium to the subendocardium. The labeled mitochondria were found peripheral to but in close proximity to the myocytes but were not observed in the myocytes. Counterstaining with mitochondria-specific MitoFluor Green showed that <0.05% of MitoTracker Orange CMTMRos-labeled mitochondria were nonviable (Fig. 9). There was no detectable staining of mitochondria in RI-vehicle hearts injected with vehicle incubated with MitoTracker Orange CMTMRos.
Fig. 9.
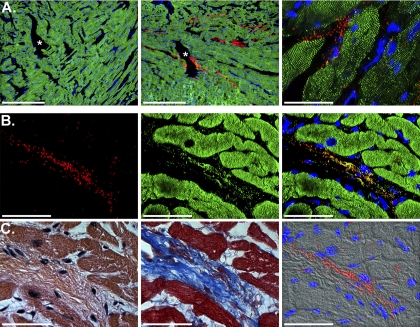
Mitochondrial viability and biodistribution after 120 min of reperfusion. A: representative confocal-microscopic images of tissue sections (5–7 μm) from AAR of RI-vehicle and RI-Mito hearts after 120 min of reperfusion. Left: sham control tissue section from AAR injected with MitoTracker Orange CMTMRos-labeled injection vehicle. No labeling of mitochondria was observed in RI-vehicle hearts. Middle: tissue section from AAR of RI-Mito heart injected with vehicle containing mitochondria (7.7 × 106 ± 1.5 × 106/ml). Injected mitochondria labeled with MitoTracker Orange CMTMRos were viable after 120 min reperfusion. Right: higher-power view of stained mitochondria. Note labeled mitochondria >2–3 mm from site of injection in subendocardium. Red, injected mitochondria; green, immunofluorescently stained microfilament protein α-actinin-2. Scale bars, 500 μm (left and middle) and 50 μm (right). *, Injection sites. B: RI-Mito heart tissue section from AAR shown in A. Left: injected mitochondria labeled with MitoTracker Orange. Middle: same tissue section stained with MitoFluor Green. Right: combined image. Injected mitochondria were viable and present within the myocardium after 120 min of reperfusion; <0.05% of MitoTracker Orange CMTMRos-labeled mitochondria were nonviable. Scale bars, 50 μm. C: adjacent serial sections stained with hematoxylin-eosin or Masson's trichrome (left and middle) and stained mitochondria overlaid on a differential interference contrast (DIC II) image (right). In fluorescently stained panels, nuclei were stained blue with 4′,6-diamidino-2-phenylindole. Scale bars, 50 μm.
Results from hearts injected, separately, with isolated mitochondria (7.7 × 106 ± 1.5 × 106/ml) that had been frozen overnight at −20°C (RI-frozen), mitochondrial components isolated by freeze-thaw (RI-FT), mitochondrial DNA + RNA (RI-DNA + RNA), or vehicle containing 1 mM ATP and 1 mM ADP (RI-ATP; ultra-pure, Sigma) into the RI zone are shown in Fig. 10.
Fig. 10.
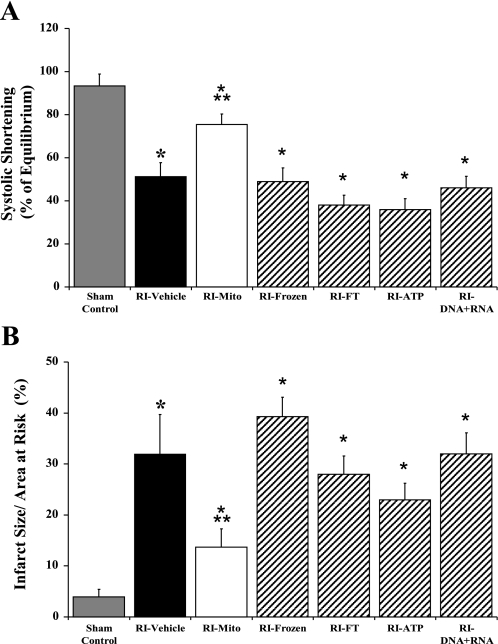
SS (A) and IS/AAR (B) after 30 min of RI and 120 min of reperfusion in RI-frozen (n = 3), RI-FT (n = 3), RI-ATP (n = 3), RI-DNA + RNA (n = 3), sham control (n = 6), RI-vehicle (n = 7), and RI-Mito (n = 7) hearts. Values are means ± SE. *P < 0.05 vs. sham control. **P < 0.05 vs. all other RI groups.
There was no significant difference in LVPDP or LVEDP after 30 min of equilibrium between sham-control and RI-Mito, RI-vehicle, RI-frozen, RI-FT, RI-ATP, or RI-DNA + RNA hearts (results not shown). After 120 min of reperfusion, LVPDP was significantly decreased (P > 0.05) and LVEDP was significantly increased (P < 0.05) in RI-frozen, RI-FT, RI-ATP, and RI-DNA + RNA hearts compared with RI-Mito hearts (results not shown). There was no significant difference in LVPDP or LVEDP between RI-vehicle and RI-frozen, RI-FT, RI-ATP, and RI-DNA + RNA hearts after 120 min of reperfusion (results not shown). SS was significantly decreased (P > 0.05) after 120 min of reperfusion in RI-frozen, RI-FT, RI-ATP, and RI-DNA + RNA hearts compared with RI-Mito hearts (Fig. 10A). There was no significant difference in SS between RI-vehicle and RI-frozen, RI-FT, RI-ATP, and RI-DNA + RNA hearts after 120 min of reperfusion (Fig. 10A).
IS after 30 min of RI and 120 min of reperfusion was significantly increased (P < 0.05) in RI-frozen, RI-FT, RI-ATP, and RI-DNA + RNA hearts compared with RI-Mito hearts (Fig. 10B). There was no significant difference in IS between RI-vehicle and RI-frozen, RI-FT, RI-ATP, and RI-DNA + RNA hearts (Fig. 10B). There was no significant difference in AAR between groups (results not shown).
Mitochondrial state 3 oxygen consumption and RCI in frozen mitochondria (RI-frozen hearts) were significantly decreased (P < 0.05) compared with freshly isolated mitochondria (Fig. 11, A and B). Complex I, II, III, IV, and V activities were detectable in RI-frozen and RI-FT hearts (Fig. 11C). No intact mitochondria were detected in mitochondrial DNA + RNA preparations as determined by MitoFluor Green staining (results not shown).
Fig. 11.
Mitochondrial oxygen consumption (A) and RCI (B) in frozen mitochondria and complex I, II, III, IV, and V activity in mitochondria that had been frozen and then thawed (C).
DISCUSSION
Previously, we showed that, after ischemia and reperfusion, there are significant alterations in mitochondrial structure and function and that these changes are associated with significantly decreased postischemic functional recovery and significantly increased IS (15, 18, 30). We also showed that the preservation of mitochondrial structure and function is associated with significantly enhanced postischemic functional recovery and significantly decreased IS (15, 18, 30).
The protocols we and others utilized to preserve mitochondrial structure and function have, for the most part, provided only limited cardioprotection, and mitochondrial injury continues to be apparent (9, 15, 26, 27, 29, 34). These studies suggested that alternatives to current cardioprotective protocols needed to be explored to enhance the preservation of mitochondrial structure and function and cardioprotection. One approach to enhance cardioprotection is replacement of organelles damaged during ischemia and reperfusion. We hypothesized that the injection of mitochondria isolated from remote tissue unaffected by ischemia during early reperfusion would enhance postischemic functional recovery and cellular viability during reperfusion.
In this report, we have isolated mitochondria from nonischemic hearts. The isolation process required ∼90 min; however, we expect that this time can be decreased by automation. Our results demonstrate that isolated mitochondria are respiration competent and maintain membrane potential. Our data also show that injection of these viable, respiration-competent mitochonria into the ischemic zone of RI hearts just before reperfusion significantly enhances regional and global postischemic functional recovery and significantly decreases IS, caspase-3-like activity, and release of CK-MB and cTnI.
We have injected mitochondria into the ischemic zone just before reperfusion to limit damage during ischemia that would result in decreased mitochondrial function. We reasoned that injection of mitochondria just before reperfusion would allow for enhanced or augmented mitochondrial function during early reperfusion, a period previously shown to be critical for the salvage of myocardial tissue and the restoration/enhancement of myocardial function (9, 28).
Injection of mitochondria just before reperfusion has clinical relevance for use in coronary artery bypass grafting, when mitochondria could be injected into the ischemic zone after bypass and just before aortic cross-clamp removal, and in percutaneous coronary intervention (PCI) for coronary revascularization for ST segment elevation myocardial infarction (STEMI), when mitochondria could be injected endocardially, via a needle-tipped catheter placed retrograde through the sheath used for the PCI, under fluoroscopic guidance, into the ventricular segment that had been revascularized. Similarly, for right ventricular revascularization, a needle-tipped catheter could be placed retrograde through the femoral vein to provide access to the right side of the heart, and mitochondria could be injected as soon as the obstructed coronary artery is opened by PCI.
Our data show that, after injection into the ischemic zone, mitochondria are viable for ≥120 min of reperfusion and are widely distributed from the epicardium to the subendocardium >2–3 mm from the injection site. We have extended the reperfusion period to 4 h and have found injected mitochondria to be viable and well distributed; however, extension of the reperfusion period beyond 2 h leads to diminished functional recovery kinetics in the isolated perfused heart model (results not shown).
The ability of injected mitochondria to distribute within the myocardium is most likely due to their small size. In contrast to cells, the small size of mitochondria (4–6 nm) would minimize damage as a result of injection and would enhance distribution within the heart after ischemia. In our studies, we have used 7.7 × 105 ± 1.5 × 105/0.1 ml mitochondria for injection. This concentration was arbitrarily determined on the basis of initial observations that showed equal distribution of viable mitochondria within the ischemic zone. However, we examined 1.0 × 105 and 1.0 × 104/0.1 ml mitochondria in preliminary studies and found similar cardioprotective effects (personal communication, data not shown).
An additional means for the distribution of mitochondria within the myocardium may be associated with alterations in myocardial structure that occur after myocardial ischemia. In a previous report, using histochemical and quantitative light-microscopic spatial analysis, we demonstrated a significant increase in myocardial interfibrillar space after ischemia (33). Our data demonstrated increased longitudinal and transverse myocardial interfibrillar separations after ≥15 min of ischemia (33). These structural changes were not associated with alterations in conduction velocity anisotropy or in tissue edema but occurred coincident with previously observed significant decreases in postischemic functional recovery, as well as increased myocardial apoptosis and necrosis (1, 17, 18, 23, 38). We speculate that the increased longitudinal and transverse interfibrillar separation after myocardial ischemia provides a pathway for mitochondrial distribution within the ischemic zone that facilitates distribution. This distribution would be aided by myocardial contraction, which would pump the injected mitochondria through the interfibrillar separations. The time frame for distribution within the ischemic zone remains to be determined; however, our data demonstrate significant improvements in LVPDP and SS after 30 min of reperfusion in RI-Mito hearts.
Our data show that injected mitochondria are found near the epicardial surface but remain within the interfibrillar space and do not enter the myocyte. The following question arises: How do injected mitochondria provide for enhanced cardioprotection while outside the myocyte?
Our protocol does not replicate any of the methods used in pre- and postconditioning, and we have used isolated mitochondria to provide cardioprotection on the basis of our studies of the mechanisms of cardioplegia cardioprotection (15–18, 30, 33, 35, 36, 38). In the present study, mitochondria were injected just before reperfusion, and no short ischemic or reperfusion interval or set of ischemia-reperfusion intervals, other than index ischemia, was utilized. The mechanisms through which this cardioprotection occurs may be similar to that of pre- and postconditioning or cardioplegia (4, 14, 37, 40).
Our studies show that cardioprotection requires mitochondria that are freshly isolated, maintain viability, and are respiration competent. The use of frozen mitochondria failed to provide cardioprotection. In frozen mitochondria, mitochondrial oxygen consumption was significantly decreased compared with freshly isolated mitochondria. This decrease in oxygen consumption has been previously described (23, 39). These reports demonstrated that mitochondrial bioenergetic function is decreased to ∼10–15% of normal after the mitochondria are frozen, even when preservatives are used (23, 39). These data suggest that mitochondrial respiration may play a role in the cardioprotection observed in our studies. Our data showing that tissue ATP levels are significantly increased in RI-Mito compared with RI-vehicle hearts support this hypothesis. However, we also show that the use of exogenous ATP and ADP was not cardioprotective. These data agree with previous reports demonstrating that exogenous ATP supplementation and/or ATP synthesis promoters do not restore high-energy phosphate stores and have no beneficial effects on postischemic functional recovery (2, 31). The inability of exogenous supplementation and/or ATP synthesis promoters to enhance cardioprotection is believed to be due primarily to the lability of ATP and its short half-life in vivo (32).
Mitochondrial components or mitochondrial DNA + RNA provided no cardioprotection in our studies. This finding contradicts previous findings of limited protection by administration of exogenous mitochondrial proteins. In the septic mouse, administration of exogenous cytochrome c (800 μg iv) has been shown to improve cardiac function, modestly through cytochrome oxidase modulation (25, 27). In addition, mitochondrial coenzyme Q10 has been shown to have beneficial effects on endothelial function and extracellular superoxide dismutase in patients with ischemic heart disease (26, 29, 34). In these studies, rather high oral doses of coenzyme Q10 (100–300 mg/day for 1 mo) were required for beneficial effects (26, 29, 34). We have not measured the levels of coenzyme Q10 or cytochrome c, but it is unlikely that the levels shown in these earlier studies were attained using our protocol.
Our data also show that ROS (TBARS) are significantly decreased in RI-Mito compared with RI-vehicle hearts. However, the ROS scavenger MPG, when used throughout reperfusion or added to mitochondria, failed to block the cardioprotection afforded by injection of mitochondria into the ischemic zone. These data must be examined carefully, inasmuch as it has been previously shown that ROS and nitric oxide are required for cardioprotection (9, 10). Although controversy exists as to the interaction of these messengers with other mechanisms, our data suggest that injection of mitochondria into the ischemic zone just before reperfusion enhances cardioprotection through mechanisms that are not modified by ROS. Whether our observations represent primary or secondary effects is beyond the scope of the present study, and more in-depth studies are required to elucidate the exact mechanism(s) of cardioprotection.
These data indicate that freshly isolated, intact, viable, respiration-competent mitochondria are required for cardioprotection and may act as mediators or as intermediates for mechanisms modulating cardioprotection. We hypothesize that viable, respiration-competent mitochondria act through mechanisms that would include modulation of intracellular calcium, synthesis of high-energy phosphates, and/or signal transduction mechanisms that include the adenosine receptors, the ATP-sensitive potassium channels, and/or ROS and/or nitric oxide (9, 24, 30).
Our data show that necrosis and apoptosis are significantly decreased in RI-Mito hearts. This reduction in myocardial injury was confirmed by CK-MB and cTnI analysis. The reduction in necrosis compared with apoptosis is more apparent in the RI model than in the global ischemic heart model, and the present data are in agreement with our earlier reports (16–18, 35).
In our previous studies, we and others showed that apoptosis is modulated principally through the intrinsic mitochondria-mediated pathway and that apoptosis is initiated during ischemia and effected during reperfusion (6, 18, 30, 38). We were unable to show any significant decrease in TUNEL-positive cells; however, there was a significant decrease in caspase-3-like activity in RI-Mito compared with RI-vehicle hearts. These data suggest that the apoptosis mechanism is decreased by injection of mitochondria into the RI zone.
The induction of apoptosis follows an ordered pathway that proceeds from the loss of mitochondrial membrane potential and the translocation of bax from the cytosol to the mitochondria and then leads to the eventual formation of the apoptosome and caspase-3 activation (8, 18, 39). Our data do not show any increase in apoptosis with the injection of mitochondria. The reason for this is that we have injected mitochondria just before reperfusion and, therefore, limited any possible induction during ischemia (8, 18, 32, 41). Our previous studies showed that a ≥15-min period of ischemia is required to induce apoptosis in this model (18). Our results show that the injected mitochondria maintain membrane potential and, thus, are unlikely to proceed to apoptosome formation (41). In addition, the injected mitochondria are found near the epicardial surface but remain within the interfibrillar space and do not enter the myocyte and, thus, would not be subject to bax activation (7, 18).
We have performed only preliminary studies (n = 2 each) in the in situ blood-perfused rabbit heart model for RI-vehicle and RI-Mito hearts using 30 min of RI and 2 h of reperfusion. The AAR was 16.2% and 18.3% of LV mass in RI-vehicle and 27.4% and 19.8% of LV mass in RI-Mito hearts. IS/AAR was 26.9% and 28% in RI-vehicle and 11.7% and 17.3% in RI-Mito hearts. SS after 120 min of reperfusion was 54.6% and 50.0% of equilibrium in RI-vehicle hearts and 72.2% and 66.9% of equilibrium in RI-Mito hearts. AAR was greater in RI-Mito than RI-vehicle hearts; however, IS was significantly decreased in RI-Mito hearts, and SS was significantly increased compared with RI-vehicle hearts. These data demonstrate that injection of isolated mitochondria into the ischemic zone enhances postischemic functional recovery and decreases IS in the blood-perfused heart (personal communication, data not shown).
In conclusion, our data show that mitochondria can be isolated and prepared for injection in <90 min and that isolated mitochondria are viable (as demonstrated by MitoTracker Orange and MitoFluor Green) and are respiration competent (as demonstrated by Clark-type electrode). Our results also demonstrate that injection of viable, respiration-competent mitochondria isolated from tissue unaffected by ischemia directly into the ischemic zone during early reperfusion significantly enhances global and regional postischemic myocardial functional recovery and significantly enhances cellular viability in the ischemic zone (significantly decreases IS, CK-MB, cTnI, TUNEL, and caspase-3 activity). Our results also show that the injected mitochondria maintain membrane potential and are present in the myocardium after 120 min of reperfusion and are distributed from the epicardium to the subendocardium.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-29077 and HL-068915.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM. An evaluation of the measurement of the activities of complex I–IV in the respiratory chain of human skeletal muscle mitochondria. Biochem Med Metab Biol 51: 35–42, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Bolling SF, Bies LE, Bove EL. Effect of ATP synthesis promoters on postischemic myocardial recovery. J Surg Res 49: 205–211, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, Graichen RE, Kay GL, Jyrala AJ, Colman A, Davidson BP, Pera M, Kloner RA. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol 43: 504–516, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dost T, Cohen MV, Downey JM. Redox signaling triggers protection during the reperfusion rather than the ischemic phase of preconditioning. Basic Res Cardiol 103: 378–384, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinberg H, Levitsky S, Lee SL. The quiescent heart: excitability, compliance, and vascular resistance. Am J Physiol Heart Circ Physiol 251: H1085–H1089, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Freude B, Masters TN, Robicsek F, Fokin A, Kostin S, Zimmermann S, Ulmann C, Lorenz-Meyer S, Schaper U. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol 32: 197–208, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Hagège AA, Carrion C, Menasché P, Vilquin JT, Duboc D, Marolleau JP, Desnos M, Bruneval P. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet 361: 491–492, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Lasley RD, Keith BJ, Kristo G, Yoshimura Y, Mentzer RM Jr. Delayed adenosine A1 receptor preconditioning in rat myocardium is MAPK dependent but iNOS independent. Am J Physiol Heart Circ Physiol 289: H785–H791, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Lebuffe G, Schumacker PT, Shao ZH, Anderson T, Iwase H, Vanden Hoek TL. ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am J Physiol Heart Circ Physiol 284: H299–H308, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tander B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol 287: H258–H267, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Levitsky S, Laurikka J, Stewart RD, Campos CT, Lahey SJ, McCully JD. Mitochondrial DNA deletions in coronary artery bypass grafting patients. Eur J Cardiothorac Surg 24: 777–784, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Levy RJ, Deutschman CS. Cytochrome c oxidase dysfunction in sepsis. Crit Care Med 35: S468–S475, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Yang XM, Iliodromitis EK, Kremastinos DT, Dost T, Cohen MV, Downey JM. Redox signaling at reperfusion is required for protection from ischemic preconditioning but not from a direct PKC activator. Basic Res Cardiol 103: 54–59, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCully JD, Rousou AJ, Parker RA, Levitsky S. Age and gender differences in mitochondrial oxygen consumption and free matrix calcium during ischemia/reperfusion and with cardioplegia and diazoxide. Ann Thorac Surg 83: 1102–1109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCully JD, Toyoda Y, Wakiyama H, Rousou AJ, Parker RA, Levitsky S. Age and gender related differences in ischemia/reperfusion injury and cardioprotection: effects of diazoxide. Ann Thorac Surg 82: 117–123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCully JD, Wakiyama H, Cowan DB, Federman M, Levitsky S. Diazoxide amelioration of myocardial injury and mitochondrial damage during cardiac surgery. Ann Thorac Surg 74: 2138–2146, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol 286: H1923–H1935, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113: 1287–1294, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Murry CE, Field LJ, Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation 112: 3174–3183, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animals tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358, 1979. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic, 1972, p. 151–156.
- 23.Olson MS, Von Korff RW. Changes in endogenous substrates of isolated rabbit heart mitochondria during storage. J Biol Chem 242: 325–332, 1967. [PubMed] [Google Scholar]
- 24.O'Rourke B Mitochondrial ion channels. Annu Rev Physiol 69: 19–49, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol 41: 879–888, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion 7: Suppl S154–S167, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Piel DA, Gruber PJ, Weinheimer CJ, Courtois MR, Robertson CM, Coopersmith CM, Deutschman CS, Levy RJ. Mitochondrial resuscitation with exogenous cytochrome c in the septic heart. Crit Care Med 35: 2120–2127, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Piper HM, Abdallah Y, Schafer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res 61: 365–371, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, Watts GF. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens 21: 297–306, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Diazoxide and cardioplegia ameliorate ischemia/reperfusion cell death through the modulation of mitochondrial volume and calcium accumulation and mitochondrial respiratory control index. Am J Physiol Heart Circ Physiol 287: H1967–H1976, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Soncul H, Ersoz A, Gokgoz L, Karasu C, Ayrancioglu K, Sinci V, Altan M. Cardioplegia with adenosine and adenosine triphosphate in the isolated guinea pig heart. Jpn Heart J 33: 843–850, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Stark G, Domanowits H, Sterz F, Stark U, Bachernegg M, Kickenweiz E, Decrinis M, Laggner AN, Tritthart HA. Action of ATP on ventricular automaticity. J Cardiovasc Pharmacol 24: 740–744, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Tansey EE, Kwaku Hammer PE KF, Cowan DB, Federman M, Levitsky S, McCully JD. Reduction and redistribution of gap and adherens junction proteins following ischemia/reperfusion. Ann Thorac Surg 82: 1472–1479, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J 28: 2249–2255, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Toyoda Y, Di Gregorio V, Parker RA, Levitsky S, McCully JD. Anti-stunning and anti-infarct effects of adenosine enhanced ischemic preconditioning. Circulation 102: 326–331, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda Y, Levitsky S, McCully JD. Opening of mitochondrial ATP-sensitive potassium channels enhances cardioplegic protection. Ann Thorac Surg 71: 1281–1289, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Vinten-Johansen J Postconditioning: a mechanical maneuver that triggers biological and molecular cardioprotective responses to reperfusion. Heart Fail Rev 12: 235–244, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Wakiyama H, Cowan DB, Toyoda Y, Federman M, Levitsky S, McCully JD. Selective opening of mitochondrial ATP-sensitive potassium channels during cardiopulmonary bypass decreases apoptosis and necrosis in a model of acute myocardial infarction. Eur J Cardiothorac Surg 21: 424–433, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wechsler MB Studies on oxidative phosphorylation and ATPase activity of fresh and frozen brain mitochondria. Arch Biochem Biophys 95: 494–498, 1961. [DOI] [PubMed] [Google Scholar]
- 40.Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang N, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL, Vinten-Johansen J. Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol 294: H1444–H1451, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Lu C, Whiteman M, Chance B, Armstrong JS. The mitochondrial permeability transition regulates cytochrome c release for apoptosis during endoplasmic reticulum stress by remodeling the cristae junction. J Biol Chem 283: 3476–3486, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol 130: 1115–1123, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]