Abstract
Circadian rhythms offer probably the best understanding of how genes control behavior, and much of this understanding has come from studies in Drosophila. More recently, genetic manipulation of clock neurons in Drosophila has helped identify how daily patterns of activity are programed by different clock neuron groups. Here, we review some of the more recent findings on the fly molecular clock and ask what more the fly model can offer to circadian biologists.
INTRODUCTION
A forward genetic screen conducted by Ron Konopka while a Ph.D. student in Seymour Benzer's lab more than 35 years ago identified three different mutations that dramatically affected circadian (∼24 hours) rhythms in pupal eclosion (Konopka and Benzer 1971). All three mutations mapped to a single gene that was named period (per) because the mutations either affected the length of the behavioral period or completely abolished rhythmicity altogether. At the time, the isolation of these mutants was a surprise to many because it was not clear that mutation of a single gene could have such a profound effect on complex animal behavior. Indeed, this is not always the case: Mice in which only one of the three mouse Per genes are eliminated are initially rhythmic, although mutation of mPer1 and mPer2 together mimics the arrhythmicity of Drosophila per null mutants (for review, see Stanewsky 2003).
Work during the next three decades led to the cloning of Drosophila per and an understanding of PER protein's integral role in an intracellular molecular clock. The importance of the Drosophila work cannot be underestimated: During the sequencing of human chromosome 17, one cDNA was identified that had sequence homology with fly per (Sun et al. 1997). This of course suggested a role in the human clock, but without flies, the human Per genes could still be in search of a function. Instead, a polymorphism in hPer2 has been associated with a human sleep disorder (Toh et al. 2001), and mice lacking Per2 show increased tumor rates, suggesting a hierarchical relationship between the circadian clock and the cell cycle (Fu et al. 2002). The multiple functions associated with mammalian Per genes make sense given the presence of molecular clock genes in many tissues. In turn, this is of great interest to basic scientists and clinicians alike and has implications for the daily timing of therapeutic interventions, discussed by Hunt and Sassone-Corsi (2007).
In contrast to mammals, the Drosophila per gene and its associated intracellular clocks are largely confined to peripheral sensory neurons and a small subset of central brain neurons. Thus, circadian studies in Drosophila have a neurobiological perspective as fly clocks mainly regulate rhythms, sensory perception, and behavior. We start with a discussion of the already detailed understanding of the molecular clock in Drosophila. We then discuss how recent findings about clock neuron coordination of daily rhythms in locomotor activity are changing the way we view the fly circadian clock.
MOLECULAR CLOCKS: TRANSCRIPTIONAL REGULATION IN THE FIRST LOOP
Our current view of the Drosophila molecular clock has two interconnected transcriptional feedback loops (Fig. 1). The first loop, involving per, is a simple negative feedback loop (for review, see Blau 2001). Two transcriptional activators, Clock (CLK) and Cycle (CYC) heterodimerize and bind to specific E-box sequences in the per and timeless (tim) promoters. PER and TIM proteins interact in the cytoplasm and then enter the nucleus. Once inside the nucleus, PER inhibits CLK/CYC activity by removing them from DNA (Yu et al. 2006); thus, PER protein inhibits further expression of per and tim. PER is progressively phosphorylated and then degraded, allowing CLK/CYC to reactivate per and tim expression and start the next cycle of the clock. Delays that slow down PER accumulation and PER/TIM nuclear entry help make each cycle of a conceptually simple feedback loop last for 24 hours and presumably help the clock oscillate as opposed to come to equilibrium.
Figure 1.
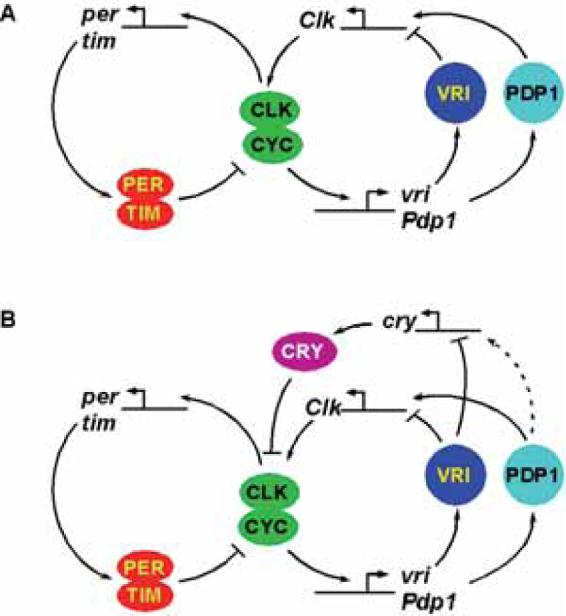
Model of the molecular clock feedback loops in Drosophila. The clock is composed of two interconnected transcriptional-translational feedback loops. (A) In s-LNVs, cry is not required for rhythms and is omitted from the model, although it acts as a cell-autonomous photoreceptor in these cells. (B) In peripheral clocks, CRY has an important clock role and acts as a transcriptional repressor of CLK/CYC activity in conjunction with PER. Because cry expression is regulated by VRI and because VRI- and PDP1-binding sites are very similar, cry is probably also regulated by PDP1 (dashed line).
Originally, PER and TIM were thought to enter the nucleus together because their subcellular localization showed essentially identical timing in flies, first appearing in the cytoplasm and then translocating into the nucleus. Furthermore, TIM is largely cytoplasmic in per null mutant flies, and a stable PER-fusion protein is cytoplasmic in tim null mutants. However, this simple model has been challenged recently.
The first upset was when two studies showed that PER could be detected in pacemaker neuron nuclei before TIM (Shafer et al. 2002, 2004). The viability of the simple model was partly rescued when Ashmore et al. (2003) showed that although TIM could shuttle in and out of the nucleus, even in a per null mutant, it seems to have a shorter dwelling time in the nucleus. Unfortunately, for the simple model, we showed that PER is constitutively nuclear in the absence of its tightly associated protein kinase, Doubletime (DBT), even in a tim null mutant (Cyran et al. 2005). The final nail in the simple PER/TIM translocation model was experiments in Drosophila S2 cells in culture transfected with per and tim expression plasmids that found that although PER and TIM associate for a considerable time in the nucleus, they often dissociate just before nuclear entry (Meyer et al. 2006).
TIM, however, is certainly required for PER's cytoplasmic accumulation (which necessarily precedes PER nuclear entry) and tim is an essential clock gene because tim null mutant flies are molecularly and behaviorally arrhythmic. Many different tim alleles carrying single point mutations profoundly affect period length (ranging from 20 to 33 hours) and hence the timing of the molecular clock. The longest of these tim mutants (timUL; Rothenfluh et al. 2000) appears to increase the association of PER and TIM in the nucleus. One interpretation of this finding is that PER and TIM normally interact in the nucleus, and this is magnified in timUL mutants. Thus, the dissociation of PER and TIM observed in S2 cells before nuclear entry may only be a transient event or could even represent a peculiarity of this system. Ultimately, it will be necessary for the fine-resolution imaging of PER and TIM described in S2 cells to be applied to fly clock neurons to answer these mechanistic details.
Importantly, the delay in nuclear entry of PER and TIM observed in clock cells seems to be an intrinsic feature of these proteins because it occurs even in S2 cells in vitro (Meyer et al. 2006). Furthermore, the perLong mutation originally identified by Konopka and Benzer (1971) lengthens the time taken for nuclear entry in S2 cells (Meyer et al. 2006), accurately reflecting what happens in perL pacemaker neurons in vivo (Siwicki et al. 1988).
MOLECULAR CLOCKS: POSTTRANSCRIPTIONAL REGULATION IN THE FIRST LOOP
A substantial amount of posttranscriptional regulation exists in this first clock loop and presumably contributes to delay in nuclear entry of PER and TIM. The DBT and casein kinase II (CK2) protein kinases probably regulate PER stability and determine the timing of its nuclear entry (Price et al. 1998; Suri et al. 2000; Lin et al. 2002; Akten et al. 2003). DBT forms a stable interaction with PER and translocates to the nucleus along with PER. Inside the nucleus, PER is progressively phosphorylated, presumably by DBT.
Inside the nucleus, maximal PER phosphorylation takes several hours and is probably so slow because of a balance between DBT phosphorylating PER and protein phosphatase 2A (PP2A) dephosphorylating PER. There is excellent genetic evidence supporting this balancing model, hidden in the supplementary data of a paper by Sathyanarayanan et al. (2004). These authors found that overexpression in clock cells of Widerborst (Wdb), a nuclear PP2A regulatory subunit, lengthens the period by about 0.8 hours. Flies heterozygous for a dominant dbt allele (dbtg) have a period almost 4 hours longer than wild type (27.6 hours). But overexpression of wdb in a dbtg background adds another 3 hours to the period (now 30.3 hours), a synergistic interaction. The well-described biochemical functions of DBT and PP2a and the numerical output of locomotor assays allowed Sathyanarayanan et al. (2004) to make a clear biochemical prediction on the basis of this genetic interaction: The synergy arises from increasing PP2A activity via increased Wdb levels at the same time as decreasing DBT kinase activity via dbtg. Overall, this supports the idea that PER stability and hence period length is a balance between PER phosphorylation via DBT and dephosphorylation via PP2A.
A second substrate for DBT and PP2A is the CLK transcription factor itself, whose activity is decreased when phosphorylated (Kim and Edery 2006; Yu et al. 2006). This adds extra significance to the stable interaction of DBT and PER: One function of PER may be to bring DBT into close proximity with CLK, leading to CLK phosphorylation and inactivation, presumably an integral part of the repression mechanism.
Shaggy (Sgg) is a third protein kinase that regulates clock speed. Overexpression of sgg in clock cells speeds up the clock, whereas reduced sgg levels slow down the clock (Martinek et al. 2001). Although sgg clearly regulates period length, it is unknown if sgg is required for rhythmicity because sgg is essential for fly development and animals without sgg function do not survive until adulthood. However, sgg is of great interest to the circadian field for at least two reasons: (1) extracellular signals regulate Sgg activity, suggesting that Sgg could link external stimuli to the intracellular clock (supported by the work of Yuan et al. 2005), and (2) Sgg is the major target of Lithium, one of the most effective agents against bipolar disorder, suggesting that bipolar disorder could even be a disorder of the circadian system, at least in part.
Martinek et al. (2001) proposed that increased Sgg levels speed up the clock by directly promoting TIM phosphorylation, leading to earlier nuclear entry of both PER and TIM. However, in a recent paper, Stoleru et al. (2007) revealed that the dramatic period-shortening associated with increased sgg expression requires the circadian photoreceptor Cryptochrome (CRY): sgg overexpression in a cryb mutant background gives only a 1-hour period-shortening compared to 3.5 hours in a cry+ background. Why should a photoreceptor have such a large effect on the period length of flies in constant darkness?
MOLECULAR CLOCKS: WHEN IS A PHOTORECEPTOR MORE THAN JUST A PHOTORECEPTOR?
Before the observations of Stoleru et al. (2007), we had asked the same question for two reasons: (1) one of the major differences between the Drosophila and mammalian clocks is the function of CRY: a photoreceptor in flies, but a transcriptional repressor in mammals and (2) if CRY functions solely as a photoreceptor, then it was unclear why the molecular clocks in the majority of clock-containing cells in the fly should become arrhythmic in cry mutants. One could imagine that light is required to start the clock. However, this is not the case, at least for the brain clocks driving behavior, because flies kept in constant darkness for their entire development are rhythmic as adults (Sehgal et al. 1992).
Although the usual criteria for a gene to be considered a core clock component are that mutations affect adult locomotor behavior in constant darkness, it was already striking that cry mutants had lost circadian rhythms in antennal sensitivity (Krishnan et al. 2001). In addition, because these rhythms are driven by clocks within the antenna, this suggested that cry is required for the peripheral antennal clocks to function (Tanoue et al. 2004) and, by analogy to the mammalian clock, could involve CRY functioning as a transcriptional repressor. We tested this idea by examining in which state the clock had stopped in the eye peripheral clocks and found that it was similar to the clocks in per mutants: Four direct CLK/CYC targets were constitutively derepressed. To balance this, we performed a gain-of-function experiment and found that overexpression of CRY together with PER stopped the clock with constitutively low levels of CLK/CYC activity, although overexpression of either CRY alone or, perhaps surprisingly, PER alone had little effect on molecular oscillations (Collins et al. 2006).
Taken together, this indicates that CRY can be considered a clock component at least in some clock cells. Perhaps understandably, our bias in the field is to focus on mutations that alter adult locomotor rhythms and these usually affect the molecular clock in the major pacemaker neurons: the small ventral lateral neurons (s-LNVs). The behavior of cry mutants in constant darkness (DD) is very similar to wild-type fly behavior (Stanewsky et al. 1998), suggesting that molecular rhythms in the s-LNVs are largely unaffected by mutating cry. Indeed, the paper by Stanewsky et al. (1998) was one piece of evidence that pointed to the s-LNVs as the major pacemaker neurons because their clocks were still rhythmic in cry mutants. However, some other clocks in brain neurons are stopped in cry mutants, suggesting that cry functions as a transcriptional repressor in a subset of central brain clock cells that excludes the s-LNVs. This fits with the observation that the effects of Sgg on rhythms in constant light (LL) are stronger in flies in which Sgg is overexpressed in all clock neurons except LNVs (Stoleru et al. 2007). However, it is not yet clear how Sgg functions via CRY to regulate the clock.
MOLECULAR CLOCKS: A SECOND CLOCK LOOP
cry RNA levels peak around dawn, perhaps to make the cells in which CRY is produced most sensitive to light as the sun rises. Clk RNA levels also oscillate in-phase with cry, and these rhythms are antiphase to those of per and tim, whose RNA levels peak close to dusk. How are these antiphase rhythms generated?
Using a now Stone Age technique called differential display, we identified a rhythmically expressed transcriptional repressor—vrille (vri)—which is a direct CLK/CYC target like per and tim (Blau and Young 1999). Transgenic overexpression of vri in clock cells either lengthened the period or prevented behavioral rhythms, depending on whether a weak or strong UAS-vri transgene was used. At the molecular level, the strong UAS-vri transgene stopped the clock in a state similar to that of Clk mutants.
Furthermore, in wild-type flies, VRI protein levels peaked as Clk RNA levels were at their lowest, suggesting that VRI protein could feed back to repress Clk expression. Experiments in flies and in vitro supported this model, thereby identifying one component of a second feedback loop in the clock, which Glossop et al. (1999) had previously predicted. Glossop et al. (2003) also came to similar conclusions about the regulation of Clk by VRI, and they also showed that cry was a second direct target of VRI.
If VRI is the Clk repressor, then what activates Clk expression? In 2003, we published a paper identifying Pdp1 as a transcriptional activator for Clk (Cyran et al. 2003). We showed that in vitro PDP1 and VRI compete for binding to at least one site in the Clk promoter. In vivo, we found that VRI and PDP1 protein levels are rhythmic, but PDP1 levels peak 3−6 hours after VRI. Thus, we proposed that in the late day and early evening, VRI is present and represses Clk transcription, whereas later at night as VRI levels fall, PDP1 activates Clk expression (see Fig. 1). Furthermore, although Pdp1 null mutants develop abnormally slowly and do not survive until adulthood, the clock in their larval LNVs could be analyzed. We found that the clock stops in the LNVs of Pdp1 null mutant larvae, with constitutively low levels of tim RNA and PER protein, again similar to both vri overexpression and Clk mutants and indicating that Pdp1 is an essential clock component.
Genetic interactions supported opposite roles for VRI and PDP1 in the clock. The period length in flies heterozygous for either a vri or Pdp1 null mutation is altered slightly from wild type: vri heterozygous flies have about 0.7-hour shorter periods than wild type, whereas Pdp1 heterozygotes have about 0.5-hour longer periods (Blau and Young 1999; Cyran et al. 2003). More dramatic changes were seen when combining alleles. The weakest of the UAS-vri overexpression transgenes gives 25-hour rhythms, but removing one copy of Pdp1 in addition leads to 27-hour rhythms, although with considerable variation, with some flies having as long a period as 28.5 hours (Cyran et al. 2003). Although constitutive expression of vri via the Gal4-UAS system is obviously artificial, the synergistic interaction between increased vri and decreased Pdp1 strongly supported the idea that VRI and PDP1 have opposing roles in the clock, in a manner analagous to that described earlier for DBT and PP2a for PER phosphorylation (see above).
There could be several functions for this second clock loop. One possible role is to add robustness to the molecular clock. This is supported by studies of mice lacking Rev-erbα, which has an analogous role to VRI in the second mammalian clock loop. Rev-erbα knockout mice have slightly shorter periods than wild-type mice overall, but the period is much more variable from mouse to mouse compared to wild-type controls (Preitner et al. 2002). A second role of the second clock loop could be to express genes important for outputs of the molecular clock with phase rhythms opposite those controlled by CLK/CYC (discussed below).
MOLECULAR CLOCKS: A CHALLENGE TO THE STATUS OF PDP1 IN THE SECOND LOOP
The importance of PDP1 in the second clock loop was recently challenged by Benito et al. (2007), who made UAS-Pdp1ε and UAS-Pdp1ε RNA interference (RNAi) transgenes to respectively overexpress or knock down PDP1ε levels specifically in clock neurons, allowing the analysis of adult behavior in flies. These authors found that either increasing or reducing PDP1ε levels only in the Pdf-expressing sLNVs made flies largely arrhythmic. However, one surprising finding was that the molecular clock in the s-LNVs keeps running in DD.
These results led Benito et al. (2007) to suggest that whereas Pdp1 is important for circadian behavior, its major function is in the regulation of outputs from the clock, rather than in regulating the core clock because this self-sustains even with dramatically altered Pdp1 levels. What could account for the different interpretations resulting from analyzing a Pdp1 null mutant that prevents normal development (Cyran et al. 2003) and Pdp1 RNAi in clock neurons (Benito et al. 2007)? There are at least two possibilities:
Because RNAi often produces knockdowns rather than complete null phenotypes, the manipulations of Benito et al. (2007) may have removed most but not all PDP1. Because this still produces behavioral arrhythmicity, it may be that PDP1-regulated behavioral output genes are indeed more sensitive to PDP1 reductions than the molecular clock, because multiple transcriptional and posttranslational controls support molecular clock rhythms (discussed earlier). A little PDP1 goes a long way within the clock by this argument, as long as there is enough to give some Clk expression to allow the clock to run. In support of the idea that a Pdp1 null mutant is stronger than Pdp1 RNAi, we noticed that Pdf RNA is absent from s-LNVs in both Clk and Pdp1 mutants (Cyran et al. 2003), whereas Benito et al. (2007) found that Pdp1 RNAi did not dramatically affect the level of PDF peptide immunostaining, which was used as a control to quantify molecular clock oscillations.
There may be other defects associated with development in Pdp1 null mutants that affect s-LNV function. This idea was raised by Benito et al. (2007) who suggested that our inability to detect molecular clock oscillations in Pdp1 null mutant LNVs might be due to defective development. However, in the first submission of our manuscript, we had included a figure showing that in light/dark cycles (LD), it is possible to detect TIM correctly localized to the nucleus at night in LNVs, even without Pdp1 (included here as Fig. 2). This indicates that LNVs are present and even function in LD; however, this is a light-driven rhythm because tim RNA oscillations stop on the first day in DD (Cyran et al. 2003). Thus, we concluded that Pdp1 is required for the molecular clock in DD, although the strong effects of light somehow bypass the requirement for Pdp1. This is reminiscent of the light-driven molecular clocks that stop in DD in a strong dbt hypomorph (Price et al. 1998) and in electrically silenced neurons (Nitabach et al. 2002). The molecular basis for any of these light-driven rhythms remains unclear. The effect of light on the clock can also be seen in the peripheral clocks in the eye, which show strong rhythms in LD but damp rapidly in DD.
Figure 2.
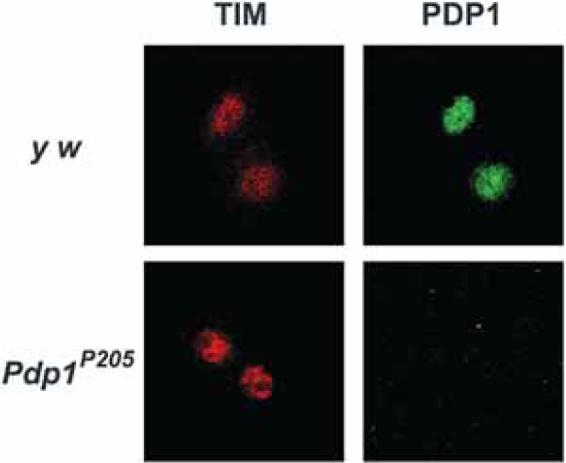
The molecular clock is at least partially functional in light/dark cycles in Pdp1 null mutants. Control (y w) and Pdp1P205 null mutant larvae were entrained in LD cycles, dissected at ZT22 and stained for TIM (red) and PDP1 (green) proteins. Larval LNVs were identified by their characteristic clustering of four cells in the center of the brain. Images were taken by confocal microscopy. LNVs in both genotypes show TIM in the nucleus, although PDP1 is missing from Pdp1P205 mutants. Note that TIM cannot be detected in Pdp1P205 mutant LNVs on the first day in constant darkness (Cyran et al. 2003).
In summary, it is clear that Pdp1 is important for circadian rhythms, and further research on this topic will aim to explain current discrepancies. One important point to note is that in flies in which Pdp1ε RNAi is driven by timgal4, the rhythmic flies have approximately 2-hour longer periods than wild type (Benito et al. 2007), again consistent with a central clock regulatory role for Pdp1. However, the idea that Pdp1 is very important in regulating behavioral outputs from s-LNVs (Benito et al. 2007) is certainly interesting because PDP1's maximal activity at the end of the night coincides with the time at which s-LNVs drive the onset of morning locomotor activity (see below). Thus, transcriptional regulation of clock outputs by VRI and PDP1 may be key to understanding how a molecular clock is linked to behavioral rhythms.
NEUROBIOLOGY AND THE DROSOPHILA CIRCADIAN CLOCK
The majority of clocks in flies are found in neurons. In contrast, many different mammalian tissues possess clocks leading to daily physiological rhythms in addition to behavioral rhythms. Thus, studies of Drosophila circadian rhythms have a largely neurobiological focus, and it is in this arena that Drosophila is likely to make key contributions to the circadian field in the future.
Some of the fly molecular clocks are found in sensory neurons, for example, in the photoreceptors that make up the adult eye. However, these clocks do not maintain rhythmicity under constant conditions. To our knowledge, it has not been established whether they simply lose synchrony from one another and so the overall population loses rhythmicity or whether the individual clocks run down and stop. Thus, although the clocks in the photoreceptor cells run in light/dark, they cannot really be considered bona fide circadian clocks because at best they drift out of phase under constant conditions, and the hallmark of a circadian clock is to keep accurate 24-hour rhythms in DD. Nevertheless, the ease of obtaining large amounts of tissue from these photoreceptor cells made possible biochemical studies of the fly clock.
In contrast, flies can keep precise rhythms of locomotor activity under constant conditions for several weeks, and the molecular clocks in the central brain neurons continue to show strong rhythms. This has often been taken to mean that the brain neurons driving this behavior have very precise cell-autonomous circadian clocks. However, the recent identification of circadian neural networks in the brain (Grima et al. 2004; Stoleru et al. 2004, 2005) means that we may have to reconsider this idea. Indeed, mammalian pacemaker neurons in the suprachiasmatic nucleus (SCN) show strong self-sustaining rhythms in electrical activity even when dissociated in culture (Welsh et al. 1995). However, these electrical rhythms are quite variable in period when comparing cells isolated from one SCN. This contrasts with the very similar behavioral periods found when comparing different animals and suggested that the precision of circadian behavior in mammals comes partly from the coupling of pacemaker neurons.
Is there any evidence for equivalent coupling in Drosophila? There have not yet been any reports of rhythms in isolated fly clock neurons to test their ability to sustain accurate rhythms autonomously. However, a number of in vivo experiments implicate strong effects of one clock neuron group on another. Indeed, it is even possible that the difference between the damping clocks in the eye in DD and the sustained central brain clocks lies in these non-cell-autonomous effects on the clock. As described below, signals from other clock neurons either may reinforce the molecular clock loops to keep them running accurately or may even be required for the clock to keep ticking.
NEUROBIOLOGY: ARE THERE CELL-AUTONOMOUS CIRCADIAN CLOCKS IN DROSOPHILA?
One example of cell nonautonomy involves the neuropeptide pigment dispersing factor (PDF), which is produced in the LNVs and is likely released from s-LNV termini in a rhythmic manner to signal circadian time of day information to downstream neurons. In Pdf null mutants, or in the absence of the LNVs themselves, flies do not anticipate dawn and instead show a startle response to the abrupt lights-on transition (Renn et al. 1999). Hence, in LD cycles, the LNVs signal via PDF to drive increased locomotor activity in anticipation of light at dawn, and the PDF cells are also known as morning (M) cells.
At the other end of the day, wild-type flies also anticipate lights-off at dusk by increasing their activity, and this behavior requires a second set of clock neurons that probably includes the dorsal lateral neurons (LNDs), dorsal neuron group 1 (DN1s), and one PDF-ve s-LNV—collectively known as evening or E cells (Grima et al. 2004; Stoleru et al. 2004). Anticipation of dusk indicates a circadian response, and this was confirmed by the requirement for per expression in these E cells (Grima et al. 2004; Stoleru et al. 2004).
Interestingly, the peak of activity at dusk is advanced by approximately 1 hour Pdf in null mutants (Renn et al. 1999). This is accompanied by an advanced phase of the molecular clock in the LND subgroup of E cells in Pdf null mutants (Lin et al. 2004). Because PDF is not produced in LNDs, this is a non-cell-autonomous effect of PDF on their clock. However, the initial reports localizing the PDF receptor (PDFR) found that most LNDs do not synthesize PDFR (Hyun et al. 2005; Mertens et al. 2005), so how the PDF signal is transmitted to LNDs remains to be identified.
Nonautonomy of the clocks in E cells in general was further supported in experiments by Stoleru et al. (2005) in which only the LNV clock was accelerated by overexpression of sgg. When the phase of the clock in all brain clock neurons was measured via tim RNA, these authors found that the clocks in the LNDs, DN1s, and DN3s were also advanced, coming into phase with the s-LNV clock. Thus, the LND, DN1, and DN3 clocks were altered in a non-cell-autonomous manner.
In the opposite experiment, Stoleru et al. (2005) tested the effect of accelerating the phase of the clock in non-Pdf-expressing cells again via overexpression of sgg in all clock neurons except s-LNVs. Here, they found that the molecular clock in the LNDs, DN1s, and DN3s was largely unaffected by this manipulation, presumably because the dominant s-LNV clock was still running with a 24-hour period. Because sgg expression in all clock neurons speeds up the phase of all neuronal clocks, the pace of the clocks in the LNDs, DN1s, and DN3s is capable of being increased at least when the s-LNV clock is accelerated (Stoleru et al. 2005).
Finally, when Stoleru et al. (2005) overexpressed sgg only in the DN2 clock neurons, they accelerated both the DN2 clock and the large-LNV (l-LNV) clock. Although this had no effect on the period of locomotor behavior, it again demonstrates a non-cell-autonomous clock effect in this case with the DN2s determining the timing of the l-LNV clock.
Taking all of this together, one could make the case that the s-LNVs and perhaps the DN2s are the only cells that possess cell-autonomous clocks in Drosophila and that they determine clock time for the rest of the brain clock neurons. However, a careful study by Lin et al. (2004) showed that the s-LNV clocks drift out of synchrony from one another in Pdf null mutants in DD. Because PDF is a secreted molecule, this provides evidence for noncell autonomy of even the s-LNV clock. Potentially, PDF could synchronize the s-LNVs via an autocrine signal back to the cell from which it was released, via cell–cell communication between neighboring s-LNVs or perhaps most likely via the clock neural circuit.
So if the s-LNV clock is not completely cell-autonomous either, is there any evidence for non-PDF clock neurons communicating with s-LNVs? Yes. In their original classification of M and E cells, Grima et al. (2004) and Stoleru et al. (2004) found that rescue of per only to E cells was sufficient to drive both morning and evening peaks of activity, even though the M cells did not have a functional clock. In contrast, ablation of M cells leads to complete loss of morning activity (Renn et al. 1999). The simplest interpretation of these experiments is that E cells communicate to M cells to drive the release of PDF at the correct time for morning activity, and anatomical studies support the idea of communication between E and M cells (Stoleru et al. 2004; Shafer et al. 2006). However, a functional clock only in E cells cannot support long-term rhythmicity in DD, underscoring the importance of M cells in DD (Grima et al. 2004; Stoleru et al. 2004). So although the PDF+ve M cells seem to have the most cell-autonomous of all of the clocks in the brain, there is evidence for communication between E and M cells (see Fig. 3).
Figure 3.
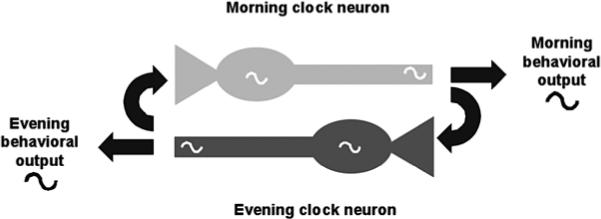
A model for cell–cell interactions that promote precision of behavioral rhythms and morning and evening activity. A highly simplified view of the clock neural circuit involving two neurons. Each cell has a functional clock that helps drive rhythmic neuronal signals. The morning (light gray) neuron promotes morning activity, and its output signals also modify the phase of the evening (dark gray) neuron. Similarly, signals from the evening neuron regulate both evening activity and input to the morning neuron. The behavioral output signals and the input signals to the other neuron could either be the same or they could be different.
NEUROPEPTIDES AS SIGNALS IN BRAIN CLOCK NEURONS
It will be important to identify not only which signals clock neurons use to communicate with one another, but also how these signals are transduced to the molecular clock to alter phase. So far, we know of only three neurotransmitters among all the different clock neuron groups: PDF in the LNVs, IPNamide in a subgroup of DN1 cells (DN1a; Shafer et al. 2006), and neuropeptide F (NPF) in a subset of LNDs, although only in male flies (Lee et al. 2006). Of these, only PDF has so far been assigned a circadian role (Renn et al. 1999), but there is also evidence that LNVs use an alternative unidentified signal(s) to mediate larval light avoidance (Mazzoni et al. 2005) and to regulate adult cocaine sensitivity (Tsai et al. 2004).
Intriguingly, all of the identified signals are neuropeptides, which are also important in signaling within the SCN. Mice lacking a key neuropeptide receptor in the SCN (the VPAC2 receptor) are arrhythmic (Harmar et al. 2002) and individual cells in the SCN of these mutants either have lost rhythms completely or are desynchronized from one another (Aton et al. 2005; Maywood et al. 2006). Thus, communication among clock neurons via neuropeptides seems to be conserved across species. The demonstration that coupling among clock neurons in the SCN can override genetic defects in their individual clocks (Liu et al. 2007) supports the importance of understanding clock neuron communication.
CONCLUSIONS
Molecular genetic analysis has made circadian rhythms the best understood behavior at the molecular level. New approaches that more accurately treat the clock cell as a neuron should also make circadian rhythms the best understood behavior at the neuronal and circuit levels. Signaling between clock neurons seems to augment the molecular loops and keep their precision in the absence of environmental signals. We believe that the ability to manipulate gene expression in precisely defined subsets of clock neurons in Drosophila and to measure changes at molecular and behavioral levels means that the fly still has a major role in the circadian field.
ACKNOWLEDGMENTS
We thank Esteban Mazzoni for help with confocal microscopy in Figure 2.
REFERENCES
- Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, Jackson FR. A role for CK2 in the Drosophila circadian oscillator. Nat. Neurosci. 2003;6:251. doi: 10.1038/nn1007. [DOI] [PubMed] [Google Scholar]
- Ashmore LJ, Sathyanarayanan S, Silvestre DW, Emerson MM, Schotland P, Sehgal A. Novel insights into the regulation of the Timeless protein. J. Neurosci. 2003;23:7810. doi: 10.1523/JNEUROSCI.23-21-07810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circa-dian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005;8:476. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito J, Zheng H, Hardin PE. PDP1 epsilon functions downstream of the circadian oscillator to mediate behavioral rhythms. J. Neurosci. 2007;27:2539. doi: 10.1523/JNEUROSCI.4870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau J. The Drosophila circadian clock: What we know and what we don't know. Semin. Cell Dev. Biol. 2001;12:287. doi: 10.1006/scdb.2001.0256. [DOI] [PubMed] [Google Scholar]
- Blau J, Young MW. Cycling vrille expression is required for a functional Drosophila clock. Cell. 1999;99:661. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- Collins B, Mazzoni E, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr. Biol. 2006;16:441. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Cyran SA, Yiannoulos G, Buchsbaum AM, Saez L, Young MW, Blau J. The Double-Time protein kinase regulates the subcellular localization of the Drosophila clock protein Period. J. Neurosci. 2005;25:5430. doi: 10.1523/JNEUROSCI.0263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyran SA, Buchsbaum AM, Reddy KL, Lin M-C, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329. doi: 10.1016/s0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Glossop NR, Lyons LC, Hardin PE. Interlocked feedback loops within the Drosophila circadian oscillator. Science. 1999;286:766. doi: 10.1126/science.286.5440.766. [DOI] [PubMed] [Google Scholar]
- Glossop NR, Houl JH, Zheng H, Ng FS, Dudek SM, Hardin PE. VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron. 2003;37:249. doi: 10.1016/s0896-6273(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Hunt T, Sassone-Corsi P. Riding tandem: Circadian clocks and the cell cycle. Cell. 2007;129:461. doi: 10.1016/j.cell.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, Bae E, Kim J. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Kim EY, Edery I. Balance between DBT/CKIepsilon kinase and protein phosphatase activities regulate phosphorylation and stability of Drosophila CLOCK protein. Proc. Natl. Acad. Sci. 2006;103:6178. doi: 10.1073/pnas.0511215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. 1971;68:2112. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Levine JD, Sisson K, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for Cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- Lee G, Bahn JH, Park JH. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc. Natl. Acad. Sci. 2006;103:12580. doi: 10.1073/pnas.0601171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R. A role for casein kinase 2α in the Drosophila circadian clock. Nature. 2002;420:816. doi: 10.1038/nature01235. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 2004;24:7951. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, III, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circa-dian clock network. Cell. 2007;129:605. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity pene shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol. 2006;16:599. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Meyer P, Saez L, Young MW. PER-TIM interactions in living Drosophila cells: An interval timer for the circadian clock. Science. 2006;311:226. doi: 10.1126/science.1118126. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circa-dian rhythms in Drosophila. Cell. 1999;99:791. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Young MW, Saez L. A TIMELESS-independent function for PERIOD proteins in the Drosophila clock. Neuron. 2000;26:505. doi: 10.1016/s0896-6273(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S, Zheng X, Xiao R, Sehgal A. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell. 2004;116:603. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Price J, Young MW. Ontogeny of a biological clock in Drosophila melanogaster. Proc. Natl. Acad. Sci. 1992;89:1423. doi: 10.1073/pnas.89.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, Truman JW. Sequential nuclear accumulation of the clock proteins Period and Timeless in the pacemaker neurons of Drosophila melanogaster. J. Neurosci. 2002;22:5946. doi: 10.1523/JNEUROSCI.22-14-05946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Förster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J. Comp. Neurol. 2006;498:180. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Levine JD, Truman JW, Hall JC. Flies by night: Effects of changing day length on Drosophila's circadian clock. Curr. Biol. 2004;14:424. doi: 10.1016/j.cub.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Eastman C, Petersen G, Rosbash M, Hall JC. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988;1:141. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Stanewsk R. Genetic analysis of the circadian system in Drosophila melanogaster and mammals. J. Neurobiol. 2003;54:111. doi: 10.1002/neu.10164. [DOI] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies Cryptochrome as an essential circadian photoreceptor in Drosophila. Cell. 1998;95:681. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernandez de la Paz M, Menet JS, Ceriani MF, Rosbash M. The Drosophila circa-dian network is a seasonal timer. Cell. 2007;129:207. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- Suri V, Hall JC, Rosbash M. Two novel doubletime mutants alter circadian properties and eliminate the delay between RNA and protein in Drosophila. J. Neurosci. 2000;20:7547. doi: 10.1523/JNEUROSCI.20-20-07547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr. Biol. 2004;14:638. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Toh KL, Jones C, He Y, Eide EJ, Hinz WA, Virshup DM, Ptác̆ek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- Tsai L, Bainton RJ, Blau J, Heberlein U. Lmo mutants reveal a novel role for circadian pacemaker neurons in cocaine-induced behaviors. PLoS Biol. 2004;2:2122. doi: 10.1371/journal.pbio.0020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yu W, Zheng H, Houl JH, Dauwalder B, Hardin PE. PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev. 2006;20:723. doi: 10.1101/gad.1404406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]


