Abstract
BACKGROUND
Cellular markers help identify different components of a pathological process and may contribute to the diagnosis, prognostic assessment, and management of patients with suspected syndromes. Flow cytometry can be used to accurately assess markers of platelet and leukocyte activation and cellular aggregation in whole blood. To use cell markers as predictors of disease requires that they be measured reliably and show modest within-individual, day-to-day variation.
METHODS
We used whole blood flow cytometry to analyze monocyte and platelet markers in the Atherosclerosis Risk in Communities (ARIC) Carotid MRI study. We estimated laboratory variability using 20 split samples, process variation using replicate blood tubes taken from 112 subjects, and biologic plus process variation using replicate blood samples taken 4-8 weeks apart from 55 people.
RESULTS
For most analytes, the laboratory CV was <10% (mean 3.6%, range 0%-14.5%) and reliability was excellent (75% of analytes had R > 0.90). Reliability coefficients based on repeat-visit data indicated substantial to high repeatability (R > 0.60) for CD14, Toll-like receptor (TLR)-2, CD162, CD61, CD41, CD62P, CD154, and platelet-leukocyte aggregates. In contrast, TLR-4, CD45, myeloperoxidase (MPO), and cyclooxygenase (COX)-2 had slight to moderate repeat visit reliability.
CONCLUSIONS
The high repeatability results for selected platelet and monocyte markers indicate that they can be reliably measured in multicenter studies with delayed sample processing, provided that rigorous standardization of sample collection, shipping, and flow cytometry procedures is applied.
Large prospective cohort studies have been critical in identifying major risk factors for cardiovascular disease, including increased blood pressure, dyslipidemia, smoking, and diabetes. In recent years, the focus of basic and epidemiologic investigations has shifted to factors or mechanisms involved in the early subclinical phases of atherosclerosis. This research suggests that many types of inflammatory cells and their activation markers play an important role in the formation and progression of atherosclerosis (1-4).
Patients with carotid atherosclerosis were reported to have an increased expression of cyclooxygenase 2 (COX-2)5 simultaneously in peripheral blood mononuclear cells and in the vulnerable plaque regions (5). It was suggested that myeloperoxidase (MPO), a leukocyte enzyme that promotes oxidative damage of host tissues at sites of inflammation, including atherosclerotic lesions, might help in risk stratification for cardiovascular disease (5-7). Recent studies have demonstrated the presence of the Toll-like receptors (TLRs) in both human plaques and murine models of atherosclerosis (8). Members of the TLR family play a critical role in the inflammatory components of atherosclerosis (9, 10).
Increased platelet activation is central to the pathophysiology of arterial thrombosis (11), and several platelet-derived factors may be involved in the inflammatory interaction between platelets, leukocytes, and endothelial cells (12, 13). Platelet P-selectin interacts with P-selectin glycoprotein ligand 1 (PSGL-1) on leukocytes to form platelet-leukocyte aggregates. It has been suggested that platelet-leukocyte aggregates may be a sensitive marker of platelet activation and a good reflection of ongoing vascular thrombosis and inflammation (14, 15).
Immunophenotyping by flow cytometry has become standard practice for identification of human leukocyte subpopulations (16-18) and is increasingly used to characterize the phenotypic alterations of platelets (19-21). Methods for measuring monocyte and platelet markers are relatively easy to apply in a single-center study. A major problem in assessing blood platelet and leukocyte markers in multicenter studies is that activation can occur due to delays in processing. Many clinical laboratories, particularly reference facilities, face this same problem. To prevent activation, careful blood collection, storage/shipment, and processing conditions are important. Studies and recommendations have been published addressing the sources of variation in multicenter studies (22-26), but they largely relate to flow cytometric lymphocyte immunophenotyping for hematologic malignancies or HIV/AIDS. The major focus of the Atherosclerosis Risk in Communities (ARIC) Carotid MRI study was the reliability of flow cytometric platelet and monocyte measures.
To test the possibility that increased levels of monocyte activation and increased leukocyte-platelet interactions may increase the risk of atherosclerosis, one needs the ability to accurately rank individuals on these characteristics. Analytes with large variability involving blood collection, processing, and analysis and within-person variability are unlikely to show significant associations with risk factors or disease outcomes. In addition, biased regression coefficients may result from modeling the association between analytes with high variability (27).
The aim of this study was to quantify the sources of variation in flow cytometry measurements of platelet and monocytes (variation in the drawing or processing of blood, in the quality of the blood sample after shipment to the central laboratory, in reagents, between technicians performing the measurements, and over time within an individual). In this study, we refer to sources of variability that involve blood collection, processing, and analysis as “method variability,” the magnitude of which has important implications for detecting association with disease. We collected replicate blood samples during the course of the ARIC Carotid MRI study to assess the magnitude of variation in flow cytometry measurements and enable correction of measurement error during data analysis.
Materials and Methods
STUDY POPULATION
The ARIC study is a multicenter, cohort study of atherosclerosis and its sequelae among African American and white men and women from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland (28). The ARIC Carotid MRI study, which enrolled participants from the ARIC cohort, was designed to further improve our understanding of cellular factors by examining the association of markers of platelet and monocyte activation and cellular aggregation with atherosclerotic plaque characteristics and early pathologic changes in the carotid arterial wall. We used flow cytometry to measure platelet and monocyte markers and cell aggregates in stabilized whole blood.
The study population consisted of 2066 men and women recruited from the ARIC study under a stratified sampling plan designed to enrich for the presence of visible plaque while allowing for population-based parameter estimates. The study was approved by the Institutional Review Boards of the 4 participating centers.
BLOOD SAMPLING FOR FLOW CYTOMETRY
All technicians performing venipuncture were centrally trained using a common protocol, followed by a period of supervised practice and certification (29). Samples for flow cytometry were collected in Cyto-Chex® BCT Vacutainer Tubes (Streck) and mixed with anticoagulant by inverting gently 8 times. Cyto-Chex tubes, blood collection tubes for immunotyping by flow cytometry, contain a combination of EDTA and a cell membrane stabilizer for blood cells (30, 31). The samples were shipped on the day of blood draw in insulated styrofoam containers with temperature-stabilizing packages (approximate specimen temperature 10-15 °C) by overnight courier to the flow cytometry laboratory (ARIC Carotid MRI Study Manual 2: Field Center Procedures. Biospecimen Collection and Processing, p. 55, www.cscc.unc.edu/carmri). Before initiation of the ARIC Carotid MRI study, we carried out multiple protocol-development studies and documented the stability of the flow cytometry measurements when the samples were shipped and received within 24 h. Immediately on receipt, samples were prepared for analysis according to the procedures described below.
MONOCLONAL ANTIBODIES
We used 11 monoclonal antibodies to label monocyte and platelet markers: phycoerythrin-cyanin 5.1-conjugated CD14 (CD14-PC5), CD45-PC5, fluorescein isothiocyanate-conjugated CD41 (CD41-FITC), CD61-FITC, phycoerythrin-conjugated CD62 (CD62P-PE, or P-selectin), CD154-PE (CD40 ligand, or CD40L), MPO-FITC (all Beckman Coulter), CD162-PE (PSGL-1; BD Biosciences), TLR-2-FITC, TLR-4-PE (both Ebioscience), and COX-2-FITC (Cayman Chemical).
THREE-COLOR STAINING FOR MONOCYTE/LEUKOCYTE SURFACE MARKERS
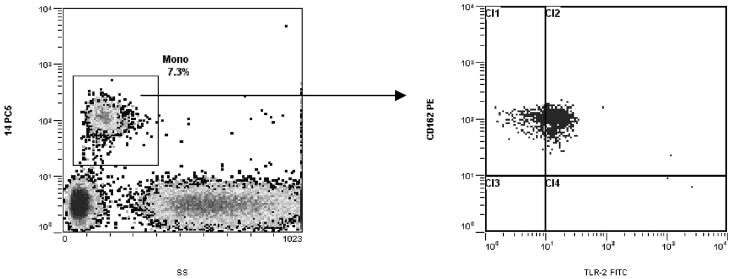
We prepared samples using the PrepPlus™ 2 Workstation (Beckman Coulter). Briefly, for the CD14/CD41/TLR-4 protocol, we placed 100 μL whole blood, 10 μL CD14-PC5, 20 μL CD41-FITC, and 20 μL TLR-4-PE into a 12-by-75-mm tube (Falcon; Fisher Scientific) and incubated them for 20 min in the dark at room temperature. We then placed the tube into a Coulter TQ-Prep™ Workstation for addition of lysing, stabilizing, and fixing reagents (ImmunoPrep™; Beckman Coulter), according to the manufacturer’s instructions. The lysed sample tube was kept in the dark and analyzed within 1 h. For the CD14/TLR-2/CD162 protocol, we mixed 100 μL blood with 10 μL CD14-PC5, 20 μL TLR-2-FITC, and 20 μL CD162-PE and processed them in the same manner. We used a tube containing the unstained patient sample and an isotype control tube to evaluate autofluorescent vs nonspecific binding properties of antibodies. Two thousand monocyte events, defined as cells with respective side scatter (SSC) and CD14-PC5 staining characteristics, were acquired in the list mode file from each sample, and corresponding levels of TLR-2, TLR-4, and PSGL-1 were obtained from the CD14+ cell gate (Fig. 1). Results were expressed as percentage of positive events gated and median fluorescence intensity (MFI).
Fig. 1. Peripheral blood monocyte surface expression of PSGL-1 and TLR-2.
The blood sample was stained with TLR-2-FITC, CD162-PE, and CD14-PC5. Monocytes were identified by their respective SSC and CD14-PC5 staining characteristics. The gated events in the monocyte region were then analyzed in a dual-fluorescence dot plot of CD162-PE vs TLR-2-FITC.
TWO-COLOR MONOCYTE/LEUKOCYTE SURFACE AND INTRACELLULAR STAINING
We processed blood samples in parallel for the CD14/COX-2 and CD45/MPO protocols. Briefly, 50 μL blood and 10 μL of either CD14-PC5 or CD45-PC5 antibody were mixed and incubated for 20 min in the dark at room temperature. The sample was then fixed with 100 μL Reagent 1 (IntraPrep™; Beckman Coulter) for 15 min, washed twice with fetal bovine serum (FBS) (5 min, 300g), permeabilized, and lysed with 100 μL Reagent 2 (IntraPrep; Beckman Coulter) for 5 min at room temperature. Either 20 μL MPO-FITC or 8 μL COX-2-FITC antibody was added, and samples were incubated for 45 min in the dark at room temperature, washed twice with PBS, and resuspended in 500 μL FBS containing 0.5% formaldehyde. Samples were analyzed within 30 min of preparation. Monocytes were identified by CD14+ and CD45+ staining, and lymphocytes and granulocytes by CD45+ staining and light-scatter characteristics. Intracellular MPO and COX-2 were detected by positive staining with anti-MPO and anti-COX-2 antibodies gated on CD14+ and CD45+ monocytes and CD45+ granulocytes, respectively. Two thousand monocyte events were acquired in the list mode file from each sample. Results were expressed as percentage of positive events and MFI for COX-2 and MFI for CD14, CD45, and MPO.
TWO-COLOR SAMPLE STAINING FOR PLATELET MARKERS
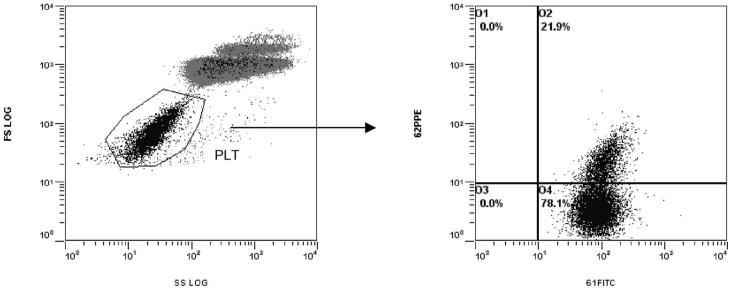
To prepare samples, we pipetted 2.5 μL well-mixed blood, 47.5 μL filtered FBS, and 10 μL CD61-FITC and 10 μL CD62P-PE or 10 μL CD41-FITC and 10 μL CD154-PE. Samples were mixed gently and kept in the dark at room temperature for 15 min, then diluted with 1.5 mL FBS. Flow cytometric analysis was performed immediately. Ten thousand platelet events were acquired in the list mode file from each sample. Platelets were identified and distinguished from nonplatelet events (red blood cells and leukocytes) by their characteristic forward light scatter (FS) vs SSC characteristics (log FS; log SSC) and their fluorescence from FL1 (CD61 and CD41), in the log-log dot plot of FS vs CD61 or CD41. Results for CD61 and CD41 were expressed as MFI. Results for CD62P and CD154 were expressed as percentage of positive events and MFI gated on CD61+ platelets and CD41+ platelets, respectively (Fig. 2A and B).
Fig. 2. Flow cytometric analysis of platelets.
(A) Platelets were identified and distinguished from nonplatelet events (red blood cells and leukocytes) by their respective FS vs SSC characteristics. (B) The gated events in region PLT were displayed in a dual-fluorescence dot plot of CD61-FITC vs CD62P-PE. Events in the region O2 that are double positive are considered to be activated. SS, side scatter.
HETEROTYPIC PLATELET-LEUKOCYTE AGGREGATES
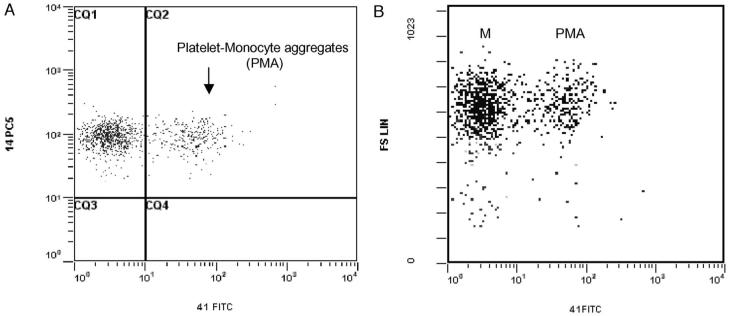
Monocytes were identified by a specific CD14-PC5 staining, and platelet-monocyte aggregates (PMAs) were defined as CD14+ monocytes that are also CD41+. Results were expressed as percentages of positive cells and MFI. Lymphocytes and granulocytes were identified by their characteristic FS and SSC characteristics, and their aggregates with platelets (PLAs, PGAs) defined by their positivity for CD41 (Fig. 3A and B). Results were expressed as percentages of positive cells and MFI.
Fig. 3.
(A) Flow cytometric analysis of platelet-monocyte aggregates (PMAs) in whole blood displayed in a dual-fluorescence dot plot of CD14-PC5 and CD41-FITC gated on monocytes. Events in region CQ2 that are double positive are considered to be PMAs. Single-positive events are platelet-free monocytes. (B) Comparison of light scatter characteristics of CD14+CD41- and CD14+CD41+ populations. Population M represents platelet-free monocytes and PMA platelet-monocyte aggregates.
FLOW CYTOMETRY
Flow cytometric analysis was performed on an Epics™ XL™ (Beckman Coulter). A range of strict internal quality assurance procedures were applied. The flow cytometer was calibrated and verified daily with Flow Check™ and Flow Set™ fluorospheres (Beckman Coulter). A stabilized whole blood sample (Immuno-Trol™ cells) was used weekly to verify 2- and 3-color compensation settings; it also served as a positive procedure and reagent control for leukocyte markers to confirm the stability of MFI measurements over time. Antibodies with the same lot number and with the longest expiration dates were used to minimize between-batch variability. On rare occasions when lots were changed, Immuno-Trol™ cells and at least 10 participants’ blood samples were used to test new reagents and compare with the previous lot. Sample data were obtained and analyzed using EXPO32™ ADC software (Applied Cytometry Systems).
Data for the proportion of cells expressing the antigen of interest and the relative level of antigen expression assessed by median fluorescence intensity were extracted from the acquired flow cytometry list mode data files and transferred into Microsoft Excel files.
STUDY DESIGN
Three substudies were designed to evaluate sources of variation in flow cytometry measurements. In the first (substudy 1), to estimate laboratory variability, blood samples from 20 subjects were selected by the laboratory for replicate testing on the same day. Each sample was split into 2 aliquots, and measures obtained from independent flow cytometry analyses were compared. Measurement variation estimated from these data cannot be attributed to variation in blood drawing, local processing, shipment procedures, laboratory handling and analysis, or within-subject variation over time. In the within-visit reliability substudy (substudy 2), each field center drew duplicate blood tubes using a single venipuncture from a subset of participants. The duplicate samples were sent to the flow cytometry laboratory under a blinded quality control (QC) ID that was indistinguishable from other IDs. After the laboratory had analyzed the paired samples (112 pairs), the results were compared to estimate the method variability (i.e., variability in blood processing, shipping, and laboratory handling and analysis). In the between-visit reliability substudy (substudy 3), each field center was asked to recruit 15 volunteers to repeat the entire clinic visit within 4-8 weeks of their original visit. Volunteers generally reflected the age, sex, and racial composition of the overall study. Again, duplicate samples were submitted to the flow cytometry laboratory under a blinded QC ID. Results from the paired samples (n = 55) were compared to estimate the method variability as well as within-person variability over time.
STATISTICAL ANALYSIS
By treating the pair as a random effect in a linear mixed-effects model, we were able to partition the total variance (σ2TOT) into a between-pair (or between-person, σ2BP) and within-pair component of variance. The within-pair variance derived from the first split-sample substudy 1 corresponds to the laboratory variability. The within-pair component of variance derived from the within-visit reliability substudy 2, in which duplicate samples were obtained from participants on the same day, corresponds to an estimate of variation due to blood collection, processing, and laboratory analysis (σ2e). In contrast, the within-pair component of variance derived from the between-visit reliability substudy 3, in which duplicate samples were obtained from participants at 2 separate visits, corresponds to an estimate of the within-person (biologic) variation over time plus method variation (σ2WP + σ2e).
The proportion of the total variance attributable to between-person variability, or reliability coefficient (R = σ2BP/σ2TOT), can be interpreted as the correlation between paired measurements. Benchmarks for characterizing the adequacy of reliability were as follows (32): slight reliability, 0-0.2; fair reliability, 0.21-0.4; moderate reliability, 0.41-0.6; substantial reliability, 0.61-0.8; almost perfect reliability, 0.81-1.0. Based on our sample sizes of 112 and 55 for the 2 substudies, the 95% CI assuming a moderate reliability of 0.60 will have lower limits of 0.48 and 0.44, respectively.
The CV was derived as the square-root of the within-pair component of variance divided by the mean of the paired observations multiplied by 100. CV values >10% were considered cause for concern.
Results
Laboratory variability was found to be low based on results from substudy 1 (see “Study Design”) in which 20 split samples were analyzed on the same day. The laboratory CVs were <10% for all monocyte and platelet markers (mean 3.6%, range 0%-14.5%) except for MPO. In addition, reliability coefficients for all but 5 of the 35 flow cytometry parameters were >0.80.
The demographic characteristics of participants who contributed to the within-visit and between-visit reliability substudies 1, 2, and 3 and to the overall ARIC Carotid MRI study are listed in Table 1. The demographic composition of the within-visit reliability substudy was similar to that of the ARIC Carotid MRI study participants: mean age was 71.7 years; 48% were male; and 24% were African American. The volunteers in the between-visit reliability substudy did not differ in age from the main study sample but included a higher percentage of African American and men than the study sample (58% vs 48% male; 31% vs 25% African American, respectively).
Table 1.
Demographic characteristics of participants who contributed to the within-visit and between-visit replicate samples, ARIC Carotid MRI Study 2005-2006
| Within-visit replicates | Between-visit replicates | ARIC Carotid MRI participants | |
|---|---|---|---|
| n | 112 | 55 | 2066 |
| Mean age, years (SD) | 71.7 (5.7) | 71.2 (5.5) | 71.1 (5.6) |
| Male, % | 48 | 58 | 48 |
| African American, % | 24 | 31 | 25 |
Table 2 gives summary QC duplicate sample results from the within-visit reliability substudy 2. In general, the percentage of positive events gated was highly reliable (R > 0.80) unless the mean percentage gated was very near 0% or 100%, as was the case for COX-2 (98%), which was intermediate in reliability (R = 0.69). All platelet markers had high reliability, with CD61 having the highest R value (R > 0.99). Ten percent was set as a goal for the upper limit of processing CV. This goal was met for all but the percentage gated for CD62P and CD154, with estimated CVs of 20% and 26%, respectively. Analysis of the 3 types of cell aggregates showed low CV (<7%) and high reliability (R > 0.90).
Table 2.
Sample means, within-visit standard deviations, reliability coefficients (R), and CVs for replicate samples drawn from a single venipuncture (substudy 2), ARIC Carotid MRI Study 2005-2006
| Mean on QC pairs |
Within-visit SD |
R |
CV |
|||||
|---|---|---|---|---|---|---|---|---|
| % gated | MFI | % gated | MFI | % gated | MFI | % gated | MFI | |
| Leukocyte markers | ||||||||
| Monocytes | ||||||||
| CD14+ | 100.0 | 113.1 | 0.0 | 4.1 | 0.35 | 0.97 | 0.0 | 3.7 |
| CD14+/TLR-2+ | 66.0 | 14.2 | 2.9 | 0.3 | 0.93 | 0.92 | 4.4 | 1.9 |
| CD14+/TLR-4+ | 64.6 | 16.1 | 1.3 | 0.4 | 0.89 | 0.85 | 2.1 | 2.6 |
| CD14+/CD41+/TLR-4+ | 10.9 | 47.4 | 1.0 | 3.3 | 0.80 | 0.63 | 9.5 | 6.9 |
| CD14+/CD162+ | 100.0 | 116.1 | 0.0 | 3.8 | 0.35 | 0.94 | 0.0 | 3.3 |
| CD14+/TLR-2+/CD162+ | 67.6 | 14.0 | 2.9 | 0.3 | 0.92 | 0.92 | 4.3 | 2.0 |
| CD14+/COX-2+ | 98.0 | 16.7 | 1.3 | 1.3 | 0.69 | 0.73 | 1.3 | 7.8 |
| CD45+ | 94.4 | 77.9 | 1.1 | 2.9 | 0.87 | 0.91 | 1.1 | 3.8 |
| CD45+/MPO+ | 94.4 | 92.8 | 1.1 | 7.7 | 0.87 | 0.90 | 1.1 | 8.3 |
| Granulocytes | ||||||||
| CD162+ | 99.9 | 81.2 | 0.1 | 2.6 | 0.67 | 0.97 | 0.1 | 3.2 |
| MPO+ | — | 845.2 | — | 32.7 | — | 0.96 | — | 3.9 |
| Lymphocytes | ||||||||
| CD162+ | 88.8 | 55.1 | 1.5 | 1.7 | 0.97 | 0.97 | 1.7 | 3.1 |
| Platelet markers | ||||||||
| Total platelets CD61+ | 5.5 | 64.7 | 0.5 | 1.9 | 0.91 | 0.99 | 8.8 | 3.0 |
| Single platelets CD61+ | 85.5 | — | 1.5 | — | 0.81 | — | 1.8 | — |
| CD61+/CD62P+ | 29.8 | 21.8 | 6.0 | 1.5 | 0.82 | 0.87 | 20.2 | 6.7 |
| CD41+ | 95.1 | 80.8 | 1.1 | 3.5 | 0.88 | 0.91 | 1.1 | 4.3 |
| CD41+/CD154+ | 3.4 | 12.0 | 0.9 | 0.4 | 0.80 | 0.71 | 25.6 | 3.6 |
| Cell aggregates | ||||||||
| Platelet-monocyte | 17.4 | 48.4 | 1.2 | 2.9 | 0.89 | 0.76 | 7.1 | 6.0 |
| Platelet-granulocyte | 16.8 | 50.3 | 1.2 | 1.8 | 0.89 | 0.91 | 7.0 | 3.5 |
| Platelet-lymphocyte | 16.8 | 49.2 | 1.1 | 2.6 | 0.91 | 0.85 | 6.6 | 5.3 |
n = 112 replicate pairs. Total platelets include single (activated and nonactivated) platelets and platelet-platelet aggregates. —, measures not available.
The indices of repeatability for the QC replicates estimated from the between-visit reliability substudy 3 are given in Table 3. The reliability coefficients derived from these repeat-visit QC samples were 25% lower on average than those derived from the within-visit QC samples. The reliability coefficients were substantially to highly repeatable (R > 0.60) for CD14, TLR-2, CD162, CD61, CD62P, CD41, CD154, and all cell aggregates. In contrast, measurement of TLR-4, COX-2, CD45, and MPO had slight to moderate reliability. The greatest decline in reliability from the within-visit reliability to between-visit reliability substudy was for TLR-4, COX-2, CD45, and MPO. The mean difference in CVs derived from the repeat-visit QC samples and those derived from the within-visit QC samples was approximately 5%, with a mean CV for the within- and between-visit reliability substudies of 6.7% and 11.6%, respectively. As in the within-visit reliability substudy, CVs were <10%, with the exception of the percentage of positive events for CD62P and CD154 and cell aggregates.
Table 3.
Sample means, between-visit SDs, reliability coefficients (R), and CVs for replicate samples drawn from 2 venipunctures taken 4-8 weeks apart (substudy 3), ARIC Carotid MRI Study 2005-2006
| Mean on QC pairs |
Between-visit SD |
R |
CV |
|||||
|---|---|---|---|---|---|---|---|---|
| % gated | MFI | % gated | MFI | % gated | MFI | % gated | MFI | |
| Leukocyte markers | ||||||||
| Monocytes | ||||||||
| CD14+ | 100.0 | 114.0 | 0.0 | 9.0 | 0.00 | 0.76 | 0.0 | 7.9 |
| CD14+/TLR-2+ | 65.0 | 14.1 | 4.7 | 0.5 | 0.84 | 0.90 | 7.3 | 3.4 |
| CD14+/TLR-4+ | 64.4 | 16.3 | 3.1 | 0.9 | 0.39 | 0.21 | 4.8 | 5.5 |
| CD14+/CD41+/TLR-4+ | 11.1 | 46.7 | 1.6 | 3.8 | 0.77 | 0.64 | 14.8 | 8.2 |
| CD14+/CD162+ | 100.0 | 115.0 | 0.0 | 7.0 | 0.00 | 0.75 | 0.0 | 6.1 |
| CD14+/TLR-2+/CD162+ | 66.2 | 14.0 | 4.6 | 0.4 | 0.85 | 0.92 | 6.9 | 3.2 |
| CD14+/COX-2+ | 98.2 | 16.7 | 2.5 | 2.2 | 0.00 | 0.20 | 2.5 | 13.3 |
| CD45+ | 94.1 | 73.9 | 1.5 | 6.3 | 0.67 | 0.43 | 1.6 | 8.5 |
| CD45+/MPO+ | 94.1 | 90.6 | 1.5 | 16.3 | 0.67 | 0.52 | 1.6 | 18.0 |
| Granulocytes | ||||||||
| CD162+ | 99.9 | 81.6 | 0.2 | 10.7 | 0.17 | 0.72 | 0.2 | 13.1 |
| MPO+ | — | 850.9 | — | 136.2 | — | 0.39 | — | 16.0 |
| Lymphocytes | ||||||||
| CD162+ | 88.1 | 54.4 | 2.5 | 3.7 | 0.73 | 0.86 | 2.8 | 6.9 |
| Platelet markers | ||||||||
| Total platelets CD61+ | 5.2 | 61.6 | 0.6 | 5.1 | 0.94 | 0.93 | 11.6 | 8.2 |
| Single platelets CD61+ | 83.4 | — | 3.9 | — | 0.86 | — | 4.6 | — |
| CD61+/CD62P+ | 31.5 | 23.3 | 8.9 | 3.0 | 0.71 | 0.91 | 28.2 | 13.1 |
| CD41+ | 95.3 | 80.0 | 1.5 | 8.0 | 0.48 | 0.60 | 1.5 | 10.1 |
| CD41+/CD154+ | 3.3 | 12.0 | 1.2 | 0.6 | 0.54 | 0.62 | 37.3 | 5.3 |
| Cell aggregates | ||||||||
| Platelet-monocyte | 17.4 | 47.8 | 2.2 | 3.6 | 0.84 | 0.70 | 12.8 | 7.6 |
| Platelet-granulocyte | 17.0 | 49.7 | 2.0 | 3.6 | 0.86 | 0.71 | 11.6 | 7.2 |
| Platelet-lymphocyte | 17.0 | 48.5 | 2.1 | 3.7 | 0.84 | 0.75 | 12.5 | 7.5 |
n = 55 replicate pairs. Total platelets include single (activated and nonactivated) platelets and platelet-platelet aggregates. —, measures not available.
Discussion
We estimated various components of variation in whole blood monocyte and platelet markers analyzed by flow cytometry within 24 h of blood collection, and we estimated repeatability of measuring these analytes within the ARIC Carotid MRI population.
Results from substudy 2 of within-visit variability showed that both the percentage of positive events and MFI were highly repeatable (R > 0.80) for monocyte and platelet markers and for the heterotypic cell aggregates, when measured from replicate samples drawn from a single venipuncture. The indices of repeatability from the between-visit reliability substudy 3, i.e., from the replicate samples drawn from 2 venipunctures taken 4-8 weeks apart, were 25% lower on average than those derived from the within-visit QC replicates. The R values were substantially to highly repeatable (R > 0.60) for all cell aggregates, platelet markers, and monocyte CD14, TLR-2, and CD162. In contrast, measurements of monocyte TLR-4, COX-2, and MPO had moderate reliability.
Results from the between-visit reliability substudy 3 show that sources of variation and repeatability vary among the markers selected for the ARIC Carotid MRI study. Within-individual variability can be in part explained by its natural biologic variation, but also to some extent by shipping conditions, storage, and reagent variation. Our results show that among monocyte markers, CD14, CD162, and TLR-2 had the highest R values. In view of the crucial role of monocytes in atherosclerosis and PSGL-1 in hemostasis and inflammation, the high reliability of these markers warrants their measurement in multicenter population studies. On the other hand, COX-2, TLR-4, and MPO had the greatest within-individual variability. Several factors may have contributed to this outcome. Both COX-2 and MPO are intracellular enzymes, and their detection requires multiple steps of cell processing, permeabilization, multiple washing, and centrifugation. It was expected that these methodological steps would contribute to increased variability. Flow cytometric detection of intracellular MPO was shown to be reliable using the same permeabilization protocol (33). The higher variability of monocyte COX-2 is possibly the reflection of the high inducibility of this enzyme (34). It is interesting that TLR-4 had a moderate R value, whereas both CD14 and TLR2 were highly repeatable. Both TLR receptors function to signal a ligand-binding event across a membrane, activating NF-κB; TLR4 acts in conjunction with CD14, and it has been reported that there is cross-talk between TLR4 and TLR2. It is possible that the greater variability of TLR4 may be in part because it appears to function both at the membrane and intracellularly, whereas TLR2 functions at the cell membrane only (34).
Among the 4 selected platelet markers, CD61 had the highest R value (0.94). Platelet P-selectin had good repeatability, whereas platelet CD40L had moderate R and high variance. Both P-selectin and CD40L are released during platelet activation and cleaved from the membrane. CD62P measurement appears to be more reliable than CD40L. The higher variance of CD40L is likely because platelets do not contain significant amounts of CD40L. Importantly, the rigorous standardization used for sample collection and shipping in our study was crucial for generating reliable platelet measures.
In this study, the measurement of surface and intracellular antigen expression was assessed by MFI. To minimize the influence of instrument settings and antibody staining variability on MFI measurement, we applied strict QC approaches during the course of the study, which resulted in high reliability of the relative staining intensities of analyzed markers. In terms of quantitative flow cytometry, the higher level of standardization should include the use of fluorescent bead calibration standards for antigen quantification (35-39).
In conclusion, high repeatability results for a selected platelet and monocyte markers in the present study indicate that they can be reliably measured in multicenter studies with delayed sample processing, when rigorous standardization of sample collection, shipping, and flow cytometry procedures is applied. In view of the multiple roles of circulating platelets and leukocytes and heterotypic cell aggregates in thrombosis and inflammation, their high reliability supports ARIC’s analysis of the relationship between blood cells and cell aggregates and atherosclerosis. It is reasonable to expect that their measurement will contribute to an improved understanding of atherosclerotic disease.
Acknowledgement
The authors thank the staff and participants of the ARIC study for their important contributions.
Grant/Funding Support: The ARIC Carotid MRI study is supported by National Heart, Lung, and Blood Institute grant 5U01HL075572. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
Footnotes
- COX
- cyclooxygenase
- MPO
- myeloperoxidase
- TLR
- Toll-like receptor
- PSGL-1
- P-selectin glycoprotein ligand 1
- ARIC
- Atherosclerosis Risk in Communities
- PC5
- phycoerythrin-cyanin 5.1
- FITC
- fluorescein isothiocyanate
- PE
- phycoerythrin
- L
- ligand
- MFI
- mean fluorescence intensity
- FBS
- fetal bovine serum
- FS
- forward scatter
- SSC
- side scatter
- PMA
- platelet-monocyte aggregate
- PLA
- platelet-lymphocyte aggregate
- PGA
- platelet-granulocyte aggregate.
Financial Disclosures: None declared.
References
- 1.Furman MI, Barnard MR, Krueger LA, Fox ML, Shilale EA, Lessard DM, et al. Circulating monocyte-platelet aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38:1002–6. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 2.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–8. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- 3.Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiological Rev. 2003;83:1069–112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature (Lond) 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Brennan M-L, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14:353–9. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hazen S. Myeloperoxidase and plaque vulnerability. Arterioscler Thromb Vasc Biol. 2004;24:1143–6. doi: 10.1161/01.ATV.0000135267.82813.52. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Brennan M-L, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001;286:2136–42. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 8.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–56. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immun. 2004;5:975–9. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 10.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173:5901–7. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 11.Wu KK. Platelet activation and arterial thrombosis. Lancet. 1994;344:991–5. [PubMed] [Google Scholar]
- 12.Steinhubl SR. Platelets as mediators of inflammation. Hematol Oncol Clin North Am. 2007;21:115–21. doi: 10.1016/j.hoc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Michiels JJ, Gawaz M. Platelets in inflammation and atherothrombosis. Semin Thromb Hemost. 2007;33:119–22. doi: 10.1055/s-2007-969024. [DOI] [PubMed] [Google Scholar]
- 14.Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 15.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104:1533–7. doi: 10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 16.Baumgarth N, Roederer A. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 17.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry. 1993;14:702–14. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy DA, Macey MG. Cytometric Analysis of Cell Phenotype and Function. Cambridge University Press; UK: 2001. [Google Scholar]
- 19.Michelson AD, Barnard MR, Krueger LA, Frelinger AL, Furman MI. Evaluation of platelet function by flow cytometry. Methods. 2000;21:259–70. doi: 10.1006/meth.2000.1006. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Clementson KJ, et al. for the European Working Group on Clinical Cell Analysis European Working Group on Clinical Cell Analysis: consensus protocol for flow cytometric characterization of platelet function. Thromb Haemost. 1998;79:885–96. [PubMed] [Google Scholar]
- 21.Shattil SJ, Cunningham M, Hoxie JA. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987;70:307–15. [PubMed] [Google Scholar]
- 22.Barnett D, Storie I, Granger V, Whitby L, Reilly JT, Brough S, et al. Standardization of lymphocyte antibody binding capacity: a multi-centre study. Clin Lab Haematol. 2000;22:89–96. doi: 10.1046/j.1365-2257.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 23.Brando B, Sommaruga E. Nationwide quality control trial on lymphocyte immunophenotyping and flow cytometer performance in Italy. Cytometry. 1993;14:294–306. doi: 10.1002/cyto.990140310. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute . Approved Guideline. Second Edition. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. Enumeration of immunologically defined cell populations by flow cytometry. CLSI document H42-A2. [Google Scholar]
- 25.Gratama JW, Kraan J, Van den Beemd R, Hooibrink B, Van Bockstaele DR, Hooijkaas H. Analysis of variation in results of flow cytometric lymphocyte immunophenotyping in a multicenter study. Cytometry. 1997;30:166–77. doi: 10.1002/(sici)1097-0320(19970815)30:4<166::aid-cyto2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Owens MA, Vall HG, Hurley AA, Wormsley SB. Validation and quality control of immunophenotyping in clinical flow cytometry. J Immunol Methods. 2000;243:33–50. doi: 10.1016/s0022-1759(00)00226-x. [DOI] [PubMed] [Google Scholar]
- 27.Fuller WA. Measurement Error Models. Wiley; New York: 1987. [Google Scholar]
- 28.The ARIC investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute . Approved Standard. Fifth Edition. Clinical and Laboratory Standards Institute; Wayne, PA: 2003. Procedures for the collection of diagnostic blood specimens by venipuncture. CLSI document H3-A5. [Google Scholar]
- 30.Schumacher MJ, Burkhead T. Stability of fresh and preserved fetal and adult lymphocyte cell surface markers. J Clin Lab Anal. 2000;14:320–6. doi: 10.1002/1098-2825(20001212)14:6<320::aid-jcla12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Warrino DE, DeGennaro LJ, Hanson M, Swindells S, Pirruccello SJ, Ryan WL. Stabilization of white blood cells and immunologic markers for extended analysis using flow cytometry. J Immunol Methods. 2005;305:107–19. doi: 10.1016/j.jim.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Fleiss J. Statistical Methods for Rates and Proportions. 2nd ed. John Wiley; New York: 1981. [Google Scholar]
- 33.Kappelmayer J, Gratama JW, Karaszi E, Menendez P, Ciudad J, Rivas R, Orfao A. Flow cytometric detection of intracellular myeloperoxidase, CD3 and CD79a: interaction between monoclonal antibody clones, fluorochromes and sample preparation protocols. J Immunol Methods. 2000;242:53–65. doi: 10.1016/s0022-1759(00)00220-9. [DOI] [PubMed] [Google Scholar]
- 34.Tobias P, Curtiss LK. Paying the price for pathogen protection: Toll receptors in atherogenesis. J Lipid Res. 2005;46:404–11. doi: 10.1194/jlr.R400015-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute . Approved Guideline. Clinical and Laboratory Standards Institute; Wayne, PA: 2004. Fluorescence calibration and quantitative measurement of fluorescence intensity. CLSI document I/LA24-A. [Google Scholar]
- 36.Lenkei R, Gratama JW, Rothe G, Schmitz G, D’hautcourt JL, Arekrans A, Mandy F. Performance of calibration standards for antigen quantitation with flow cytometry. Cytometry. 1998;33:188–96. doi: 10.1002/(sici)1097-0320(19981001)33:2<188::aid-cyto13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Montes M, Jaensson EA, Orozco AF, Lewis DE, Corry DB. A general method for bead-enhanced quantitation by flow cytometry. J Immunol Methods. 2006;317:45–55. doi: 10.1016/j.jim.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz A, Gaigalas AK, Wang L, Marti GE, Vogt RF, Fernandez-Repollet E. Formalization of the MESF unit of fluorescence intensity. Cytometry B Clin Cytom. 2004;57:1–6. doi: 10.1002/cyto.b.10066. [DOI] [PubMed] [Google Scholar]
- 39.Vogt RF, Jr, Whitfield WE, Henderson LO, Hannon WH. Fluorescence intensity calibration for immunophenotyping by flow cytometry. Methods. 2000;21:289–96. doi: 10.1006/meth.2000.1009. [DOI] [PubMed] [Google Scholar]