Abstract
The effects of pantethine, glutathione, and selected chemical reagents on the anti-aggregation activity of α-crystallin was evaluated. Protein aggregation was monitored by light scattering of solutions of denatured βL-crystallin or alcohol dehydrogenase (ADH). The ratios of βL-crystallin/α-crystallin and ADH/α-crystallin were adjusted so that partial inhibition of protein aggregation at 60°C or 37°C, respectively, was observed and modulation of the chaperone action of α-crystallin could be evaluated easily with selected endogenous metabolites. Enhancement of the anti-aggregation activity in the βL-crystallin assay was strongest with pantethine, which appeared to interact with α-crystallin. Enhancement of the anti-aggregation activity in the ADH assay was strongest with glutathione which appeared to interact with ADH. The results indicated that the products of common metabolic pathways can modulate the chaperone-like effects of α-crystallin on protein aggregation.
α-Crystallin, which can account for as much as 35% of the proteins in lens cytoplasm, has been identified as a small heat shock protein having chaperone-like activity (1–8). α-Crystallin has been found in a variety of non-lens cells and tissues including kidney, heart, skeletal muscle, brain, placenta, and lung, and may be up-regulated in response to physiological or pathological stress associated with multiple sclerosis and neurodegenerative diseases (9–24). The protective chaperone activity of α-crystallin and other molecular chaperones has been evaluated in vitro using optical density as a measure of protein aggregation that occurs following heat or urea induced denaturation (1–4). The first step in the protective action of a molecular chaperone is thought to involve interaction with the denatured unfolded protein to provide an environment that favors normal refolding or inhibits the formation of large protein aggregates that scatter light (2, 3, 5, 25). In the absence of α-crystallin the temporal progress of aggregation of crystallins and various enzymes is characterized by an initial period of low light scattering before a steep increase in scattering that slows as the aggregation process is saturated (1–5). The rate of aggregation and the protection provided by molecular chaperones varies with protein concentration, temperature, enzyme cofactors, or posttranslational modification (1, 2, 4, 26–28). Approximately one α-crystallin monomer is thought to interact with one target protein to protect against aggregation (1, 2, 4, 5). Proteins are found at very high concentrations in lens cytoplasm. It might be expected that rather high α-crystallin concentrations are crucial for protection against aggregation unless chaperone activity was enhanced by endogenous cellular constituents and metabolic products. In this study, the effects of two well known cellular metabolites, pantethine and glutathione, were studied on the chaperone-like activity of α-crystallin in vitro.
Previous studies of the protective effects of α-crystallin used βL-crystallin, a major cytoplasmic constituent of lens cells that has a tertiary structure formed largely by β-sheet. βL-crystallin formed by denatures and aggregates at temperatures near 60°C (1–5). While previous studies emphasized the thermal stability of α-crystallin (29–31), recent reports suggested α-crystallin structure was altered at nonphysiological temperatures above 37°C, which complicated the interpretation of its chaperone effects on βL-crystallin at 60°C (32–34). In contrast to βL-crystallin, denaturation and aggregation of alcohol dehydrogenase (ADH) occurs at 37°C in the presence of EDTA, which chelates Zn, a stabilizer of the structure of ADH. ADH is ≈60% α-helix and may resemble ζ-crystallin found in guinea pig lenses (39). α-Crystallin protects against aggregation of ADH (1, 25, 28), and most studies in this report were conducted using the ADH plus α-crystallin at 37°C to avoid alterations of α-crystallin at high temperatures.
METHODS
The standard methods used to evaluate chaperone-like activity of α-crystallin are summarized as follows. Aggregation of the βL-crystallin was measured spectroscopically as apparent optical density, OD, at 60°C and the aggregation of ADH was measured spectroscopically at 37°C for up to 60 min. The apparent OD, which is proportional to turbidity, was measured at 360 nm using a Beckman model DU 70, multisample UV/Vis spectrometer fitted with a Peltier temperature regulator (MJ Research, Watertown, MA). All experiments used standard 1.0 cm path length cuvettes. The temperature of the samples was monitored by using a bead thermistor installed in a cuvette within the sample chamber. The apparent OD was recorded approximately every 20 sec. To compare the effects of selected reagents on aggregation, the background was first subtracted from each value of OD recorded using the spectrophotometer. Next, the values of OD for each sample were normalized using the expression: (ODt − ODmin)/(ODmax − ODmin), where ODt = the OD for a sample at each timepoint, ODmin = minimum OD, and ODmax = maximum OD of the standards for each sample. The normalized values of the OD were used in all figures and tables. The α-crystallin (≈8 × 105 kDa molecular weight) and the βL-crystallins (≈6 × 104 kDa) were prepared from fresh bovine eyes obtained from a local slaughterhouse as described (1). Equine liver ADH (8 × 104 kDa) was obtained from Sigma. All test reagents were obtained from Sigma, and all solutions were prepared using 150 mM PBS of the following composition: 50 mM sodium phosphate buffer, pH 7.0 in 0.1 M NaCl, except for experiments with ADH, which included 2 mM EDTA. Stock solutions were stored on ice until they were mixed at room temperature and quickly placed in the temperature-controlled sample chamber of the spectrophotometer. For experiments with added reagents, the ratio of βL/α was approximately 20:1 by weight and ADH/α was approximately 2:1 by weight. Final concentrations of modulating reagents in the test solutions are listed in the legends of the figures and tables.
RESULTS
The apparent optical density, OD, was a direct measure of the aggregation of βL-crystallin or ADH over 60 min (Fig. 1). After placing a sample in the spectrophotometer at 60°C, the OD of the solution remained at a minimum value for 5–8 min before increasing steeply over the next 30–50 min and slowing to a maximum OD at 60 min. In solutions of βL-crystallin, the OD often decreased after reaching a maximum because of the formation of huge protein aggregates that fell out of solution and formed a white layer of precipitate at the bottom of the sample cuvette. The aggregation of ADH at 37°C resulted in a similar plot of OD versus time, although the decrease in OD due to formation of extremely large aggregates was not observed (Fig. 1).
Figure 1.
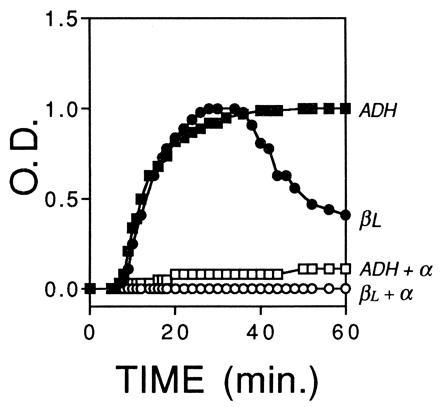
Action of α-crystallin on the aggregation of βL-crystallin or ADH. At 60°C, the apparent OD of a solution of 0.05 mg βL-crystallin (•) increased rapidly to a maximum before decreasing as a result of the formation of very large high molecular weight aggregates. At 37°C, the OD of 0.5 mg ADH (▪) increased rapidly to a maximum. Addition of 0.015 mg α-crystallin to the solution of βL-crystallin (○) or to 1.0 mg α-crystallin to the solution of ADH (□) completely suppressed protein aggregation and the increase in OD. Thus complete suppression of aggregation occurred in solutions of 0.05 mg βL containing 0.015 mg α-crystallin (3.3:1, βL/α) and 0.5 mg ADH containing 1.0 mg α-crystallin (1:2, ADH/α). All solutions were in 150 mM PBS (pH 7.0), at a final volume of 0.4 ml. The OD was normalized as described.
The addition of α-crystallin suppressed completely the aggregation at ratios of approximately 3.3:1 (βL/α) and 1:2 (ADH/α) by weight (Fig. 1). At these high levels, the effect of the α-crystallin was so strong that any modulating effect of added reagents was overwhelmed and difficult to detect. At lower levels of α-crystallin [20:1 (βL/α) and 2:1 (ADH/α) by weight], partial inhibition of the aggregation of βL-crystallin or ADH was observed and the modulating effects of pantethine, glutathione, and other reagents on the anti-aggregation activity of α-crystallin were evaluated using these conditions.
The addition of 1.0 mM and 10 mM pantethine enhanced the inhibitory action of α-crystallin on aggregation of βL-crystallin at 60°C (Fig. 2A). In the presence of 1.0 mM pantethine, the OD was only 0.1 when the standard without pantethine increased to 0.6. At 60 min, when the OD of the standard reached the maximum value of 1.0, the OD of the 1.0 mM pantethine sample was only about 0.5. In the presence of 10 mM pantethine, the OD remained below 0.2 at 60 min when the OD of the standard reached the maximum. In contrast, the addition of 1.0 mM and 10 mM glutathione resulted in a small enhancement of the chaperone effect of α-crystallin. The maximum OD for samples containing glutathione was slightly below the maximum OD without glutathione (Fig. 2A). The effects of the pantethine and glutathione on βL-crystallin at 60°C in the absence of α-crystallin are presented in Fig. 2B. At concentrations as high as 20 mM pantethine, the aggregation of βL-crystallin was similar to the aggregation without pantethine. In contrast, the addition of 10 mM and 20 mM glutathione accelerated the aggregation of βL-crystallin. With 10 mM glutathione the OD increased to nearly 0.7 in the first 15 min, when the OD of the βL-crystallin alone was only about 0.1. The effect of the 20 mM glutathione on aggregation was even stronger, and at 10 min the OD of the βL-crystallin with glutathione reached a maximum of 1.9, nearly twice the maximum OD observed in a solution of βL-crystallin alone.
Figure 2.
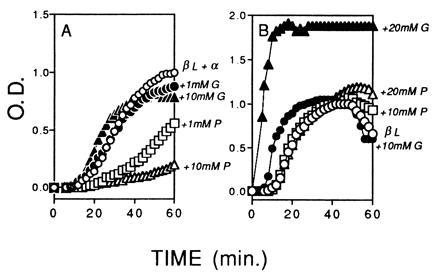
Effect of pantethine (P) and glutathione (G) on chaperone-like activity of α-crystallin on the aggregation of βL-crystallins at 60°C. (A) Protein concentrations of βL- and α-crystallin were adjusted to approximately 20:1 (βL/α) to provide partial protection against aggregation and to permit observation of the effects of the added reagents. ○, βL plus α standard without additions; □, plus 1.0 mM pantethine; ▵, plus 10 mM pantethine; •, plus 1.0 mM glutathione; ▴, plus 10 mM glutathione. In the presence of α-crystallin, pantethine had a stronger protective effect than glutathione on aggregation of βL-crystallin. (B) Effect of pantethine and glutathione on βL-crystallin alone. ○, βL standard without additives; □, plus 10 mM pantethine; ▵, plus 20 mM pantethine; •, plus 10 mM glutathione; ▴, plus 20 mM glutathione. In the absence of α-crystallin, pantethine had a weak effect on aggregation of βL-crystallin and glutathione increased aggregation of βL-crystallin. All solutions were in 150 mM PBS (pH 7.0), at a final volume of 0.4 ml. The OD was normalized as described.
To evaluate the chaperone effects of α-crystallin at a physiological temperature of 37°C, experiments were conducted using ADH, which aggregates at 37°C (Fig. 3). The glutathione had a stronger effect than pantethine on aggregation of ADH plus α-crystallin. At a concentration of 20 mM pantethine, the OD reached a maximum of nearly 0.4 at 60 min when the OD of the ADH plus α-crystallin was 1.0 (Fig. 3A). In contrast, the OD in the presence of 10 mM glutathione was only 0.1 after 60 min, and 20 mM glutathione completely suppressed the aggregation (Fig. 3A). The effects of glutathione and pantethine were evaluated on the aggregation of ADH in the absence of α-crystallin (Fig. 3B). When the OD of ADH alone was 0.8, the OD with 20 mM pantethine was only 0.1, and then the OD increased rapidly to a maximum of 0.97. The addition of 10 mM pantethine also delayed the initiation of aggregation, but then the OD increased sharply to a maximum of 1.2 at 60 min, which exceeded the maximum of the ADH alone. The 10 mM and 20 mM concentrations of glutathione were strong inhibitors of the aggregation of ADH, and the 20 mM glutathione provided nearly complete inhibition of aggregation of ADH alone at 37°C (Fig. 3B).
Figure 3.
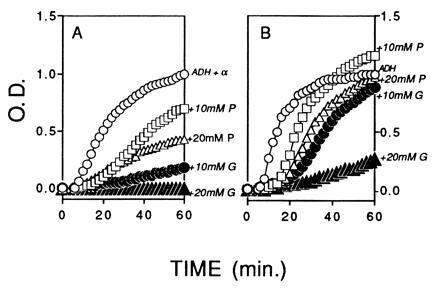
Effect of pantethine (P) and glutathione (G) on chaperone-like activity of α-crystallin on the aggregation of ADH at 37°C. (A) Protein concentrations of ADH and α-crystallin were adjusted to approximately 2:1 (ADH/α) to provide partial protection against aggregation and permit observation of the effects of the added reagents. ○, ADH plus α-crystallin standard; □, plus 10 mM pantethine; ▵, plus 20 mM pantethine; •, plus 10 mM glutathione; ▴, plus 20 mM glutathione. In the presence of α-crystallin, glutathione had a stronger effect on aggregation of ADH than pantethine. (B) Effect of pantethine and glutathione on ADH only. ○, ADH only; □, plus 10 mM pantethine; ▵, plus 20 mM pantethine; •, plus 10 mM glutathione; ▴, plus 20 mM glutathione. In the absence of α-crystallin, glutathione had a stronger effect on aggregation of ADH than pantethine. All solutions were in 150 mM PBS and 2 mM EDTA (pH 7.0), at a final volume of 0.4 ml. The OD was normalized as described.
Pantethine is composed of pantothenic acid, pantoic acid plus β-alanine, and cystamine, and glutathione is a tripeptide consisting of cystine, glutamate, and glycine (Fig. 4). The effects of selected chemical constituents of pantethine and glutathione were tested on the α-crystallin chaperone assays, and the results are listed in Tables 1 and 2. In each table each test reagent is listed in column 1. In each row, the OD value of the test solution in the presence of each experimental reagent is listed for comparison with the corresponding OD of the standard reaction in the absence of the reagent (top row). In Table 1, it is interesting to note that the effect of 1 mM β-alanine, a constituent of pantethine, was nearly as strong as the effect of 1 mM pantethine. In the presence of 1 mM cystamine and 1 mM pantothenic acid the OD increased to levels as high as the standard. The OD in the presence of 1 mM and 10 mM oxidized glutathione was similar to that of the standard. To evaluate the possible effect of sulfhydryl reduction, reduced glutathione (GSH), cysteine, cysteamine, DTT, as well as the thiophosphate, cysteamine phosphate, were tested. In the presence of 20 mM GSH the OD increased to 1.14, and addition of the reducing agents cysteine, cysteamine, cysteamine phosphate, and DTT at 20 mM concentrations also resulted in OD values greater than those of the standard βL plus α-crystallin solution.
Figure 4.

Chemical constituents of pantethine and glutathione. The pantethine consists of a dimer of pantothenic acid linked through a disulfide cystamine. Glutathione is a dimer of the tripeptide glycine, glutamate, and cysteine linked through the disulfide cystine.
Table 1.
Effect of selected constituents of pantethine and glutathione on the aggregation of βL crystallin in the presence of α crystallin
| Standard (βL + α) | OD(apparent)
|
|||
|---|---|---|---|---|
| 0.1 | 0.5 | 0.9 | Max = 1.0 | |
| +1 mM pantethine | 0.04 | 0.12 | 0.32 | 0.48 |
| +10 mM pathethine | 0.01 | 0.02 | 0.04 | 0.18 |
| +1 mM glutathione | 0.10 | 0.50 | 0.81 | 0.95 |
| +10 mM glutathione | 0.18 | 0.63 | 0.81 | 0.82 |
| +1 mM pantothenic acid | 0.16 | 0.64 | 1.00 | 1.00 |
| +1 mM cystamine | 0.41 | 0.75 | 0.93 | 0.92 |
| +1 mM β-alanine | 0.06 | 0.21 | 0.50 | 0.63 |
| +20 mM GSH | 0.29 | 0.67 | 1.00 | 1.14 |
| +20 mM cysteine (-SH) | 0.81 | 1.21 | 1.43 | 1.62 |
| +20 mM cysteamine (-SH) | 0.26 | 0.55 | 0.93 | 1.10 |
| +20 mM cysteamine (-SPO4) | 0.29 | 0.55 | 1.00 | 1.14 |
| +20 mM DTT | 0.17 | 0.57 | 1.00 | 2.00 |
In row 1, the values for the OD during opacification of a standard solution of βL plus α-crystallin at 60°C without added reagents are listed. Pantothenic acid (1.0 mM) had a weak negative effect and the OD values were higher than the standard. The disulfide cystamine had a very weak positive effect at a concentration of 1.0 mM. The effect of 1.0 mM β-alanine was quite strong, decreasing the OD values at each part of the opacification curve. In comparison with pantethine, 1.0 mM of the disulfide glutathione had weak activity and the OD values were higher than with pantethine. The reduced form of glutathione (GSH) was not protective against aggregation. Tests of several other sulfur compounds, including cysteine, cysteamine, cysteamine-PO4, and dithiothreitol (DTT), demonstrated that the −SH form of the reagents increased aggregation resulting in OD values that were higher than the standard. All of the studies above were conducted at 60°C. The OD was normalized as described.
Table 2.
Effect of selected constituents of pantethine and glutathione on the aggregation of ADH in the presence and absence of α crystallin.
| Standard (ADH + α) | OD(apparent)
|
|||
|---|---|---|---|---|
| 0.1 | 0.5 | 0.9 | Max = 1.0 | |
| +10 mM pantethine | 0.08 | 0.13 | 0.54 | 0.72 |
| +10 mM glutathione | 0.00 | 0.03 | 0.12 | 0.18 |
| +20 mM cystamine (-SS-) | 0.02 | 0.05 | 0.18 | 0.33 |
| +20 mM cysteamine (-SH-) | 0.07 | 0.44 | 0.85 | 0.94 |
| +20 mM cystine (-SS-) | 0.03 | 0.09 | 0.26 | 0.44 |
| +20 mM cysteine (-SH-) | 0.19 | 0.86 | 1.26 | 1.40 |
| +20 mM cysteamine (-SPO4) | 0.04 | 0.09 | 0.28 | 0.50 |
| +20 mM β-alanine | 0.07 | 0.38 | 0.62 | 0.66 |
| +20 mM glycine | 0.09 | 0.45 | 0.76 | 0.83 |
| +20 mM pantothenate | 0.08 | 0.51 | 1.00 | 1.10 |
| +20 mM glutamate | 0.14 | 0.62 | 1.03 | 1.14 |
| Standard (ADH alone) | ||||
| +20 mM pantethine | 0.02 | 0.03 | 0.59 | 0.97 |
| +20 mM glutathione | 0.01 | 0.02 | 0.07 | 0.26 |
| +20 mM GSH | 0.15 | 0.61 | 1.25 | 1.43 |
| +20 mM cysteine (-SH) | 1.61 | 1.72 | 1.89 | 1.89 |
| +20 mM cysteamine (-SH) | 0.10 | 0.50 | 0.93 | 1.03 |
| +20 mM cysteamine (-SPO4) | 0.01 | 0.02 | 0.34 | 0.84 |
We were most interested in the action of the test reagents at physiological temperatures, and further studies were conducted using ADH which undergoes aggregation and opacification at physiological temperatures of 37°C. The OD values for the standard solution of ADH plus α-crystallin without added reagents are listed in row 1 (standard). In the ADH plus α-crystallin assay, glutathione had a much stronger effect than the pantethine. The effects of the constituent molecules, glycine and β-alanine, glutamate and pantothenate, cystine and cystamine were analyzed. With the exception of the acids, glutamate and pantothenate, the constituent molecules of glutathione and pantethine enhanced the action of the α-crystallin. The strongest effects were observed with sulfur containing peptides. A study of selected -SH and -SS- forms of cystamine and cystine found that the oxidized -SS- forms were more protective than the reduced -SH forms and resulted in lower values for OD. The effects of selected reagents on aggregation of ADH without α-crystallin were evaluated. Both pantethine and glutathione inhibited aggregation of ADH alone although the effect of the glutathione was much stronger than the pantethine. The sulfhydryl reducing agents increased OD and aggregation of ADH alone. The OD was normalized as described.
Table 2 summarizes the effects of selected reagents on the ADH plus α-crystallin system conducted at 37°C. Under these conditions, the addition of oxidized glutathione had a stronger effect than pantethine on aggregation in the ADH plus α-crystallin solution. A study of cystamine and cystine found the -SS- forms decreased the OD to 0.33 and 0.44, respectively, while the addition of cysteamine (-SH) decreased the OD to 0.94 only, and the addition of cysteine (-SH) increased the OD to 1.40. The thiophosphate, cysteamine phosphate, was observed to enhance the activity of α-crystallin in the ADH assay much more than in the βL-crystallin assay. As observed in the study using βL-crystallin, the action of the β-alanine constituent of pantethine resembled the effect of the pantethine. These preliminary results may suggest that the action of the pantethine was due, in large part, to the β-alanine, while the action of the glutathione may be due to the cystine. In separate experiments, the effect of delayed addition of glutathione to the ADH plus α-crystallin was evaluated. Addition of glutathione at times up to 10 min after placing the ADH plus α-crystallin solution at 37°C to start the aggregation resulted in a delay in the increase in the OD and the maximum was not reached at 60 min. When glutathione was added at times later than 10 min, the glutathione had no effect on the increase in OD (data not shown).
In the lower part of Table 2 the effects of selected reagents on aggregation of ADH alone were compared at 20 mM concentrations. Even in the absence of α-crystallin, 20 mM oxidized glutathione was most effective in protecting against aggregation of ADH. Pantethine delayed the increase in OD slightly, although the maximum OD was similar to the maximum without pantethine. The effect of cysteamine phosphate was similar to the effect of pantethine. In the presence of the thiol reagents, reduced glutathione and cysteine, the OD increased faster and to a higher maximum than the ADH alone. At 20 mM concentrations, cysteamine had no observed effect on aggregation of ADH alone.
DISCUSSION
The results demonstrated that common metabolites, including pantethine and glutathione, can influence the protective action of α-crystallin on protein aggregation. The glutathione appeared to act directly on the aggregating target proteins, βL-crystallin and ADH, while the action of the pantethine required the presence of α-crystallin. Previous studies indicated that the metabolite, NADPH, stabilized ζ-crystallin against aggregation (2), while posttranslational modification by oxidants and glycating agents decreased the protective activity of α-crystallin (2, 5, 26, 27). The effects of chemical constituents of pantethine and glutathione indicated that the β-alanine constituent of the pantethine resembled and accounts for much of the action of the pantethine, while the cystine constituent resembled the action of the glutathione on chaperone assay. The results suggested that the pantethine and glutathione may act at different molecular sites to modulate the chaperone effect. The studies of the oxidized and reduced sulfhydryl reagents suggested that the reduced (-SH) forms had a lesser effect than the oxidized (-SS-) forms on aggregation of βL plus α or ADH plus α and under some conditions may favor aggregation. Clearly the action of -SH and -SS- reagents on α-crystallin and on the target proteins is complex. This study is preliminary, and a systematic and thorough investigation is needed. These results lend additional support to the concept that the physiological action of common metabolic products in lens cells such as pantethine and glutathione have direct effects on protein interactions that are important for maintenance of transparent lens cell structure. Reagents acting on molecular interactions between lens proteins to inhibit protein aggregation and maintain transparent lens cell microstructure (35, 36) may modulate the chaperone-like activity of α-crystallin. To understand the molecular basis for the action of cellular metabolites on lens cell transparency it will be necessary to characterize the chemical nature of the interactions with the crystallins.
Models for the effects of chaperone proteins on protein aggregation and protein folding include numerous intermediates, I, in the action on an unfolded peptide chain, U, which can aggregate, A, or fold to its functional native conformation, N: A↔IU↔IN (37). Our results are consistent with previous studies which demonstrated that the plot of aggregation versus time is an S-shaped curve (1–5). In a lens cell, the α-crystallin may form soluble complexes with partially unfolded proteins to stabilize the cytoplasmic structure in the transparent state (2, 3, 32, 38). The action of modulating reagents on either the partially denatured proteins or the molecular chaperone itself can enhance the protective effect of α-crystallin against aggregation. Different reagents have different activities, and some may delay the initiation of aggregation, others may inhibit the rate of aggregation, and others may inhibit the maximum size of the aggregates. While complicated models can describe the chaperone-like activity of α-crystallin (5), new and simpler models are needed to describe the kinetic, thermodynamic, and molecular parameters important for modulation of the aggregation process by pantethine and glutathione.
It is difficult to relate the effects of the various test reagents on aggregation in vitro with their effects on protein aggregation and opacification in vivo. It is of interest that the oxidized forms of the pantethine and glutathione were effective inhibitors of lens opacification in the selenite and other animal models for lens opacification (35, 36). In the selenite model, protection against protein aggregation was limited to the earliest stages of opacification process in vivo and the effectiveness of modulators of the chaperone-like activity of α-crystallin was limited to the early stages of the aggregation process in vitro. Further characterization of the chemical basis for the action of the endogenous metabolites and other modulating reagents on the molecular interactions between α-crystallin and other proteins in vitro will contribute to understanding the importance of chaperone activity in the maintenance of lens cell transparency.
CONCLUSIONS
Studies of the anti-aggregation activity of α-crystallin found that the action of pantethine was on α-crystallin and the action of glutathione was on the target proteins, βL or ADH. β-Alanine may be the active element of the pantethine and cystine the active element of glutathione. The enhancement observed with these common cellular metabolites in the aggregation assays may resemble their action on protein aggregations during cataract formation in vivo.
Acknowledgments
We appreciate the invaluable advice and assistance of L. Ding and Dr. J. Horwitz. We appreciate the assistance of T. Cranick with this manuscript. This work was supported by Grants EY04542 and EY03897 from the National Eye Institute.
Footnotes
Abbreviations: ADH, alcohol dehydrogenase; DTT, dithiothreitol; GSH, reduced glutathione.
References
- 1.Horwitz J. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao P V, Horwitz J, Zigler J S., Jr J Biol Chem. 1994;269:13266–13272. [PubMed] [Google Scholar]
- 3.Rao P V, Huang Q L, Horwitz J, Zigler J S., Jr Biochim Biophys Acta. 1995;1245:439–447. doi: 10.1016/0304-4165(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Spector A. Invest Ophthalmol Visual Sci. 1995;36:311–321. [PubMed] [Google Scholar]
- 5.Wang K, Spector A. J Biol Chem. 1994;269:13601–13608. [PubMed] [Google Scholar]
- 6.Merck K B, Groenen P J, Voorter C E, de Haard-Hoekman W A, Horwitz J, Bloemendal H, deJong W W. J Biol Chem. 1993;268:1046–1052. [PubMed] [Google Scholar]
- 7.Jakob U, Gaestel M, Engel K, Buchner J. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 8.de-Jong W W, Leunissen J A, Voorter C E. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- 9.Bhat S P, Nagineni C N. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 10.Bhat S P, Horwitz J, Srinivasan A, Ding L. Eur J Biochem. 1991;102:775–781. doi: 10.1111/j.1432-1033.1991.tb16432.x. [DOI] [PubMed] [Google Scholar]
- 11.Iwaki T, Kume-Iwaki A, Liem R K, Goldman J E. Cell. 1989;57:71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Shinohara H, Kurobe N, Goto S, Inaguma Y, Ohshima K. Biochim Biophys Acta. 1991;1080:173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- 13.Klemenz R, Frohli E, Aoyama A, Hoffmann S, Simpson R J, Moritz R L, Schafer R. Mol Cell Biol. 1991;11:803–812. doi: 10.1128/mcb.11.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee D C, Kim R Y, Wistow G J. J Mol Biol. 1993;232:1221–1226. doi: 10.1006/jmbi.1993.1476. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan A N, Nagineni C N, Bhat S P. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- 16.Nagineni C-N, Bhat S P. FEBS Lett. 1989;249:89–94. doi: 10.1016/0014-5793(89)80022-5. [DOI] [PubMed] [Google Scholar]
- 17.Dubin R A, Wawrousek E F, Piatigorsky J. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaguma Y, Shinohara H, Goto S, Kato K. Biochem Biophys Res Commun. 1992;182:844–850. doi: 10.1016/0006-291x(92)91809-5. [DOI] [PubMed] [Google Scholar]
- 19.Dasgupta S, Hohman T C, Carper D. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-z. [DOI] [PubMed] [Google Scholar]
- 20.Chiesi M, Longoni S, Limbruno U. Mol Cell Biochem. 1990;97:129–136. doi: 10.1007/BF00221054. [DOI] [PubMed] [Google Scholar]
- 21.Lowe J, Landon M, Pike I, Spendlove I, McDermott H, Mayer R J. Lancet. 1990;336:515–516. doi: 10.1016/0140-6736(90)92075-s. [DOI] [PubMed] [Google Scholar]
- 22.Murano S, Thweatt R, Shmookler-Reis R J, Jones R A, Moerman E J, Goldstein S. Mol Cell Biol. 1991;11:3905–3914. doi: 10.1128/mcb.11.8.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagineni C N, Bhat S P. Exp Eye Res. 1992;54:193–200. doi: 10.1016/s0014-4835(05)80208-8. [DOI] [PubMed] [Google Scholar]
- 24.Steinman L. Nature (London) 1995;375:739–740. doi: 10.1038/375739b0. [DOI] [PubMed] [Google Scholar]
- 25.Marini I, Bucchioni L, Voltarelli M, Del-Corso A, Mura U. Biochem Biophys Res Commun. 1995;212:413–420. doi: 10.1006/bbrc.1995.1985. [DOI] [PubMed] [Google Scholar]
- 26.Cherian M, Abraham E C. Biochem Biophys Res Commun. 1995;212:184–189. doi: 10.1006/bbrc.1995.1954. [DOI] [PubMed] [Google Scholar]
- 27.Cherian M, Abraham E C. Biochem Biophys Res Commun. 1995;208:675–679. doi: 10.1006/bbrc.1995.1391. [DOI] [PubMed] [Google Scholar]
- 28.Sharma K K, Ortwerth B J. Exp Eye Res. 1995;61:413–421. doi: 10.1016/s0014-4835(05)80136-8. [DOI] [PubMed] [Google Scholar]
- 29.Maiti M, Kono M, Chakrabarti B. FEBS Lett. 1988;236:109–114. doi: 10.1016/0014-5793(88)80295-3. [DOI] [PubMed] [Google Scholar]
- 30.Castoro J A, Bettelheim F A. Lens Eye Toxic Res. 1989;6:781–793. [PubMed] [Google Scholar]
- 31.Carver J A, Aquilina J A, Truscott R J. Biochim Biophys Acta. 1993;1164:22–28. doi: 10.1016/0167-4838(93)90107-3. [DOI] [PubMed] [Google Scholar]
- 32.Surewicz W K, Olesen P R. Biochemistry. 1995;34:9655–9660. doi: 10.1021/bi00030a001. [DOI] [PubMed] [Google Scholar]
- 33.Raman B, Ramakrishna T, Rao C M. FEBS Lett. 1995;365:133–136. doi: 10.1016/0014-5793(95)00440-k. [DOI] [PubMed] [Google Scholar]
- 34.Das K P, Surewicz W K. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 35.Hiraoka T, Clark J I. Invest Ophthalmol Visual Sci. 1995;36:2550–2555. [PubMed] [Google Scholar]
- 36.Hiraoka T, Clark J I, Li X Y, Thurston G M. Exp Eye Res. 1996;62:11–19. doi: 10.1006/exer.1996.0002. [DOI] [PubMed] [Google Scholar]
- 37.Jaenicke R. FASEB J. 1996;10:84–92. doi: 10.1096/fasebj.10.1.8566552. [DOI] [PubMed] [Google Scholar]
- 38.Carver J A, Guerreiro N, Nicholls K A, Truscott R J. Biochim Biophys Acta. 1995;1252:251–260. doi: 10.1016/0167-4838(95)00146-l. [DOI] [PubMed] [Google Scholar]
- 39.Persson B, Zigler J S, Jornvall H. Eur J Biochem. 1994;226:15–22. doi: 10.1111/j.1432-1033.1994.tb20021.x. [DOI] [PubMed] [Google Scholar]


