Abstract
Aprotinin is a serine protease inhibitor with diverse biological effects, and up until recently was utilized in the context of ischemia/reperfusion (I/R). It has been hypothesized, that a signaling pathway modulated by aprotinin in the context of I/R is the tumor necrosis factor-alpha receptor (TNFR) pathway. An intact mouse model of I/R (30 min-I/60 min-R) was used and LV peak + dP/dt was measured in wild type mice (WT, C57BL/6; n=10), WT mice with aprotinin (4mL/kg; n=10), transgenic mice devoid of the TNFRI (TNFRInull; n=10), and TNFRInull with aprotinin (n=10). Following I/R, LV peak + dP/dt decreased in both WT groups, but remained similar to baseline values in the TNFRInull group. In contrast, aprotinin caused a marked reduction in LV peak + dP/dt in the TNFRInull group following I/R. Soluble plasma TNF levels increased in the WT and TNFRInull mice with I/R, and was reduced with aprotinin. Soluble TNFRI and TNFRII levels, indicative of TNF activation increased in the WT mice following I/R and remained elevated with aprotinin. Soluble TNFRII levels were increased in the TNFRInull mice following I/R and remained elevated with aprotinin. The new and unique findings of this study were two fold. First, aprotinin failed to improve LV function after I/R despite a reduction in circulating TNF levels. Second, genetic ablation of the TNFRI uncovered a negative inotropic effect of aprotinin. These findings demonstrate that complex biological pathways and interactions are affected with broad spectrum serine protease inhibition, which are relevant to myocardial function in the context of I/R.
INTRODUCTION
For over a decade, aprotinin, a global serine protease inhibitor, was commonly used in the context of cardiac surgery with ischemia and reperfusion (I/R) for hemostatic purposes.1–2 Despite the beneficial effects on blood loss, recent outcomes based morbidity and mortality studies have called into question the mechanisms of action and safety of aprotinin, which ultimately resulted in complete discontinuation of this serine protease inhibitor.3–4 However, the underlying biological basis for the potential deleterious effects of aprotinin remain poorly understood. Aprotinin likely imparts multiple effects on a number of pathways, one of which includes the tumor necrosis factor-alpha (TNF) receptor pathway. Past studies have suggested that aprotinin may affect TNF levels.5–6 TNF is synthesized and in initially bound to the membrane requiring proteolytic cleavage to yield a soluble form which can then bind to the TNF receptor I (TNFRI) and/or TNFRII.7–8 Recent studies have shown the importance of TNFRI in mediating cardiac dysfunction and left ventricular (LV) myocardial injury following I/R. 8–11 Accordingly, the overall goal of the present study was to examine the potential effect of aprotinin on LV function in the presence and absence of TNFRI in the context of I/R. Prior studies have characterized a transgenic mouse genetically devoid of TNFRI (TNFRInull) which has been used in the context of I/R.8,10 Thus the first objective was to examine LV function in WT and TNFRInull mice in the context of I/R with and without aprotinin. A number of cytokines are up-regulated with I/R and can be potentially modified by aprotinin, particularly TNF.12–14 TNF binding to TNFRI or TNFRII results in proteolytic cleavage of the extracellular domain of the receptor. Thus, plasma levels of TNF, TNFRI and TNFRII provide an index of overall TNF receptor activation. Accordingly, the second objective was to examine these biomarkers of TNF receptor activation in WT and TNFRInull mice following I/R, and in the presence and absence of aprotinin.
METHODS
Experimental Design
Instrumentation and Animal Model
In this study, TNFRInull mice (C57BL/6-Tnfrsf1atm1Imx/J, 10–16 wk, 24–30 gm) were obtained from Jackson Laboratory (Bar Harbor, Me), along with strain and aged matched WT.15 The presence of the specific transgene indicative of TNFRI ablation was confirmed by polymerase chain reaction using extracted DNA from a tail clip sample.9–11 The mice were induced, intubated with a twenty gauge Jelco needle (Medex Medical Ltd., Rossendale, UK), and maintained under isoflurane anesthesia (2%) using a MiniVent Type 845 ventilator (Hugo Sachs Elektronik) with tidal volumes of 250μL, at a rate of 250 strokes/min, and a FiO2 of 27%. Temperature was monitored via a rectal probe during the length of the procedure, and maintained by a feedback loop to a heating pad within the operating table, as well as a heating lamp. The mice received a one time intraperitoneal normal saline bolus of 0.5mL. The ventilator settings provided a pH of 7.35 ±0.01, pCO2 of 29 ±2, and a pO2 of 453 ±34. The right carotid was exposed and a pre-calibrated Millar catheter (1.4 F, SPR-839, Millar Instruments, Houston, TX) was placed in the LV for continuous pressure measurements.
A left thoracotomy was then performed, the posterolateral aspect of the LV free wall visualized, and a purse-string placed around the left anterior descending artery just distal to the bifurcation of the left main coronary artery using 6.0 Prolene and an atraumatic needle (Ethicon, Somerville, NJ). The suture was exteriorized and the wound was closed in layers. The ligature was tightened to induce ischemia (30 minutes) and then released for reperfusion (60 minutes). In a preliminary set of studies (n=6), fluorescent microspheres (F-8838, Molecular Probes, 15μm diameter, 7.5×104) of different emission spectra were injected at baseline, at 30 minutes of ischemia, and at 60 minutes of reperfusion by LV injection methods described previously.16 LV regional myocardial blood flow fell to approximately 50% of baseline values with peak ischemia and returned to within baseline values with reperfusion. Thus, this murine model provided a transient period of low myocardial blood flow followed by a restoration of blood flow, and therefore allowed for the study of LV function in the context of ischemia-reperfusion (I/R). It has been established previously that this site of coronary occlusion in the mouse resulted in a uniform area at risk of 50%.17 At the end of the reperfusion period, the LV catheter was removed, and the heart re-exposed, and 200 Units of heparin systemically delivered. Blood was then collected from the right carotid artery for cytokine and aprotinin analysis. A 1% alcian blue solution was then delivered in a retrograde fashion, into the aorta and visualization of the entire LV was performed to ensure uniform and complete coronary perfusion had been achieved in all preparations. The total procedure time was 120 minutes. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, 1996). This protocol was reviewed and approved by the MUSC Institutional Animal Care & Use Committee (AR# 2451).
Experimental Protocol
Following instrumentation, baseline LV function measurements were performed as described in a subsequent paragraph in WT mice (n=24) and TNFRInull mice (n=24). Following these measurements, mice were randomized to one of four treatment groups: WT mice with normal saline, WT mice with aprotinin (4 mL/kg) TNFRI knockout mice with normal saline, and TNFRInull mice with aprotinin (4 mL/kg). Randomization was accomplished by blindly drawing group assignments from an envelope. Thus, all baseline measurements were obtained prior to any drug administration or treatment protocol. After baseline hemodynamic measurements were taken, the assigned dose of saline or aprotinin was given by intra-peritoneal injection using an equivalent final volume (0.5 mL). Ischemia was initiated 5 to 10 minutes after the injection.
In order to provide a referent control group for the purposes of cytokine measurements, WT mice (n=25) were instrumented and treated in identical fashion to the experimental groups with the exception of I/R. An additional group of WT mice (n=10) were administered aprotinin and also maintained in identical fashion with the exception of I/R induction.
Aprotinin Protocol
The selected aprotinin dose of 4 mL/kg (10,000 Kallikrein Inhibiting Units (KIU)/mL) was utilized to simulate a clinical dosing algorithm of 4×104 KIU/kg, which would achieve a plasma concentration corresponding to a clinical full Hammersmith dose. 18–20 Our approach was to utilize this clinical dosing regimen using the weight-based initial bolus. Furthermore, this is similar to weight-based dosing regimens used in previous animal models.21–22 Nevertheless, the present study administered aprotinin in a murine model of I/R and therefore a procedure was developed to measure relative plasma levels of aprotinin. For this approach, a fluorogenic substrate cleaved by the serine protease plasmin was utilized in an ex-vivo assay system. Specifically, the peptide sequence D-ala-leu-lys-7-amido-4-methylcomarin (Sigma, A8171) at a fixed concentration of 10 nM, was mixed in a reaction buffer containing a 1:33 dilution of normal mouse plasma and incubated at 37degC for 15 min in the presence and absence of 7 μg/mL of plasmin (Sigma, P1876, 3 U/mg). The fluorescence of this reaction was detected in continuous fashion (Fluostar Galaxy, BMG Labtech, NC) at an excitation/emission wavelength of 365/440 nm. The plasmin substrate, plasmin concentrations, and the incubation conditions were determined from preliminary dilution studies in order to yield peak performance as defined as that which yielded a consistent and stable fluorescence signal. This reaction solution was then incubated in the presence and absence of increasing concentrations of aprotinin (range 0–560 KIU/mL) in order to generate a standardized enzyme activity-inhibition curve (y=4071 +7643e−0.0028x, r=0.98, p<0.001). The intra-assay coefficient of variation was 5% and an inter-assay coefficient was 9%. The intraperitoneal aprotinin dose of 4mL/kg was found to result in a plasma concentration of 334±102 KIU/mL which recapitulated a full Hammersmith dose used clinically.
LV Function Assessment
Following stabilization from the instrumentation, LV function and geometry were measured. LV pressures were obtained from the microtransducer in order to obtain peak pressure and peak +dP/dt. Transthoracic echocardiography was performed in order to measure LV geometry and function. 23–24 Two-dimensional M-mode echocardiographic recordings were obtained using a 40 MHz scanning head with a spatial resolution of 30 um (Vevo 660, VisualSonics, Toronto). Using short-axis views, LV end-diastolic dimension, posterior wall thickening, and fractional shortening were computed. Heart rate was determined from a surface electrocardiogram. Through rigorous maintenance of normotheria, ventilatory status and fluid balance, ambient heart rate remained between 400–500 bpm throughout the experimental procedures.
Cytokine Measurements
Mouse plasma samples taken after 60 minutes of reperfusion or following a referent control period were utilized for measuring soluble TNF, TNFRI and TNFRII levels. For soluble TNF, a multiplex array was utilized according to manufacturer’s instructions (#LUM410; R &D Systems, Minneapolis, MN). The relative fluorescence detected was compared to a 5 parameter logistic calibration curve generated for TNF (Bio-Plex Manager 4.1.1). The sensitivity for TNF was 0.42 pg/mL. Plasma TNFRI and TNFRII were assessed by enzyme-linked immunoassay (Cat. # MRT10, MRT20, R &D Systems, Minneapolis, MN).
Data Analysis
LV function and plasma cytokine levels and the effects of aprotinin dosing on these parameters were first compared between the groups using analysis of variance (ANOVA). If the ANOVA revealed significant differences, post-hoc mean separation was performed using Tukey-adjusted mean square differences (Module prcomp, STATA Intercooled, v8, College Station, TX). Following this multiway ANOVA approach, data transformation was also performed in which changes with I/R on the indices of LV function from baseline were computed and expressed as a percentage. These transformed computations were then examined using an adjusted t-score. In addition, plasma cytokine levels determined in the reference control mice for each cytokine and ANOVA with means separation was performed.. Results are presented as mean ± standard error of the mean (SEM). Values of p<0.05 were considered to be statistically significant.
RESULTS
A total of 48 mice were enrolled in the ischemia-reperfusion (I/R) protocol, with 8 mice dying prior to the final set of measurements. These mice died of arrhythmias during reperfusion, and were equally distributed among both strains.
LV Function
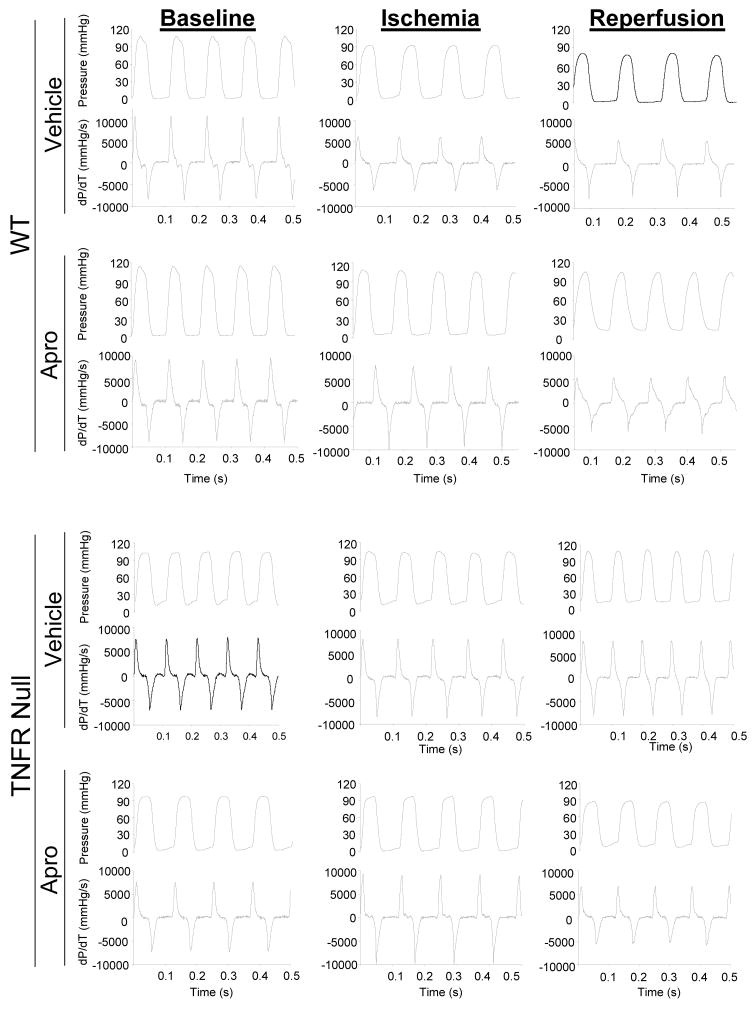
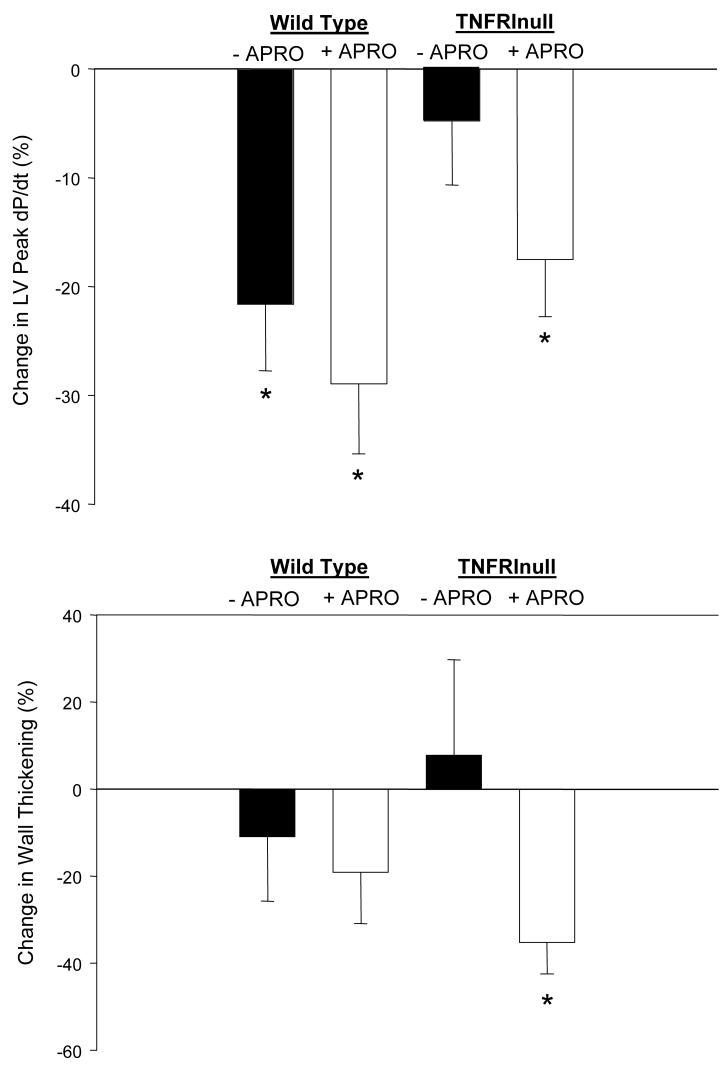
LV function at baseline, at peak ischemia, and following reperfusion are shown in Table 1. Representative LV pressure traces for these periods in both the WT and TNFRInull mice are shown in Figure 1. LV peak pressure fell in the WT with ischemia and reperfusion, but was abrogated with aprotinin. In the TNFRInull mice, LV peak pressure remained within baseline values with I/R, but was reduced with aprotinin treatment. LV peak +dP/dt was reduced from baseline after 60 minutes of reperfusion in both WT groups. Following I/R, LV peak +dP/dt remained within baseline values in the TNFRInull group, but was reduced with aprotinin treatment. The respective changes in LV peak +dP/dt with respect to individual baseline values are summarized in Figure 2. While LV peak +dP/dt fell by over 20% in both the vehicle and aprotinin WT groups following I/R, this effect was not observed in the TNFRInull vehicle group. However, in marked contrast with aprotinin administration in the TNFRInull group, LV peak +dP/dt fell to levels similar to WT values following I/R. LV fractional shortening fell at peak ischemia in the WT group with aprotinin administration, but returned to baseline values with reperfusion. LV fractional shortening remained within baseline values following I/R in both TNFRInull groups. Following I/R, LV wall thickening returned to baseline values in both WT groups, and in the vehicle TNFRInull group. However, LV wall thickening was reduced in the aprotinin TNFRI group. These relative changes in LV wall thickening are summarized in Figure 2. Specifically, LV wall thickening was reduced by approximately 40% following I/R in the aprotinin and TNFRInull group.
Table 1.
LV Function Following Ischemia/Reperfusion Effects of Aprotinin and TNFRI Deletion
| WT | TNFRInull | |||||
|---|---|---|---|---|---|---|
| Baseline | Peak Ischemia | Reperfusion | Baseline | Peak Ischemia | Reperfusion | |
| LV Peak Pressure (mmHg) | ||||||
| Vehicle | 104±4 | 83±3* | 80±3* | 106±3 | 105±4 | 99±6# |
| Aprotinin | 101±5+ | 95±5+ | 103±3 | 93±4* | ||
| dP/dt max (mmHg/s) | ||||||
| Vehicle | 8143± 499 | 6398±434* | 6382±497* | 7731±368 | 7988±426 | 7360± 454 |
| Aprotinin | 6910±516 | 5784±522* | 7555±369 | 6375± 405* | ||
| Wall Thickening (%) | ||||||
| Vehicle | 29.5± 3.0 | 33.3±5.9 | 26.3±4.4 | 39.3±3.6 | 25.8±2.9* | 42.4±8.6 |
| Aprotinin | 20.5±2.4*+ | 23.8±3.4 | 32.1±4.2 | 25.4±2.8* | ||
| Fractional Shortening (%) | ||||||
| Vehicle | 28.2± 0.8 | 26.6±1.3 | 28.0±1.5 | 33.9±1.1 | 36.9±1.8 | 37.0±2.0# |
| Aprotinin | 23.8±1.1* | 25.8±1.2 | 32.7±1.4 | 35.4±1.8# | ||
p <0.05 versus baseline values
p <0.05 versus respective vehicle values
p<0.05 versus respective WT values
Data are reported as Mean ± SEM, (n=10/group)
Figure 1.
Representative recordings of LV pressure and peak +dP/dt for wild type (WT) and tumor necrosis factor-α receptor I knockout (TNFRInull) mice under baseline conditions, at peak ischemia, and with reperfusion (I/R). In addition, mice were administered either saline (vehicle) or aprotinin prior to the induction of I/R. LV peak +dP/dt fell following I/R in the WT groups, but appeared to be reduced in the TNFRInull group. A summary of LV function during and following I/R ion the different treatment groups is presented in Table 1. The relative changes in LV peak +dP/dt as a function of a baseline values is summarized in Figure 2.
Figure 2.
LV peak +dP/dt and LV wall thickening were computed as relative changes from baseline values in both the wild type (WT) and the tumor necrosis factor-α receptor I knockout (TNFRInull) mice following I/R, with or without aprotinin (APRO) administration. LV peak +dP/dt fell in all groups following I/R with the exception of the TNFRInull group receiving vehicle only. LV wall thickening in the TNFRInull with APRO group was significantly reduced as compared to baseline, while the other groups did not change significantly. (* = p<0.05 vs Baseline)
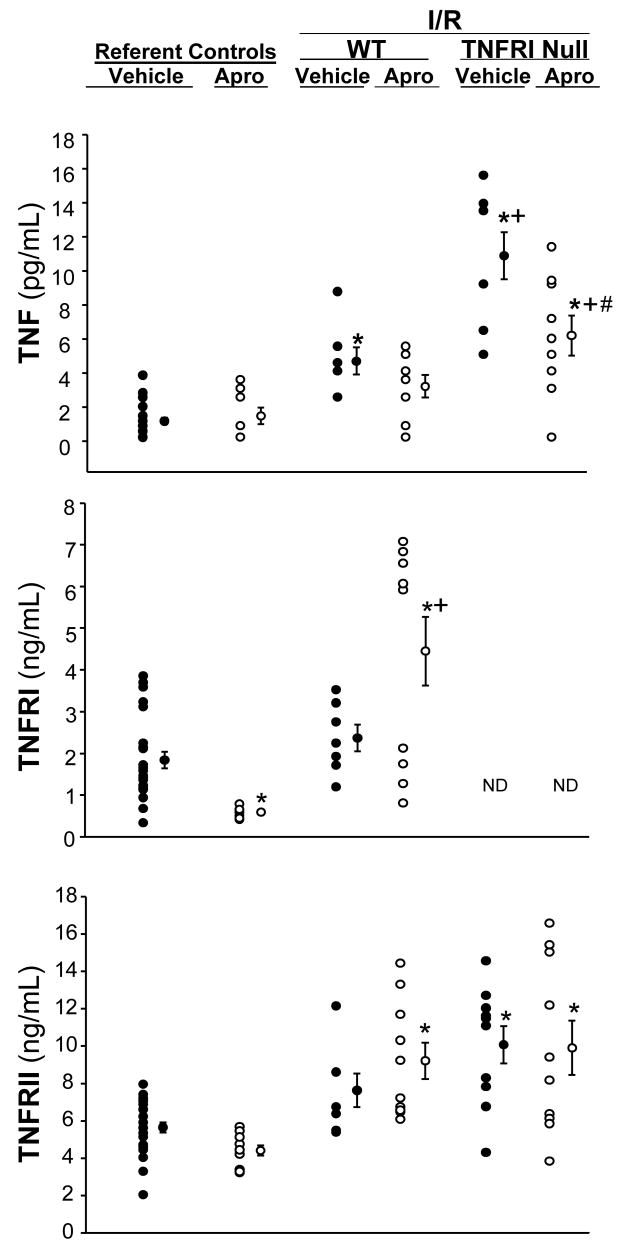
Plasma Cytokine Levels
Plasma levels for soluble TNF, TNFRI and TNFRII are summarized in Figure 3. For these measurements, a group of WT mice in which identical experimental conditions were maintained, with the absence of I/R, served as referent controls. Aprotinin treatment in the absence of I/R did not affect plasma TNF levels. Following I/R, plasma TNF was increased by approximately 2-fold from referent control values in the vehicle WT group, and was attenuated with aprotinin. Following I/R, plasma TNF was increased significantly in both TNFRInull groups, and was increased from respective WT values. With aprotinin administration, plasma TNF levels were reduced in the TNFRInull group when compared to respective vehicle values, but remained elevated when compared to respective WT or referent control values. Soluble plasma TNFRI levels were reduced from referent control values in the aprotinin only group. However, soluble TNFRI levels were significantly increased following I/R in the aprotinin treated WT mice. As expected, soluble TNFRI levels were not detected in all TNFRInull mice. TNFRII was significantly increased in the WT with aprotinin group, and in both TNFRInull groups. Thus, while aprotinin administration appeared to blunt soluble TNF release, these effects were not associated with a relative reduction in soluble TNF receptor levels.
Figure 3.
Plasma tumor necrosis factor-α (TNF), TNF receptor I (TNFRI) and TNFRII were computed in referent wild type (WT) control mice (maintained under equivalent conditions without I/R) receiving vehicle or aprotinin (APRO), in WT mice following I/R with or without aprotinin, and in TNFRInull mice following I/R with or without aprotinin. In both WT and TNFRInull mice following I/R, plasma TNF levels increased, with a more robust increase observed in the TNFRInull mice. Aprotinin administration prior to I/R blunted TNF release. TNFRI levels were reduced with aprotinin administration only, in the absence of I/R. However, TNFRI levels significantly increased with aprotinin administration following I/R. As expected, TNFRI levels were not detectable in the TNFRInull mice. Plasma TNFRII levels were significantly elevated across all treatment groups as compared to referent vehicle controls. (*p<0.05 vs referent vehicle controls; +p<0.05 vs respective WT values, #p<0.05 vs respective vehicle values)
DISCUSSION
Up until recently, aprotinin was routinely administered during complex cardiovascular surgical cases in order to improve hemostasis and minimize blood loss post-operatively.1–2 However, retrospective studies of large numbers of patients receiving aprotinin, as well as a prospective study utilizing a high dose of aprotinin in cardiac surgery patients, has resulted in the discontinuation of this non-selective serine protease inhibitor. 3–4 Specifically, using a dose of aprotinin, defined clinically as the full Hammersmith dose, retrospective studies suggested a negative association between aprotinin treatment and post-operative renal dysfunction.3 In a recently completed prospective trial utilizing a full Hammersmith dose of aprotinin in cardiac surgery patients, 4 a higher postoperative mortality was reported when compared to other hemostatic agents. This untoward effect on early post-operative mortality with aprotinin occurred despite a significant reduction in peri-operative blood loss. Thus, while aprotinin favorably affects hemostasis, which is an important determinant of morbidity and mortality in the context of I/R and cardiac surgery, aprotinin likely influences other biological processes which impart deleterious effects. Aprotinin has been shown, particularly at the full Hammersmith dose, to modify cytokine levels, such as TNF.5–6 However, the inter-relationship between aprotinin, LV functional recovery following a period of I/R, and cytokine receptor activation remained unknown. The current study utilized an aprotinin dosing strategy in mice, which yielded similar plasma concentrations to those achieved in clinical studies utilizing a full Hammersmith dosing protocol. Moreover, the present study examined the effects of aprotinin in the presence and absence of the TNFRI. Using this murine construct and a period of myocardial I/R, there were two unique findings from the present study. First, aprotinin did not improve LV function in WT mice following I/R despite a relative decrease in TNF plasma concentrations. Second, aprotinin administered in TNFRInull mice actually worsened LV function following I/R. The findings of this study underscore the fact that aprotinin independently modifies several pathways which lead to a decrease in TNF plasma concentrations without providing a protective effect through the TNF receptor, and at a clinically relevant dose, failed to improve LV function.
Past studies have reported that aprotinin can reduce circulating levels of TNF. 5–6,12 For example, Bull et al demonstrated a decrease in uptake and generation of TNF in an in vitro myocardial model, following aprotinin administration. 5–6 The current study is the first to examine a decrease in circulating plasma TNF concentration and its interrelationship with TNFR in the context of I/R, following aprotinin administration. Using an aprotinin concentration which achieved plasma levels consistent with the full-Hammersmith dosing, 20–22 aprotinin failed to modify TNFR activation despite blunting TNF levels. TNF is synthesized initially as a membrane-bound protein which when cleaved by TNF converting enzyme and released in labile form, converts to soluble TNF. Soluble TNF then forms a trimeric complex which then binds to TNFRI or TNFRII, resulting in the activation of the receptor.25 Accordingly, the current study measured plasma concentrations of TNFRI and TNFRII as an index of TNF activation. 26–27 In the WT mouse with intact TNFRI and TNFRII, robust increases of soluble TNFRI and TNFRII occurred following I/R. Importantly, aprotinin failed to decrease the emergence of soluble TNFRI and TNFRII. Therefore while aprotinin reduced plasma concentrations of TNF, these results demonstrate that aprotinin failed to inhibit TNFR activation at the local level.
The present study moved beyond past findings regarding aprotinin and TNF, through the use of the TNFRInull mouse. Past studies have clearly demonstrated the protective effects of TNFRI gene deletion with respect to myocardial function following I/R. 8,10–11 Using the same TNFRInull genotype as the current study, Maekawa et al performed in vivo I/R studies, and showed increased LV peak +dP/dt when compared to referent wild type mice.9 The current study used a similar model of in vivo I/R in TNFRInull mice following aprotinin administration. Aprotinin negatively affected LV function in the TNFRInull group, despite decreased TNF plasma cytokine concentration. Thus, in the present study, deletion of the TNFRI receptor uncovered a negative inotropic effect of aprotinin, and further underscores the pleiotropic effects of this serine protease inhibitor in the context of I/R.
The findings from the present study challenge the canonical thought that aprotinin provides protective effects on myocardial function in the context of I/R through interruption of TNF production and signaling. While the present study demonstrated a blunting of soluble TNF release with aprotinin treatment, there was no concomitant reduction in soluble TNF receptors, indicative that local TNF receptor activation may have been unabated. There are several potential mechanisms that have contributed to this observation. First, the emergence of soluble TNF into the plasma results in the spillover from local release by inflammatory cells as well as by endogenous cells. It has been demonstrated previously that aprotinin can affect neutrophil function,27–28 which may in turn reduce TNF release by this inflammatory cell type. However, whether and to what degree aprotinin affects TNF release within local tissue compartments remains unknown. Second, while aprotinin is a non-selective serine protease inhibitor, the inhibitory constants for different proteolytic enzymes can vary widely and by 100 fold. 5,29 Moreover, while the critical enzyme for processing of membrane bound TNF is a metalloprotease 30–31, whether and to what degree aprotinin inhibits this proteolytic pathway remains to be established. Third, activation of the TNF receptor may not necessarily require a soluble TNF ligand, as membrane bound TNF may also activate the receptor, and in turn cause a soluble TNF receptor to be formed. 12,32–33 While the underlying mechanisms remain speculative, the findings from the present study clearly demonstrated that aprotinin does not provide a protective effect through reducing soluble TNF levels and subsequently reducing TNFRI activation.
While the present study provided insight on the effect of aprotinin on the TNFR pathway in the setting on I/R, it must be placed in context and limitations recognized. First, the murine I/R model does not necessarily recapitulate the transient myocardial I/R that may occur in the context of cardiac surgery. Moreover, the study only examined LV function and relative cytokine levels at one point in time following I/R. In a study by Buerke et al using a rodent model of I/R, aprotinin administration was demonstrated to alter a number of biological signaling cascades for up to 24 hours.34 Second, the aprotinin dosing was based upon a clinical weight based algorithm that may not be translatable to a mouse model. However, plasma aprotinin measurements were directly measured in this study, rather than inferred, and correlated with a full-Hammersmith dosing protocol.20–22 It must be recognized that the volume of distribution, pharmacokinetics and serine protease inhibitory profiles are likely to be different in the murine system than that of man. Nevertheless, the present study reinforces the findings from past studies that the effects of aprotinin on LV function and inflammatory pathways are likely to be concentration/dose dependent.18–22,29 These limitations notwithstanding, the unique findings of the present study demonstrated the multiplicity of the effects of aprotinin on both LV function and the TNFR pathway. In light of the findings of the current study, future mechanistic investigation would be appropriate given the current concerns regarding the safety and efficacy of serine protease inhibition in the clinical context of cardiac surgery. 3,4,35–36
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants PO1-HL-48788 (FG Spinale), RO1-HL-59165 (FG Spinale), Research Service of the Department of Veterans Affairs (FG Spinale), Research Fellowships from Bayer Pharmaceuticals (MD McEvoy), and the Foundation for Anesthesia Education and Research (MD McEvoy).
List of Abbreviations
- LV
Left ventricular
- I/R
Ischemia-reperfusion
- TNF
Tumor necrosis factor-α
- TNFRI/TNFRII
Tumor necrosis factor-α I/II
- TNFRInull
Tumor necrosis factor-α receptor I knockout
- WT
Wild Type
- KIU
Kallikrein inhibiting units
- ANOVA
Analysis of variance
References
- 1.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in a coronary arterty bypass graft surgery: A systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–448. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briet E, Buller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940–7. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 3.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. NEJM. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 4.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008 Feb 21;358(8):771–83. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 5.Bull D, Connors R, Albanil A, Reid B, et al. Cardiopulmonary support and physiology. Aprotinin preserves myocardial biochemical function during cold storage through suppression of tumor necrosis factor. J Thorac Cardiovasc Surg. 2000;119:242–250. doi: 10.1016/S0022-5223(00)70179-6. [DOI] [PubMed] [Google Scholar]
- 6.Bull D, Maurer J. Aprotinin and preservation of myocardial function after ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S735–739. doi: 10.1016/s0003-4975(02)04702-1. [DOI] [PubMed] [Google Scholar]
- 7.Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007 Sep 1;179(5):2686–9. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Tumor necrosis factor receptor 1 signaling resistance in the female myocardium during ischemia. Circulation. 2006 Jul 4;114(1 Suppl):I282–9. doi: 10.1161/CIRCULATIONAHA.105.001164. [DOI] [PubMed] [Google Scholar]
- 9.Maekawa N, Wada H, Kanda T, Niwa T, Yamada Y, Saito K, Fujiwara H, Sekikawa K, Seishima M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J Am Coll Cardiol. 2002 Apr 3;39(7):1229–35. doi: 10.1016/s0735-1097(02)01738-2. [DOI] [PubMed] [Google Scholar]
- 10.Reil JC, Gilles S, Zahler S, Brandl A, Drexler H, Hültner L, Matrisian LM, Welsch U, Becker BF. Insights from knock-out models concerning postischemic release of TNFalpha from isolated mouse hearts. J Mol Cell Cardiol. 2007 Jan;42(1):133–41. doi: 10.1016/j.yjmcc.2006.09.020. Epub 2006 Nov 13. [DOI] [PubMed] [Google Scholar]
- 11.Ramani R, Mathier M, Wang P, Gibson G, Tögel S, Dawson J, Bauer A, Alber S, Watkins SC, McTiernan CF, Feldman AM. Inhibition of tumor necrosis factor receptor-1-mediated pathways has beneficial effects in a murine model of postischemic remodeling. Am J Physiol Heart Circ Physiol. 2004 Sep;287(3):H1369–77. doi: 10.1152/ajpheart.00641.2003. [DOI] [PubMed] [Google Scholar]
- 12.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–95. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 13.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 14.Ren G, Dewald O, Frangogiannis NG. Inflammatory mechanisms in myocardial infarction. Curr Drug Targets Inflamm Allergy. 2003;2:242–56. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 15.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998 Jan 15;160(2):943–52. [PubMed] [Google Scholar]
- 16.Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, Ichinose F, Zapol WM, Bloch KD, Picard MH, Scherrer-Crosbie M. In vivo characterization of murine myocardial perfusion with myocardial contrast echocardiography: validation and application in nitric oxide synthase 3 deficient mice. Circulation. 2007 Sep 11;116(11):1250–7. doi: 10.1161/CIRCULATIONAHA.107.707737. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy RJ, Tuman K, O’Connor C, Ivankovish AD. Aprotinin pretreatment diminished postischemic myocardial contractile dysfunction in dogs. Anesth Analg. 1999;89:1096–1101. [PubMed] [Google Scholar]
- 18.van Oeveren W, Jansen NJ, Bidstrup BP, Royston D, Westaby S, Neuhof H, Wildevuur CR. Effects of aprotinin on hemostatic mechanisms during cardiopulmonary bypass. Ann Thorac Surg. 1987;44:640–5. doi: 10.1016/s0003-4975(10)62153-4. [DOI] [PubMed] [Google Scholar]
- 19.Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987;2:1289–91. doi: 10.1016/s0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 20.Royston D, Cardigan R, Gippner-Steppert C, Jochum M. Is perioperative plasma aprotinin concentration more predictable and constant after a weight-related dose regimen? Anesth Analg. 2001;92:830–6. doi: 10.1097/00000539-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Khan TA, Bianchi C, Araujo E, Voisine P, Xu SH, Feng J, Li J, Sellke FW. Aprotinin preserves cellular junctions and reduces myocardial edema after regional ischemia and cardioplegic arrest. Circulation. 2005;112:I196–201. doi: 10.1161/CIRCULATIONAHA.104.526053. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H149–58. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 23.Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography. 2007 Jan;24(1):83–9. doi: 10.1111/j.1540-8175.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.El-Barbary M, Khabar KS. Soluble tumor necrosis factor receptor p55 predicts cytokinemia and systemic inflammatory response after cardiopulmonary bypass. Crit Care Med. 2002 Aug;30(8):1712–6. doi: 10.1097/00003246-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Steele IC, Nugent AM, Maguire S, Hoper M, Campbell G, Halliday MI, Nicholls DP. Cytokine profile in chronic cardiac failure. Eur J Clin Invest. 1996 Nov;26(11):1018–22. doi: 10.1046/j.1365-2362.1996.2560587.x. [DOI] [PubMed] [Google Scholar]
- 27.Mojcik CF, Levy JH. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745–54. doi: 10.1016/s0003-4975(00)02218-9. [DOI] [PubMed] [Google Scholar]
- 28.Wachtfogel YT, Kucich U, Hack CE, Gluszko P, Niewiarowski S, Colman RW, Edmunds LH., Jr Aprotinin inhibits the contact, neutrophil, and platelet activation systems during simulated extracorporeal perfusion. J Thorac Cardiovasc Surg. 1993;106:1–9. [PubMed] [Google Scholar]
- 29.Englberger L, Kipfer B, Berdat PA, Nydegger UE, Carrel TP. Aprotinin in coronary operation with cardiopulmonary bypass: does “low-dose” aprotinin inhibit the inflammatory response? Ann Thorac Surg. 2002;73:1897–904. doi: 10.1016/s0003-4975(02)03535-x. [DOI] [PubMed] [Google Scholar]
- 30.Smookler DS, Mohammed FF, Kassiri Z, Duncan GS, Mak TW, Khokha R. Tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J Immunol. 2006 Jan 15;176(2):721–5. doi: 10.4049/jimmunol.176.2.721. [DOI] [PubMed] [Google Scholar]
- 31.Grell M, Douni E, Wajant H, Löhden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995 Dec 1;83(5):793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 32.Weiss T, Grell M, Siemienski K, Mühlenbeck F, Dürkop H, Pfizenmaier K, Scheurich P, Wajant H. TNFR80-dependent enhancement of TNFR60-induced cell death is mediated by TNFR-associated factor 2 and is specific for TNFR60. J Immunol. 1998 Sep 15;161(6):3136–42. [PubMed] [Google Scholar]
- 33.Wajant H, Grell M, Scheurich P. TNF receptor associated factors in cytokine signaling. Cytokine Growth Factor Rev. 1999 Mar;10(1):15–26. doi: 10.1016/s1359-6101(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 34.Buerke M, Pruefer D, Sankat D, Carter JM, Buerke U, Russ M, Schlitt A, Friedrich I, Börgermann J, Vahl CF, Werdan K. Effects of aprotinin on gene expression and protein synthesis after ischemia and reperfusion in rats. Circulation. 2007 Sep 11;116(11 Suppl):I121–6. doi: 10.1161/CIRCULATIONAHA.106.680249. [DOI] [PubMed] [Google Scholar]
- 35.Shaw AD, Stafford-Smith M, White WD, Phillips-Bute B, Swaminathan M, Milano C, Welsby IJ, Aronson S, Mathew JP, Peterson ED, Newman MF. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008 Feb 21;358(8):784–93. doi: 10.1056/NEJMoa0707768. [DOI] [PubMed] [Google Scholar]
- 36.Ray WA. Learning from aprotinin--mandatory trials of comparative efficacy and safety needed. N Engl J Med. 2008 Feb 21;358(8):840–2. doi: 10.1056/NEJMe0800268. [DOI] [PubMed] [Google Scholar]