Abstract
The isothiocyantes (ITCs) have long been known to possess chemopreventive activities for varieties of neoplasms including breast cancer, but the molecular mechanism by which ITCs prevent breast cancer development has not been established. In this study, we investigated the effects of benzyl and phenethyl isothiocyanate (BITC and PEITC) on the estrogen-stimulated growth of estrogen receptor alpha (ERα)-positive breast cancer MCF7 and T-47D cells. BITC and PEITC inhibited estrogen-stimulated cell growth and reduced the expression levels of ERα in MCF7 and T-47D cells in a dose and time -dependent and reversible manner. In addition, BITC and PEITC also abrogated the transcriptional activity of ERα and hence inhibited estrogen-stimulated expression of the estrogen responsive gene, pS2. These results demonstrated that BITC and PEITC function as potent ERα disruptors to abrogate mitogenic estrogen signaling in ER-positive breast cancer cells, which provides a molecular explanation for the growth inhibitory function of ITCs in breast cancer development, and a rational for further exploration of ITCs as chemopreventive agents for human mammary carcinogenesis.
Keywords: isothiocyanate, breast cancer, estrogen receptor
Introduction
Breast cancer is a leading cause of cancer-related deaths among women in the United States. Despite the fact that significant advances have occurred in the understanding of the biology and etiology of breast cancer development, there is still no effective treatment and prevention for this deadly disease. Experimental evidence for the role of endogenous in breast cancer etiology has been well documented. It is believed that estrogen signaling is involved in mammary epithelial cell proliferation and differentiation. Dysregulated estrogen signaling increases the rate of cell proliferation and thus the risk of breast cancer.
The growth-promoting function of estrogen is mediated by specific receptors designated estrogen receptors (ERs). ERs belong to the nuclear receptor superfamily and have two isoforms (ERα and ERβ) encoded by independent genes (1). Both ERs are composed of three independent but interacting functional domains: the N-terminal or A/B domain, the C or DNA-binding domain, and the D/E/F or ligand-binding domain. In cell lines and some tissues, 17β-estrodial (E2β) elicits proliferation in the presence of ERα, but inhibits proliferation in presence of ERβ.1 Recently, loss of tumorigenesis was observed in ERβ transfected ER-positive breast cancer MCF7 cells (2). Therefore, ERα is considered to be responsible for the estrogen-stimulated cell proliferation while ERβ has protection activity. The binding of estrogen to ERα leads to receptor phosphorylation, dimerization, and recruitment of co-activators to the estrogen-bound receptor complex, which in turn binds the promoter regions of target genes via direct interaction with the DNA binding site known as estrogen response element (ERE) and activates transcription of target genes. Activated ERα can also transactivate additional target genes through protein-protein interaction with other transcription factors such as the Jun/Fos activator protein 1 (AP-1) and Sp1 (3, 4). Stimulation of target gene expression by ERα in response to estrogen is prevailingly thought to be responsible for estrogen-stimulated cell proliferation. Thus, targeting ERα itself and ERα-mediated mitogenic estrogen signaling provides an effective approach to treat or prevent ER-positive breast cancer.
Epidemiological and pharmacological evidences indicate that isothiocyanates (ITCs) have substantial chemopreventive activities against various types of cancer including breast cancer, lung cancer, bladder cancer, pancreatic cancer, and prostate cancer (5–10). ITCs exist as conjugates in the genus Brassica of cruciferous vegetables (e.g., cabbage, cauliflower, brussels sprouts, watercress, broccoli, mustard) and the genus Raphanus (radishes and daikons) (11, 12). More than 25 natural and synthetic ITCs have been demonstrated to block chemically induced carcinogenesis effectively by blocking carcinogen metabolic activation and enhancing carcinogen detoxification (13–16). In addition to their roles in cancer prevention, isothiocyanates also potently inhibit the malignant growth in cancer cells by inducing cell cycle arrest and apoptosis with little or no toxicity toward normal cells (17–19). Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC) (Figure 1) are two well studied ITCs for their inhibitory functions in different cancer cells. BITC and PEITC were potent in inducing apoptosis and activating caspases in bladder cancer cells (20). It was also reported that BITC effectively suppressed growth of human breast cancer cells by causing G2-M phase cell cycle arrest and apoptosis induction.7 Hence, Tseng et al also examined the potency of BITC and PEITC as chemotherapy reagents for human breast cancer cells (21).
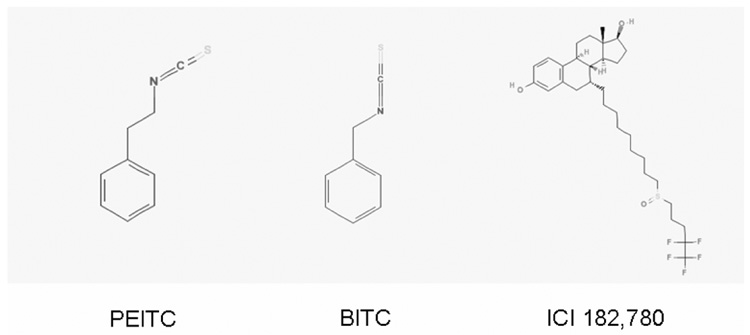
Figure 1. The chemical structures of phenethyl isothiocyanate (PEITC), benzyl (BITC), and ICI 182,780.
Recently, epidemiologic evidence indicated that dietary intake of dietary ITC- containing cruciferous vegetables may have preventive function against breast cancer development (9, 10). However, the molecular mechanisms by which dietary ITCs prevent human mammary carcinogenesis are not well established.
In the present study, we have demonstrated that two well studied ITCs, PEITC and BITC, significantly downregulate ERα expression, reduce ERα transcriptional activity and inhibit growth of ER-positive breast cancer MCF7 and T-47D cells.
Materials and methods
Reagents
PEITC and BITC were purchased from Sigma-Aldrich (St. Louis, MO, USA). ICI 182,780 was purchased from TOCRIS (Ellisville, MO, USA). The Fugene/HD transfection reagent was purchased from Roche Diagnostics (Indianapolis, IN, USA). The Luciferase Assay System was purchased from Promega Corporation (Madison, WI, USA). The ERα polyclonal antibody was obtained from Lab Vision Corporation (Fremont, CA, USA). The ERβ antibody, the actin antibody, the goat anti-rabbit IgG-HRP and the donkey anti-goat IgG-HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). MG-132 was purchased from CalBiochem (San Diego, CA, USA). The ECL Western Blotting Detection Reagents were purchased from GEHealthcare (Little Chalfont, Buckinghamshire, UK). The “Concert” cytoplasmic RNA purification reagent was from Invitrogen (Carlsbad, CA, USA), and the ProtoScript II RT-PCR kit was purchased from New England BioLabs (Ipswich, MA, USA).
Cell Culture
ER-positive breast cancer cell line, MCF7 and T-47D from ATCC (Manassas, VA, USA) were maintained in Improved Minimal Essential Medium (IMEM) from Invitrogen (Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% non-essential amino-acids, 1% HEPES buffer, 1% antibiotic-antimycotic from Invitrogen (Carlsbad, CA, USA), and 2µg/ml bovine insulin (Sigma, St. Louis, MO, USA) at 37°C under 5% CO2 in a humidified incubator.
Cell growth inhibition assays
MCF7 and T-47D cells were changed to medium containing 5% charcoal-dextran stripped FBS for two days, and then seeded in Φ35mm dishes at a density of 5×104 cells/dish in IMEM supplemented with 2.5% charcoal-dextran stripped FBS. After twenty-four hours, cells were treated with vehicle (DMSO) and different concentrations of PEITC, BITC or ICI 182,780 for 3 days. Cells were then trypsinized and counted with a hemocytometer. Three dishes were used for each concentration point and the experiments were repeated three times.
To test the effects of the ITCs on estrogen-stimulated growth, MCF7 and T-47D cells were changed to medium containing 5% charcoal-dextran stripped FBS for two days, and then seeded in Φ35mm dishes at a density of 5×104 cells/dish in IMEM containing 2.5% charcoal-dextran stripped FBS for another twenty-four hours. Cells were treated with 1nM of 17β–estradiol (E2) alone or together with different concentrations of PEITC, BITC or ICI 182,780 for 3 days. The cells were then trypsinized and counted with a hemocytometer.
Western blot analysis
Cells were washed with PBS twice and lysed with RIPA buffer with 1% proteinase inhibitor cocktail solution and 1% phosphatase inhibitor cocktail solution (Sigma, St. Louis, MO, USA). The cell lysates were boiled for 5 minutes in SDS gel-loading buffer and separated on a 10% SDS-PAGE gel. After electrophoresis, the proteins were transferred to a PVDF membrane (BioRad Laboratories, Hercules, CA, USA). The membranes were probed with appropriate primary antibodies and visualized with corresponding secondary antibodies and the ECL Western Blotting Detection Reagents. The same membranes were stripped and reprobed with an antibody against β-actin as loading controls.
DNA transfection and luciferase assay
MCF7 and T-47D cells maintained in IMEM medium containing 10% FBS were changed to medium containing 5% charcoal-dextran stripped FBS for two days, and then were seeded in Φ35mm dishes at a density of 2×105 cells/dish for another day. Cells were transfected with the p2×ERE-Luc reporter plasmid (a kind gift from Dr. Katarine Pettersson at Karolinska Institute, Sweden) and a β-galactosidase expression plasmid, pCMV-β (Clontech Laboratories, Inc. Palo Alto, CA), to establish transfection efficiency. Twelve hours after transfection, cells were treated with 1 nM of E2 alone or together with different concentrations of PEITC, BITC or ICI 182,780. Twenty-four hours after transfection, cell extracts were prepared and aliquots were normalized for transfection efficiency by assay of β-galactosidase activity. Luciferase assays were performed using the luciferase reporter system in a TD 20/20 Luminometer as instructed by the manufacture.
RNA purification and RT-PCR
To examine the downregulation of ERα mRNA by ITCs, MCF7 and T-47D cells cultured in IMEM containing 2.5% charcoal-dextran stripped FBS were treated with different concentrations of PEITC, BITC or ICI 182,780 for 12 hours. To assess the effects of ITCs on estrogen-stimulated gene expression, MCF7 and T-47D cells maintained in medium containing 2.5% charcoal-dextran stripped FBS were treated with 1 nM of E2β alone or together with different concentrations of PEITC, BITC or ICI 182,780 for 12 hours. Total RNA was prepared with the “Concert” cytoplasmic RNA purification reagent. One µg of total RNA was reverse transcribed using ProtoScript II RT-PCR kit with random primers. The reverse transcription reaction was performed at 42°C for 1 hour. Semi-quantitative RT-PCR of ERα , β-Actin and pS2 were done using gene specific primers synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA). The following are the primer sequences for ERα, β-actin and pS2.
ERα: forward primer: 5’- CAC TCA ACA GCG TGT CTC CGA-3’; reverse primer: 5’- CCAATC TTT CTC TGC CAC CCT G-3’. β-actin: forward primer: 5’-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3’; reverse primer: CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3’. pS2: forward primer: 5’- TGG AGA ACA AGG TGA TCT GC-3’; reverse primer: 5’- ATC TGT GTT GTG AGC CGAGG-3’.
The procedure of PCR for ERα and β-actin was carried out as following: first a denaturing at 95°C for 1 minute, then the remaining PCR was performed at 94 °C for 30 seconds, 58 °C for 30 seconds, and 68 °C for 1 minute (35 cycles for ERα, and 25 cycles for β-actin). PCR for pS2 was started with a denaturing at 94°C for 3 minute, then 94 °C for 45 seconds, 55 °C for 45 seconds, and 72 °C for 45 seconds (30 cycles). At the end, there was a final elongation at 72 °C for 7 minutes. PCR products were analyzed by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining under UV illumination.
Statistical analysis
Data were summarized as the mean ± standard error (SE) using GraphPad InStat software program. Tukey-Kramer Multiple Comparisons Test was used, and the significance was accepted for P values of less than 0.05.
Results
PEITC and BITC repress ERα expression in MCF7 and T-47D cells
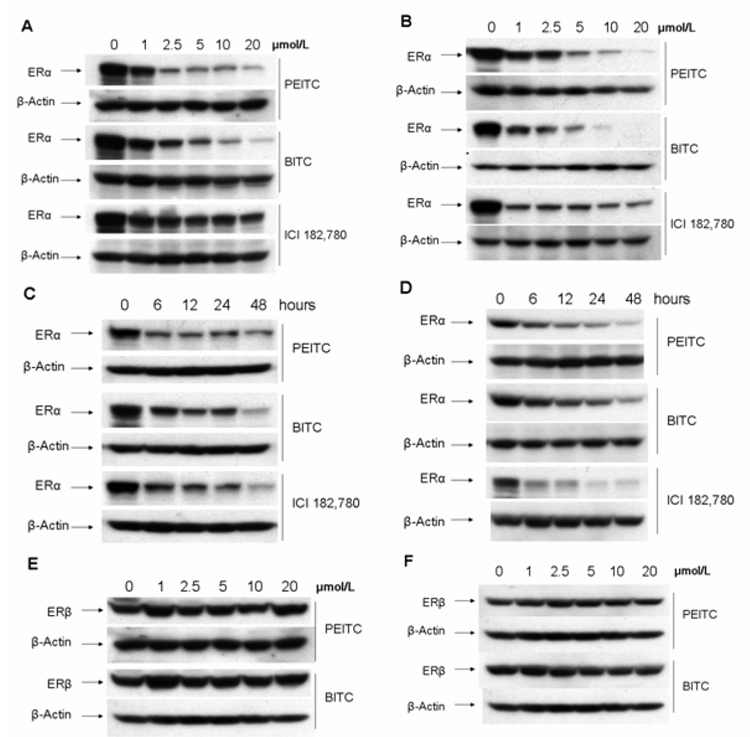
MCF7 and T-47D cells express high levels of ERα and strongly response to mitogenic estrogen stimulation. Previously, it was reported that BITC and PEITC are potent growth inhibitors in breast cancer MCF7 cells (21), and PEITC repressed the expression of androgen receptor and inhibited growth of prostate cancer cells (22). Hence, we decided to examine the ERα expression in PEITC-or BITC-treated MCF7 and T-47D cells. Cells were incubated with different concentrations of PEITC or BITC for 12 hours. Western blot analysis was performed to assess ERα expression. We found that the expression levels of ERα were dramatically reduced in PEITC- or BITC-treated MCF7 and T-47D cells in a dose-dependent manner (Figure 2 A, B). The well-known estrogen receptor disruptor, ICI 182,780 (Figure 1) was also able to reduce levels of ERα expression but with much less efficiency compared to both BITC and PEITC at 12 hours under higher concentrations (Figure 2 A, B). Furthermore, the expression levels of ERα in MCF7 and T-47D cells treated with 10µM of PEITC or BITC for different time-points were also examined (Figure 2 C, D). ERα expression levels were started to reduce after treatment of PEITC and BITC for 6 hours, and continued to decrease to a very low level at 48 hours (Figure 2 C, D). As a positive control, ICI 182,780 treatment for 48 hours, exhibited a strong reduction of ERα expression to a level comparable to the levels in cells treated with PEITC or BITC (Figure 2 C, D). However, both PEITC and BITC had no effect on ERβ expression (Figure 2 E, F).
Figure 2. PEITC and BITC reduce the expression level of ERα protein as demonstrated by Western blot analysis.
MCF7 (A) and T-47D (B) cells were treated with vehicle (DMSO, 0), 1, 2.5, 5, 10, and 20µM of PEITC, BITC or ICI 182,780 for 12 hours and lysed for Western blot analysis with anti-ERα and anti-β-actin antibodies.
MCF7 (C) and T-47D (D) cells were treated with 10µM of PEITC, BITC or ICI 182,780 for indicated time points, and lysed for Western blot analysis with anti-ERα and anti-β-actin antibodies.
MCF7 (E) and T-47D (F) cells were treated with vehicle (DMSO, 0), 1, 2.5, 5, 10, and 20µM of PEITC or BITC for 12 hours, and lysed for Western blot analysis with anti-ERβ and anti-β-actin antibodies.
PEITC and BITC repress ERα expression at the transcription level
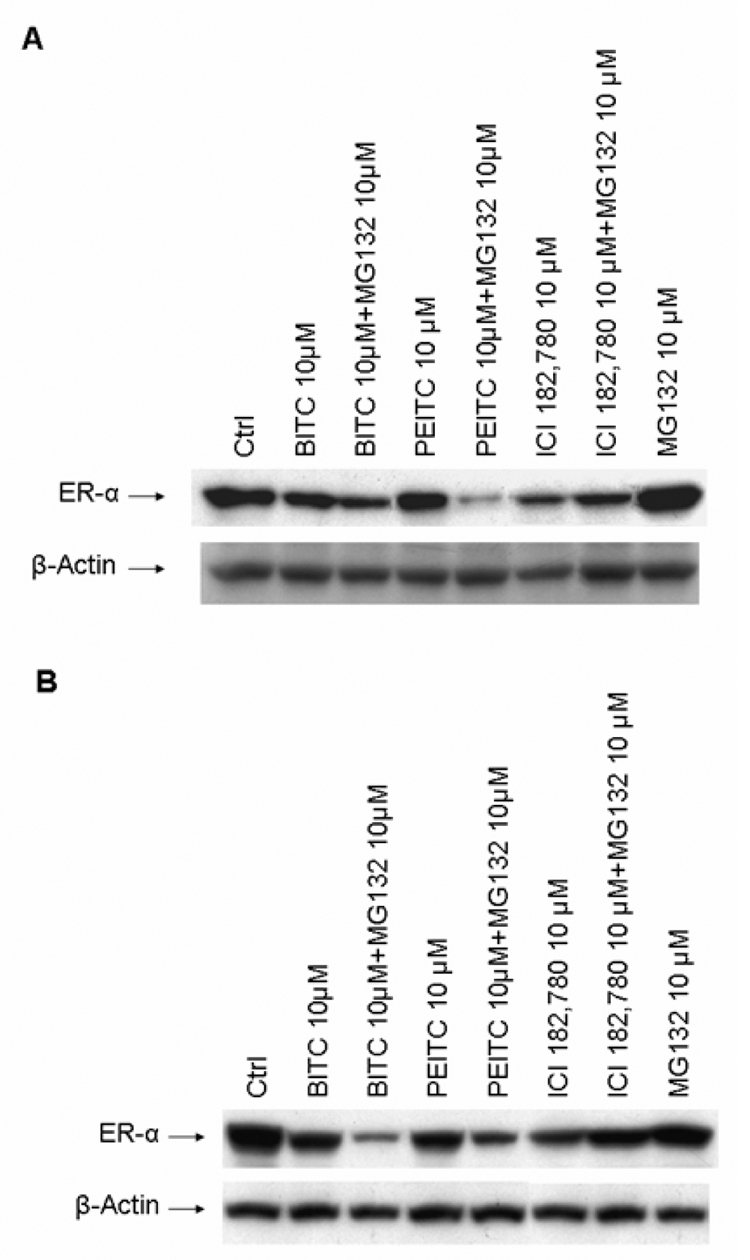
It is well established that ICI 182,780 triggers the degradation of ERα protein through proteasome-dependent proteolysis (23). We decided to test whether the downregulation of ERα protein by BITC and PEITC was via the same mechanism. MCF7 and T-47D cells were treated with 10µM of BITC or PEITC alone or together with proteasome inhibitor MG-132 10µM respectively for 12 hours. Western blot analysis revealed that proteasome inhibitor MG-132 was unable to reverse the expression levels of ERα protein downregulated by PEITC and BITC (Figure 3 A, B). On the contrary, a further reduction of ERα protein expression was observed in the cells treated with ITCs and MG-132 (Figure 3 A, B). However, the proteasome inhibitor MG132 efficiently blocked ICI 182,780 induced downregulation of ERα protein (Figure 3 A, B), consistent with the previous reports that ICI 182,780 induces degradation of ERα protein through the proteasome system (23, 24). Our results thus suggested that PEITC and BITC functions through a different mechanism to repress ERα expression.
Figure 3. Effects of proteasome inhibitors on ERα down-regulation induced by PEITC, BITC or ICI 182,780 in MCF7 and T-47D cells.
A. MCF7 cells were incubated for 12 hours in the vehicle (DMSO, Ctrl), 10 µM PEITC, BITC or ICI 18,780 with or without MG 132 10 µM and lysed for Western blot analysis with anti-ERα and anti-β-actin antibodies.
B. T-47D cells were incubated for 12 hours in the vehicle (DMSO, Ctrl), 10 µM PEITC, BITC or ICI 18,780 with or without MG 132 10 µM and lysed for Western blot analysis with anti-ERα and anti-β-actin antibodies.
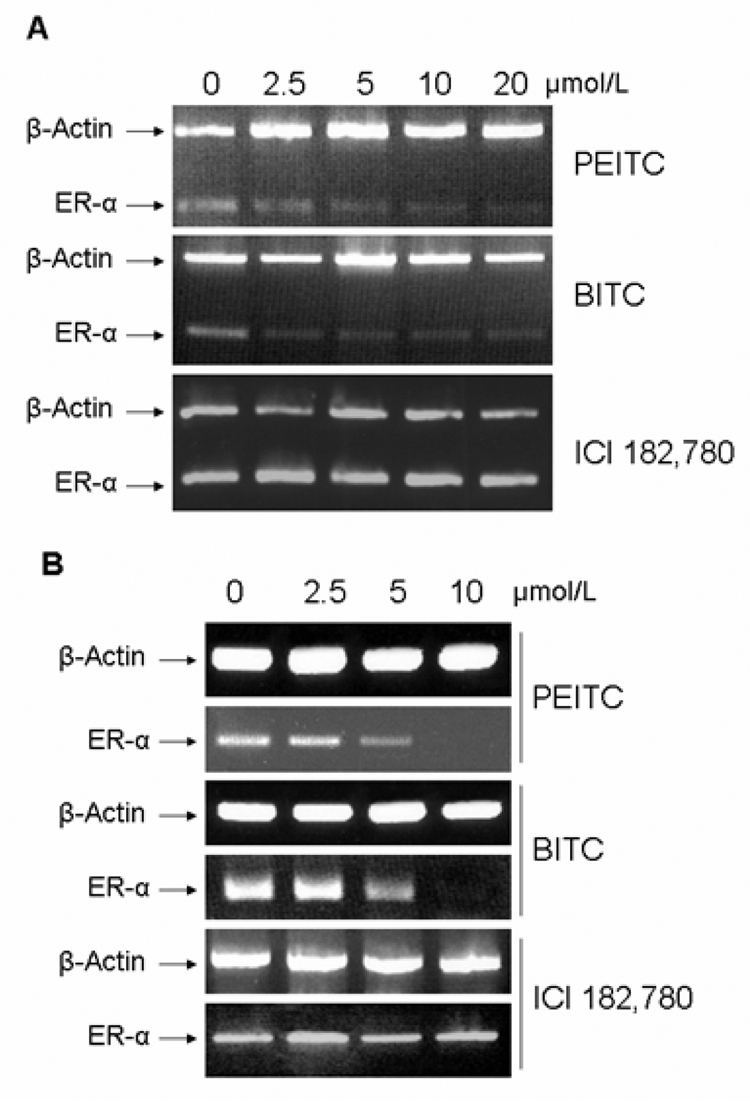
We then examined the mRNA levels of ERα in MCF7 and T-47D cells treated with different concentrations of PEITC or BITC for 12 hours by RT-PCR analysis. We found that mRNA levels of ERα were dramatically reduced after the treatment of PEITC or BITC starting at 5 µM (Figure 4 A, B), indicating that the suppression of ERα expression by PEITC and BITC occurs at the transcription level. While in ICI 182,780 treated cells, no change of ERα mRNA level was observed (Figure 4 A, B), consistent with the previous report that ICI 182,780 has no effect on ERα transcription (23).
Figure 4. Effects of PEITC, BITC or ICI 182,780 on the mRNA levels of ERα.
MCF7 (A) and T-47D (B) cells were incubated for 12 hours in vehicle (DMSO, 0), 2.5, 5, 10, and 20 µM of PEITC, BITC or ICI 182,780. Total RNA were extracted and 1 µg of total RNA were used for semi-quantitative RT-PCR using specific primers for ERα and β-actin as described in methods. RT-PCR products were separated by 1.5% agarose gels and stained with ethidium bromide.
Downregulation of ERα expression by PEITC and BITC is reversible
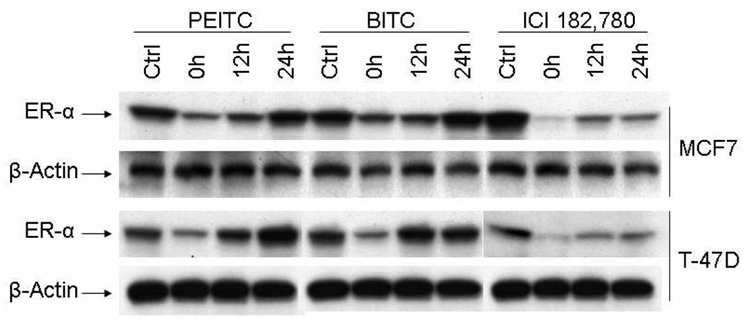
To further determine if the down-regulation of ERα by PEITC and BITC is a reversible process, the MCF7 and T-47D cells were treated with 10µM of PEITC or BITC for 12 hours, then washed with PBS twice and changed to normal medium without PEITC and BITC. The cells were harvested at different time points after PEITC and BITC withdrawal and examined for ERα expression with Western blot analysis. The levels of ERα expression were almost fully recovered after 24 hours (Figure 5), indicating the suppression activities of PEITC and BITC is reversible. However, ERα downregulation mediated by ICI 182,780 was not fully recovered by 24 hours (Figure 5).
Figure 5. PEITC and BITC inhibitory effects on ERα expression are reversible.
MCF7 and T-47D cells were incubated for 12 hours in the absence (Ctrl) or presence of 10 µM PEITC, BITC or ICI 182,780. Then the medium were removed, washed cells with PBS twice, and replaced with fresh medium. Cells were then harvested after different time intervals, Western blot analysis were then performed with anti-ERα and anti-β-actin antibodies.
PEITC and BITC inhibit ERα transcriptional activity
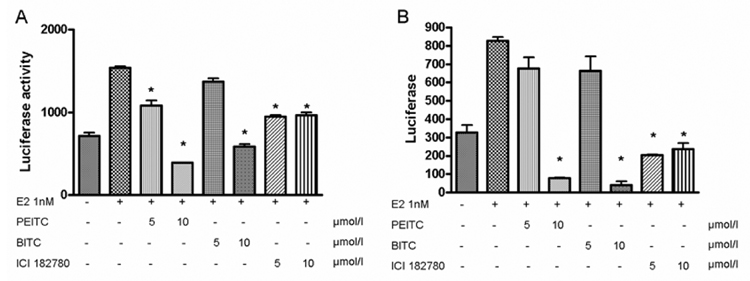
To further determine if PEITC and BITC would inhibit transcriptional signaling mediated by ERα. Transient transfection assays were conducted in MCF7 and T-47D cells using a luciferase reporter plasmid containing two copies of ERE binding sites. We found that E2β induced the luciferase reporter activity about 3 folds, which was then attenuated by PEITC or BITC in a dose-dependent manner (Figure 6 A, B). As a positive control, the pure antagonist ICI 182,780 repressed the luciferase activity induced by estrogen treatment (Figure 6 A,B).
Figure 6. PEITC and BITC inhibit ERα transcriptional activity.
MCF7 (A) and T-47D (B) cells maintained in IMEM containing 2.5% charcoal-stripped FBS were transfected with the 2×ERE-TK-Luc plasmid and treated with vehicle (DMSO), 1nM of 17β-estradiol (E2 1nM) or together with 5 and 10 µM of PEITC, BITC or ICI 182,780, respectively for 12 hours. Cells were harvested for luciferase assay. The data were based on the mean of 3 independent experiments with the mean ± SE shown. * P<0.001 (compare to the E2 1nM group).
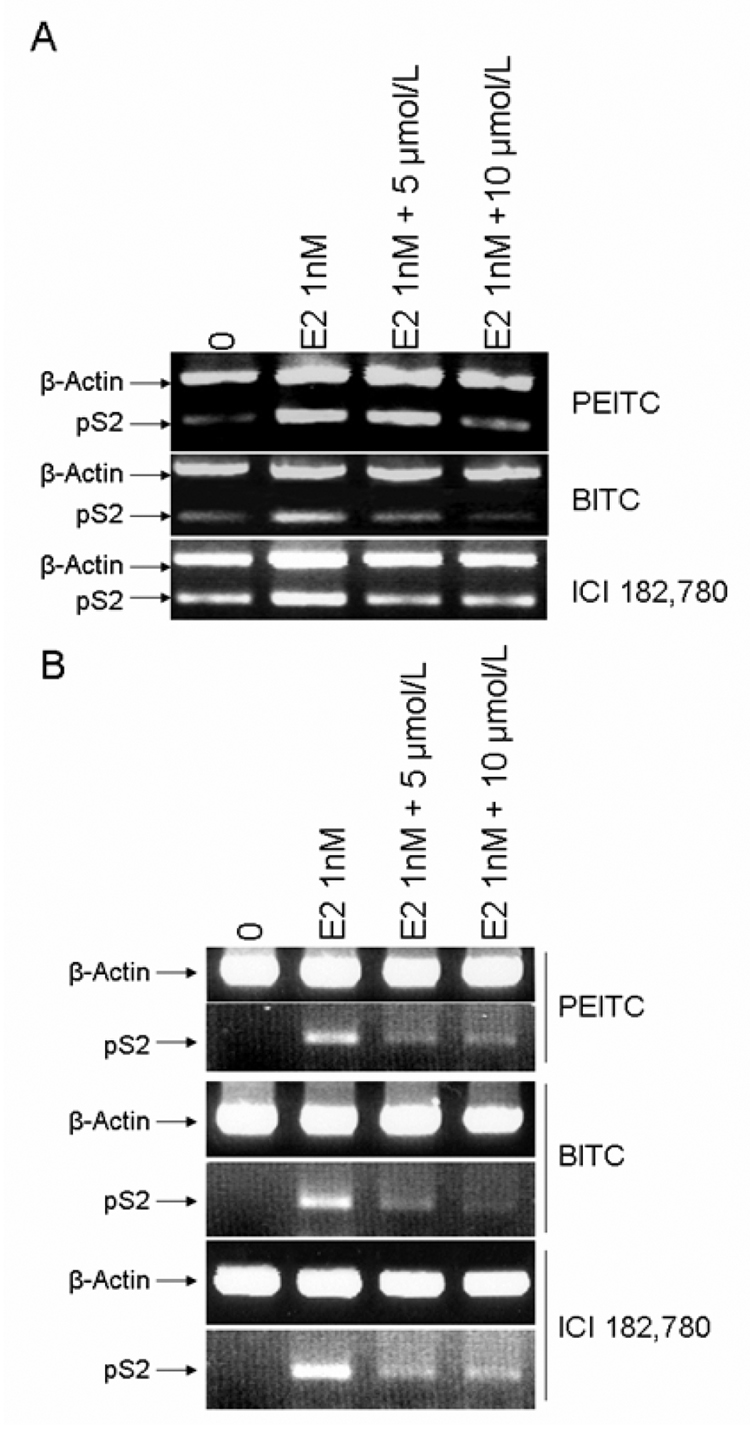
We then examined the effects of PEITC and BITC on the estrogen-induced expression of pS2, an ERα targeting gene, in MCF7 and T-47D cells using RT-PCR analysis. We found that E2β at 1 nM induced pS2 expression in MCF7 and T-47D cells, which was strongly inhibited by both PEITC and BITC (Figure 7 A, B). ICI 182,780 also antagonized the stimulatory effect of E2 in MCF7 and T-47D cells (Figure 7 A, B). These results strongly indicated that PEITC and BITC are able to function as potent estrogen antagonists as the pure antiestrogen ICI 182,780.
Figure 7. Effects of PEITC, BITC or ICI 182,780 on ERα targeting gene pS2.
MCF7 (A) and T-47D (B) cells maintained in IMEM with 2.5% charcoal-stripped FBS were treated with vehicle (DMSO), 1nM of 17β-estradiol (E2 1nM) alone or together with 5µM and 10µM of PEITC, BITC or ICI 182,780 for 12 hours. Total RNA was extracted and 1 µg of total RNA was used for semi-quantitative RT-PCR using specific primers for pS2 and β-actin. RT-PCR products were separated in 1.5% agarose gels and stained with ethidium bromide.
Discussion
A number of studies have shown that ERα expression is elevated from very early stages of mammary carcinogenesis and thus is a risk factor for progression to invasive breast cancer (25–27). The ERα expression level in ER-positive tumors is greatly increased as compared to the neighboring normal tissues (28). This strong correlation between ERα expression and breast cancer development had made ERα an important target for drug development.
Antiestrogens designed to target ERα can be classified into two major groups: analogs of tamoxifen and its metabolites such as 4-hydroxytamoxifen that have mixed estrogenic/antiestrogenic actions, and pure antiestrogens such as ICI 182,780. Tamoxifen is commonly used for adjuvant therapy of breast cancer and is a key drug for breast cancer chemoprevention in high-risk women. ICI 182,780 impairs ERα dimerization and inhibits nuclear localization of ER (29, 30). In addition, ICI 182,780 also accelerates degradation of the ERα protein without a reduction of ERα mRNA level (24). Thus, ICI 182,780 functions as an estrogen receptor disruptor to enhance degradation of ERα protein, resulting in an inhibition of mitogenic estrogen signaling.
In this report, we have demonstrated that PEITC and BITC, two well-studied ITCs, strongly suppress ERα expression. The exposure of ER-positive breast cancer MCF7 and T-47D cells to PEITC or BITC resulted in a reduced level of ERα expression as demonstrated by Western blot analysis. The ERα expression was down-regulated by PEITC or BITC at a level comparable to the levels in cells treated with the pure estrogen antagonist ICI 182,780. We also found that proteasome inhibitor MG132 failed to reverse the downregulation of ERα expression mediated by PEITC and BITC while efficiently restored the levels of ERα protein expression downregulated by ICI 182,780. Thus, our results demonstrated that PEITC and BITC downregulate ERα expression through a mechanism other than enhanced protein degradation through the proteasome system. A similar phenomenon was reported in the downregulation of androgen receptor (AR) expression mediated by PEITC in prostate cancer cells; MG132 could not reverse the AR downregulation after a 24 hours’ incubation with PEITC (22). This result together with ours suggested that PEITC and BITC may function at the transcriptional level to negatively regulate gene expression.
We then used semi-quantitative RT-PCR analysis to demonstrate that PEITC and BITC could reduce the ERα transcripts in ER-positive breast cancer cells while ICI 182,780 had no effect on ERα mRNA as reported before (23,24). Thus, PEITC and BITC function as a negative regulator of ERα transcription. However, the molecular mechanism by which PEITC and BITC inhibit ERα transcription was not clear right now. Recently, it was reported that PEITC repress AR expression in prostate cancer cells at the transcriptional level through inhibition of the transcription factor Sp1 expression (22). There are several Sp1 binding sites in the proximal promoter region of ERα that are essential for maximum ERα transcription (31). It is thus possible that PEITC or BITC also inhibits Sp1 expression in ER-positive breast cancer MCF7 and T-47D cells, which then in turn attenuates ERα transcription. In addition, it was reported that ERα can activate transcription via formation of an ER-Sp1 complex on the GC-rich Sp1 binding sites in the promoter region of target genes and ERα enhances Sp1 DNA binding in a hormone independent manner (32). In ERα promoter region, the formation of a multi-protein complex composed of Sp1, ERα and upstream stimulating factor 1 (USF-1) is essential for optimum ERα transcription (31). ERα might autoregulate the expression of itself through interaction with Sp1 bound to its promoter. Downregulation of both ERα and Sp1 by PEITC and BITC may abrogate this positive feedback of ERα transcription regulation and further downregulate ERα promoter activity.
As a result of ERα downregulation, the transcriptional activities of ERα were reduced in ER-positive breast cancer MCF7 and T-47D cells treated with PEITC or BITC. Estrogen stimulated promoter activity of the ERE containing reporter plasmid and expression of the endogenous ERα targeting gene pS2 were dramatically reduced in the presence of PEITC or BITC. These results further confirm that PEITC and BITC repress ERα expression and its transcriptional activities. These data also indicate that PEITC and BITC may function as an inhibitor of the mitogenic estrogen signaling.
In humans, the maximal plasma concentration of PEITC after ingestion of 100g watercress can reach as high as 1155 nM (ranges between 673 and 1155 nM, mean 928.5±250.7 nM) within 1.5 hours (ranges between 1.5 and 4 hours, mean 2.6±1.1 hours) (33), and 1.04±0.22 µM concentration can be reached after a single 40-mg dose of PEITC (34). In animal experiments, the maximal plasma concentration of PEITC can reach 9.2±0.6 µM or 42.1±11.4 µM after doses of 10 µmol/kg and 100 µmol/kg in rats (35). Therefore, the PEITC and BITC concentrations required to produce statistically significant inhibition of ER-α expression in human breast cancer cells may be achievable in vivo.
It was reported that BITC induces the G2-M phase cell cycle arrest and cell apoptosis in ER-negative breast cancer MDA-MB-231 cells and ER-positive breast cancer MCF7 cells (7). The cell cycle arrest was associated with a decrease in expression levels of proteins involved in regulation of G2-M transition, including cyclin B1, cyclin-dependent kinase 1, and cell division cycle 25C (7). The apoptosis correlated with induction of proapoptotic proteins Bax and Bak, and downregulation of anti-apoptotic proteins Bcl-2 and Bcl-xL (7). It was also reported that ITC treatment causes mitochondrial damage and caspase-9 activation (8). PEITC could also downregulate JNK-specific phosphatase and function to activate JNK through suppression of JNK dephosphorylation (36). Thus, ITCs such as PEITC and BITC may regulate many cellular processes that acts together to result in growth inhibition and apoptosis induction. However, the cytotoxic effects of PEITC and BITC could not account for the downregulated levels of ERα expression observed, since the doses of PEITC and BITC that effectively suppress ERα expression did not promote significant cell death in 12 hours. Moreover, because ERα expression was totally restored after the withdrawal of PEITC and BITC for 24 hours, the possibility that the apoptotic cell death induced by PEITC and BITC contributed to the down-regulation of ERα expression could be excluded.
Our findings, that the reversible downregulation of ERα expression and mitogenic estrogen signaling by PEITC and BITC revealed that another important signaling pathway that is influenced by ITCs. Our experimental results thus strongly support the idea that the ITCs from cruciferous vegetables are important dietary factors for breast cancer chemoprevention.
Acknowledgement
This work was supported by NIH grant DK070016 (Z.Y. Wang).
References
- 1.Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod. 2005;73:866–871. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- 2.Behrens D, Gill JH, Fichtner I. Loss of tumorigenicity of stably ERβ-transfected MCF7 breast cancer cells. Mol Cell Endocrinol. 2007;274:19–29. doi: 10.1016/j.mce.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 4.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 5.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiology Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 7.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 8.Tang L, Zhang Y. Mitochondria are the primary target in isothiocyanate-induced apoptosis in human bladder cancer cells. Mol Cancer Ther. 2005;4:1250–1259. doi: 10.1158/1535-7163.MCT-05-0041. [DOI] [PubMed] [Google Scholar]
- 9.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, Brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- 10.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast Cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 11.Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 12.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomark Prev. 1999;8:447–451. [PubMed] [Google Scholar]
- 13.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahéo K, Morel F, Langouët S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochrome P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- 16.Dick RA, Kensler TW. Chemoprotective potential of phase 2 enzyme inducers. Expert Rev Anticancer Ther. 2002;2:581–592. doi: 10.1586/14737140.2.5.581. [DOI] [PubMed] [Google Scholar]
- 17.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 18.Hu R, Kim BR, Chen C, Hebbar C, Kong AN. The role of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cell. Carcinogenesis. 2003;24:1361–1367. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava SK, Singh SV. Cell cycle arrest and apoptosis-induced by benzyl isothiocyanate are associated with inhibition of nuclear factor kappa B activation in human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- 20.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 21.Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and Mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood) 2004;229:835–842. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- 22.Wang LG, Liu XM, Chiao JW. Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis. 2006;27:2124–2132. doi: 10.1093/carcin/bgl075. [DOI] [PubMed] [Google Scholar]
- 23.Preisler-Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor-alpha. Am J Physiol Endocrinol Metab. 2002;282:E891–E898. doi: 10.1152/ajpendo.00353.2001. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson RI, Gee JM, Manning DL, Wakeling AE, Montano MM, Katzenellenbogen BS. Responses to pure antiestrogens (ICI 164384, ICI 182780) in estrogen-sensitive and -resistant experimental and clinical breast cancer. Ann NY Acad Sci. 1995;761:148–163. doi: 10.1111/j.1749-6632.1995.tb31376.x. [DOI] [PubMed] [Google Scholar]
- 25.Khan SA, Rogers MA, Obando JA, Tamsen A. Estrogen receptor expression of benign breast epithelium and its association with breast cancer. Cancer Res. 1994;54:993–997. [PubMed] [Google Scholar]
- 26.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst. 1998;90:37–42. doi: 10.1093/jnci/90.1.37. [see comments]. [DOI] [PubMed] [Google Scholar]
- 27.Allegra JC, Lippman ME, Green L, Barlock A, Simon R, Thompson EB, Huff KK, Griffin W. Estrogen receptor values in patients with benign breast disease. Cancer. 1979;44:228–231. doi: 10.1002/1097-0142(197907)44:1<228::aid-cncr2820440137>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 28.van Agthoven T, Timmermans M, Dorssers LC, Henzen-Logmans SC. Expression of estrogen, progersterone and epidermal growth factor receptors in primary and metastatic breast cancer. Int J Cancer. 1995;63:790–793. doi: 10.1002/ijc.2910630607. [DOI] [PubMed] [Google Scholar]
- 29.Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG. Inhibition of estrogen receptor-DNA binding by the "pure" antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci USA. 1990;87:6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106:1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- 31.deGraffenried LA, Hopp TA, Valente AJ, Clark RA, Fuqua SA. Regulation of the estrogen receptor alpha minimal promoter by Sp1, USF-1 and ER alpha. Breast Cancer Res Treat. 2004;85:111–120. doi: 10.1023/B:BREA.0000025398.93829.78. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 33.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem. 2003;323(1):39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Liebes L, Conaway CC, Hochster H, Mendoza S, Hecht SS, Crowell J, Chung FL. High-performance liquid chromatography-based determination of total isothiocyanate levels in human plasma: application to studies with 2-phenethyl isothiocyanate. Anal Biochem. 2001;291(2):279–289. doi: 10.1006/abio.2001.5030. [DOI] [PubMed] [Google Scholar]
- 35.Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharm Res. 2005;22(10):1658–1666. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 36.Chen YR, Han J, Kori R, Kong AN, Tan TH. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem. 2002;277:39334–39342. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]