Abstract
Although safe in most cases, ancient treatments are ignored because neither their active component nor their molecular targets are well defined. This is not the case, however, with curcumin, a yellow-pigment substance and component of turmeric (Curcuma longa), which was identified more than a century ago. For centuries it has been known that turmeric exhibits anti-inflammatory activity, but extensive research performed within the past two decades has shown that the this activity of turmeric is due to curcumin, a diferuloylmethane. This agent has been shown to regulate numerous transcription factors, cytokines, protein kinases, adhesion molecules, redox status and enzymes that have been linked to inflammation. The process of inflammation has been shown to play a major role in most chronic illnesses, including neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. In the current review, we provide evidence for the potential role of curcumin in the prevention and treatment of various pro-inflammatory chronic diseases. These features, combined with the pharmacological safety and negligible cost, render curcumin an attractive agent to explore further.
Keywords: Curcumin, NSAIDs, Diabetes, Inflammation, Arthritis, Allergy, CVDs, Psoriasis
1. Introduction
Within the past half-century, there has been a major breakthrough in our understanding of the cellular, molecular, genetic, and biochemical mechanisms of most chronic diseases. The discovery of growth factors, hormones, and cytokines; their receptors; protein kinases; and transcription factors have provided the basis for signal transduction at the cellular level. How these signals mediate different diseases, has also become apparent. It is now common knowledge that the products of approximately 25,000 different genes regulate the human body and that most diseases are caused by dysregulation of multiple gene products. Using microarray technology, it has been estimated that as many as 300–500 different genes may control any given chronic illness. Until now, few of these genes have been targeted for therapy. Tumor necrosis factor (TNF), cyclo-oxygenase 2 (COX-2) inhibitor, vascular epithelial growth factor (VEGF), CD20, and epidermal growth factor receptor are perhaps the best-known examples (Aggarwal et al., 2007). Another intriguing revelation is that most chronic illnesses are caused by dysregulated inflammation. For instance, inflammation has been found to play a major role in cancer, cardiovascular diseases (CVDs), pulmonary diseases, metabolic diseases, neurologic diseases, and even psychological diseases (Aggarwal et al., 2006a); (Hansson et al., 2006); (Garodia et al., 2007) (Khanna et al., 2007) (Libby, 2007) (Odrowaz-Sypniewska, 2007) (Robinson et al., 2007) (Selmi et al., 2007) (Packard and Libby, 2008) (Hold and El-Omar, 2008) (Dantzer et al., 2008).
Almost 2 decades ago, our laboratory was the first to isolate 2 different cytokines (TNF-α and TNF-β) as antitumor agents (Aggarwal et al., 1985a) (Aggarwal et al., 1985b). It has now become clear, however, that TNF-α is a major mediator of inflammation in most diseases, and this effect is regulated by the activation of a transcription factor, nuclear factor (NF)-κB. Whereas TNF is the most potent NF-κB activator yet described, the expression of TNF-α is also regulated by NF-κB (Aggarwal, 2003). Besides TNF, NF-κB is activated by most inflammatory cytokines; gram-negative bacteria; various disease-causing viruses; environmental pollutants; chemical, physical, mechanical, and psychological stress; high glucose; fatty acids; ultraviolet radiation; cigarette smoke; and other disease-causing factors (Aggarwal, 2004) (Kumar et al., 2004) (Sethi et al., 2008); (Tergaonkar, 2006) (Karin and Greten, 2005) (Ahn and Aggarwal, 2005). Interestingly, most mediators of inflammation that have been identified up to now are also regulated by NF-κB, including inflammatory cytokines, chemokines, adhesion molecules, enzymes, and kinases (see Fig. 1). Thus, NF-κB and NF-κB–regulated gene products have been closely linked with most chronic illnesses. Therefore, agents that downregulate NF-κB and NF-κB–regulated gene products have potential efficacy against several of these diseases.
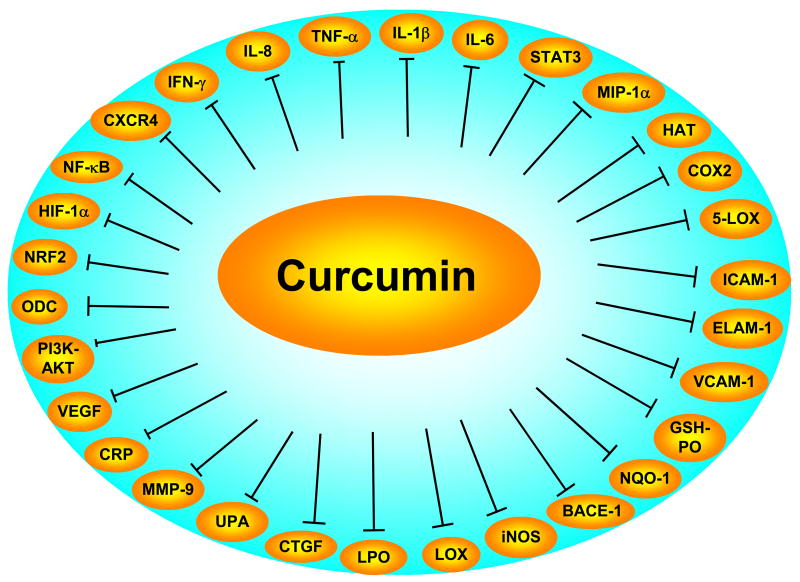
Fig. 1.
Inhibition of inflammatory pathways by curcumin.
BACE-1, beta-site APP-cleaving enzyme 1; CRP, C-reactive protein; CTGF, connective tissue growth factor; ELAM-1, endothelial leukocyte adhesion molecule-1; HAT, histone acetyl transferase; HIF, hypoxia inducible factor; ICAM-1, intracellular adhesion molecule-1; LPO, lipid peroxidation; MMP, matrix metalloprotease ; NF-κB, nuclear factor kappa B; ODC, ornithine decarboxylase; STAT, signal transducers and activator of transcription protein; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
Suppression of NF-κB activation is a topic actively being pursued in the academic and industrial settings. Our laboratory was the first to demonstrate that curcumin is a potent blocker of NF-κB activation induced by different inflammatory stimuli (Singh and Aggarwal, 1995). We and others subsequently showed that curcumin blocks NF-κB activation through inhibition of IκBα kinase and AKT (Aggarwal et al., 2005) (Shishodia et al., 2005) (Siwak et al., 2005) (Aggarwal et al., 2006c) (Kamat et al., 2007) (Deeb et al., 2007) (Aoki et al., 2007), thus resulting in the suppression of NF-κB–dependent gene products that suppress apoptosis and mediate proliferation, invasion, and angiogenesis. Our laboratory more recently showed that curcumin also suppresses NF-κB activation in most tumor cells, leading to suppression of anti-apoptotic proteins and resulting in apoptosis (Aggarwal et al., 2004); (Kunnumakkara et al., 2007). We also showed that curcumin could downregulate the expression of interleukin (IL)-6 protein, TNF, and various other chemokines (Jagetia and Aggarwal, 2007). Abe et al (Abe et al., 1999) showed that curcumin inhibited the production of IL-8, MIP-1α, MCP-1, IL-1α, and TNF-α induced by inflammatory stimuli in human peripheral blood monocytes and alveolar macrophages. We and others subsequently showed that curcumin downregulates the expression of the NF-κB –regulated gene products such as COX-2, TNF, 5-LOX, IL-1, IL-6, IL-8, MIP-1α, adhesion molecules, c-reactive protein (CRP), CXCR-4, and others (Skommer et al., 2007) (Shakibaei et al., 2005) (Shishodia et al., 2005) (Li et al., 2004) (see Fig. 1). Curcumin has also been reported to bind to COX-2 and 5-LOX and to inhibit their activity (Hong et al, 2004). Recent work from our laboratory has shown that curcumin directly binds to IkBα kinase needed for NF-κB activation (Aggarwal et al., 2006c). Our laboratory was the first to demonstrate that curcumin is a potent inhibitor of STAT 3, another transcription factor through which proinflammatory cytokine IL-6 mediates its effects (Bharti et al., 2003a). Thus curcumin could suppress inflammation through multiple pathways.
The effect of curcumin against various pro-inflammatory diseases is discussed in detail in this report.
2. Role of Curcumin in the Treatment of Chronic Inflammatory Diseases
In various chronic illnesses in which inflammation is known to play a major role, curcumin has been shown to exhibit therapeutic potential. These diseases include Alzheimer’s disease (AD), Parkinson’s disease, multiple sclerosis, epilepsy, cerebral injury, CVDs, cancer, allergy, asthma, bronchitis, colitis, rheumatoid arthritis, renal ischemia, psoriasis, diabetes, obesity, depression, fatigue, and AIDS (see Table 1). How curcumin mediates its activity against these diseases is described in this section.
Table 1.
Effect of curcumin on neurodegenerative, cardiovascular, neoplastic, pulmonary, metabolic, and autoimmune diseases
| Disease | Dose | Effects | References |
|---|---|---|---|
| Neurodegenerative diseases | |||
| Alzheimer Disease: | |||
| In vitro | Protects nerve cells and EC from A beta-induced cytotoxicity. | Kim et al, 2001 | |
| In vitro | 0.1–1μM | Inhibits A beta fibril formation | Ono et al, 2004 |
| In vitro | Curcumin interacts with Cu and Fe | Baum et al, 2004 | |
| In vitro | 0.1–10μM | Inhibits the peroxidase activity of A beta-heme complex | Atamna et al, 2006 |
| In vitro | 0.1μM | Enhances uptake of A beta by macrophages from AD | Zhang et al, 2006 |
| In vitro | 0.1μM | Enhances Abeta uptake by increasing exp of MGAT3 and TLRs | Fiala et al, 2007 |
| Tg mice | 160, 5000 ppm, diet | Reduces oxidative damage and oxidative pathology | Lim et al, 2001 |
| Tg mice | 500 ppm, diet, 50μM, i.v | Inhibits A beta oligomers & fibrils, binds plaques and reduces amyloid1 | Yang et al, 2005 |
| Mice | 7.5, i.v | Clears and reduces existing plaques; reversed changes in dystrophic dendrities, abnormal curvature and dystrophy size | Garcia-Alloza et al, 2007 |
| Patients | Capsule or diet | No side effects, increased the level of A beta in serum | Baum et al, 2008 |
| Multiple sclerosis: | |||
| In vitro | Modulates IFN-β and IL-12 signaling | Fahey et al, 2007 | |
| Mice | 50,100μg, i.p | Decreased EAE and IL-12 production, Inhibited IL-12 induced JAK2 and TYK2 phosphorylation | Natarajan et al, 2002 |
| Mice | 2.5 mg/mL, d.w | Delays recovery from EAE | Verbeek et al, 2005 |
| Parkinson Disease: | |||
| In vitro | 50μM | Reverses peroxynitrate mediated inhibition brain mitochondria complex I | Mythri et al, 2007 |
| Rats | 50mg/kg, p.o | Attenuated the loss of dopaminergic neurons, protects rats from 6-OHDA induced Parkinson disease | Zbarsky et al, 2005 |
| Epilepsy: | |||
| Mice | 3,30 mg/kg, i.p | Decrease the severity of epilepsy, attenuated kainate induced histone modifications | Sng et al, 2006 |
| Rats | 50 mg/kg, i.p | Protects from KA induced neuronal damage, reduced the level of NO, decrease the expression of c-jun, COX-2, BDNF, and iNOS2 | Sumanont et al, 2006, 2007 |
| Cerebral injury: | |||
| Rat | 50,100,200 mg/kg, ip | Protects rat brain against I/R injury through modulation of XO, O2−, MDA, GPx SOD and LDH | Ghoneim et al, 2002 |
| Rats | 100, 300, mg/kg, ip | Protects rat brain from cerebral ischemia, modulate the activity of GPx, and SOD | Thiyagarajan et al, 2004 |
| Mangolian | 30 mg/kg, ip or | Protects I/R-induced neuronal cell death and glial activation; decreased LPO | Wang, et al, 2005 |
| Gerbils | 2 g/kg, diet | mitochondrial dysfunction and the apoptosis; curcumin levels goes up in plasma and brain within 1h | |
| Rats | 200mg/kg, i.p | Reduced the neuronal damage, decreased the level of LPO, increased the level of GSH and activities of SOD and CAT | Al-Omar et al, 2006 |
| Rats | 500mg/kg, i.p | Delayed neuronal death, increase antioxidant system and levels of peroxynitrite3 | Rathore et al, 2008 |
| Rats | 1,2 mg/kg, i.v | Prevent blood-brain barrier damage, improved neurological deficit, decreased mortality and the level of iNOS, | Jiang et al, 2008 |
| Cardiovascular diseases: | |||
| In vitro | 10 μmol/L | Inhibits high glucose-induced foam cell formation by inhibition of NF-κB | Li et al, 2004 |
| In vitro | 1–25 uM | Inhibited PDGF-induced migration, proliferation and collagen synthesis in cultured VSMCs | Yang et al, 2006 |
| In vitro | 10μM | Inhibited CRP-induced PAI-1 mRNA expression in HCAEC | Chen et al, 2007 |
| In vitro | 100μg | Improved blood compatibility of rapamycin-eluting stent | Pan et al, 2007 |
| Mice | 25–100 mg/kg, i.p | Antithrombotic effects | Srivastava et al, 1985 |
| Rats | 200 mg/kg, p.o | Prevents isoproterenol-induced myocardial infaraction | Nirmala et al, 1996 a&b |
| Rats | 200 mg/kg, po | Protects from adriamycin-induced myocardial toxicity | Venkatesan et al, 1998 |
| Rabbits | 1.6,3.2 mg/kg, p.o | Decreases the LPO of liver microsomes and mitochondria4 | Quiles et al, 1998 |
| Rabbits | 1.6,3.2 mg/kg, p.o | Inhibits LDL oxidation & has hyocholesteromic effects4 | Ramirez-Tortosa et al, 1999 |
| Rabbits | 1.6 mg/kg, p.o | Reduces oxidative stresss and reduces aortic fatty streak4 | Quiles et al, 2002 |
| Rats | 15 mg/kg, p.o | Decreases the levels of O2−, XO, MPO. LPO in myocardium elevated the levels of GPX, SOD, CAT and GST | Manikandan et al, 2004 |
| Mice | 0.3mg/day, diet | Inhibits the development of atherosclerosis in apoE/LDLR-DKO mice | Olszanecki et al, 2005 |
| Rabbits | 7, 70 mM/kg, i.p | Attenuate global cardiac I/R injury; decreases myocardial MMP-9, IL-6, MCP-1, TNF-α | Yeh et al, 2005a |
| Rabbits | 70,100 mM/kg, i.p | Decreased plasma IL-8, IL-10, TNF-α and cardiac troponin 1, decreased apoptosis in cardiomyocytes & myocardial MPO | Yeh et al, 2005b |
| Mice | 100 mg/kg, p.o | Decreased AP-1, NF-κB, IL-1, IL-6. MCP-1, MMP-9 in aortic tissue; inhibits | Parodi et al, 2006 |
| Rats | 72 μg in gel | Inhibit VSMC function; attenuated carotid artery neointima formation destructive connective tissue remodeling in experimental AAAs | Yang et al, 2006 |
| Mice | 75 mg/kg, p.o | Blocked phenylephrin (PE)-induced cardiac hypertrophy, prevented and reversed mouse cardiac hypertrophy induced by AB and PE infusion, abrogated histone acetylation, GATA4 acetylation, and DNA-binding by blocking p300-HAT | Li et al, 2008 |
| Rats | 50 mg/kg, p.o | Inhibited the hypertrophy-induced acetylation and DNA-binding abilities of GATA4, disrupted the p300/GATA4 complex and repressed hypertrophic responses. Prevented deterioration of systolic function and heart failure-induced increase in both myocardial wall thickness and diameter | Morimoto et al, 2008 |
| Ex-vivo | 5 μmol/L | Blocks homocystein induced endothelial dysfunction & O2− production, | Ramaswami et al, 2004 |
| Allergy, Asthma and Bronchitis: | |||
| In vitro | 10μM | Inhibited the allergen-induced lymphocyte (from bronchial asthmatics), proliferation and production of IL-2, IL-5, GM-CSF and IL-4 | Kobayashi et al, 1997 |
| In vitro | 1μM | Inhibits IL-1β induced chemokine release from human airway HASMC | Wuyts et al, 2003 |
| In vitro | 100μg/ml | Caused a marked decrease in histamine release from basophils | Suzuki et al, 2005 |
| In vitro | Reverses steroid resistance in asthma and COPD by inducing HDAC2 Biswas et al, 2008 | Marwick et al, 2007; | |
| Rats | 0.5%; diet | Lowered the IgE-mediated degranulation of intestinal mast cells | Ju et al, 1996 |
| Rats | 1,2,40 mg/kg, diet | Enhanced IgG levels | South et al, 1997 |
| Guinea pigs | 10, 24,40 mg/kg, p.o | Attenuates OVA-induced airway constriction & hyperresponsiveness | Ram et al, 2003 |
| Mice | 250μg, i.g | Diminished Th2 response, reduction in lung inflammation, reduced eosinephilia; decrease in expression of CD80,CD86, OX40L, MMP9, OAT, TSLP in latex allergy model | Kurup et al, 2007 |
| Inflammatory Bowel Disease and Colitis: | |||
| Mice | 0.25%, diet | Attenuates DNB-induced colitis; reduces macroscopic damage and NF-κB activation reduces MPO, IL-1β, p38 activation | Salh et al, 2003 |
| Mice | 50 mg/kg, i.g, | Decreased TNBS-induced colitis, decreased diarrhea and disruption of colonic architecture, reduction in MPO, LPO, serine protease activity, iNOS and O2− | Ukil et al, 2003 |
| Mice | 2%, diet | Prevents TNBS-induced downregulation of Phex gene expression in osteoblasts involved bone formation | Uno et al, 2006 |
| Mice | 2%, diet | Prevents development of DSS-induced colitis; inhibits NF-κB in mucosa | Deguchi et al, 2007 |
| Mice | 2%, diet | Protects BALB/c but not SJL/J mice from TNBS-induced colitis | Billerey-Larmonier et al, 2008 |
| Rats | 2%, diet | Prevents and treats TNBS-induced colitis; suppresses NF-κB and NF-κB-regulated inflammatory cytokine expression in colonic mucosa | Jian, 2005 |
| Rats | 25,50,100 mg/kg, p.o | Inhibits DNCB-induced ulcerative colitis through inhibition of NF-κB and iNOS. Effects were comparable with sulfasalazine | Venkataranganna et al, 2007 |
| Rheumatoid Arthritis: | |||
| In vitro | 0, 1,10 μM | Inhibits MMIF-induced MMP expression in synovial fibroblasts from RA | Onodera et al, 2000 |
| In vitro | 10,15 μM | Suppressed TNF-induced MMP-13 expression in primary chondrocytes | Liacini et al, 2003 |
| In vitro | 0–20 μM | Potentiates the apoptotic effect of celecoxib on synovial fibroblast | Lev-Ari et al, 2006 |
| In vitro | 0–100 μM | Curcumin induces apoptosis and reduces PGE2 production from synovial fibroblasts of RA pts. | Park et al, 2007 |
| Rats | 30 mg/kg, p.o | Lowered the levels of acidic glycoprotein in serum and paw inflammation of arthritis rats | Joe et al, 1997 |
| Rats | 100 mg/kg, p.o | Reduced adjuvant-induced CRP, haptoglobin, IL-1β | Banerjee et al, 2003 |
| Rats | 0.5–1 L/g, p.o, i.p | Reduces streptococcal cell wall-induced arthritis (joint swelling) 5 | Funk et al, 2006 |
| Renal Ischemia: | |||
| Rats | 30 mg/kg, i.p | Prevents ischemic renal injury; decreased the elevation of RANTES, MCP-1 and AIF | Shoskes et al, 1998 |
| Rats | 30 mg/kg, i.p | Inhibits renal ischemia reperfusion injury; prolongs skin graft survival | Jones et al, 2000 |
| Rat | 30 mg/kg, i.p | Upregulates antioxidant gene expression in rat kidney after ureteral obstruction or I/R injury | Shahed et al, 2001 |
| Rats | 30 mg/kg, i.p | Potentiates the effect of mycophenolate mofetil in prevention of immune & ischemic injury | Shokes et al, 2000 |
| Rat | 200 mg/kg, p.o | Protects kidneys against I/R injury via modulation of antioxidant system | Bayrak et al, 2008 |
| Psoriasis: | |||
| In vitro | Acts as a photosensitizer for skin cells | Tonnesen et al, 1987 | |
| In vitro | 0.1–10 μM | Inhibits keratinocyte proliferation associated with psoriasis | Pol et al, 2003 |
| Pateints | 1%, topical | Suppresses phosphorylase kinase activity and keratinocyte transferring receptor connected with psoriasis | Heng et al, 2000 |
| Patients | 4.5g/d, p.o | 16.7% patients responded | Kurd et al, 2008 |
| Scleroderma: | |||
| In vitro | 10μM | Induced apoptosis in lung fibroblasts from scleroderma pts but not from normal. mediated through PKC-e regulated GST-PI expression | Tourkina et al, 2004 |
| Diabetes and metabolic disorders: | |||
| Rats | 0.1,0.25,0.5%, diet | Decreased cholesterol level in serum and liver | Rao SD et al, 1970 |
| Rats | 0.5%, diet | Ameliorate renal lesions associated with diabetes in diabetic rats | Babu et al, 1998 |
| Rats | 200 mg/kg, p.o | Inhibits AGE and cross-linking of collagen in diabetic rats | Sajithlal et al, 1998 |
| Mice, rats | 40 mg/kg, p.o | Enhances wound healing in diabetic rats and genetically diabetic mice | Sidhu et al, 1999 |
| Rats | 0.002, 0.01% diet | Prevents the loss of chaperone-like activity of alpha-crystallin in the lens of diabetic rats | Kumar et al, 2005 |
| Rats | 0.002, 0.01% diet | Delays diabetic cataract | Suryanarayana et al, 2005 |
| Mice | 0.2,1.0 g/100 g of diet | Induces hypoglycemia in genetically diabetic KK-Ay mice via binding to PPAR-γ | Kuroda et al, 2005, Nishiyama et al, 2005 |
| Rats | 80 mg/kg, p.o | Reduces the accumulation and cross-linking of collagen in diabetic rats | Pari and Murugan, 2005, 2007 |
| Mice | 15,30,60 mg/kg, i.p | Attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain | Sharma et al, 2006 |
| Rats | 80 mg/kg, p.o | Antihyperlipidemic: reduced the blood glucose and increased in plasma insulin indiabetic rats; significant reduction in LPO and lipids in serum and tissues | Murugan and Pari, 2006 |
| Rats | 150 mg/kg, i.p | Modulates vasoactive factors in the diabetic rat heart; reduces eNOS, iNOS oxidative DNA and protein damage; increased vasoconstrictor ET-1 in the heart | Farhangkhoee et al, 2006 |
| Rats | 15,30 mg/kg, p.o | Ameliorates diabetic nephropathy in rats | Sharma et al, 2006 |
| Rats | 80 mg/kg, p.o | Reduces blood glucose and increase plasma insulin | Pari, and Murugan 2007a; Murugan and Pari 2007a |
| Rats | 80 mg/kg, p.o | Decreases blood glucose, glycosylated haemoglobin and erythrocyte TBARS and increases plasma insulin, HG, erythrocyte antioxidants and the activities of membrane bound enzymes | Murugan and Pari, 2007b |
| Rats | 80 mg/kg, p.o | Reduces serum and liver lipid levels, HMG CoA reductase activity, and increased HDL | Pari, and Murugan, 2007b |
| Rats | 0.002 or 0.01% diet | Prevents diabetes-induced oxidative stress | Suryanarayana et al, 2007 |
| Rats | 80 mg/kg, p.o | Prevents brain lipid peroxidation in diabetic rats | Pari, and Murugan 2007b |
| Rats | 60 mg/kg, p.o | Attenuate cognitive deficit, cholinergic dysfunction, oxidative stress and inflammation | Kuhad et al, 2007 |
| Rats | 0.002, 0.01% diet | Inhibits hyperglycemia-induced VEGF expression in diabetic retina | Mrudula et al, 2007 |
| Rats | 0.05%, diet | Suppresses retinal oxidative stress and inflammation. | Kowluru et al, 2007 |
| Rats | 60 mg/kg, p.o | Suppresses diabetic neuropathic pain through inhibition of NO and TNF | Sharma et al, 2007 |
| Mice | 3% diet | Inhibits diabetes and inflammation in murine models of insulin-resistant obesity | Weisberg et al, 2008 |
| Patients | 5mg, diet | Lowers blood glucose level | Srinivasan, 1970 |
| Patients | 10mg, oral | Lowers plasma fibrinogen levels | Ramirez-Bosca et al, 2000 |
| Depression : | |||
| Mice | 5,10 mg/kg, p.o | Reduce the depressive like behaviour in mice, increase the levels of Serotonin and dopamine and decrease monoamine oxidase activity | Xu et al, 2005 |
| Rats | 1.25,2.5, mg/kg, p.o | Demosnstrate antidepressant effect the forced swimming test and bilateral olfactory bulbectomy models of depression in rats | Xu et al, 2005 |
| Rats | 5,10,20 mg/kg, p.o | Decreased the stress, reverses impaired hippocampal neurogenesis, Elevated the expression of serotonin receptor 1A mRNA and brain-derived neurotrophic factor | Xu et al, 2007, 2006 |
| Rats | 60 mg/kg, p.o | Inhibuts diabetic encephalopathy | Kuhad et al, 2007 |
| Mice | 10 mg/kg, p.o | Shows antidepressant-like effect in the forced swimming test | Wang et al, 2008 |
| Fatigue : | |||
| Mice | 20μg/kg, i,p | Stimulates muscle regeneration after traumatic injury, inhibits NF-κB | Thaloor et al, 1999 |
| Mice | 10mg, diet | Reduces inflammation, decreased the expression of IL-1, IL-6 and TNF | Davis et al, 2007 |
| AIDS/HIV: | |||
| In vitro | 40μM | Inhibits HIV integrase | Mazumder et al, 1995 |
| In vitro | 10–100μM | Inhibits tat transactivation | Barthelemy et al, 1998 |
| In vitro | 50, 100, 200, 300 μM | Inhibits acetylation of Tat-1 protein and replication of HIV in culture | Balasubramanyam et al, 2004 |
| In vitro | Binds to HIV protease and integrase | Vajragupta et al, 2005 | |
| In vitro, | 10μM, | Enhance IDV antiretroviral activity in HIV-1 persistently infected cells | Riva et al, 2008 |
mice were given 500ppm curcumin in diet and on day of perfusin 50μM curcumin was given i.v ;
used manganese complex of curcumin;
curcuma oil was used;
Used hydroalcoholic extract of rhizome of Curcuma longa (~ 10% concentration of curcumin);
Turmeric extract is used; AAAs, Abdominal aortic aneurysms; AB, aortic banding; A-beta, amyloid beta; AGE, advanced glycation; CRP, C-reactive protein; DSS, dextran sulfate sodium; ET-1, endothelin-1; GST-PI, glutathions-S-transferase; HASMC, human airway smooth muscle cells, i.p, intra peritoneal; I/R, ischemia-reperfusion; i.v, intra venous; IDV, idinavir; IL, interleukin; LPO, lipid peroxidation; MCP-1, macrophage chemotactic protein-1; MMIF, macrophage migration inhibitory factor; MPO, myeloperoxidase; MVEC, microvascular endothelial cells; NF-κB, nuclear factor kappa B; NOS, nitric oxide synthese; p.o, orally; RA, rheumatoid arthritis; RANTES, regulated upon activation, normal T cell expressed and secreted; TBARS, thiobarbituric acid reacting substances; TNBS, 2,4,6-trinitrobenzene sulphonic acid; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
2.1. Neurodegenerative diseases
The brain is a highly oxidative organ that consumes 20% of the body’s oxygen despite accounting for only 2% of the total body weight. With normal aging, the brain accumulates metals ions such as iron (Fe), zinc (Zn), and copper (Cu). Consequently, the brain is abundant in antioxidants that control and prevent the detrimental formation of reactive oxygen species (ROS) generated via Fenton chemistry involving redox-active metal-ion reduction and activation of molecular oxygen (Smith et al., 2007). Curcumin has been shown to exhibit activity against various neurologic diseases, including AD (Lim et al., 2001), multiple sclerosis (Natarajan and Bright, 2002), Parkinson disease (Zbarsky et al., 2005), epilepsy (Sumanont et al., 2006), cerebral injury (Ghoneim et al., 2002), age-associated neurodegeneration (Calabrese et al., 2003), schizopherenia (Bishnoi et al., 2008), Spongiform encephalopathies (Creutzfeld-Jakob disease) (Hafner-Bratkovic et al., 2008), neuropathic pain (Sharma et al., 2006a), and depression (Xu et al., 2005) (Fig. 2).
Fig. 2.
Effect of curcumin on various proinflammatory diseases.
AD is a neurodegenerative disease that involves inflammation, oxidative damage, and Abeta accumulation. Inhibition of the accumulation of Abeta and the formation fibrillar (f) Abeta (fAbeta) from Abeta and the destabilization of preformed fAbeta in the central nervous system are potential therapeutic targets for the treatment of AD. In AD, an over-accumulation of Abeta is due to either an elevated generation of amyloid precursor protein (APP) or inefficient clearance of Abeta from the brain. Abeta can efficiently generate ROS in the presence of the transition metals (Cu and Fe) in vitro. Under oxidative conditions, Abeta will form stable dityrosine cross-linked dimers that are generated from free-radical attack on the tyrosine residue. There are elevated levels of urea and SDS-resistant, stable-linked Abeta oligomers as well as dityrosine cross-linked peptides and proteins in AD brain (Huang et al., 2004) (Tschape and Hartmann, 2006) (Smith et al., 2007) (Annaert and De Strooper, 2002) (Atwood et al., 2004).
Extensive research has revealed that curcumin may mediate its effects against AD through the 8 mechanisms:
Kim et al (2001) found that curcumin and its analogues demethoxycurcumin (DMC) and bis-demethoxycurcumin (BDMC) can protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from Abeta-induced oxidative stress, and these compounds were better antioxidants than alpha-tocopherol (Kim et al., 2001).
Inflammation in AD patients is characterized by increased expression of inflammatory cytokines and activated microglia. Lim et al (Lim et al., 2001) investigated whether curcumin could affect AD-like pathology in the APPsw mice, as suggested by inflammation, oxidative damage, and plaque pathology. Curcumin significantly lowered levels of oxidized proteins and IL-1β elevated in the brains of these mice. Interestingly, with low-dose curcumin, but not with high-dose curcumin, the astrocytic marker glial fibrillary acidic protein was reduced, and insoluble Abeta, soluble Abeta, and plaque burden were significantly decreased (by 43%–50%). However, levels of APP in the membrane fraction were not reduced. Microgliosis was also suppressed in neuronal layers but not adjacent to plaques.
In studies conducted to determine whether curcumin can modulate the formation, extension, and destabilization of fAbeta(1–40) and fAbeta(1–42) in vitro, curcumin was found to inhibit the formation and extension of fAbeta from Abeta(1–40) and Abeta(1–42); curcumin also destabilized preformed fAbeta (Ono et al., 2004).
In animal models of AD, curcumin reduced levels of amyloid and oxidized proteins and prevented cognitive deficits. Metals can induce Abeta aggregation and toxicity and are concentrated in AD brain. Thus chelation of metals can reduce amyloid aggregation and oxidative neurotoxicity. Each Cu2+ or Fe2+ ion can bind at least 2 curcumin molecules. The interaction of curcumin with Cu and Fe suggested another potential mechanism by which it could mediate its effects against AD animal models (Baum and Ng, 2004). Because curcumin binds the redox-active metals Fe and Cu, it may also not only protect against Abeta toxicity but may also suppress inflammatory damage by preventing metal induction of NF-κB.
That curcumin can suppress oxidative damage, inflammation, cognitive deficits, and amyloid accumulation has been established. Yang et al (Yang et al., 2005) showed that curcumin could inhibit aggregated as well as disaggregated fAbeta40. For this application, curcumin was found to be a better Abeta40 aggregation inhibitor than ibuprofen or naproxen. Curcumin decreased Abeta formation. This effect did not depend on Abeta sequence but rather on fibril-related conformation. AD and Tg2576 mice brain sections incubated with curcumin revealed preferential labeling of amyloid plaques. In vivo studies showed that curcumin injected peripherally into aged Tg2576 mice crossed the blood-brain barrier and bound plaques. When fed to aged Tg2576 mice with advanced amyloid accumulation, curcumin labeled plaques and reduced amyloid levels and plaque burden. Hence, curcumin directly binds small beta-amyloid species to block aggregation and fibril formation in vitro and in vivo. These data suggest that low-dose curcumin effectively disaggregates Abeta and prevents fibril and oligomer formation.
Atamna and Boyle (Atamna and Boyle, 2006) showed that beta-amyloid peptide binds with heme to form a peroxidase. This action plays a major role in the cytopathologies of AD, and curcumin inhibits this peroxidase.
Patients with AD have defects in phagocytosis of Abeta by the macrophages and in clearance of Abeta plaques. Curcumin was found to enhance Abeta uptake by macrophages of patients with AD (Zhang et al., 2006). How curcumin enhances the phagocytosis of Abeta was examined by Fiala et al (Fiala et al., 2007) who found that macrophages of a majority of patients with AD do not transport Abeta into endosomes and lysosomes and that AD monocytes do not efficiently clear Abeta from the sections of AD brain, although they phagocytize bacteria. In contrast, macrophages of normal subjects transport Abeta to endosomes and lysosomes, and monocytes of these subjects clear Abeta in AD brain sections. Upon Abeta stimulation, mononuclear cells of normal subjects upregulate the transcription of beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase (MGAT3) and other genes, including Toll-like receptors (TLRs), whereas mononuclear cells of patients with AD generally downregulate these genes. Defective phagocytosis of Abeta may be related to downregulation of MGAT3, as suggested by inhibition of phagocytosis by using MGAT3 siRNA and correlation analysis. Transcription of TLR3, bditTLR4, TLR5, bditTLR7, TLR8, TLR9, and TLR10 upon Abeta stimulation is severely depressed in mononuclear cells of AD patients in comparison to those of control subjects. In mononuclear cells of some AD patients, the BDMC may enhance defective phagocytosis of Abeta, the transcription of MGAT3 and TLRs, and the translation of TLR2-4. Thus, BDMC may correct immune defects in patients with AD and provide a previously uncharacterized approach to AD immunotherapy.
Garcia-Alloza et al (Garcia-Alloza et al., 2007) also showed that curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an AD mouse model. They found that curcumin crosses the blood-brain barrier and labels senile plaques and cerebrovascular amyloid angiopathy in APPswe/PS1dE9 mice. Moreover, systemic treatment of mice with curcumin for 7 days cleared and reduced the existing plaques, suggesting a potent disaggregation effect. Curcumin also led to a limited, but significant reversal of structural changes in dystrophic dendrites, including abnormal curvature and dystrophy size.
These studies led to a 6-month randomized, placebo-controlled, double-blind, clinical pilot study of curcumin in patients with AD (Baum et al., 2008). Thirty four subjects started the six-month trial and 27 completed (8 subjects on 0 g, 9 on 1g, 11 on 4g curcumin per day). The serum levels of curcumin reached maximum (250 nM) at 1.5h when given with food and 270 nM at 4h when given with water. No difference was observed between 1 and 4g groups. The inability to detect any relative protective effect of curcumin, was assigned to the lack of cognitive decline in the placebo group in this 6-month trial. Curcumin group when compared with placebo control, however, showed increased plasma levels of Vitamin E and increased serum Aβ40. The latter reflects an ability of curcumin to disaggregate Aβ-deposits in the brain, thus releasing Aβ for circulation and disposal. The authors recommended, longer and larger trials to determine the efficacy of curcumin in AD patients.
2.2. Cardiovascular Diseases
Numerous reports have indicated that inflammation plays a major role in most CVDs (Hansson et al., 2006). First, it is widely appreciated that inflammation and oxidant stress contribute to atherogenesis. Atherosclerosis is characterized by oxidative damage, which affects lipoproteins, the walls of blood vessels, and subcellular membranes. The oxidation of low-density lipoproteins (LDLs) plays an important role in the development of atherosclerosis (Ansell, 2007) (Mach, 2005). Second, following cardiopulmonary bypass (CPB) and cardiac global ischemia and reperfusion (I/R), pro-inflammatory cytokines regulated by NF-κB are activated and cause cardiomyocytic injury (Yeh et al., 2005). Third, chronic transmural inflammation and proteolytic destruction of medial elastin are key mechanisms in the development of abdominal aortic aneurysms (AAAs) (McCormick et al., 2007). Fourth, CRP, which is also regulated by NF-κB, is an inflammatory marker and a well-known predictor of CVD (Kawanami et al., 2006). Numerous lines of evidence suggest that curcumin mediates its effects against CVDs through diverse mechanisms, several of which are discussed in this report.
Several studies have suggested that curcumin protects the heart from I/R injury (Srivastava et al., 1985) (Manikandan et al., 2004) (Yeh et al., 2005). Perhaps one of the earliest reports about the effects of curcumin against CVD was by Srivastava et al (Srivastava et al., 1985). They examined the effect of curcumin on myocardial ischemia induced by the ligation of the left descending coronary artery. Curcumin was administered 30 min before ligation, and the hearts were removed 4 h prior to coronary artery ligation and examined for glutathione (GSH), malonaldialdehyde (MDA), myeloperoxidase (MPO), superoxide dismutase (SOD), catalase (CAT), and lactate dehydrogenase (LDH). Curcumin protected the animals against decreases in the heart rate and blood pressure following ischemia. Curcumin also prevented the ischemia-induced elevation in MDA contents and LDH release. Manikandan et al (Manikandan et al., 2004) investigated the protective effect of curcumin against isoprenaline-induced myocardial ischemia in rat myocardium. The effect of a single oral dose of curcumin, administered 30 min before and/or after the onset of ischemia, was investigated. Curcumin given before and after treatment decreased the levels of xanthine oxidase, superoxide anion, lipid peroxides (LPs), and myeloperoxidase and the levels of SOD, CAT, GSH peroxidase, and GSH-S-transferase activities were significantly increased after curcumin treatment. Thus curcumin was found to protect rat myocardium against ischemic insult.
Following CPB and cardiac global I/R, proinflammatory genes are upregulated, and NF-κB is involved in this regulation. Whether inactivation of NF-κB could decrease myocardial I/R injury with cardioplegia during CPB, attenuate matrix metalloproteinase (MMP) activation and prevent cardiac mechanical dysfunction in rabbits was examined by Yeh et al (Yeh et al., 2005). Postoperative expression of myocardial mRNA levels of IL-6, MCP-1, and TNF-α; post-reperfusion plasma level of troponin I; and cardiac mechanical dysfunction were significantly decreased in the curcumin groups. The myocardial levels of activated MMP-2 and -9 were also significantly reduced compared with the levels in the control group. Thus inhibition of NF-κB activation by curcumin led to suppression of the upregulation of cardiac proinflammatory genes and activation of MMPs during CPB, thereby lessening the severity of the cardiac mechanical dysfunction after global cardiac I/R injury. In another study, the same group examined whether curcumin could decrease myocardial I/R injury with cardioplegia during CPB and attenuate the apoptosis of cardiomyocytes in rabbits (Yeh et al., 2005). They showed that curcumin significantly decreased plasma levels of IL-8, IL-10, TNF-α, and cardiac troponin I. The appearance of apoptotic cardiomyocytes significantly decreased in the curcumin groups. Thus curcumin ameliorated the surge of pro-inflammatory cytokines during CPB and decreased the occurrence of cardiomyocytic apoptosis after global cardiac I/R injury.
Nirmala and Puvanakrishnan (1996) showed that isoproterenol-induced myocardial infaraction in rats is prevented by curcumin (Nirmala and Puvanakrishnan, 1996) (Nirmala and Puvanakrishnan, 1996). Histopathologic studies of the infarcted rat heart also showed a decrease in necrosis after curcumin treatment. Cardiotoxicity induced by chemotherapeutic agents in cancer patients is a common problem. Venkatesan (Venkatesan, 1998) showed that curcumin decreased acute Adriamycin (ADR)-induced myocardial toxicity in rats. Treatment with curcumin treatment 7 days before and 2 days following administration of ADR ameliorated the cardiotoxicity, prevented the rise in serum and LDH, and induced a significant inhibition of lipid peroxidation and augmentation of endogenous antioxidants.
Another line of evidence suggested by Quiles et al showed that curcumin exhibits a potential effect against CVD where they examined the effect of curcumin in atherosclerotic rabbits (Quiles et al., 1998). The researchers showed that curcumin exhibited protective effects as indicated by inhibition of lipoperoxidation of subcellular membranes. In another study, they showed that oral curcumin inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis (Ramirez-Tortosa et al., 1999). Interestingly, they found that rabbits treated with 1.6 mg/kg of curcumin had lower levels of cholesterol, phospholipids, and triglycerides in LDL than those treated at a higher dose (3.2 mg/kg). In a more recent study, these researchers showed that curcumin reduces oxidative stress and attenuates aortic fatty streak development in rabbits (Quiles et al., 2002), as indicated by lower plasma lipid peroxide and significantly higher α-tocopherol and coenzyme Q levels. Histologic results for the fatty streak lesions revealed damage in the thoracic and abdominal aorta that was significantly lower in the curcumin-treated group than in the control group.
Olszanecki et al (2005) examined the effect of low-dose curcumin on atherosclerosis in apoE/LDL receptor (LDLR)-double knockout mice. The mice were fed a Western diet (21% fat, 0.15% cholesterol w/w, without cholic acid). Curcumin (purity ± 98%) premixed with diet was given to each mouse for 4 months at a dose of 0.3 mg/per day. In this model, curcumin inhibited atherogenesis but did not influence the concentrations of cholesterol or triglycerides in blood or animal body weight (Olszanecki et al., 2005).
Vascular smooth muscle cell (VSMC) migration, proliferation, and collagen synthesis are key events in the pathogenesis of CVD. Growth factors, such as platelet-derived growth factor (PDGF) and fibroblast growth factor, which are released during vascular injury, play a pivotal role in regulating these events. Yang et al (2006) assessed whether curcumin could inhibit PDGF-stimulated migration, proliferation, and collagen synthesis in cultured VSMCs and neointima formation after carotid artery injury in rats (Yang et al., 2006). Curcumin inhibited PDGF-elicited VSMC migration, proliferation, and collagen synthesis assessed by chemotaxis, [3H]thymidine incorporation, and [3H]-L-proline incorporation, respectively. Curcumin also blocked PDGF-induced VSMC actin-cytoskeleton reorganization, attenuated PDGF signal transduction, and inhibited the binding of PDGF to its receptors. Carotid artery neointima formation was significantly attenuated by perivascular curcumin compared with vehicle controls 14 days after injury, characterized by reduced DNA synthesis, collagen synthesis, and PDGF receptor phosphorylation. Thus curcumin is a potent inhibitor of PDGF-stimulated VSMC functions and may play a critical role in regulating these events after vascular injury.
Ramaswami et al (2004) showed that curcumin blocks homocysteine (HE)-induced endothelial dysfunction in porcine coronary arteries (Ramaswami et al., 2004). They found that curcumin could effectively block HC-induced impairment of endothelium-dependent vasorelaxation, inhibit the HC-induced epithelial nitric oxide synthase (NOS) expression, and block the effect of homocysteine on superoxide anion production.
Parodi et al (2006) examined the effect of oral administration of curcumin on proinflammatory cytokines and destructive connective tissue remodeling in experimental AAAs. Curcumin-treated mice exhibited relative decreases in aortic tissue activator protein (AP)-1 and NF-κB DNA binding activities and significantly lower aortic tissue concentrations of IL-1β, IL-6, MCP-1, and MMP-9. Curcumin suppressed the development of experimental AAAs along with the structural preservation of medial elastin fibers and reduced aortic wall expression of several cytokines, chemokines, and proteinases known to mediate aneurismal degeneration. (Parodi et al., 2006)
That curcumin plays an important role in the hypertrophy of the heart is evidenced by the results of 2 recent reports (Li et al., 2008) (Morimoto et al., 2008). This activity is based on the ability of curcumin to inhibit histone acetyltransferase (HAT), also called p300, which plays a critical role in the progression of pathologic cardiac hypertrophy and heart failure. Li et al (2008) showed that curcumin-blocked phenylephrin (PE) induces cardiac hypertrophy in vitro. Curcumin also prevented and reversed mouse cardiac hypertrophy induced by aortic banding (AB) and PE infusion and abrogated histone acetylation, GATA4 acetylation, and DNA-binding activity through blocking p300-HAT activity. Curcumin also blocked AB-induced inflammation and fibrosis through disrupting p300-HAT–dependent-signaling pathways (Li et al., 2008). Similarly, Morimoto et al (2008) showed that curcumin inhibited the hypertrophy-induced acetylation and DNA-binding abilities of GATA4, a hypertrophy-responsive transcription factor, in rat cardiomyocytes. Curcumin also disrupted the p300/GATA4 complex and repressed agonist- and p300-induced hypertrophic responses in these cells. Both the acetylated form of GATA4 and the relative levels of the p300/GATA4 complex markedly increased in rat hypertensive hearts in vivo. In 2 different heart-failure models, hypertensive heart disease in salt-sensitive Dahl rats and surgically induced myocardial infarction in rats, curcumin prevented deterioration of systolic function and heart failure-induced increases in both myocardial wall thickness and diameter (Morimoto et al., 2008). Thus curcumin can protect against cardiac hypertrophy, inflammation, and fibrosis through suppression of p300-HAT activity and downstream GATA4, NF-κB, and other signaling pathways. Inhibition of p300-HAT activity by the nontoxic dietary compound curcumin may provide a novel therapeutic strategy for heart failure in humans.
Curcumin has also been shown to improve the blood compatibility of the rapamycin-eluting stent (Pan et al., 2007b) (Pan et al., 2007a). The rapamycin- and rapamycin/curcumin-loaded poly (dl-lactic acid-co-glycolic acid (PLGA) coatings were fabricated. The data showed that incorporating curcumin in rapamycin-loaded PLGA coating can significantly decrease platelet adhesion and activation, prolong clotting time, and decrease fibrinogen adsorption, thus improving the blood compatibility of rapamycin-eluting stents. This ability of curcumin can be used to fabricate a drug-eluting stent for use in preventing thrombosis formation (Pan et al., 2007b).
2.3. Diabetes
Diabetes is a hyperglycemic disorder that affects the brain, kidney, heart, liver, and other organs. Inflammation has been shown to play a major role in development of type II diabetes (Pillarisetti and Saxena, 2004). The role of various inflammatory cytokines and transcription factors (such as NF-κB, NRF2, PPAR-γ) and various enzymes have been implicated in this process. Both TNF and NF-κB activation have been linked with insulin resistance (Moller and Berger, 2003). In diabetes, curcumin can suppress blood glucose levels, increase the antioxidant status of pancreatic β-cells, and enhance the activation of PPAR-γ (Nishiyama et al., 2005). That curcumin can modulate blood sugar levels in human subjects with diabetes was shown almost 35 years ago (Srinivasan, 1972). Years later. Babu and Srinivasan (1995) showed in curcumin feeding in rats improves the metabolic status in diabetic conditions (Babu and Srinivasan, 1995). That curcumin treatment can induce hypoglycemia in rats with streptozotocin (STZ)-induced diabetes has been confirmed by others (Mahesh et al., 2004). The mechanism by which curcumin improves this situation is probably its hypocholesterolemic influence, antioxidant nature, and free-radical scavenging property. In another study, Babu and Srinivasan (1997) showed that curcumin exhibits hypolipidemic activity in rats with STZ-induced diabetes (Babu and Srinivasan, 1997). The decrease in cholesterol level was due exclusively to the LDL-very LDL (VLDL) fraction. A significant decrease in blood triglyceride and phospholipids was also brought about by dietary curcumin in diabetic rats. When the mechanism of hypocholesterolemic activity in dietary curcumin was examined, it was found that hepatic cholesterol-7α-hydroxylase activity was markedly higher in curcumin-fed diabetic animals, suggesting a higher rate of cholesterol catabolism in these animals (Babu and Srinivasan, 1997). Curcumin was found to be more effective in rats than turmeric in attenuating diabetes mellitus–related changes (Arun and Nalini, 2002). Hyperlipidemia is a complication of diabetes mellitus. The ability of curcumin to modulate the lipid profile in rats with STZ-nicotinamide-induced diabetes was investigated by Pari and Murugan (Pari and Murugan, 2007a). Curcumin caused a significant reduction in the blood glucose levels and a significant increase in the plasma insulin levels in these rats and a significant reduction in serum and liver cholesterol, triglycerides, free fatty acids, phospholipids, HMG coenzyme A reductase activity, VLDL, and LDL cholesterol levels. The decreased serum high-density lipoprotein (HDL) cholesterol in diabetic rats was also reversed toward normalization after the treatment (Murugan and Pari, 2006).
Obesity is a major risk factor for type 2 diabetes, and it is now recognized that significant inflammatory components underly the pathophysiologies of both of these conditions (Vazquez et al., 2007). The ability of curcumin to ameliorate diabetes and inflammation in murine models of insulin-resistant obesity was examined by Weisberg et al (2008). Curcumin ameliorated diabetes in high-fat diet-induced obese and leptin-deficient ob/ob male C57BL/6J mice as determined by glucose and insulin tolerance testing and hemoglobin A1c percentages. Curcumin treatment also significantly reduced macrophage infiltration of white adipose tissue, increased adipose tissue adiponectin production, and decreased hepatic nuclear NF-κB activity, hepatomegaly, and markers of hepatic inflammation. Thus orally ingested curcumin reverses many of the inflammatory and metabolic dearrangements associated with obesity and improves glycemic control in mouse models of type 2 diabetes (Weisberg et al., 2008).
Both curcumin and its metabolite tetrahydrocurcumin (THC) have been shown to decrease blood glucose levels, increase plasma insulin levels, and modulate hepatic key enzyme levels in STZ-induced diabetic rats(Murugan and Pari, 2005) through modulation of oxidative stress (Murugan and Pari, 2006) and reduction in lipids and lipid peroxidation (Murugan and Pari, 2006). In another study, these authors showed that oral curcumin decreased the blood glucose and plasma glycoprotein levels in diabetic rats (Murugan and Pari, 2007a). The levels of plasma insulin and tissue sialic acid were increased, whereas the levels of tissue hexose, hexosamine, and fucose were near normal in diabetic rats treated with curcumin. These findings show that the effect of THC is more prominent than that of curcumin (Murugan and Pari, 2007b).
Studies have been conducted to determine whether curcumin’s direct stimulatory effect on the pancreatic beta-cell can contribute to the hypoglycemic activity of this compound. In a study by Best et al (2007), curcumin induced electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel f. Single-channel studies have indicated that activation is the result of increased channel open probability. This effect was accompanied by depolarization of the cell membrane potential, the generation of electrical activity, and enhanced insulin release (Best et al., 2007). Curcumin also decreased beta-cell volume, presumably reflecting loss of Cl(−), and hence water, as a result of anion channel activation. These findings are consistent with the suggestion that Cl(−) fluxes play an important role in regulating beta-cell function the stimulation of beta-cell function by curcumin might contribute to the hypoglycemic actions of this compound.. Additionally, curcumin was found to induce heme oxygenase-1 expression, which has been reported to have cytoprotective effects in mouse pancreatic beta-cells (Pugazhenthi et al., 2007). These effects were mediated through the activation of NF-E2-related factor 2 (Nrf2). Another report indicated that in addition to heme ozygenase-1, curcumin treatment enhances islet recovery by inducing heat-shock protein 70, a response protein, during cryopreservation (Kanitkar and Bhonde, 2008).
Pancreatic islet cell death is the cause of deficient insulin production in diabetes mellitus. Approaches to preventing cell death have prophylactic significance in the management of hyperglycemia. Generation of oxidative stress is implicated in STZ, a beta-cell–specific, toxin-induced islet cell death. The role of curcumin in STZ-induced islet damage was examined in vitro by Meghana et al (2007). Curcumin retarded islet ROS generation and inhibited apoptosis, indicating that curcumin protects islets against STZ-induced oxidative stress by scavenging free radicals (Meghana et al., 2007).
How curcumin mediates its hypoglycemic effects has also been examined. Curcumin was found to suppress an increase in blood glucose levels in KK-AY mice with type 2 diabetes through PPAR-γ ligand-binding activity (Kuroda et al., 2005) (Nishiyama et al., 2005). Increased oxidative stress and hyperglycemia has been postulated to contribute to the accelerated accumulation of advanced glycation end-products (AGEs) and the cross-linking of collagen in diabetes mellitus. Curcumin administration for the prevention of AGE-induced complications of diabetes mellitus (Sajithlal et al., 1998). Sidhu et al showed that curcumin enhances wound healing in genetically engineered rats with STZ-induced diabetes. Curcumin was effective via both oral and topical administration and enhanced wound repair in diabetic-impaired healing (Sidhu et al., 1999, Sidhu et al., 1998). Another study showed that curcumin inhibits protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels (Jain, 2006). This finding provides evidence for a novel mechanism by which curcumin supplementation may prevent the cellular dysfunction associated with diabetes. Administration of curcumin to diabetic rats showed a significant beneficial effect on erythrocyte membrane-bound enzymes and antioxidant defense in addition to its antidiabetic effect (Murugan and Pari, 2007a).
Diabetic neuropathic pain, an important microvascular complication in diabetes mellitus, is recognized as one of the most difficult types of pain to treat. In a study by Sharma et al (2006), curcumin attenuated thermal hyperalgesia in a diabetic mouse model of neuropathic pain. (Sharma et al., 2006a) Curcumin also inhibited TNF-α and NO release in a dose-dependent manner. These results indicate an anti-nociceptive activity of curcumin, possibly through its inhibitory action on NO and TNF-α release and point to its potential to attenuate diabetic neuropathic pain. In a later study, the same authors showed the antinociceptive activity of curcumin in combination with insulin in attenuating diabetic neuropathic pain through the participation of NO and TNF-α (Sharma et al., 2007b).
Diabetic retinopathy is one of the most devastating microvascular complications of longstanding type 1 and type 2 diabetes. Oxidative stress and inflammation are implicated in the pathogenesis of retinopathy in diabetes (Haidara et al., 2006) (Kowluru and Chan, 2007). Curcumin administration was found to prevent a diabetes-induced decrease in the antioxidant capacity and an increase in 8-OHdG and nitrotyrosine (Kowluru and Kanwar, 2007). Curcumin also inhibited diabetes-induced elevation in the levels of IL-1β, VEGF, and NF-κB. The effects of curcumin were achieved without amelioration of the severity of hyperglycemia. In another study, curcumin was found to prevent the development of STZ-induced diabetic cataracts in rats by inhibition of hyperglycemia-induced aggregation and insolubilization of lens proteins (Suryanarayana et al., 2005, Suryanarayana et al., 2007). Interestingly, these authors showed that turmeric was the ocular lens, is composed of 2 subunits: αA and αB. Of these, αB-crystallin has been shown to present widely in nonlenticular tissues, whereas αA-crystallin is largely lens specific. Kumar et al (2005) showed an elevated expression of αA- and αB-crystallins in rats with STZ-induced diabetes, and feeding curcumin to these rats attenuated the enhanced expression of αB-crystallin (Kumar et al., 2005). Curcumin was also found to protect endothelial dysfunction in the iris tissues of STZ-induced diabetic rats (Patumraj et al., 2006). Curcumin decreased the blood glucose, glycosylated hemoglobin, dyslipidemia, and MDA levels significantly. Neovascularization stimulated by hyperglycemia-mediated induction of VEGF has been implicated in the pathogenesis of diabetic retinopathy. The ability of curcumin to inhibit VEGF expression in rats with STZ-induced diabetic retina was examined by (Mrudula, 2007). Curcumin induced a decrease in VEGF expression in diabetic retina compared to control retina at both the transcription and protein levels.
Chronic hyperglycaemia in diabetes leads to the development of diabetic nephropathy. Sharma et al (2006) showed that treatment with curcumin for 2 weeks significantly attenuated both renal dysfunction and oxidative stress in diabetic animals (Sharma et al., 2006b). Curcumin was also found to improve hepatic and renal function markers and protein levels in experimental type 2 diabetic rats (Murugan and Pari, 2007b). Curcumin reversed the diabetes-induced total protein, albumin, globulin, and albumin/globulin ratio; the activities of hepatic and renal markers; and the levels of urea, uric acid, and creatinine. Tikoo et al (2008) examined the changes in histone modification by curcumin treatment, which prevents development of type I diabetic. At the nuclear level, curcumin prevented the decrease in dephosphorylation and the increase in acetylation of histone H3 suggesting that protection against the development of diabetic nephropathy by curcumin treatment involves changes in post-translational modifications of histone H3 (Tikoo et al., 2008).
Cardiomyopathy has been associated with the pathogenesis of chronic diabetic complications. Treatment of STZ-induced diabetic rats with curcumin reduced eNOS and inducible NOS levels in association with reduced oxidative DNA and protein damage in the heart (Farhangkhoee et al., 2006). Curcumin prevented NOS alteration and oxidative stress, which was mediated by NF-κB and AP-1. Exposure to curcumin also increased ET-1 levels in the microvascular endothelial cells. These studies indicate the differential effects of curcumin in vasoactive factor expression in the heart and indicate the importance of the tissue microenvironment in the treatment of diabetic complications.
Diabetic cardiomyopathy, structurally characterized by cardiomyocyte hypertrophy, eventually leads to heart failure. Transcriptional co-activator p300 and its interaction with myocyte enhancer factor 2 (MEF2) play a major role in diabetes-induced cardiomyocyte hypertrophy. Whether curcumin, a p300 blocker, can prevent these abnormalities, was examined by Feng et al (2008). Treatment with curcumin prevented diabetes-induced upregulation of these transcripts, suggesting the existence in curcumin of a novel glucose-induced epigenetic mechanism regulating gene expression and cardiomyocyte hypertrophy in diabetes (Feng et al., 2008).
Emerging epidemiologic data indicate that diabetes is a potential predisposing factor for neuropsychiatric deficits such as stroke, cerebrovascular diseases, diabetic encephalopathy, depression, and anxiety. Diabetic encephalopathy, characterized by impaired cognitive functions and neurochemical and structural abnormalities, involves direct neuronal damage caused by intracellular glucose. In a study by Kuhad and Chopra (2007), chronic treatment with curcumin significantly attenuated cognitive deficit, cholinergic dysfunction, oxidative stress and serum levels of TNF in diabetic rats (Kuhad and Chopra, 2007). Thus, curcumin could be used as an adjuvant therapy to conventional anti-hyperglycemic regimens for the prevention and treatment of diabetic encephalopathy. The ability of curcumin to affect the occurrence of oxidative stress in the brains of rats with diabetes was also examined. Curcumin was found to prevent brain lipid peroxidation in rats with STZ-induced diabetes (Pari and Murugan, 2007b).
Altogether, these studies reveal that curcumin plays an important role in attenuating diabetes and diabetes-associated symptoms.
2.4. Allergy, asthma, and bronchitis
That allergy is a proinflammatory disease is indicated by the fact that this disease is normally mediated through inflammatory cytokines and is treated with steroids. Asthma is also an inflammatory disease in which eotaxin, MCP-1, and MCP-3 play a crucial role. These chemokines have been shown to be expressed and produced by IL-1β-stimulated human airway smooth muscle cells in culture. For instance, house dust mite is a common allergen of allergic asthma. Eosinophils are principal effector cells of allergic inflammation and their adhesion onto human bronchial epithelial cells is mediated by a CD18-intracellular adhesion molecule (ICAM)-1-dependent interaction. As shown in experiments in vivo (in guinea pigs) and in vitro (basophils), curcumin can help clear constricted airways and increase antioxidant levels. Ju et al (1996) examined the effect of dietary fats and curcumin on immunoglobulin E (IgE)-mediated degranulation of intestinal mast cells in brown Norway rats (Ju et al., 1996). Rats were primed intraperitoneally with β-lactoglobulin for 3 weeks to induce reaginic antibody, during which time they were fed diets containing 10% each of coconut oil (CO), high oleic safflower oil, safflower oil (SO), or fish oil and were then challenged for 3 hours orally with the antigen. The dietary SO, compared to other dietary fats, resulted in lower circulatory release of rat chymaseII (RChyII), an indicator of degranulation of mucosal mast cells in the intestine, in response to the antigen. The addition of 0.5% curcumin to the CO or SO diets lowered the release. The SO diet, compared to the CO diet, tended to increase the concentration of reaginic antibody, but the influence of curcumin was not prominent, suggesting that dietary ingredients differently influence the synthesis of IgE and degranulation of mast cells.
South et al (1997) examined the effects of dietary curcumin (1, 20 or 40 mg/kg) for 5 weeks on antibody (IgG) production, delayed-type hypersensitivity, and natural killer cell activity in rats. The highest doses of curcumin, but not the lower doses, significantly enhanced IgG levels. Neither delayed-type hypersensitivity nor natural killer cell activity was different from control values at any dietary concentration of curcumin (South et al., 1997).
To clarify the potential effect of curcumin against allergic diseases, Kobayashi et al (1997) examined the effect of curcumin on the production of IL-2, IL-5, granulocyte macophage-colony stimulating factor (GM-CSF), and IL-4 by lymphocytes from atopic asthmatics in response to house dust mites (Dermatophagoides farinea: Df). Curcumin inhibited Df-induced lymphocyte proliferation and production of IL-2. Furthermore, curcumin inhibited IL-5, GM-CSF, and IL-4 production. These results indicate that curcumin may have a potential effect on controlling allergic diseases through inhibiting the production of cytokines affecting eosinophil function and IgE synthesis (Kobayashi et al., 1997).
Ram et al (2003) examined the anti-asthma property of curcumin in a guinea pig model of airway hyper-responsiveness (Ram et al., 2003). Guinea pigs sensitized with ovalbumin (OVA) develop certain features characteristic of asthma: allergen-induced airway constriction and airway hyper-reactivity to histamine. Treatment with curcumin was done during sensitization (preventive) or after developing impaired airway features (therapeutic). Curcumin treatment (20 mg/kg body weight) significantly inhibited OVA-induced airway constriction and airway hyper-reactivity. The results demonstrate that curcumin is effective in improving the impaired airway features in OVA-sensitized guinea pigs.
Kurup et al used a murine model of latex allergy to investigate the role of curcumin as an immunomodulator. BALB/c mice were exposed to latex allergens and developed latex allergy with a thyroid hormone (Th)2-type immune response. These animals were treated with curcumin and the immunologic and inflammatory responses were evaluated. Animals exposed to latex showed enhanced serum IgE, latex-specific IgG1, IL-4, IL-5, IL-13, and eosinophils; and inflammation in the lungs. Intragastric treatment of latex-sensitized mice with curcumin demonstrated a diminished Th2 response with a concurrent reduction in lung inflammation. Eosinophilia in curcumin-treated mice was markedly reduced, co-stimulatory molecule expression (CD80, CD86, and OX40L) on antigen-presenting cells was decreased, and expression of MMP-9, OAT, and TSLP genes was also attenuated. These results suggest that curcumin has potential therapeutic value for controlling allergic responses resulting from exposure to allergens (Kurup and Barrios, 2008, Kurup et al., 2007).
In patients with severe asthma or chronic obstructive pulmonary disease (COPD), an inflammatory condition exists that leads to activation of the NF-κB pathway. A change also occurs in the histone acetylation and deacetylation balance via post-translational modification of histone deacetylases (HDACs). HDAC2 plays a major role in insensitivity to corticosteroid treatment in asthma and COPD. It has been shown that curcumin can restore HDAC activity, thereby restoring corticosteroid function (Marwick et al., 2007) (Biswas and Rahman, 2008).
2.5. Inflammatory bowel disease
Inflammatory bowel disease (IBD), a major risk factor for colon cancer, is characterized by oxidative and nitrosative stress, leucocyte infiltration, and upregulation of proinflammatory cytokines (Jess et al., 2005) (Danese, 2008). Numerous therapies used for IBD target NF-kB, which is involved in the production of cytokines and chemokines integral for inflammation. Curcumin has been shown to attenuate colitis in the dinitrobenzene sulfonic acid (DNB)-induced murine model of colitis (Salh et al., 2003). This was accompanied by a reduction in MPO activity, IL-1β expression, and reduction of p38 MAPK. Additionally, an immunohistochemical signal was dramatically attenuated at the level of the mucosa by curcumin. Together, these results suggest that curcumin may have therapeutic implications for human IBD. Ukil et al (2003) investigated the protective effects of curcumin on 2,4,6- trinitrobenzene sulphonic acid (TNBS)-induced colitis in mice, a model for IBD (Ukil et al., 2003). Intestinal lesions were associated with neutrophil infiltration, increased serine protease activity, and high levels of malondialdehyde. Pretreatment of mice with curcumin (50 mg/kg daily intragastrically, for 10 days) significantly ameliorated the appearance of diarrhea and the disruption of colonic architecture. Higher doses (100 and 300 mg/kg) had comparable effects. In curcumin-pretreated mice, there was a significant reduction in the degree of both neutrophil infiltration and lipid peroxidation in the inflamed colon as well as decreased serine protease activity. Curcumin also reduced the levels of NO and O2(−) associated with the favorable expression of Th1 and Th2 cytokines and inducible NOS. Consistent with these observations, NF-κB activation in colonic mucosa was suppressed in the curcumin-treated mice, suggesting that curcumin can exert beneficial effects in experimental colitis and may, therefore, be useful in the treatment of IBD.
Jian et al (2005) also assessed the use of curcumin in the prevention and treatment of TNBS-induced colitis in rats (Jian et al., 2005). Sixty rats with TNBS-induced colitis were treated with 2.0% curcumin in the diet. Thirty positive control rats were treated with 0.5% sulfasalazine (SASP). Thirty negative control rats and 30 model rats were treated with a general diet. Treatment with curcumin prevented and treated both wasting and histopathologic signs of rats with TNBS-induced intestinal inflammation. In accordance with these findings, NF-kB activation in colonic mucosa was suppressed in the curcumin-treated groups. Degradations of cytoplasmic IκBα protein in colonic mucosa were blocked by curcumin treatment. Proinflammatory cytokine messenger RNA expression in colonic mucosa was also suppressed (Jian et al., 2005).
Reduced bone mass is a common complication of IBD, although the mechanisms that contribute to osteopenia are not completely understood (Rodriguez-Bores et al., 2007). TNF-α is upregulated in patients with IBD and has detrimental effects on osteoblasts. Phex gene is expressed predominantly in osteoblasts, and its disruption results in defective bone mineralization. Uno (2006) examined whether TNF-α regulates Phex gene expression thus contributing to the abnormal bone metabolism observed in IBD (Uno et al., 2006). Phex gene expression was evaluated in calvaria of 6- to 7-week-old mice administered TNBS with or without neutralizing anti-TNF-alpha antibody, dietary curcumin, or systemically with recombinant TNF-α. TNF-α-treated UMR-106 osteoblasts were also examined. Compared with control animals, Phex mRNA expression decreased by 40%-50% in both TNBS colitis and TNF-alpha-injected mice. Dietary curcumin and anti-TNF-α antibody counteracted the detrimental effect of TNBS on Phex gene expression. TNF-α-treated UMR-106 cells showed a decrease in Phex mRNA and gene promoter activitis. Coinciding with decreased Phex protein level, TNF-α drastically reduced mineralization in UMR-106 osteoblasts. Acute colitis and TNF-α decrease Phex mRNA and protein expression via a transcriptional mechanism. TNF-α-mediated reduction in Phex protein is at least partially responsible for inhibition of osteoblast mineralization, and the described mechanism may contribute to the abnormal bone metabolism associated with IBD.
Deguchi et al (2007) evaluated the effects of curcumin on the development of dextran sulfate sodium (DSS)-induced experimental colitis (Deguchi et al., 2007). BALB/c mice were fed a chow containing either 3.5% (wt/wt) DSS or 3.5% DSS + 2.0% (wt/wt) curcumin. The body weight loss was more apparent in DSS-treated mice than in DSS + curcumin-treated mice. The disease activity index, histologic colitis score, and MPO activity were all significantly higher in DSS-treated mice than in DSS + curcumin-treated mice. Microscopically, mucosal edema, cellular infiltration, and epithelial disruption were more severe in DSS-treated mice than in DSS + curcumin-treated mice. In DSS + curcumin-treated mice, NF-κB activation was blocked in the mucosa. Overall, the development of DSS-induced colitis was significantly attenuated by curcumin.
TNBS-induced colitis in NKT-deficient SJL/J mice has been described as Th1-mediated inflammation, whereas BALB/c mice are believed to exhibit a Th1/Th2 response. Billerey-Larmonier et al (2008) investigated the effect of dietary curcumin in colitis induced in these 2 strains (Billerey-Larmonier et al., 2008). In the BALB/c mice, curcumin significantly increased survival, prevented weight loss, and normalized disease activity. In the SJL/J mice, curcumin demonstrated no protective effects. Genome-wide microarray analysis of colonic gene expression was employed to define the differential effect of curcumin in these 2 strains. This analysis not only confirmed the disparate responses of the 2 strains to curcumin but also indicated different responses to TNBS. Curcumin inhibited proliferation of splenocytes from naive BALB/c mice but not SJL/J mice when nonspecifically stimulated in vitro with concanavalin A (ConA). Proliferation of CD4(+) splenocytes was inhibited in both strains, albeit with about a 2-fold higher IC(50) in SJL/J mice. Secretion of IL-4 and IL-5 by CD4(+) lymphocytes of BALB/c mice but not SJL/J mice was significantly augmented by Con A and reduced to control levels by curcumin. Why BALB/c strain of mice responds to curcumin and SJL/J mouse strain does not, is not clear but suggests that the therapeutic value of dietary curcumin may differ depending on the nature of immune dysregulation in IBD.
On the basis of these study results in rodents, Holt et al (2005) performed a pilot clinical study with curcumin in patients with IBD. A pure curcumin preparation was administered in an open-label study to 5 patients with ulcerative proctitis and 5 with Crohn’s disease. All proctitis patients improved, with reductions in concomitant medications in 4, and 4 of the 5 patients with Crohn’s disease patients had lowered CDAI scores and sedimentation rates (Holt et al., 2005). The results of this encouraging pilot study suggest the need for double-blind placebo-controlled follow-up studies.
Thus curcumin, overall, exhibits a protective role in mouse models of IBD and to reduce the relapse rate in human ulcerative colitis (UC), thus making it a potentially viable supportive treatment option.
2.6. Rheumatoid arthritis (RA) and other arthritide diseases
There are more than 100 different arthritides; however, the 3 most commonly occurring subtypes in the Western world are gout, osteoarthritis (OS), and RA. Gout occurs in response to the presence of crystals of monosodium urate in joints, bones, and soft tissues, and is treated with non-steroidal anti-inflammatory agents (NSAIDs); oral or intravenous colchicines; and oral, intravenous, or intra-articular glucocorticoids (Li, 2004) (Liote and Ea, 2007) (Schlesinger et al., 2006). All are effective in aborting acute attacks of gout; however, they have severe side effects. Osteo arthritis (OA), the second most common arthritis worldwide, results from articular cartilage failure induced by a combination of genetic, metabolic, biochemical, and biomechanical factors. OA is normally treated with analgesics such as acetaminophen and opioids, NSAIDs, and intra-articular therapies such as glucocorticoids and hyaluronans.
The third most common arthritide, RA, is a chronic proinflammatory disease that is characterized by hyperplasia of the synovial fibroblasts, which is partly the result of decreased apoptosis, and joint stiffness and swelling, often manifesting in a symmetrical pattern on both sides of the body. Like most other autoimmune diseases, arthritis is more prevalent in the Western world than in other countries. Although the precise reason for this predilection is not well understood, lifestyle is known to play a major role. RA occurs in women more often than men (75% vs 25%), suggesting the role of hormones in its etiology. The roles of inflammatory cytokines, such as TNF, IL-1, IL-6, and chemokines; inflammatory enzymes such as COX-2, 5-LOX, and MMP-9; and adhesion molecules in the pathogenesis of arthritis are well documented. Almost all the mediators of inflammation linked with arthritis have been shown to be regulated by the transcription factor NF-κB. Smoking and stress are thought to contribute to RA.
The goals of management of patients with RA are to control pain and swelling, delay disease progression, minimize disability, and improve quality of life. For pain control and swelling, the treatment includes analgesics such as acetaminophen and opioids, NSAIDs, and intra-articular therapies such as glucocorticoids. In addition, diseases modifying antirheumatic drugs are used to modify the clinical and radiological courses of RA (Smolen and Aletaha, 2008a). Examples include methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, and newer therapies such as anti-TNF-α therapy (etanercept, infliximab, and adalimumab), anti-CD20 therapy (rituximab), and abatacept. All of these agents are associated with numerous side effects (Smolen and Aletaha, 2008b). Because current treatments for arthritis are inefficient, produce substantial side effects, and tend to be expensive, natural products, which are devoid of such disadvantages, offer a novel treatment opportunities (Hak and Choi, 2008) (Sale et al., 2008). Numerous reports suggest that curcumin has potential in the treatment of arthritis. Joe et al examined the effect of curcumin on acidic glycoprotein in the sera of rats with adjuvant-induced arthritis (Joe et al., 1997). Increased levels of a glycoprotein with an apparent molecular weight of 72 kDa (Gp A72), an acidic protein with a pI of 5.1 with antitryptic activity, were observed in the sera of arthritic rats. The appearance of Gp A72 in these sera preceded the onset of paw inflammation in arthritic rats and persisted in the chronic phase. Oral administration of curcumin lowered the levels of Gp A72 by 73% with concomitant lowering of paw inflammation in arthritic rats.
Neutral matrix metalloproteinases (MMPs) are responsible for the pathologic features of RA and causes the degradation of cartilage. Onodera et al (2000) examined the effect of curcumin on the upregulation of MMP-1 and MMP-3 mRNAs on the cultured synovial fibroblasts retrieved from patients with RA in response to MIF. They showed that mRNA upregulation of MMPs was inhibited by curcumin (Onodera et al., 2000).
How curcumin could affect the immune response of the body during adjuvant-induced chronic inflammation in rats has also been investigated (Banerjee et al, 2003). Inflammatory mediators were estimated on day 21 and day 35 after adjuvant injection. The level of CRP increased to 200% on day 21 and then reduced to 50% on day 35 compared to controls. Curcumin further reduced the increased levels at both time intervals. The haptoglobin level decreased to 42% on day 21 but increased to 5 times that of controls on day 35. Curcumin reduced the increased levels at day 35. No significant change was observed in prostaglandin-E (PGE) 2 and leukotriene-B4 levels nor in lymphocyte proliferation. The level of TNF-α increased 3-fold on day 21, but reduced to 88% on day 35. Ibuprofen treatment decreased the raised level on day 21 and increased the reduced level on day 35. IL-1β increased 2-folds on day 21 and 10-fold on day 35, both of which were significantly reduced by curcumin (Banerjee et al., 2003). In another study, Liacini et al (2003) showed that curcumin suppressed TNF-α-induced MMP-13 expression in primary chondrocytes; thus curcumin may reduce cartilage breakdown by MMP-13 in arthritis (Liacini et al., 2003).
OA is the leading cause of disability in the Western world. COX-2 inhibitors are efficient anti-inflammatory agents commonly used in the treatment of OA (Chen et al., 2008). However, recent studies have shown that their long-term use may be limited due to cardiovascular toxicity (Kean and Buchanan, 2005). Whether curcumin can augment the growth-inhibitory and pro-apoptotic effects of celecoxib in OA synovial adherent cells was examined by Lev-Ari et al (2006). OA synovial adherent cells were prepared from human synovial tissue collected during total knee replacement surgery. A synergistic effect was observed in inhibition of cell growth when the cells were exposed to celecoxib combined with curcumin. The inhibitory effect of the combination of these drugs on cell growth resulted in an increased induction of apoptosis. The synergistic effect was mediated through a mechanism that involves inhibition of COX-2 activity. Thus the use of celecoxib at lower and safer concentrations in combination with curcumin may provide a novel combination treatment in OA and other rheumatologic disorders (Lev-Ari et al., 2006).
Funk et al (2006) examined the in vivo efficacy of curcumin in the prevention and treatment of arthritis using streptococcal cell wall–induced arthritis, a well-described animal model of RA (Funk et al., 2006). Arthritic index, a clinical measure of joint swelling, was used as the primary end point for assessing the effect of extracts on joint inflammation. Curcumin was found to prevent joint inflammation when treatment was started before, but not after, the onset of joint inflammation. These data document the in vivo anti-arthritic efficacy of curcumin. Jackson et al recently reported that curcumin can inhibit inflammatory processes associated with arthritis (Jackson et al., 2006).
Park et al (2006) showed that curcumin induces apoptosis and inhibits PGE(2) production in synovial fibroblasts of patients with RA. Curcumin caused the downregulation of anti-apoptotic Bcl-2 and the X-linked inhibitor of the apoptosis protein as well as the upregulation of pro-apoptotic Bax expression. Curcumin-induced apoptosis was also associated with the proteolytic activation of caspase-3 and caspase-9 and the concomitant degradation of poly(ADP-ribose) polymerase protein. Furthermore, curcumin decreased the expression levels of the COX-2 mRNA and protein without causing significant changes in the COX-1 levels, which was correlated with the inhibition of PGE (2) synthesis (Park et al., 2007).
A clinical pilot study of curcumin 1200 mg/per day administered to a small number of patients also revealed the anti-rheumatic activity of curcumin (Deodhar et al., 1980).
2.7. Renal ischemia
Nonimmune renal injury plays an important role in acute and chronic rejection by triggering an I/R injury response through cytokine and chemokine release. Shoskes (1998) examined the effect of curcumin on I/R in rats. Pretreatment with curcumin resulted in preservation of histologic integrity, with a decrease in tubular damage and interstitial inflammation and reversal of changes in creatinin levels. I/R induced RANTES, MCP-1, and AIF proteins at high levels in kidneys, but pretreatment with curcumin strongly attenuated this expression (Shoskes, 1998). Chronic renal allograft nephropathy is associated with both immune and ischemic injury, which may act synergistically to promote an inflammatory response. Whether curcumin can ameliorate such injury, was examined by Jones and Shoskes (2000). The effects of curcumin in models of ischemic renal injury and skin allograft rejection were examined (Jones and Shoskes, 2000). Curcumin decreased the tubular damage, attenuated renal inflammation, and prolonged skin graft survival; decreased serum creatinine; inhibited apoptosis at day 2; and attenuated the expression levels of RANTES, MCP-1, and AIF. Thus curcumin was found to be renoprotective. The same groups also showed that curcumin can upregulate antioxidant gene expression in rat kidney after ureteral obstruction or I/R injury (Shahed et al., 2001). Renal ischemia followed by reperfusion leads to acute renal failure in both native kidneys and renal allografts. Curcumin significantly improved the I/R-induced changes in urea and cystatin C levels and reversed changes in serum GSH-Px; however, the drug had no effect on SOD enzyme activity. Treatment with curcumin also resulted in significant reduction in serum and tissue MDA, NO, and PC. On histologic examination, the rats treated with curcumin had nearly normal morphology of the kidney (Bayrak et al., 2008). Thus it is clear that curcumin protects the kidneys against I/R injury via its antioxidant effects.
2.8. Psoriasis
Psoriasis is a proinflammatory skin disease in which the roles of NF-kB, STAT3, and TNF are well documented (Aggarwal et al., 2006b) (Liu et al., 2006) (Abdou and Hanout, 2008). TNF blockers have been approved for the treatment of psoriasis (Vamvouris and Hadi, 2006). Furthermore, skin-specific STAT3 transgenic animals are known to develop psoriasis (Sano et al., 2005). Numerous lines of evidence suggest that curcumin may be an effective treatment for psoriasis. Topical application of 1% curcumin gel to psoriatic areas reduced the density of CD8+ T cells compared to their density in untreated areas; in fact, the density of CD8+ T cells was elevated. These study results and those of others suggest that curcumin could be an effective paradigm in the treatment of psoriasis as it could also reduce the activity of PhK. Decreased PhK activity in patients with psoriasis who were treated with curcumin and calcipotriol was associated with corresponding decreases in keratinocyte transferrin receptor expression, severity of parakeratosis, and density of epidermal CD8+ T cells. These results suggest that curcumin-induced suppression of PhK activity is associated with resolution of psoriatic activity as assessed by clinical, histologic, and immunohistochemical criteria.
Curcumin, at a very low concentration, has been shown to be a photosensitizer in killing bacteria such as Salmonella typhimurium and Escherichia coli (Tonnesen et al., 1987). The photosensitizing effects of curcumin may approve effective in the treatment of psoriasis.
Pharmacological treatments for psoriasis are generally based on antiproliferative, anti-inflammatory, or differentiation-modifying activity or some combination of these actions. Like most anti-psoriatic drugs, curcumin was found to inhibit the keratinocyte proliferation (Pol et al., 2003), suggesting its potential in suppressing posriasis.
Kurd and colleagues (2008) assessed the safety and efficacy of oral curcumin in patients with psoriasis. They conducted a phase II, open-label, Simon’s two-stage trial of 4.5 g/d oral curcumin in patients with plaque psoriasis. The study end points included improvement in Physicians Global Assessment score, Psoriasis Area and Severity Index score, and safety throughout the study. The intent-to-treat analysis response rate was 16.7% (95% confidence interval 2%–48%), and both responders achieved a Psoriasis Area and Severity Index score of 75. There were no study-related adverse events that necessitated participant withdrawal. The researchers concluded that large placebo-controlled studies are necessary before recommending oral curcumin as treatment for psoriasis (Kurd et al., 2008).
2.9. Scleroderma
Because scleroderma is a disease that involves excessive collagen deposition and hyperproliferation of fibroblasts, curcumin may be able to provide a therapeutic benefit through its ability to suppress the proliferation of lung fibroblasts in a process involving the inhibition of protein kinase C epsilon (PKCε) (Tourkina et al., 2004). In the study by Tourkina and colleagues, curcumin was found to induce apoptosis in scleroderma lung fibroblasts (SLFs) but not in normal lung fibroblasts (NLF), and the effect was related to the expression of PKCepsilon. Thus PKC epsilon and phase 2 detoxification enzymes provide protection against curcumin-induced apoptosis in NLF and are defective in SLF. Curcumin may have therapeutic value in treating scleroderma, just as it has already been shown to protect rats from lung fibrosis induced by a variety of agents (Thresiamma et al., 1996) (Punithavathi et al., 2000) (Xu et al., 2007).
2.10. Acquired immunodeficiency disease (AIDS)
There are several reports indicating that curcumin may be an effective treatment for AIDS (Vlietinck et al., 1998). These effects of curcumin are mediated through suppression of replication of the human immunodeficiency virus (HIV) virus by inhibition of HIV long-terminal repeats (Barthelemy et al., 1998), HIV protease (Vajragupta et al., 2005), inhibition of HIV-1 integrase (Mazumder et al., 1995), inhibition of p300/CREB-binding protein-specific acetyltransferase, repression of the acetylation of histone/nonhistone proteins, and histone acetyltransferase-dependent chromatin transcription (Balasubramanyam et al., 2004). Thus curcumin has a great potential as a treatment for AIDS.
2.11. Cancer
Till now more than 800 reports have been published demonstrating the anticancer potential of curcumin. Various in vitro as well as in vivo studies demonstrated that curcumin can inhibit the growth of various cancer cells from different organs including blood, brain, breast, gastrointestinal system, head and neck, liver, pancreas, colon, prostate, ovary and skin cancers (Anand et al., 2008) (Kunnumakkara et al., 2008). Various clinical trials with curcumin, those that have been completed and those that are ongoing have been recently reviewed (Goel et al., 2008). These trials have shown promise in patients with familial adenomatous polyposis (Cruz-Correa et al., 2006), advanced pancreatic cancer (Dillon et al, 2007), and multiple myeloma (Vadhan et al, 2008). Another study reported by Sharma et al demonstrated that in advance colon cancer patients curcumin is well tolerated at all doses and no dose limiting toxicity was observed (Sharma et al., 2004). However no partial response to treatment was observed. They concluded that systemic pharmacological properties of a daily dose of 3.6 g of curcumin are suitable for its evaluation in the prevention of malignancies at sites other than the gastrointestinal tract.
3. Bioavailability of curcumin
That curcumin exhibits poor bioavailability is well documented (Anand et al., 2007) (Sharma et al., 2007a). The major reasons attributed to the low bioavailability of curcumin are poor absorption, rapid metabolism, and rapid systemic elimination. In humans a comprehensive pharmacokinetic data do not exist. The pilot studies summarized that low systemic bioavailability is observed in humans following oral dosing. First phase I clinical trial of curcumin was done in 25 patients with high-risk or pre-malignant lesions (Cheng et al., 2001). The starting dose was 500 mg/day and if no toxicity was noted, the dose was then escalated to another level in the order of 1,000, 2,000, 4,000, 8,000, and 12,000 mg/day. There was no treatment-related toxicity up to 8 g/day but the bulky volume of the drug was unacceptable to the patients beyond 8 g/day. The serum concentration of curcumin usually peaked at 1 to 2 hours after oral intake of curcumin and gradually declined within 12 hours. The average peak serum concentrations after taking 4,000 mg, 6,000 mg and 8,000 mg of curcumin were 0.51 +/− 0.11 μM, 0.63 +/− 0.06 μM and 1.77 +/− 1.87 μM, respectively. Urinary excretion of curcumin was undetectable. Lao et al conducted a pilot study in 24 healthy subjects and they administered curcumin up to 12g/day. They detected curcumin in the serum samples of only those who took 10 and 12g/day (Lao et al., 2006). It was reported that a daily oral dose of 3.6g of curcumin results in detectable levels in colorectal tissue, which might be sufficient to exert pharmacological activity (Sharma et al., 2004). Curcumin undergoes metabolism in the liver particularly via glucuronidation and sulfation. The metabolites of curcumin such as glucoronides appear to lack any pharmacological activity.
The systemic elimination of curcumin is another contributing factor for low bioavailability of curcumin. The initial reports by Wahlstorm and Blennow showed that after oral administration of 1g/kg curcumin to rats, more than 75% of curcumin was excreted in feces and negligible amount was detected in urine (Wahlstrom and Blennow, 1978).
How to increase the bioavailability of curcumin, is also being explored. The roles of adjuvants, which can block the metabolism of curcumin, are of great interest. Combining curcumin with piperine has been shown to increase the bioavailability in rats and in human subjects. Piperine is an inhibitor of glucuronidation of curcumin. The study conducted by Shobha et al demonstrated that concomitant administration of curcumin with piperine produced 150% increase in bioavailability in rats and 2000% increase in man (Shoba et al., 1998) (Shoba et al, 1998). Other ways to improve the bioavailability of curcumin is by making curcumin nanoparticles (Bisht et al., 2007), liposomes (Li et al., 2005), micelles and phoshoplipid complexes (Maiti et al., 2007) (Marczylo et al., 2007). The possible advantages attributed to such formulations are (a) provide longer circulation; (b) increase the cellular permeability and (c) induce resistance to metabolic processes.
4. Potential side effects of curcumin
Food and drug administration has declared curcumin as “generally regarded as safe” GRAS. Even though curcumin exhibits a wide variety of pharmacological activities and has been found to be quite safe in animals and humans, there are some reports concerning its toxicity (Lopez-Lazaro, 2008). The National Toxicology Program (NTP) evaluated the short term as well as long-term toxicity of turmeric oleoresin (79–85% curcumin) in F344/N rats and B6C3F1 mice. Animals were fed diets containing the turmeric extract at different concentrations (1000, 5000, 10, 000, 25,000 or 50,000 ppm that delivers daily doses of 50, 250, 480, 1300 or 2600 mg/kg body weight) for periods of 13 weeks or two years. In 13-week study, no death was attributed to curcumin and only toxicity noted was relative increase in liver weight, stained fur, discolored faces, and hyperplasia of the mucosal epithelium in the cecum and colon of rats that received 50,000 ppm. No sign of carcinogenic lesions was observed. In 2 years study, the turmeric administration did not have any effect on the food consumption when compared to controls and no mortality was observed in both male and female rats. In 50,000 ppm group, however, rats developed ulcers, chronic active inflammation, hyperplasia of the cecum, and forestomach, increased incidences of clitoral gland adenomas, the development of hepatocellular adenoma and intestinal carcinoma (NTP, 1993).
Although numerous reports have been published on the synergistic effects of curcumin with chemotherapy (Bharti et al., 2003b) (Aggarwal et al., 2005) (Kamat et al., 2007) (Kunnumakkara et al., 2007) (Lin et al., 2007), a report by Somasundaram et al (2002) showed that administration of curcumin (2.5% curcumin w/w) abolished the effect of cyclophosphamide on reduction of tumor size in human breast cancer xenografts in nude mice (Somasundaram et al., 2002). The major draw back of the study was very short duration (3 days) of treatment of mice with curcumin. Why curcumin exhibits such opposing effects is not clear. It has been reported that curcumin exhibit both antioxidant and prooxidant activities (Sandur et al., 2007a, Sandur et al., 2007b). It is possible that these opposing activities of curcumin are regulated by the concentration when the effect of curcumin may switches from antioxidant to prooxidant. This may correspond to the switch from anticarcinogenic to carcinogenic agent, from chemoresistant to chemosensitizer or from radioresistant to radiosensensitizer.
So far there has not been any long-term study with curcumin, which shows its toxic or adverse effects. Such studies are necessary in both rodents as well as in human subjects to determine the safety of curcumin. The available epidemiological evidence, however, shows that the incidence several types of cancer are low in populations taking curcumin around 100–200 mg/day over long periods of time.
5. Conclusions
The wisdom and scientific credentials of curcumin in the Ayurvedic and Chinese systems of medicine have been corroborated by numerous studies conducted over the past 30 years. These observations are also supported by epidemiological data suggesting lower incidence of chronic diseases in people from countries where curcumin is consumed. The various effects of curcumin has been widely studied in Western systems of medicine for decades, and has been found to possess antioxidant and anti-inflammatory activities. Considering that inflammation plays a major role in most chronic illnesses, anti-inflammatory agents are needed for prevention purposes. Although several different steroids and NSAIDS (such as celecoxib, aspirin, ibuprofen, phenylbutazole, etc) have been approved for treatment of inflammatory conditions, most of them have side effects, especially when consumed over long periods of time. Because curcumin inhibits multiple proinflammatory pathways and is affordable, this phytochemical should be further explored for prevention and treatment of various chronic diseases. Further clinical trials are needed to fully develop the potential of this “age-old NSAID”.
Acknowledgments
We would like to thank Vickie J Williams for carefully proofreading the manuscript and providing valuable comments. We would like to thank Preetha Anand and Vijayalekshmi Nair for assistance with references. Dr. Aggarwal is a Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research to B. B. A., and the National Institutes of Health core grant (CA16672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdou AG, Hanout HM. Evaluation of survivin and NF-kappaB in psoriasis, an immunohistochemical study. J Cutan Pathol. 2008;35:445–451. doi: 10.1111/j.1600-0560.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985a;318:665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, Bringman TS, Nedwin GE, Goeddel DV, Harkins RN. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985b;260:2345–2354. [PubMed] [Google Scholar]
- Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102:580–592. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006a;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Shishodia S, Takada Y, Jackson-Bernitsas D, Ahn KS, Sethi G, Ichikawa H. TNF blockade: an inflammatory issue. Ernst Schering Res Found Workshop. 2006b:161–186. doi: 10.1007/3-540-37673-9_10. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006c;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004;111:679–692. doi: 10.1002/ijc.20333. [DOI] [PubMed] [Google Scholar]
- Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Annaert W, De Strooper B. A cell biological perspective on Alzheimer’s disease. Annu Rev Cell Dev Biol. 2002;18:25–51. doi: 10.1146/annurev.cellbio.18.020402.142302. [DOI] [PubMed] [Google Scholar]
- Ansell BJ. Targeting the anti-inflammatory effects of high-density lipoprotein. Am J Cardiol. 2007;100:n3–9. doi: 10.1016/j.amjcard.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- Arun N, Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum Nutr. 2002;57:41–52. doi: 10.1023/a:1013106527829. [DOI] [PubMed] [Google Scholar]
- Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:3381–3386. doi: 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling KQ, Huang X, Moir RD, Wang D, Sayre LM, Smith MA, Chen SG, Bush AI. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry. 2004;43:560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- Babu PS, Srinivasan K. Influence of dietary curcumin and cholesterol on the progression of experimentally induced diabetes in albino rat. Mol Cell Biochem. 1995;152:13–21. doi: 10.1007/BF01076459. [DOI] [PubMed] [Google Scholar]
- Babu PS, Srinivasan K. Hypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic rats. Mol Cell Biochem. 1997;166:169–175. doi: 10.1023/a:1006819605211. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Tripathi LM, Srivastava VM, Puri A, Shukla R. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacol Immunotoxicol. 2003;25:213–224. doi: 10.1081/iph-120020471. [DOI] [PubMed] [Google Scholar]
- Barthelemy S, Vergnes L, Moynier M, Guyot D, Labidalle S, Bahraoui E. Curcumin and curcumin derivatives inhibit Tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat. Res Virol. 1998;149:43–52. doi: 10.1016/s0923-2516(97)86899-9. [DOI] [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis, 6. 2004:367–377; discussion 443–369. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca AF, Sahin S, Yildirim ME, Kaya A, Cimentepe E, Akcay A. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol. 2008;26:285–291. doi: 10.1007/s00345-008-0253-4. [DOI] [PubMed] [Google Scholar]
- Best L, Elliott AC, Brown PD. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem Pharmacol. 2007;73:1768–1775. doi: 10.1016/j.bcp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003a;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003b;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- Billerey-Larmonier C, Uno JK, Larmonier N, Midura AJ, Timmermann B, Ghishan FK, Kiela PR. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14:780–793. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishnoi M, Chopra K, Kulkarni SK. Protective effect of Curcumin, the active principle of turmeric (Curcuma longa) in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes in rat brain. Pharmacol Biochem Behav. 2008;88:511–522. doi: 10.1016/j.pbb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Rahman I. Modulation of steroid activity in chronic inflammation: A novel anti-inflammatory role for curcumin. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200700259. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Giuffrida Stella AM, Galli F, Butterfield DA. Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids. 2003;25:437–444. doi: 10.1007/s00726-003-0048-2. [DOI] [PubMed] [Google Scholar]
- Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, Taylor RS. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1–178. doi: 10.3310/hta12110. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Danese S. Inflammatory bowel disease and inflammation-associated colon cancer: partners in crime. Curr Drug Targets. 2008;9:360. doi: 10.2174/138945008784221134. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, Gautam SC. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;321:616–625. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Andoh A, Inatomi O, Yagi Y, Bamba S, Araki Y, Hata K, Tsujikawa T, Fujiyama Y. Curcumin prevents the development of dextran sulfate Sodium (DSS)-induced experimental colitis. Dig Dis Sci. 2007;52:2993–2998. doi: 10.1007/s10620-006-9138-9. [DOI] [PubMed] [Google Scholar]
- Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane) Indian J Med Res. 1980;71:632–634. [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Rurzrock R. Phase II trial of Curcumin, an NF-κB inhibitor, in patients with advanced pancreatic cancer. Clin Cancer Res (Accepted) doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Farhangkhoee H, Khan ZA, Chen S, Chakrabarti S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutr Metab (Lond) 2006;3:27. doi: 10.1186/1743-7075-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Chen S, Chiu J, George B, Chakrabarti S. Regulation of Cardiomyocyte Hypertrophy in Diabetes at the Transcriptional Level. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.00029.2008. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, Chiang B, Hui J, Mahanian M, Baghaee A, Hong P, Cashman J. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solyom AM, Timmermann BN. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46:273–279. doi: 10.1016/s1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein - a new mechanism for the inhibition of PrP(Sc) accumulation. J Neurochem. 2008;104:1553–1564. doi: 10.1111/j.1471-4159.2007.05105.x. [DOI] [PubMed] [Google Scholar]
- Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA. Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol. 2006;4:215–227. doi: 10.2174/157016106777698469. [DOI] [PubMed] [Google Scholar]
- Hak AE, Choi HK. Lifestyle and gout. Curr Opin Rheumatol. 2008;20:179–186. doi: 10.1097/BOR.0b013e3282f524a2. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- Hold GL, El-Omar ME. Genetic aspects of inflammation and cancer. Biochem J. 2008;410:225–235. doi: 10.1042/BJ20071341. [DOI] [PubMed] [Google Scholar]
- Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT. Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann N Y Acad Sci. 2004;1012:153–163. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- Jackson JK, Higo T, Hunter WL, Burt HM. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res. 2006;55:168–175. doi: 10.1007/s00011-006-0067-z. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Aggarwal BB. Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–2729. doi: 10.1111/j.1572-0241.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005;11:1747–1752. doi: 10.3748/wjg.v11.i12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe B, Rao UJ, Lokesh BR. Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol Cell Biochem. 1997;169:125–134. doi: 10.1023/a:1006877928703. [DOI] [PubMed] [Google Scholar]
- Jones EA, Shoskes DA. The effect of mycophenolate mofetil and polyphenolic bioflavonoids on renal ischemia reperfusion injury and repair. J Urol. 2000;163:999–1004. [PubMed] [Google Scholar]
- Ju HR, Wu HY, Nishizono S, Sakono M, Ikeda I, Sugano M, Imaizumi K. Effects of dietary fats and curcumin on IgE-mediated degranulation of intestinal mast cells in brown Norway rats. Biosci Biotechnol Biochem. 1996;60:1856–1860. doi: 10.1271/bbb.60.1856. [DOI] [PubMed] [Google Scholar]
- Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol Cancer Ther. 2007;6:1022–1030. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- Kanitkar M, Bhonde RR. Curcumin treatment enhances islet recovery by induction of heat shock response proteins, Hsp70 and heme oxygenase-1, during cryopreservation. Life Sci. 2008;82:182–189. doi: 10.1016/j.lfs.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Saito T, Manabe I, Imai Y, Nagai R. C-reactive protein induces VCAM-1 gene expression through NF-kappaB activation in vascular endothelial cells. Atherosclerosis. 2006;185:39–46. doi: 10.1016/j.atherosclerosis.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Kean WF, Buchanan WW. The use of NSAIDs in rheumatic disorders 2005: a global perspective. Inflammopharmacology. 2005;13:343–370. doi: 10.1163/156856005774415565. [DOI] [PubMed] [Google Scholar]
- Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci Lett. 2001;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hashimoto S, Horie T. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem Pharmacol. 1997;54:819–824. doi: 10.1016/s0006-2952(97)00220-7. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: behavioral and biochemical evidences. Eur J Pharmacol. 2007;576:34–42. doi: 10.1016/j.ejphar.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Haseeb A, Suryanarayana P, Ehtesham NZ, Reddy GB. Elevated expression of alphaA- and alphaB-crystallins in streptozotocin-induced diabetic rat. Arch Biochem Biophys. 2005;444:77–83. doi: 10.1016/j.abb.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT, Gelfand JM. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: A prospective clinical trial. J Am Acad Dermatol. 2008;58:625–631. doi: 10.1016/j.jaad.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Mimaki Y, Nishiyama T, Mae T, Kishida H, Tsukagawa M, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol Pharm Bull. 2005;28:937–939. doi: 10.1248/bpb.28.937. [DOI] [PubMed] [Google Scholar]
- Kurup VP, Barrios CS. Immunomodulatory effects of curcumin in allergy. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200700293. [DOI] [PubMed] [Google Scholar]
- Kurup VP, Barrios CS, Raju R, Johnson BD, Levy MB, Fink JN. Immune response modulation by curcumin in a latex allergy model. Clin Mol Allergy. 2007;5:1. doi: 10.1186/1476-7961-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Ari S, Strier L, Kazanov D, Elkayam O, Lichtenberg D, Caspi D, Arber N. Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology (Oxford) 2006;45:171–177. doi: 10.1093/rheumatology/kei132. [DOI] [PubMed] [Google Scholar]
- Li EK. Gout: a review of its aetiology and treatment. Hong Kong Med J. 2004;10:261–270. [PubMed] [Google Scholar]
- Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, Huang Y, He CW, Shi Y, Chen X, Nghiem MP, Liu Y, Chen M, Dawood F, Fukuoka M, Maekawa Y, Zhang L, Leask A, Ghosh AK, Kirshenbaum LA, Liu PP. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest. 2008;118:879–893. doi: 10.1172/JCI32865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- Liacini A, Sylvester J, Li WQ, Huang W, Dehnade F, Ahmad M, Zafarullah M. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288:208–217. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65:S140–146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, Lutgendorf SK, Aggarwal BB, Sood AK. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- Liote F, Ea HK. Recent developments in crystal-induced inflammation pathogenesis and management. Curr Rheumatol Rep. 2007;9:243–250. doi: 10.1007/s11926-007-0039-5. [DOI] [PubMed] [Google Scholar]
- Liu H, Moroi Y, Yasumoto S, Kokuba H, Imafuku S, Nakahara T, Dainichi T, Uchi H, Tu Y, Furue M, Urabe K. Immunohistochemical localization of activated Stat3 and hTERT protein in psoriasis vulgaris. Eur J Dermatol. 2006;16:205–207. [PubMed] [Google Scholar]
- Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- Mach F. Inflammation is a crucial feature of atherosclerosis and a potential target to reduce cardiovascular events. Handb Exp Pharmacol. 2005:697–722. doi: 10.1007/3-540-27661-0_26. [DOI] [PubMed] [Google Scholar]
- Mahesh T, Sri Balasubashini MM, Menon VP. Photo-irradiated curcumin supplementation in streptozotocin-induced diabetic rats: effect on lipid peroxidation. Therapie. 2004;59:639–644. doi: 10.2515/therapie:2004110. [DOI] [PubMed] [Google Scholar]
- Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Manikandan P, Sumitra M, Aishwarya S, Manohar BM, Lokanadam B, Puvanakrishnan R. Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int J Biochem Cell Biol. 2004;36:1967–1980. doi: 10.1016/j.biocel.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Marwick JA, Ito K, Adcock IM, Kirkham PA. Oxidative stress and steroid resistance in asthma and COPD: pharmacological manipulation of HDAC-2 as a therapeutic strategy. Expert Opin Ther Targets. 2007;11:745–755. doi: 10.1517/14728222.11.6.745. [DOI] [PubMed] [Google Scholar]
- Mazumder A, Raghavan K, Weinstein J, Kohn KW, Pommier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol. 1995;49:1165–1170. doi: 10.1016/0006-2952(95)98514-a. [DOI] [PubMed] [Google Scholar]
- McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:461–469. doi: 10.1161/01.ATV.0000257552.94483.14. [DOI] [PubMed] [Google Scholar]
- Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007;577:183–191. doi: 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Moller DE, Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S17–21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008;118:868–878. doi: 10.1172/JCI33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan P, Pari L. Effect of tetrahydrocurcumin on erythromycin estolate-induced lipid peroxidation in rats. J Basic Clin Physiol Pharmacol. 2005;16:1–15. doi: 10.1515/jbcpp.2005.16.1.1. [DOI] [PubMed] [Google Scholar]
- Murugan P, Pari L. Effect of tetrahydrocurcumin on lipid peroxidation and lipids in streptozotocin-nicotinamide-induced diabetic rats. Basic Clin Pharmacol Toxicol. 2006;99:122–127. doi: 10.1111/j.1742-7843.2006.pto_447.x. [DOI] [PubMed] [Google Scholar]
- Murugan P, Pari L. Influence of tetrahydrocurcumin on erythrocyte membrane bound enzymes and antioxidant status in experimental type 2 diabetic rats. J Ethnopharmacol. 2007a;113:479–486. doi: 10.1016/j.jep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Murugan P, Pari L. Influence of tetrahydrocurcumin on hepatic and renal functional markers and protein levels in experimental type 2 diabetic rats. Basic Clin Pharmacol Toxicol. 2007b;101:241–245. doi: 10.1111/j.1742-7843.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168:6506–6513. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- Nirmala C, Puvanakrishnan R. Effect of curcumin on certain lysosomal hydrolases in isoproterenol-induced myocardial infarction in rats. Biochem Pharmacol. 1996;51:47–51. doi: 10.1016/0006-2952(95)02118-3. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53:959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- NTP. NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%–85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1993;427:1–275. [PubMed] [Google Scholar]
- Odrowaz-Sypniewska G. Markers of pro-inflammatory and pro-thrombotic state in the diagnosis of metabolic syndrome. Adv Med Sci. 2007;52:246–250. [PubMed] [Google Scholar]
- Olszanecki R, Jawien J, Gajda M, Mateuszuk L, Gebska A, Korabiowska M, Chlopicki S, Korbut R. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol. 2005;56:627–635. [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Onodera S, Kaneda K, Mizue Y, Koyama Y, Fujinaga M, Nishihira J. Macrophage migration inhibitory factor up-regulates expression of matrix metalloproteinases in synovial fibroblasts of rheumatoid arthritis. J Biol Chem. 2000;275:444–450. doi: 10.1074/jbc.275.1.444. [DOI] [PubMed] [Google Scholar]
- Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- Pan CJ, Shao ZY, Tang JJ, Wang J, Huang N. In vitro studies of platelet adhesion, activation, and protein adsorption on curcumin-eluting biodegradable stent materials. J Biomed Mater Res A. 2007a;82:740–746. doi: 10.1002/jbm.a.31108. [DOI] [PubMed] [Google Scholar]
- Pan CJ, Tang JJ, Shao ZY, Wang J, Huang N. Improved blood compatibility of rapamycin-eluting stent by incorporating curcumin. Colloids Surf B Biointerfaces. 2007b;59:105–111. doi: 10.1016/j.colsurfb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Pari L, Murugan P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren Fail. 2007a;29:881–889. doi: 10.1080/08860220701540326. [DOI] [PubMed] [Google Scholar]
- Pari L, Murugan P. Tetrahydrocurcumin prevents brain lipid peroxidation in streptozotocin-induced diabetic rats. J Med Food. 2007b;10:323–329. doi: 10.1089/jmf.2006.058. [DOI] [PubMed] [Google Scholar]
- Park C, Moon DO, Choi IW, Choi BT, Nam TJ, Rhu CH, Kwon TK, Lee WH, Kim GY, Choi YH. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med. 2007;20:365–372. [PubMed] [Google Scholar]
- Parodi FE, Mao D, Ennis TL, Pagano MB, Thompson RW. Oral administration of diferuloylmethane (curcumin) suppresses proinflammatory cytokines and destructive connective tissue remodeling in experimental abdominal aortic aneurysms. Ann Vasc Surg. 2006;20:360–368. doi: 10.1007/s10016-006-9054-7. [DOI] [PubMed] [Google Scholar]
- Patumraj S, Wongeakin N, Sridulyakul P, Jariyapongskul A, Futrakul N, Bunnag S. Combined effects of curcumin and vitamin C to protect endothelial dysfunction in the iris tissue of STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2006;35:481–489. [PubMed] [Google Scholar]
- Pillarisetti S, Saxena U. Role of oxidative stress and inflammation in the origin of Type 2 diabetes--a paradigm shift. Expert Opin Ther Targets. 2004;8:401–408. doi: 10.1517/14728222.8.5.401. [DOI] [PubMed] [Google Scholar]
- Pol A, Bergers M, Schalkwijk J. Comparison of antiproliferative effects of experimental and established antipsoriatic drugs on human keratinocytes, using a simple 96-well-plate assay. In Vitro Cell Dev Biol Anim. 2003;39:36–42. doi: 10.1290/1543-706x(2003)039<0036:coaeoe>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Akhov L, Selvaraj G, Wang M, Alam J. Regulation of heme oxygenase-1 expression by demethoxy curcuminoids through Nrf2 by a PI3-kinase/Akt-mediated pathway in mouse beta-cells. Am J Physiol Endocrinol Metab. 2007;293:E645–655. doi: 10.1152/ajpendo.00111.2007. [DOI] [PubMed] [Google Scholar]
- Punithavathi D, Venkatesan N, Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles JL, Aguilera C, Mesa MD, Ramirez-Tortosa MC, Baro L, Gil A. An ethanolic-aqueous extract of Curcuma longa decreases the susceptibility of liver microsomes and mitochondria to lipid peroxidation in atherosclerotic rabbits. Biofactors. 1998;8:51–57. doi: 10.1002/biof.5520080110. [DOI] [PubMed] [Google Scholar]
- Quiles JL, Mesa MD, Ramirez-Tortosa CL, Aguilera CM, Battino M, Gil A, Ramirez-Tortosa MC. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler Thromb Vasc Biol. 2002;22:1225–1231. doi: 10.1161/01.atv.0000020676.11586.f2. [DOI] [PubMed] [Google Scholar]
- Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003;26:1021–1024. doi: 10.1248/bpb.26.1021. [DOI] [PubMed] [Google Scholar]
- Ramaswami G, Chai H, Yao Q, Lin PH, Lumsden AB, Chen C. Curcumin blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2004;40:1216–1222. doi: 10.1016/j.jvs.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Ramirez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baro L, Ramirez-Tortosa CL, Martinez-Victoria E, Gil A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/s0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Robinson LE, Buchholz AC, Mazurak VC. Inflammation, obesity, and fatty acid metabolism: influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:1008–1024. doi: 10.1139/H07-087. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bores L, Barahona-Garrido J, Yamamoto-Furusho JK. Basic and clinical aspects of osteoporosis in inflammatory bowel disease. World J Gastroenterol. 2007;13:6156–6165. doi: 10.3748/wjg.v13.i46.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal GB, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998;56:1607–1614. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- Sale JE, Gignac M, Hawker G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J Rheumatol. 2008;35:335–342. [PubMed] [Google Scholar]
- Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007a;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007b;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database Syst Rev. 2006:CD006190. doi: 10.1002/14651858.CD006190. [DOI] [PubMed] [Google Scholar]
- Selmi C, Montano N, Furlan R, Keen CL, Gershwin ME. Inflammation and oxidative stress in obstructive sleep apnea syndrome. Exp Biol Med (Maywood) 2007;232:1409–1413. doi: 10.3181/0704-MR-103. [DOI] [PubMed] [Google Scholar]
- Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- Shahed AR, Jones E, Shoskes D. Quercetin and curcumin up-regulate antioxidant gene expression in rat kidney after ureteral obstruction or ischemia/reperfusion injury. Transplant Proc. 2001;33:2988. doi: 10.1016/s0041-1345(01)02283-7. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187:487–497. doi: 10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007a;595:453–470. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007b;21:278–283. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006a;536:256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006b;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- Shoskes DA. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: a new class of renoprotective agents. Transplantation. 1998;66:147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999;7:362–374. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–177. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–890. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- Skommer J, Wlodkowic D, Pelkonen J. Gene-expression profiling during curcumin-induced apoptosis reveals downregulation of CXCR4. Exp Hematol. 2007;35:84–95. doi: 10.1016/j.exphem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim Biophys Acta. 2007;1768:1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Smolen J, Aletaha D. The burden of rheumatoid arthritis and access to treatment: a medical overview. Eur J Health Econ, 8 Suppl. 2008a;2:S39–47. doi: 10.1007/s10198-007-0087-9. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D. Strengths and limitations of a systematic review on DMARDs for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008b doi: 10.1038/ncprheum0813. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- South EH, Exon JH, Hendrix K. Dietary curcumin enhances antibody response in rats. Immunopharmacol Immunotoxicol. 1997;19:105–119. doi: 10.3109/08923979709038536. [DOI] [PubMed] [Google Scholar]
- Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26:269–270. [PubMed] [Google Scholar]
- Srivastava R, Dikshit M, Srimal RC, Dhawan BN. Anti-thrombotic effect of curcumin. Thromb Res. 1985;40:413–417. doi: 10.1016/0049-3848(85)90276-2. [DOI] [PubMed] [Google Scholar]
- Sumanont Y, Murakami Y, Tohda M, Vajragupta O, Watanabe H, Matsumoto K. Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci. 2006;78:1884–1891. doi: 10.1016/j.lfs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46:2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit, 13. 2007:BR286–292. [PubMed] [Google Scholar]
- Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Thresiamma KC, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J Exp Biol. 1996;34:845–847. [PubMed] [Google Scholar]
- Tikoo K, Meena RL, Kabra DG, Gaikwad AB. Change in post-translational modifications of histone H3, heat-shock protein-27 and MAP kinase p38 expression by curcumin in streptozotocin-induced type I diabetic nephropathy. Br J Pharmacol. 2008;153:1225–1231. doi: 10.1038/sj.bjp.0707666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen HH, de Vries H, Karlsen J, Beijersbergen van Henegouwen G. Studies on curcumin and curcuminoids. IX: Investigation of the photobiological activity of curcumin using bacterial indicator systems. J Pharm Sci. 1987;76:371–373. doi: 10.1002/jps.2600760506. [DOI] [PubMed] [Google Scholar]
- Tourkina E, Gooz P, Oates JC, Ludwicka-Bradley A, Silver RM, Hoffman S. Curcumin-induced apoptosis in scleroderma lung fibroblasts: role of protein kinase cepsilon. Am J Respir Cell Mol Biol. 2004;31:28–35. doi: 10.1165/rcmb.2003-0354OC. [DOI] [PubMed] [Google Scholar]
- Tschape JA, Hartmann T. Therapeutic perspectives in Alzheimer’s disease. Recent Patents CNS Drug Discov. 2006;1:119–127. doi: 10.2174/157488906775245354. [DOI] [PubMed] [Google Scholar]
- Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno JK, Kolek OI, Hines ER, Xu H, Timmermann BN, Kiela PR, Ghishan FK. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology. 2006;131:497–509. doi: 10.1053/j.gastro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Vadhan Raj S, Weber D, Giralt S, Alexanian R, Thomas S, Zhou X, patel P, Bueso-Ramos C, Newman R, Aggarwal B. Curcumin downregulates NF-κB and related genes in patients with multiple myeloma: Results of a phase1/2 study. American Society of Hematology. 2007 [Google Scholar]
- Vajragupta O, Boonchoong P, Morris GM, Olson AJ. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg Med Chem Lett. 2005;15:3364–3368. doi: 10.1016/j.bmcl.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Vamvouris T, Hadi S. A review of the treatment of psoriasis with infliximab. Rev Recent Clin Trials. 2006;1:201–205. doi: 10.2174/157488706778250122. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol. 1998;124:425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlietinck AJ, De Bruyne T, Apers S, Pieters LA. Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med. 1998;64:97–109. doi: 10.1055/s-2006-957384. [DOI] [PubMed] [Google Scholar]
- Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Leibel R, Tortoriello DV. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology. 2008 doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Deng B, Chow YL, Zhao ZZ, Hu B. Effects of curcumin in treatment of experimental pulmonary fibrosis: a comparison with hydrocortisone. J Ethnopharmacol. 2007;112:292–299. doi: 10.1016/j.jep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol. 2005;518:40–46. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yang X, Thomas DP, Zhang X, Culver BW, Alexander BM, Murdoch WJ, Rao MN, Tulis DA, Ren J, Sreejayan N. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Chen TP, Wu YC, Lin YM, Jing Lin P. Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res. 2005;125:109–116. doi: 10.1016/j.jss.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fiala M, Cashman J, Sayre J, Espinosa A, Mahanian M, Zaghi J, Badmaev V, Graves MC, Bernard G, Rosenthal M. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2006;10:1–7. doi: 10.3233/jad-2006-10101. [DOI] [PubMed] [Google Scholar]