Abstract
Background
The relationship between advanced glycation end products and arterial stiffness has previously been examined in highly selected groups of patients with diabetes or hypertension. Our aim was to determine whether elevated serum advanced glycation end products are associated with increased arterial stiffness in relatively healthy, community-dwelling adults.
Methods
Aortic pulse wave velocity (PWV), an index of aortic stiffness, and serum AGEs, as represented by the specific AGE, serum carboxymethyl-lysine (CML), were measured in 493 adults, aged 26-93 years, who participated in the Baltimore Longitudinal Study of Aging.
Results
Mean (SD) PWV (m/sec) was 6.6 (1.8) m/sec. Mean CML was 0.47 (0.13) μg/mL. Serum CML (per 1 Standard Deviation [S.D.]) was associated with PWV (beta = 0.16, standard error [S.E.] = 0.07, P = 0.02), adjusting for age, sex, body mass index, mean arterial pressure, fasting plasma glucose, high density lipoprotein cholesterol, smoking, and other covariates. After excluding all diabetic patients, serum CML (per 1 S.D.) was associated with PWV (beta = 0.18, S.E. = 0.07, P = 0.009), adjusting for the same covariates.
Conclusions
Elevated AGEs are associated with increased arterial stiffness, a known predictor of adverse cardiovascular outcomes, among relatively healthy community-dwelling adults. Interventions to lower levels of AGEs, such as altering the pattern of dietary intake, warrant examination as putative novel strategies to lower arterial stiffness in adults.
Keywords: advanced glycation end products, aging, arterial stiffness, cardiovascular disease, pulse wave velocity
Cardiovascular diseases are the leading cause of morbidity and mortality in industrialized countries. Aging of the arterial system is characterized by decreased elasticity, diffuse intimal thickening, endothelial dysfunction, and accumulation of vascular interstitial collagen.1 The conduit arteries provide a low resistance path for the blood supply to the visceral organs and limbs, and the compliant nature of the aorta and carotid arteries provide a buffering function by expanding to accommodate the blood ejected by the left ventricle during systole.2 With normal elasticity of the conduit arteries, the pressure wave reflected from the periphery returns to the heart during diastole. In middle-aged adults with increased arterial stiffness, the velocity of the pressure wave increases, and the reflected pressure wave reaches the heart during late-systole.3 In older adults, the reflected pressure wave arrives earlier in systole, leading to increased systolic pressure, elevated cardiac afterload, and decreased diastolic blood pressure that potentially compromises coronary blood flow.4 Increased arterial stiffness is associated with hypertension and is predictive of coronary heart disease, stroke,5 and cardiovascular mortality.1,4 The factors that contribute to the pathophysiology of arterial stiffness have been incompletely characterized.
Advanced glycation end products (AGEs), bioactive molecules formed by the non-enzymatic glycation of proteins, lipids, and nucleic acids, accumulate in arteries with age6 and increase vascular stiffness by inducing collagen cross-linking in the vessel wall, at least in diabetic rats.7 In animal models, treatment with alagebrium (formerly ALT7-111), a thiazolium derivative that can break established AGE cross-links, reversed the arterial stiffness associated with diabetes,8 reversed age-related increases in myocardial stiffness, improved cardiac function, and reduced the upregulation of collagen in the myocardium.9 In old, healthy, nondiabetic rhesus monkeys, treatment with alagebrium reduced arterial stiffness, as measured by aortic pulse wave velocity and augmentation index of the carotid arterial pressure waveform, and improved cardiac function.10 In phase 2 trials, alagebrium improved arterial compliance in elderly patients with vascular stiffening11 and improved cardiac function in older adults with diastolic heart failure.12
A recent prospective study showed that moderately to severely disabled older community-dwelling women with elevated serum AGEs were at increased risk of dying from cardiovascular disease.13 Although AGEs may be related to cardiovascular mortality in the general population, the biological mechanisms that might explain this association are unclear. Previous studies of AGEs and arterial stiffness have been conducted in highly selected patients with hypertension14 or diabetes.15 We postulated that elevated serum AGEs are associated with increased aortic pulse wave velocity, a measure of arterial stiffness, in older adults. To address this hypothesis, we examined the relation between serum advanced glycation end products and aortic pulse wave velocity in community-dwelling adults.
Methods
Study Sample
The study subjects consisted of participants in the Baltimore Longitudinal Study of Aging (BLSA) who were seen between May 2003 and June 2007. The BLSA is a prospective open cohort study of community-dwelling volunteers, largely from the Baltimore/Washington area. The inclusion criteria at the time of enrollment in the study are aged 20 years or older, in good health with no established genetic diseases, no diabetes requiring glucose lowering medication, no neurological, renal, hepatic, gastrointestinal, musculoskeletal, cardiovascular, or psychiatric diseases, no active cancer, able to perform self-care and instrumental activities of daily living without difficulties or need for help, able to walk independently for 400 meters without using assistive devices, no substantial cognitive impairment, and no important sensory deficits. Subjects are excluded if they have positive laboratory tests for HIV infection, hepatitis B or C infection, syphilis, or any abnormal laboratory tests. The study was established in 1958 and is described in detail elsewhere.16 The participants are primarily Caucasian, middle- and upper-middle-socioeconomic class volunteers who return approximately every two years to the National Institute on Aging Clinical Research Center in Baltimore, Maryland, for 2.5 days of medical, physiological, and psychological examinations.16 The study is an open cohort design, with dropouts replaced to maintain roughly equal numbers of subjects in each ten-year age stratum. A total of about 1,000 subjects are examined at each study cycle.
Height, weight, and waist circumference were determined for all participants. Body mass index was determined as kg/m2. Smoking status was ascertained by a questionnaire that classified each subject as a non-smoker, former smoker, or current smoker. Use of medications was determined at each study visit. Vasoactive medications were defined as thiazide and non-thiazide diuretics, combination thiazide drugs, nitrates, beta-blockers, calcium channel blockers, peripheral vasodilators, angiotensin converting enzyme inhibitors, central and peripheral antihypertensive agents, and drugs combining any of the previous agents. Diabetes was defined as medication-treated diabetes. Hypertension was defined as self-reported medication-treated hypertension. Written, informed consent was obtained from all study participants. The BLSA has continuing approval from the Institutional Review Board (IRB) of the MedStar Research Institute, and the protocol for the present study was also approved by the IRB of the Johns Hopkins School of Medicine.
Pulse Wave Velocity Measurements
Carotid-femoral pulse wave velocity (PWV) and oscillometric blood pressure were measured after the subjects had rested in the supine position in a quiet room for at least 10 minutes. Subjects abstained from food or from drinking coffee or other caffeine-containing beverages for at least 45 minutes before performance of the PWV measurements. After simultaneous acquisition of pressure waveforms in the right common carotid artery and right femoral artery, pulse transit time between these two sites was automatically determined by the Complior® SP device (Artech Medical, Paris, France), using the foot to foot method, as previously described and validated.17 The distance travelled by the pulse wave was measured to the nearest centimeter with an external tape measure over the body surface. This distance was measured by subtracting the distance between the manubrium and the carotid sampling site from the sum of the distances between the manubrium and the umbilicus and the umbilicus and the femoral sampling site. PWV was calculated by dividing the distance traveled by the pulse wave by the pulse transit time. Measurements of PWV were made in triplicate and averaged for the analyses. The coefficient of variation for the measurement of PWV was 8.2%, and the intraclass correlation coefficient was 0.68.
Laboratory Studies
Blood samples were drawn from the antecubital vein between 7 and 8 AM after an overnight fast. Subjects were not allowed to smoke, engage in physical activity, or take medications before the sample was collected. Concentrations of plasma triglycerides and total cholesterol were determined by an enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer, Irving, TX). The concentration of high-density lipoprotein (HDL) cholesterol was determined by a dextran sulfate-magnesium precipitation procedure.18 Low-density lipoprotein (LDL) cholesterol concentrations were estimated by using the Friedewald formula.19 The fasting plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments, Inc., Fullerton, CA). Blood samples were stored continuously at -70° C until the time of analysis of serum AGEs. The measure of serum AGEs in this study was serum carboxymethyl-lysine (CML). CML is a dominant circulating AGE, the best characterized of all the AGEs, and a dominant AGE in tissue proteins.20 CML was measured in duplicate using a competitive ELISA (AGE-CML ELISA, Microcoat, Penzberg, Germany).21 This assay has been validated,22 is specific, and shows no cross-reactivity with other compounds.21 The with-in assay and between assay coefficients of variation in the CML assay were both <5%, respectively.
Statistical Methods
Continuous variables were reported as mean ± standard deviation. Body mass index was categorized as underweight (<18.5 kg/m2), normal range (18.5-24.9 kg/m2), overweight (≥25-29.9 kg/m2) and obese (≥30 kg/m2) according to World Health Organization criteria.23 Serum CML values was normally distributed. Univariate and multivariate linear regression models were used to examine the relationship between demographic, anthropometric, laboratory, and clinical characteristics and PWV. Age and body mass index were used as categorical variables in the analyses because the relationships between age and body mass index, respectively, with PWV were not linear. Variables were included in the multivariate model if they were significant in the univariate analyses, except for cardiovascular diseases. All analyses were conducted using SAS version 9.13 (Cary, NC).
Results
Demographic, disease, and other characteristics of the 493 study subjects are shown in Table 1. Overall, mean (SD) PWV was 6.6 ± 1.8 m/sec. Among adults aged <50, 50-59, 60-69, 70-79, and ≥80 years, mean (SD) PWV was 5.3 ± 1.1, 5.8 ± 1.2, 6.7 ± 1.5, 7.6 ± 1.7, and 8.4 ± 1.8, respectively (P <0.0001). The relationships between demographic, disease, serum CML, and other factors with aortic pulse wave velocity are shown in Table 2. Age, gender, body mass index, former smoking, mean arterial pressure, fasting plasma glucose, HDL cholesterol, serum CML, serum creatinine, use of glucose-lowering, vasoactive, and lipid-lowering drug(s), hypertension, diabetes, coronary heart disease, and heart failure were associated with aortic pulse wave velocity in univariate linear regression models. Diastolic blood pressure, triglycerides, total cholesterol, LDL cholesterol, and stroke were not significantly associated with aortic pulse wave velocity. Heart rate measurements were only available in 300 of the participants, and heart rate was marginally associated with aortic pulse wave velocity (P = 0.06).
Table 1.
Characteristics of Study Subjects in the Baltimore Longitudinal Study of Aging with Aortic Pulse Wave Velocity and Serum Carboxymethyl-lysine (CML) Measurements
| Characteristic | N | Mean (SD) or Percent | |
|---|---|---|---|
| Age (years) (%) | <50 | 86 | 17.4 |
| 50-59 | 101 | 20.5 | |
| 60-69 | 113 | 22.9 | |
| 70-79 | 103 | 20.9 | |
| ≥80 | 90 | 18.3 | |
| Sex (%) | Female | 231 | 46.9 |
| Male | 262 | 53.1 | |
| Body mass index (kg/m2) (%) | <18.5 | 3 | 0.6 |
| 18.5-24.9 | 180 | 36.5 | |
| 25.0-29.9 | 201 | 40.8 | |
| ≥30 | 109 | 22.1 | |
| Smoking history (%) | Never | 270 | 53.3 |
| Former | 215 | 42.4 | |
| Current | 22 | 4.3 | |
| On glucose-lowering medication(s) | 23 | 4.7 | |
| On vasoactive medication(s) | 186 | 37.7 | |
| On lipid-lowering medication(s) | 121 | 24.5 | |
| Systolic blood pressure (mm Hg) | 493 | 124 (15) | |
| Diastolic blood pressure (mm Hg) | 493 | 69 (9) | |
| Mean arterial pressure (mm Hg) | 489 | 88 (10) | |
| Heart rate (beats/min) | 300 | 65 (10) | |
| Pulse wave velocity (m/sec) | 493 | 6.6 (1.8) | |
| Fasting plasma glucose (mg/dL) | 493 | 93 (14) | |
| Triglycerides (mg/dL) | 491 | 103 (64) | |
| Total cholesterol (mg/dL) | 491 | 194 (37) | |
| LDL cholesterol (mg/dL) | 485 | 116 (34) | |
| HDL cholesterol (mg/dL) | 491 | 59 (18) | |
| Serum CML (μg/mL) | 493 | 0.47 (0.13) | |
| Serum creatinine (mg/dL) | 492 | 1.00 (0.23) | |
| Hypertension (%) | 143 | 29.0 | |
| Diabetes (%) | 24 | 4.9 | |
| Coronary heart disease (%) | 54 | 11.0 | |
| Stroke (%) | 2 | 0.4 | |
| Heart failure (%) | 2 | 0.4 | |
Table 2.
Univariate Relationships of Demographic, Disease, Serum Carboxymethyl-lysine (CML), and Other Factors with Aortic Pulse Wave Velocity in 493 Adults in the Baltimore Longitudinal Study of Aging
| Characteristic | Beta | 95% CI | SE | P | |
|---|---|---|---|---|---|
| Age (years)1 | 50-59 | 0.44 | 0.03, 0.86 | 0.21 | 0.03 |
| 60-69 | 1.32 | 0.91, 1.72 | 0.21 | <0.0001 | |
| 70-79 | 2.30 | 1.88, 2.71 | 0.21 | <0.0001 | |
| ≥80 | 3.04 | 2.54, 3.54 | 0.26 | <0.0001 | |
| Male gender | 0.71 | 0.39, 1.01 | 0.16 | <0.0001 | |
| Body mass index2 (kg/m2) | <18.5 | -1.38 | -3.42, 0.65 | 1.03 | 0.18 |
| 25.0-29.9 | 0.34 | -0.01, 0.70 | 0.18 | 0.06 | |
| ≥30 | 0.46 | 0.03, 0.89 | 0.22 | 0.04 | |
| Smoking history2 | Former | 0.77 | 0.45, 1.09 | 0.16 | <0.0001 |
| Current | 0.14 | -0.62, 0.91 | 0.39 | 0.71 | |
| On glucose-lowering medication(s) | 0.76 | 0.018, 1.51 | 0.38 | 0.05 | |
| On vasoactive medication(s) | 1.12 | 0.81, 1.43 | 0.16 | <0.0001 | |
| On lipid-lowering medication(s) | 0.77 | 0.39, 1.11 | 0.18 | <0.0001 | |
| Systolic blood pressure (mm Hg) | 0.046 | 0.036, 0.055 | 0.004 | <0.0001 | |
| Diastolic blood pressure (mm Hg) | 0.005 | -0.012, 0.022 | 0.009 | 0.57 | |
| Mean arterial pressure (mm Hg) | 0.045 | 0.029, 0.061 | 0.008 | <0.0001 | |
| Heart rate (beats/min) | 0.017 | -0.001, 0.035 | 0.009 | 0.06 | |
| Fasting plasma glucose (mg/dL) | 0.019 | 0.008, 0.030 | 0.006 | 0.0007 | |
| Triglycerides (mg/dL) | 0.001 | -0.001, 0.003 | 0.001 | 0.34 | |
| Total cholesterol (mg/dL) | -0.0006 | -0.0048, 0.0037 | 0.002 | 0.79 | |
| LDL cholesterol (mg/dL) | 0.002 | -0.003, 0.006 | 0.002 | 0.46 | |
| HDL cholesterol (mg/dL) | -0.009 | -0.019, -0.001 | 0.004 | 0.03 | |
| Serum CML (per 1 S.D.) | 0.27 | 0.11, 0.43 | 0.08 | 0.0007 | |
| Serum creatinine (mg/dL) | 1.79 | 1.12, 2.46 | 0.34 | <0.0001 | |
| Hypertension | 0.89 | 0.55, 1.23 | 0.17 | <0.0001 | |
| Diabetes | 0.94 | 0.21, 1.67 | 0.37 | 0.02 | |
| Coronary heart disease | 1.19 | 0.69, 1.68 | 0.25 | <0.0001 | |
| Stroke | -0.03 | -2.59, 2.46 | 1.27 | 0.98 | |
| Heart failure | 3.23 | 0.75, 5.72 | 1.26 | 0.01 | |
Reference category is <50 years.
Reference category is body mass index 18.5-24.9 kg/m2.
Reference category is never smoking.
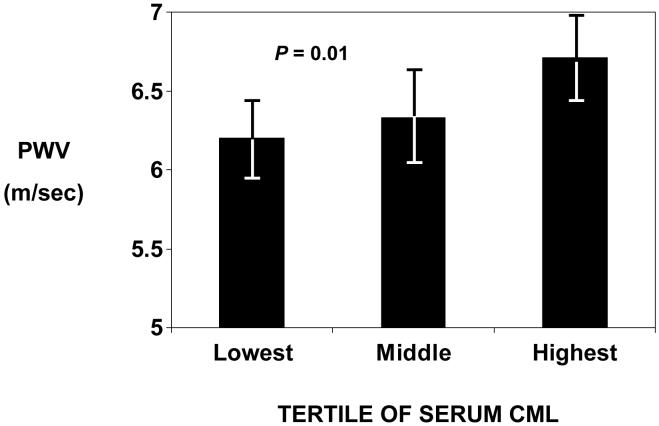
Serum CML was divided into tertiles, with tertile cut-offs at 0.41 and 0.52 μg/mL. Geometric mean aortic pulse wave velocity increased across tertiles of serum CML (P = 0.01, by ANOVA), as shown in Figure 1.
Figure 1.
Geometric mean aortic pulse wave velocity (PWV) by tertile of serum CML in adults in the Baltimore Longitudinal Study of Aging. Bars indicate 95% confidence intervals. P = 0.01 by ANOVA.
Serum CML, per 1 Standard Deviation (S.D.) was associated with aortic pulse wave velocity, after adjusting for age, gender, body mass index, and smoking, mean arterial pressure, fasting plasma glucose, HDL cholesterol, and serum creatinine as shown in Table 3. There was a weak association of PWV with heart rate (r = 0.11, P = 0.06). When heart rate was added to the same model as in Table 3, the number of subjects in the model was only 300 due to the more limited number of measurements of heart rate. In the multivariate model that included heart rate, serum CML, per 1 SD, was associated with aortic pulse wave velocity (beta = 0.16, SE = 0.08, P = 0.049).
Table 3.
Multivariate Linear Regression Models for Serum Carboxymethyl-lysine (CML) and Other Risk Factors Associated with Aortic Pulse Wave Velocity
| Characteristic* | Standardized Beta | Beta | 95% CI | SE | P | |
|---|---|---|---|---|---|---|
| Serum CML (per 1 S.D.) | 0.10 | 0.16 | 0.02, 0.30 | 0.07 | 0.02 | |
| Age (years)1 | 50-59 | 0.07 | 0.31 | -0.10, 0.72 | 0.21 | 0.14 |
| 60-69 | 0.24 | 1.00 | 0.57, 1.42 | 0.21 | <0.0001 | |
| 70-79 | 0.43 | 1.85 | 1.39, 2.31 | 0.23 | <0.0001 | |
| ≥80 | 0.47 | 2.73 | 2.19, 3.27 | 0.27 | <0.0001 | |
| Male gender | 0.009 | 0.03 | -0.29, 0.36 | 0.17 | 0.84 | |
| Body mass index2 (kg/m2) | <18.5 | -0.007 | -0.17 | -1.82, 1.49 | 0.85 | 0.84 |
| 25.0-29.9 | 0.026 | 0.10 | -0.21, 0.41 | 0.16 | 0.55 | |
| ≥30 | 0.055 | 0.24 | -0.17, 0.65 | 0.21 | 0.24 | |
| Smoking3 | Former | 0.080 | 0.29 | 0.01, 0.56 | 0.14 | 0.04 |
| Current | 0.020 | 0.18 | -0.47, 0.83 | 0.34 | 0.59 | |
| Mean arterial pressure (mm Hg) | 0.15 | 0.027 | 0.012, 0.042 | 0.007 | 0.0003 | |
| Fasting plasma glucose (mg/dL) | 0.072 | 0.009 | -0.001, 0.019 | 0.005 | 0.09 | |
| HDL cholesterol (mg/dL) | -0.041 | -0.004 | -0.012, 0.004 | 0.004 | 0.33 | |
| On glucose-lowering medications(s) | -0.020 | -0.17 | -0.85, 0.51 | 0.34 | 0.62 | |
| On vasoactive medication(s) | 0.041 | 0.15 | -0.15, 0.45 | 0.15 | 0.33 | |
| On lipid-lowering medication(s) | 0.026 | 0.11 | -0.22, 0.43 | 0.17 | 0.52 | |
| Serum creatinine (mg/dL) | 0.038 | 0.30 | -0.37, 0.97 | 0.34 | 0.38 | |
Reference category is <50 years.
Reference category is body mass index 18.5-24.9 kg/m2.
Reference category is never smoking.
There was no significant interaction term between serum CML and fasting plasma glucose. In an alternative model that included diabetes in addition to all the variables as in the multivariate model in Table 3, serum CML (per 1 S.D.) was associated with aortic pulse wave velocity (beta = 0.16, SE = 0.07, P = 0.03). After excluding all patients with diabetes, mean (SD) CML was 0.47 (0.13) μg/mL. After excluding all patients with diabetes, serum CML (per 1 S.D.) was associated with aortic pulse wave velocity (beta = 0.18, SE = 0.07, P = 0.009) in a model that included all the variables as in Table 3.
Discussion
The present study shows that in community-dwelling adults, elevated serum AGEs are independently associated with increased arterial stiffness, as indexed by increased aortic pulse wave velocity. To our knowledge, this is the first study to show that serum AGEs are associated with arterial stiffness in a cohort of relatively healthy, community-dwelling adults. Serum AGEs were also associated with arterial stiffness even after excluding all patients with diabetes. These findings are consistent with studies showing that elevated plasma carboxymethyl-lysine concentrations are associated with increased pulse wave velocity in hypertensive middle-aged adults14 and increased pulse pressure in patients with type 1 diabetes.15 These findings from human studies and those from animal models8,9 suggest that advanced glycation end products play a role in the development of arterial stiffness.
Although normative data have not yet been described for serum CML concentrations, it is possible to compare CML concentrations with other studies that utilized the same assay. The serum concentrations of CML among adults in this study were less than CML concentrations described in diabetic adults with both hypertension and nephropathy24 and in diabetic adults with retinopathy.21
AGEs alter the functional properties of important matrix molecules such as type-IV collagen and laminin.25 Cross-linking of AGEs with type-IV collagen from the basement membrane inhibits the association of these molecules into a normal complex network-like structure,26,27 and AGE cross-linking of laminin reduces polymer self-assembly.28 In addition to inducing the cross-linking of collagen in arterial walls, AGEs contribute to endothelial dysfunction by reducing nitric oxide,29 increasing the oxidation of low-density lipoprotein,30 enhancing macrophage migration across the endothelium,31 increasing production of reactive oxygen species,32 and upregulating inflammation via RAGE.33
Diet is a major source of exogenous AGEs, and AGEs are especially high in Western diets where foods are processed under elevated temperatures such as by broiling, roasting, deep frying, oven frying, or grilling.34 In addition, cola drinks, which contain large amounts of caramel additives, are rich sources of AGEs.35 The AGE content of the same food item can be increased 10-200 fold by increasing the temperature and conditions used in cooking.35 About 10% of dietary AGEs are absorbed, of which about one-third is excreted and two-thirds deposited in tissues.36 It is possible to reduce dietary intake of AGEs by avoiding foods that are processed at high temperatures and other rich sources of dietary AGEs. Intervention trials have shown that reduced dietary intake of AGEs leads to large reductions in serum AGEs, decreases in C-reactive protein and other inflammatory markers, and improvements in vascular function.37-39 Although dietary intake of AGEs was not assessed in the present study, serum CML concentrations have been shown previously to correlate well with dietary intake of AGEs.38
Our recent studies showed that older disabled community-dwelling women with elevated serum AGEs had a greatly increased risk of dying from cardiovascular diseases.13 The present study suggests that one potential biological mechanism by which AGEs could increase the risk of cardiovascular mortality is through increasing arterial stiffness. Other potential mechanisms not addressed in this study include upregulation of inflammation and worsening of atherosclerosis.
The present study suggests that AGEs may be a major risk factor for arterial stiffness. It should be noted that the values of PWV in our study may be lower than those reported in other studies because in our measurement of the distance travelled by the pulse wave, we subtracted the difference between the manubrium and the carotid sampling site from the sum of the distances between the manubrium and the umbilicus and the umbilicus and the femoral sampling sites. This correction was done to account for the fact that the centrifugal travel of the pulse wave occurs simultaneously in the aortic arch/carotid segment and the aortic arch/ascending aorta segment. However, other investigators have used different strategies for measuring the distance travelled by the pulse wave. An expert consensus panel commented that the various methods are approximations,40 and to date, none has emerged as preferred over the others.
Another limitation of the study is the cross-sectional design and the potential that arterial stiffness increases the risk of elevated serum AGEs. Serum AGE concentrations are thought to be largely determined by dietary intake of AGEs, current smoking (cured tobacco leaves contain AGEs), and glucose metabolism, and it is unclear how arterial stiffness could increase serum AGEs. Further studies are needed to corroborate these observations in other study populations and to determine whether elevated serum AGEs are predictive of incident adverse cardiovascular events, such as stroke, myocardial infarction, heart failure, and cardiovascular mortality in the general population.
In conclusion, elevated AGEs are associated with increased arterial stiffness, a known predictor of adverse cardiovascular outcomes. This suggests that interventions to lower levels of AGEs, such as altering the pattern of dietary intake, could warrant examination as putative novel strategies to lower arterial stiffness.
Acknowledgments
Sources of support: This work was supported by National Institute on Aging Grant R01 AG027012 and the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Disclosure: The authors have no conflict of interest.
References
- 1.Najjar SS, Scutieri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 5.Mattace-Raso FUS, van der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 6.Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product Nε-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004;68:132–142. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Wolffenbuttel BHR, Boulanger CM, Crijns FRL, Hiujberts MSP, Poitevin P, Swennen GNM, Vasan S, Egan JJ, Ulrich P, Cerami A, Lévy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA. 1998;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA. 2000;97:2809–2813. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaitkevicius PV, Lane M, Spurgeon H, Ingram DK, Roth GS, Egan JJ, Vasan S, Wagle DR, Ulrich P, Brines M, Wuerth JP, Cerami A, Lakatta EG. A cross-link breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci USA. 2001;98:1171–1175. doi: 10.1073/pnas.98.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 12.Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, deGroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191–195. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Ferrucci L, Sun K, Beck J, Dalal M, Varadhan R, Walston J, Guralnik JM, Fried LP. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin Exp Res. doi: 10.1007/bf03325227. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20:242–247. doi: 10.1016/j.amjhyper.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Schram MT, Schalkwijk CG, Bootsma AH, Fuller JH, Chaturvedi N, Stehouwer CDA. Advanced glycation end products are associated with pulse pressure in type 1 diabetes. The EURODIAB prospective complications study. Hypertension. 2005;46:232–237. doi: 10.1161/01.HYP.0000164574.60279.ba. [DOI] [PubMed] [Google Scholar]
- 16.Shock NW, Greulich RC, Andres RA, Arenberg D, Casta PT, Lakatta EG, Arenberg D, Tobin JD. Normal Human Aging: the Baltimore Longitudinal Study of Aging. U.S. Government Printing Office; Washington, D.C.: 1984. [Google Scholar]
- 17.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 18.Warnick G, Benderson J, Albers J. Dextran sulfate-Mg2+ precipitation procedure for quantification of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Frederikson DS. Estimation of the concentration of low-density-lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 21.Boehm BO, Schilling S, Rosinger S, Lang GE, Lang GK, Kientsch-Engel P, Stahl P. Elevated serum levels of Nε-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47:1376–1379. doi: 10.1007/s00125-004-1455-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Frischmann M, Kientsch-Engel R, Steinmann K, Stopper H, Niwa T, Pischetsrieder M. Two immunochemical assays to measure advanced glycation end-products in serum from dialysis patients. Clin Chem Lab Med. 2005;43:503–511. doi: 10.1515/CCLM.2005.089. [DOI] [PubMed] [Google Scholar]
- 23.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9(suppl 4):228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 24.Busch M, Franke S, Wolf G, Brandstädt A, Ott U, Gerth J, Hunsicker LG, Stein G, Collaborative Study Group The advanced glycation end product Nε-carboxymethyllysine is not a predictor of cardiovascular events and renal outcomes in patients with type 2 diabetic kidney disease and hypertension. Am J Kidney Dis. 2006;48:571–579. doi: 10.1053/j.ajkd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Basta G, Schmidt AM, de Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Tsilibary EC, Charonis AS, REger LA, Wohlhueter RM, Furcht LT. The effect of nonenzymatic glycosylation on the binding of the main noncollagenous NC1 domain to type IV collagen. J Biol Chem. 1988;263:4302–4308. [PubMed] [Google Scholar]
- 27.Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. J Biol Chem. 1989;264:21597–21602. [PubMed] [Google Scholar]
- 28.Charonis AS, Reger LA, Dege JE, Kouzi-Koliakos K, Furcht LT, Wohlhueter RM, Tsilibary EC. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990;39:807–814. doi: 10.2337/diab.39.7.807. [DOI] [PubMed] [Google Scholar]
- 29.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirstein M, Brett J, Radoff S, Ogawa S, Stern D, Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci USA. 1990;87:9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- 33.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196:9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BW, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc. 2004;104:1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 35.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94:6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 37.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, Vassalotti JA, Vlassara H. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis. 2003;42:532–538. doi: 10.1016/s0272-6386(03)00779-0. [DOI] [PubMed] [Google Scholar]
- 39.Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 40.Laurent S, Cockcroft J, van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson J, Struijker-Boudier H, European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]