Abstract
We describe a near-infrared spectroscopy (NIRS) method to noninvasively measure relative changes in the pulsate components of cerebral blood flow (pCBF) and volume (pCBV) from the shape of heartbeat oscillations. We present a model that is used and data to show the feasibility of the method. We use a continuous-wave NIRS system to measure the arterial oscillations originating in the brains of piglets. Changes in the animals' CBF are induced by adding CO2 to the breathing gas. To study the influence of scalp on our measurements, comparative, invasive measurements are performed on one side of the head simultaneously with noninvasive measurements on the other side. We also did comparative measurements of CBF using a laser Doppler system to validate the results of our method. The results indicate that for sufficient source-detector separation, the signal contribution of the scalp is minimal and the measurements are representative of the cerebral hemodynamics. Moreover, good correlation between the results of the laser Doppler system and the NIRS system indicate that the presented method is capable of measuring relative changes in CBF. Preliminary results show the potential of this NIRS method to measure pCBF and pCBV relative changes in neonatal pigs.
Keywords: cerebral blood flow, cerebral blood volume, near-infrared spectroscopy, arterial oscillations
1 Introduction
Oxygen deprivation, typically the result of a combination of hypoxia and hypoperfusion, is often the primary event instigating a biochemical cascade that leads to irreversible brain injury in perinatal encephalopathy. Continuous measurement of the arterial oxygenation (SaO2) with a pulse oximeter is a reliable and commonly used means to monitor systemic oxygen supply. Nevertheless, local oxygen supply and consumption cannot be evaluated by the measurement of arterial saturation alone. Additional physiological parameters such as local cerebral blood flow are needed to assess the efficiency of local oxygen supply. Especially in critically ill infants, continuous monitoring of cerebral hemodynamics and oxygenation is crucial to prompt early and specific intervention, thereby preventing the development of irreversible injury.
Although multiple methods have been developed for measurement of cerebral blood flow1—including magnetic resonance imaging (MRI), arterial spin labeling MRI,2 PET,3 Fick's law-based optical systems,4 laser Doppler,5 diffuse correlation spectroscopy,6 and Doppler ultrasound7—none of these methods is suitable for noninvasive, continuous bedside monitoring of local cerebral blood flow.
Near-infrared spectroscopy (NIRS) offers a promising alternative for continuous bedside monitoring.8 It is inexpensive, noninvasive, and applicable in almost any environment, and can easily be combined with other bedside monitoring methods. MRI is expensive, not always available, and requires the patient to remain still during measurements, which is quite difficult with awake neonates.7 PET is expensive, not always available, has poor temporal resolution, and exposes the patient to radiation.7 Laser Doppler flow meter has limited penetration depth.7 Diffuse correlation spectroscopy has shown promising results, but it is still unclear whether it is practical enough to be used for continuous monitoring in humans. Fick's law-based systems are not entirely noninvasive, since they require the injection of a chromophore, and therefore cannot be used for continuous monitoring.
Near-infrared photons can achieve sufficient penetration depth for noninvasive probing of cerebral cortex hemodynamics in humans. Noninvasive optical measurements of brain oxygenation and functional activity can be successfully performed in both adults and neonates. A few studies have demonstrated blood flow measurements with NIRS, utilizing physiological perturbations caused by changes in either the breathing gasses or body position.9,10 However, the fact that the method requires these perturbations to work makes it inappropriate for bedside monitoring. We suggest a NIRS method to noninvasively measure relative changes in the pulsatile component of cerebral blood flow (pCBF) and cerebral blood volume (pCBV) from the shape of the heartbeat oscillations.
Diffuse optical signals obtained in vivo on the human body very often contain systemic oscillations that can be used to extract important information about the physiology. Arterial pulsation is caused by the heartbeat pressure pulse, which propagates through the arterial network and locally causes expansion of the elastic arterial walls. The pressure pulse is strongly dampened when it reaches the capillary bed, and therefore is practically absent from the veins. This local expansion of the arteries causes an increase in light absorption and consequently a decrease in the light intensity measured with NIRS systems. These oscillations are often considered to be noise clutter, and can be eliminated with temporal and spatial filters. At the same time, these oscillations can be used to extract important information about the physiology.
For example, the pulse oximeter utilizes the arterial oscillations to extract arterial oxygen saturation, SaO2.11 The pulse oximeter is widely used in clinical practice and is considered the standard method for noninvasive monitoring of arterial oxygenation. Operation of the pulse oximeter is based on the fact that the detected oscillations are caused by oscillations of the arterial volume, and therefore the variations in the measured spectrum characterize arterial blood only. Consequently, by analyzing only the oscillating component of the measured spectrum, and discarding the temporally constant component, the arterial oxygenation can be calculated. In general, the pulse oximeter does not exploit all of the information from the heartbeat oscillations, since it effectively uses only the fact that these physiological variations exist and not the information contained within their shape and amplitude. Recent studies have proposed that the shape of the peripheral pulse wave measured by the pulse oximeter can be used to derive dynamic circulatory parameters such as local perfusion and blood volume.12-14 Using the pulse oximeter in the conventional manner limits these measurements of flow to the skin and the periphery (toe, finger, earlobes).
We have shown in a previous study15 that NIRS can measure SaO2 in the same way as pulse oximeters but directly on the head, by using the NIRS system in the reflection mode and large source-detector separations. We suggest a NIRS method to noninvasively measure relative changes in pulsatile components of the cerebral blood flow (pCBF) and cerebral blood volume (pCBV) based on the shape of the heartbeat pulse waveform. In this work, we report measurements in an animal model (newborn piglets) with a continuous-wave (cw) near-infrared spectroscopy system in reflectance geometry. We present the model that was used to analyze the shape of the optically measured heartbeat and calculate the blood flow and volume.
To test the validity of this model, we induced and measured changes in the blood flow by adjusting the fractions of inspired CO2 by the piglets (hypercapnia). It is known that in mammals, systemic hypercapnia induces a circulatory redistribution that favors the blood flow toward the heart, brain, and adrenals at the expense of the other organs and tissues.16 The cerebral vasodilatory response to hypercapnia is consistent, reproducible, and reversible.17 Accordingly, changes in systemic gas tensions are frequently employed as a test of essential cerebrovascular reactivity under normal and pathologic conditions.17 In humans, 5 and 7% CO2 inhalation raises CBF by approximately 50 and 100%, respectively.1 In contrast, the skin blood flow response to hypercapnia has been reported to be equivocal and significantly smaller than in the brain.18,19 Consequently, those two layers on the head (scalp and brain) exhibit different hemodynamic responses to hypercapnia. Our measurements showed that our blood flow and volume results are consistent with the known physiology for CO2 perturbation. To study the influence of signal contributions from the scalp, we performed comparative invasive measurements on one side of the head while simultaneously performing noninvasive measurements on the other side. These invasive measurements included local elimination of the scalp blood flow by surgical excision of a skin flap, followed by placement of the NIRS probe in direct contact with the skull, and comparison with laser Doppler measurements.
2 Materials and Methods
2.1 Animal Preparation
The newborn piglet has been shown to be an appropriate model to simulate neonate physiology for the study of cerebral blood flow and metabolism,16 and for this reason we used newborn piglets. Measurements were performed on eight piglets that were 7±2 days old and weighed 2.6±0.6 kg. Animals were premedicated with atropine [0.05 mg/kg, intramuscularly (IM)] and anesthetized with ketamine (15 mg/kg/IM) and xylazine (2 mg/kg/IM). Inhalant anesthesia, isoflurane (1 to 1.5%), was subsequently administered. Animals were intubated, a femoral artery line inserted for invasive blood pressure measurement, and an ear vein catheter for fluid repletion. A lactated ringers solution containing 5% dextrose was given at 5 ml/kg/hr to maintain hydration. The following physiological parameters were measured continuously: heart rate and oxygen saturation (pulse oximeter), blood pressure, rectal temperature, and respiration.
The analog outputs from a strain gauge placed around the chest of the animal to monitor respiration, the blood pressure monitor, and the pulse oximeter readings (SaO2 and heart rate) were all recorded by an analog-to-digital converter installed on a PC. Each hypercapnia cycle consisted of three steps: normocapnia baseline (50% O2, 50% N2O), hypercapnia (50% O2, 43% N2O.7% CO2), and normocapnia recovery (50% O2, 50% N2O). Measurements lasted 8 min at each step of the hypercapnia cycle. Table 1 shows statistics on the physiological parameters recorded during normocapnia and hypercapnia.
Table 1.
Physiological parameters before and during hypercapnia. All values are mean±SD; n=42 hypercapnia cycles, n'=8 animals. * shows where the difference of the population means is significantly different, p>0.05. ** shows where the difference of the population means is not significantly different, p>0.05. MABP is the mean arterial blood pressure.
| Physiological parameter |
Normocapnia | Hypercapnia | |
|---|---|---|---|
| SaO2 (%) | 98.4 ± 0.5 | 97.7 ± 1.8 | * |
| MABP (mmHg) | 47.8 ± 5.3 | 50.2 ± 8.4 | ** |
| T(°C) | 37.3 ± 0.4 | 38.4 ± 0.4 | ** |
| Heart rate (bpm) | 139.5 ± 14.2 | 132.3 ± 12.8 | * |
2.2 Near-Infrared Spectroscopy Instrument
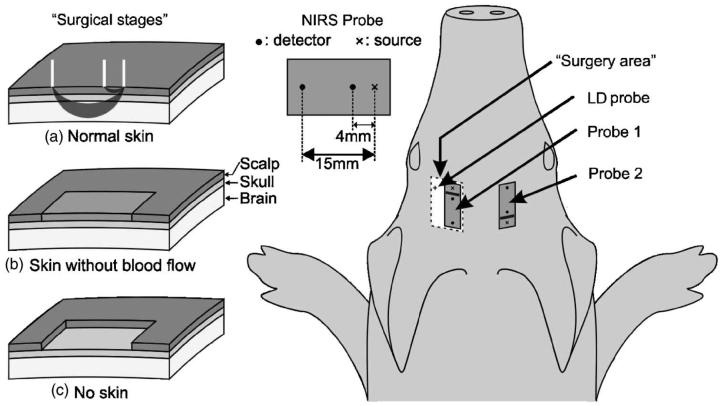
We used a cw NIRS system (NIRS 2, Techen Incorporated, Milford, Massachusetts) with four detectors (APDs C5460-01, Hamamatsu Photonics K.K., Japan) and two laser diodes, each emitting at 830 nm. The lasers are frequency encoded, thus enabling simultaneous measurement by each of the detectors. The signal detected by each detector was frequency unmixed using embedded analog electronics, while the signal from each source was calculated in real time. An analog-to-digital converter allowed parallel recording by a laptop computer. Light was delivered to the tissue using 400-μm single-core fibers. Fiber optic bundles 3 mm in diameter were used for the detectors. The sampling rate for the measurement was 200 Hz. For these measurements, sources and detectors were divided into two identical probes, each with one source and two detectors positioned along a line. In each probe, one detector was positioned 4 mm from the source and one 15 mm from the source (Fig. 1). The two different source-detector (S-D) separations were used to measure simultaneously the responses of the brain and scalp to hypercapnia.
Fig. 1.
For protocol A, the two probes were positioned symmetrically on the two sides of the head. The surgery stages (a), (b) and (c) were performed only on the left hemisphere. The skin on the right side was not touched surgically, and probe 2 was not moved until the end of the measurements. For protocol B, we used only probe 1 and surgical stage (c). The Laser Doppler probe was positioned near the optical probe in a burr hole in the skull of the piglets.
2.3 Experimental Protocols
Two groups of four piglets each were measured in two different protocols aiming to address different questions.
Protocol A: Our NIRS experimental setup can measure selectively brain-originating heartbeat oscillations.
Protocol B: The suggested method can indeed assess relative changes in blood flow.
Protocol A
This protocol was designed to study the influence of the scalp on measurements with different source-detector separations. This included the measurements after surgically eliminating the scalp to assess the influence of the scalp in the measurements of the pulsatile CBF and CBV. For this protocol, we used two identical probes symmetrically positioned on both sides of the piglet's head (Fig. 1), and measurements were obtained simultaneously. One optical probe (probe 2) was always located at the right side of the head and was not moved until the end of the experiment (Fig. 1). The other probe (probe 1) was symmetrically positioned on the left side of the head. Probe 1 was removed for each of the surgery stages and then repositioned on the same spot. The surgery stages, depicted in Figs. 1(b) and 1(c), refer to a 3 ×2-cm flap of skin on the left side of the head, under probe 1. The surgical stages were: normal skin with no surgical intervention [Fig. 1(a)], total blockage of the skin flap blood flow [surgical excision of the skin and fascia from the skull [Fig. 1(b)], and removal of the skin flap [Fig. 1(c)]. We repeated two hypercapnia cycles for each of the three different surgical stages for a total of 24 hypercapnia measurements on four animals.
Protocol B
The aim of this protocol is to correlate the results of the suggested method with the results of laser Doppler, an established method for measuring blood flow. Four piglets were measured in this protocol simultaneously, with an optical probe (probe 1 in Fig. 1) in direct contact with the skull (skin removed) and with a laser Doppler probe. The laser Doppler system used was a laser Doppler perfusion monitor (model PF5010, Perimed Incorporated), with source-detector fiber separation of 0.25 mm. The laser Doppler (LD) probe was positioned 1to 2 cm from the NIRS probe fibers (Fig. 1) to avoid saturating the detectors of the NIRS system. To ensure that the laser Doppler indeed measures cerebral blood flow, a 1.5-mm-diam area of the skull was removed, which allowed detection of cerebral blood flow. For this protocol, we measured four animals and a total of 17 hypercapnia cycles. Of these we discarded five hypercapnia cycles, where either the animal became unstable or the laser Doppler probe moved during the measurement.
2.4 Model for Estimating Pulsate Cerebral Blood Flow Changes
The suggested method for measuring relative changes of cerebral blood flow and volume is based on the analysis of the optically detected heartbeat oscillations. Heartbeat oscillations are very often present in optical signals measured in vivo on the human body. NIRS measurements of the brain in particular are very likely to contain heartbeat oscillations due to the very dense cerebral arterial network. Every heartbeat causes a pressure gradient that propagates through the arterial network and causes local changes in blood flow and volume. The local changes in blood volume alter the light absorption and therefore the detected light intensity.12 Therefore the waveform of the optical oscillations is assumed to represent the blood volume versus time and consequently the amplitude of the pulse waveform is an indicator of the local blood volume.12,14
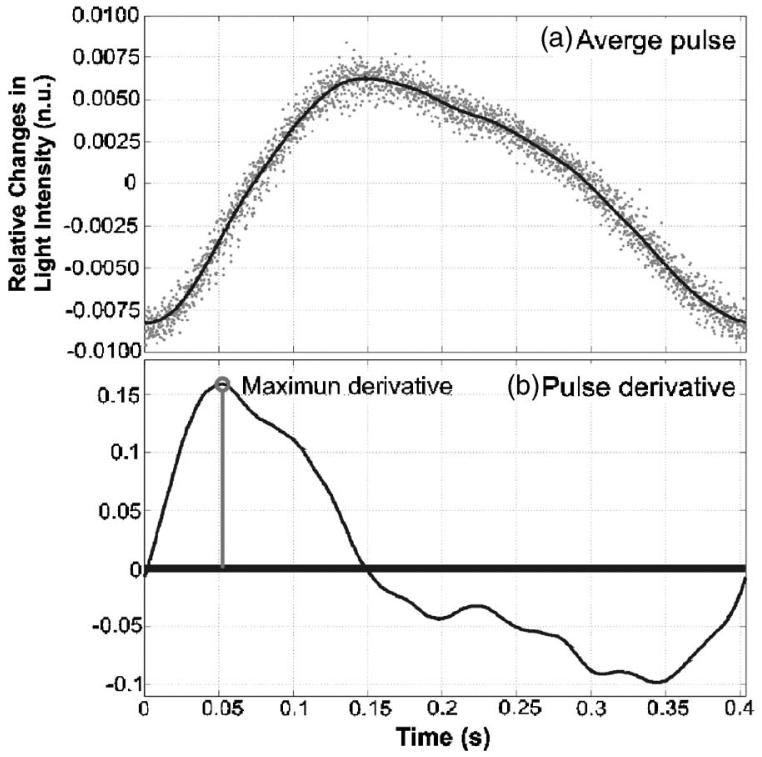
Although blood flow does not directly affect light attenuation, it is closely related to blood volume changes. A simple fluid dynamics model has been used previously to describe the relationship between local changes of blood volume and flow.13,14 In this model, the tissue volume that is probed by optical means can be modeled as an artery that expands elastically with every heartbeat, and the changes in blood volume are negatively proportional to the light intensity.13,14 Therefore, the slope at any point of the optically measured waveform represents the change of blood volume per time unit. In other words, the time derivative of the optical signal is a parameter with units of flow, and represents the pulsatile component of the flow through the tissue (Fig. 2):
where I is the intensity of light (relative changes), V is the blood volume, and Q is the pulsatile flow.12,14 It is important to clarify that the flow obtained in this way is the total pulsate flow (Qtotal), which consists of two intermingled components, inflow (Qin) and outflow (Qout): Qtotal=Qin-Qout.14 The inflow is predominant in the early part of the pulse and is discernible as a sharp rise of the blood volume.14 The outflow is the passive emptying of the vascular bed, and its influence is mostly seen at the latter part of the pulse waveform. Inflow cannot be separated from the total flow without further assumptions.14 Nevertheless, diagnostic importance lies in the change of the inflow magnitude from pulse to pulse, rather than on the variations of the inflow throughout the cardiac cycle. Therefore, we suggest using the inflow peak value to express the magnitude of the pulsatile flow for each pulse. The peak of the total flow is approximately equal to the inflow peak value, since the inflow peak occurs early in the pulse when outflow is still relatively low and can still be neglected.14 Therefore, we can assume that the peak of the pulse derivative represents the magnitude of the pulsatile flow into the local region throughout the time of one cardiac period.
Fig. 2.
(a) Thick line average of 20 pulses, dots changes in normalized intensity during the 20 pulses (nu: normalized units). (b) The derivative of the average curve.
The objective of the ensuing analysis was to obtain time-varying estimates of the arterial oscillation frequency, amplitude, and maximum positive gradient. Amplitude is representative of the blood volume, and its maximum positive gradient represents the pulsatile blood flow. The raw data for this analysis were the time-varying light intensity measured with each detector (Iraw). This light intensity signal was normalized by dividing it by its low frequency component ILP (0.6-Hz cutoff frequency) and subtracting 1 (I=Iraw/ILP-1). This allowed us to express the remaining heartbeat oscillations as a relative change in the detected light intensity, which is also proportional to changes in absorption in the case of constant scattering. The local amplitude was the average relative intensity difference between the peaks and troughs. The average of 20 pulses was used to calculate the pulse gradient (using windowed linear regression), and from there the maximum positive value was selected to represent blood flow (see Fig. 2). The temporal resolution of these results was determined by the duration of the set of 20 pulses, which is typically between 7 and 10 s, depending on the heart rate of the piglet.
3 Results and Discussion
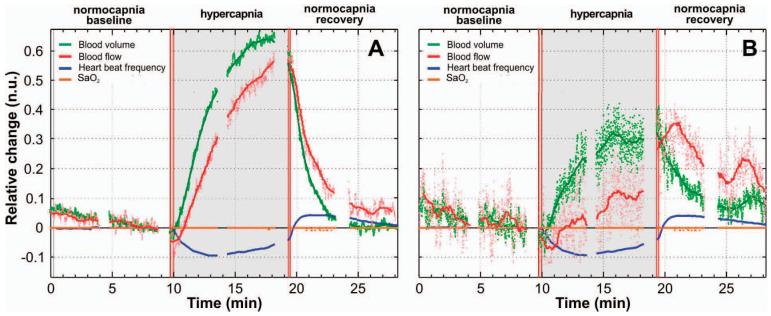
Figure 3 shows a representative measurement from a 6-day-old piglet with no surgical intervention to the skin (surgical stage A). Figures 2(a) and 2(b) correspond to different S-D separations (15 and 4 mm, respectively), which demonstrate the hemodynamics of the brain and the skin, respectively. The gray area indicates the hypercapnia period. The plotted parameters are displayed as relative changes from the baseline value. In these graphs, the light-color dots are the results without any filtering, and the solid lines are the results after removing the high frequency noise using a windowed linear regression (250 point Hanning window, 249 point overlap). The blue curves represent the changes in heart rate and are the same in both graphs, as expected. The orange line represents the changes in arterial blood oxygenation (SaO2) and shows that there were no significant changes in the peripheral blood oxygenation, which could have affected the intensity of the measured signal. The green curves represent the changes in blood volume, and the red curves show the changes in blood flow.
Fig. 3.
Representative curves derived from one hypercapnia cycle. (a) is from the large S-D distance and therefore is more representative of the hemodynamics of the brain. (b) is from the short S-D distance and represents the head skin hemodynamics.
For the large S-D separation, the pulsatile blood flow increases steadily throughout the hypercapnia period and reaches a maximum of 55% after 9 min [Fig. 3(a)]. In the posthypercapnia period, pCBF decreases exponentially. For the small S-D separation [Fig. 3(b)], blood flow initially decreases by about 7% below baseline at the beginning of hypercapnia and then slowly increases throughout this period until it reaches 35% above baseline, 2 min after the end of the CO2-breathing period. The different responses at the two S-D separations are in agreement with changes in blood flow of the brain and the skin reported in the literature. In fact, it is known that cerebral blood flow increases during hypercapnia and it is triggered by increased PCO2 in the arterial blood.20 In contrast, blood flow in the skin does not increase significantly. This supports our hypothesis that at sufficiently large S-D separations, we can measure relative changes of pulsatile cerebral blood flow. Blood volume appears to increase in similar ways in the large and short S-D distances, but by different degrees: 65 and 30%, respectively.
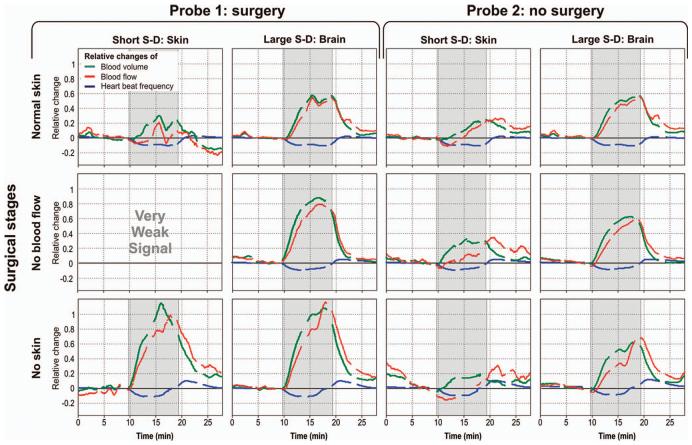
Figure 4 shows representative results from three hypercapnia measurements in one animal during the different surgical stages. The two left columns show results from probe 1, for short and large S-D separations, whereas the right two columns show results from the untouched probe 2 at short and large S-D separations. The three rows represent the three different surgical stages under probe 1. It can be seen that for all hypercapnia cycles probe 2 clearly shows different blood flow at small and large S-D separations. The same is true for probe 1 during the first surgical stage (normal skin). When the skin blood flow is eliminated (surgical stage B, middle row), the short S-D separations do not detect heartbeat oscillations and hence no blood flow and volume calculation is possible. When noninvasive, with this S-D separation the calculated parameters describe exclusively the scalp hemodynamics. When the skin is removed (surgical stage C, bottom row) and probe 1 is in direct contact with the skull, the detected heartbeat oscillations are exclusively from the brain. In this case, short and large S-D separations measure similar cerebral hemodynamic responses.
Fig. 4.
Representative results from three different surgical stages in the same piglet. The two left columns show results from the surgical area whereas the two columns on the right show results where no surgery was performed. Each line is from a different surgical stage.
A comparison between the two probes at large S-D separations shows that the shape of the measured cerebral hemodynamic response is not affected significantly by the presence of the scalp. Specifically, the pulsatile blood flow measured by large S-D of probe 2 (normal skin) is significantly correlated with the measurement of the large S-D of probe 1 (no skin) (R2=0.874) and uncorrelated with the measurement of the short S-D of probe 2 (R2=0.019). Nevertheless, the changes in pCBF and pCBV for probe 1 have larger magnitude than for probe 2, where skin blood flow or skin is not removed. This is probably because, although the large S-D separation is mostly sensitive to heartbeat oscillations within the brain, we still have a contribution of the skin pulsate flow, which partially contaminates the measured signal in probe 2.
Combining the results of the hypercapnia measurements on all of the animals for both protocols A and B at large separation distances (36 hypercapnia cycles on eight animals, probe 2 for protocol A and probe 1 for protocol B, all with p <0.05), we found the average change of the pulsatile CBF to be 50.7±21.7% (percent change from baseline, mean±standard deviation).
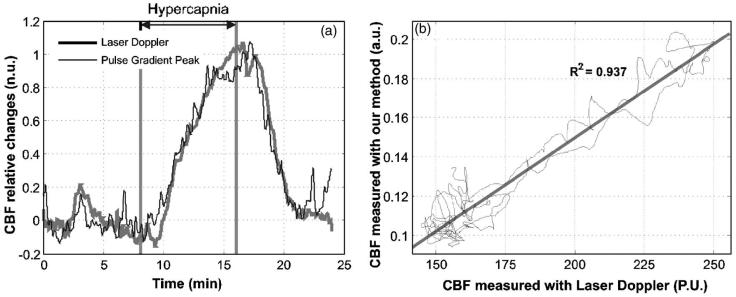
Figure 5 shows comparative measurements during a hypercapnia cycle on a 7-day-old piglet. Figure 5(a) illustrates the laser Doppler results (gray thick line) and the results of our method (black line) for the large S-D separation. Both measurements are normalized so that the baseline and the maximum change correspond to values 0 and 1. It is apparent that there is good correlation between the two methods. The pulse gradient peak parameter appears to respond slightly earlier (30 to 40 s) to changes of the breathing gasses. This could potentially be attributed to the different volumes and depths the two methods are probing. Figure 5(b) shows a plot of the results of one method against the other. There appears to be a strong linear correlation between the results of the pulse gradient peak parameter and the laser Doppler results with R2=0.937. The average correlation coefficient of 12 hypercapnia cycles on four animals was found to be 0.892±0.078 (standard deviation). Although the method presented is sensitive to changes of the pulsatile flow, the results appear to agree with the laser Doppler measurements of the total CBF, which includes both the baseline and pulsatile components.
Fig. 5.
Representative results from one hypercapnia cycle simultaneously measured with the suggested NIRS method and a laser Doppler system. (a) shows the time course results of both methods, and (b) the results of the one method against the other with a linear fit (au: arbitrary units, PU: perfusion units).
Although we have found that there is some variability in the results from piglet to piglet and even between different measurements on the same animal, the hemodynamic responses of all animals measured were found to follow the same general trend, as shown in Fig. 4. Differences between measurements could be due to altered animal physiology, deterioration of the animal health due to anesthesia and surgery, or even different sensitivity of each animal to hypercapnic stresses. The blood flow response of the skin was found to be more irregular and less reproducible than the brain response. One of the reasons could be that the pressure applied by the probe on the skin was not reproducible and could have caused partial occlusion of the local blood circulation. On the contrary, the CBF exhibits a relatively consistent and reproducible response, as shown in Fig. 4, in all four animals.
4 Conclusions and Future Work
The results presented here indicate the ability of NIRS to measure relative changes of pulsatile cerebral blood flow and volume from the shape of heartbeat oscillations. We demonstrate that different S-D separations probe heartbeat oscillations at different depths. In particular, we find that for an appropriately large S-D separation, the influence of the scalp on the measured signal is minimized and therefore the measured signal is mostly representative of the brain cortex. Good linear correlation of the results with the laser Doppler measurements of CBF indicate the potential of the method to provide information about the relative changes of CBF by measuring relative changes of the pulsatile component of the flow.
The instrumentation required for the implementation of this method is portable, inexpensive, and safe for continuous bedside monitoring in neonates. This method for measuring relative changes of CBF and CBV could be easily incorporated into a NIRS system that measures tissue optical properties (absorption and scattering) and therefore calculates oxy-and deoxy-hemoglobin concentrations, tissue oxygen saturation, and total hemoglobin concentration. Combinations of these parameters can be used to derive other physiologically and clinically interesting parameters such as the tissue metabolic rate of oxygen consumption.
Despite the encouraging preliminary results, more measurements are required to further test the method and estimate the sensitivity and accuracy. Moreover, further development of the theoretical model that correlates the waveform of the detected heartbeat oscillations and the hemodynamic parameters could improve the accuracy of the method and potentially lead to a better quantification of the measured parameters.
Acknowledgments
We would like to thank Gary Boas for editorial assistance and John Moore for technical assistance during the measurements. This research was supported by the U.S. National Institutes of Health (NIH) grant RO1-HD42908.
References
- 1.Edvinsson L, Krausse DN. Cerebral Blood Flow and Metabolism. Lippincott Williams and Wilkins; New York: Changes in the arterial gas tension; p. 384. [Google Scholar]
- 2.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc. Natl. Acad. Sci. U.S.A. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J. Nucl. Med. 1983;24(9):790–798. [PubMed] [Google Scholar]
- 4.Patel J, Marks K, Roberts I, Azzopardi D, Edwards AD. Measurement of cerebral blood flow in newborn infants using near infrared spectroscopy with indocyanine green. Pediatr. Res. 1998;43(1):34–39. doi: 10.1203/00006450-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fabricius M, Akgoren N, Dirnagl U, Lauritzen M. Laminar analysis of cerebral blood flow in cortex of rats by laser-Doppler flowmetry, a pilot study. J. Cereb. Blood Flow Metab. 1997;17(12):1326–1336. doi: 10.1097/00004647-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cheung C, Culver JP, Takahashi K, Greenberg JH, Yodh AG. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys. Med. Biol. 2001;46(8):2053–2065. doi: 10.1088/0031-9155/46/8/302. [DOI] [PubMed] [Google Scholar]
- 7.Kirkham FJ, Padayachee TS, Parsons S, Seargeant LS, House FR, Gosling RG. Transcranial measurement of blood velocities in the basal cerebral arteries using pulsed Doppler ultrasound: velocity as an index of flow. Ultrasound Med. Biol. 1986 Jan.12(1):15–21. doi: 10.1016/0301-5629(86)90139-0. [DOI] [PubMed] [Google Scholar]
- 8.Edwards AD, Wyatt JS, Richardson C, Delpy DT, Cope M, Reynolds EO. Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet. 1988;2(8614):770–771. doi: 10.1016/s0140-6736(88)92418-x. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AD, Richardson C, van der Zee P, Elwell C, Wyatt IS, Cope M, Delpy DT, Reynolds EO. Measurement of hemoglobin flow and blood flow by near-infrared spectroscopy. J. Appl. Physiol. 1993;75(4):1884–1889. doi: 10.1152/jappl.1993.75.4.1884. [DOI] [PubMed] [Google Scholar]
- 10.Skov L, Pryds O, Greisen G. Estimating cerebral blood flow in newborn infants: comparison of near infrared spectroscopy and 133Xe clearance. Pediatr. Res. 1990 Dec.30(6):570–573. doi: 10.1203/00006450-199112000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Mendelson Y. Pulse oximetry: theory and applications for noninvasive monitoring. Clin. Chem. 1992;38:1601–1607. [PubMed] [Google Scholar]
- 12.Murray WB, Foster PA. The peripheral pulse wave: information overlooked. J. Clin. Monit. 1996 Sep.12(5):365–377. doi: 10.1007/BF02077634. [DOI] [PubMed] [Google Scholar]
- 13.Wisely NA, Cook LB. Arterial flow waveforms from pulse oximetry compared with measured Doppler flow waveforms. Anaesthesia. 2001;56:556–561. doi: 10.1046/j.1365-2044.2001.01987.x. [DOI] [PubMed] [Google Scholar]
- 14.Cook LB. Extracting arterial flow waveforms from pulse oximeter waveforms. Anaesthesia. 2001;56:551–555. doi: 10.1046/j.1365-2044.2001.01986.x. [DOI] [PubMed] [Google Scholar]
- 15.Franceschini MA, Gratton E, Fantini S. Non-invasive optical method to measure tissue and arterial saturation: an application to absolute pulse oximetry of the brain. Opt. Lett. 1999;24(12):829–831. doi: 10.1364/ol.24.000829. [DOI] [PubMed] [Google Scholar]
- 16.Bauer R, Bergmann R, Walter B, Brust P, Zwiener U, Johannsen B. Regional distribution of cerebral blood volume and cerebral blood flow in newborn piglets—effect of hypoxia/hypercapnia. Brain Res. Dev. Brain Res. 1999 Jan.112(1):89–98. doi: 10.1016/s0165-3806(98)00167-9. [DOI] [PubMed] [Google Scholar]
- 17.Edvinsson L, Krause DN. Cerebral blood flow and metabolism. Lippincott Williams and Wilkins; New York: 2001. [Google Scholar]
- 18.Smielewski P, Kirkpatrick P, Minhas P, Pickard JD, Czosnyka M. Can cerebrovascular reactivity be measured with near-infrared spectroscopy. Stroke. 1995;26:2285–2292. doi: 10.1161/01.str.26.12.2285. [DOI] [PubMed] [Google Scholar]
- 19.Mengesha YA. Variability of cardiovascular responses to hypercapnia in Man. Ethiop. J. Health Dev. 2000;14(2):135–141. [Google Scholar]
- 20.Bereczki D, Wei L, Otsuka T, Hans FJ, Acuff V, Patlak C, Fenstermacher J. Hypercapnia slightly raises blood volume and sizably elevates flow velocity in brain microvessels. Am. J. Physiol. 1993;264(5 Pt 2):H1360–H1369. doi: 10.1152/ajpheart.1993.264.5.H1360. [DOI] [PubMed] [Google Scholar]