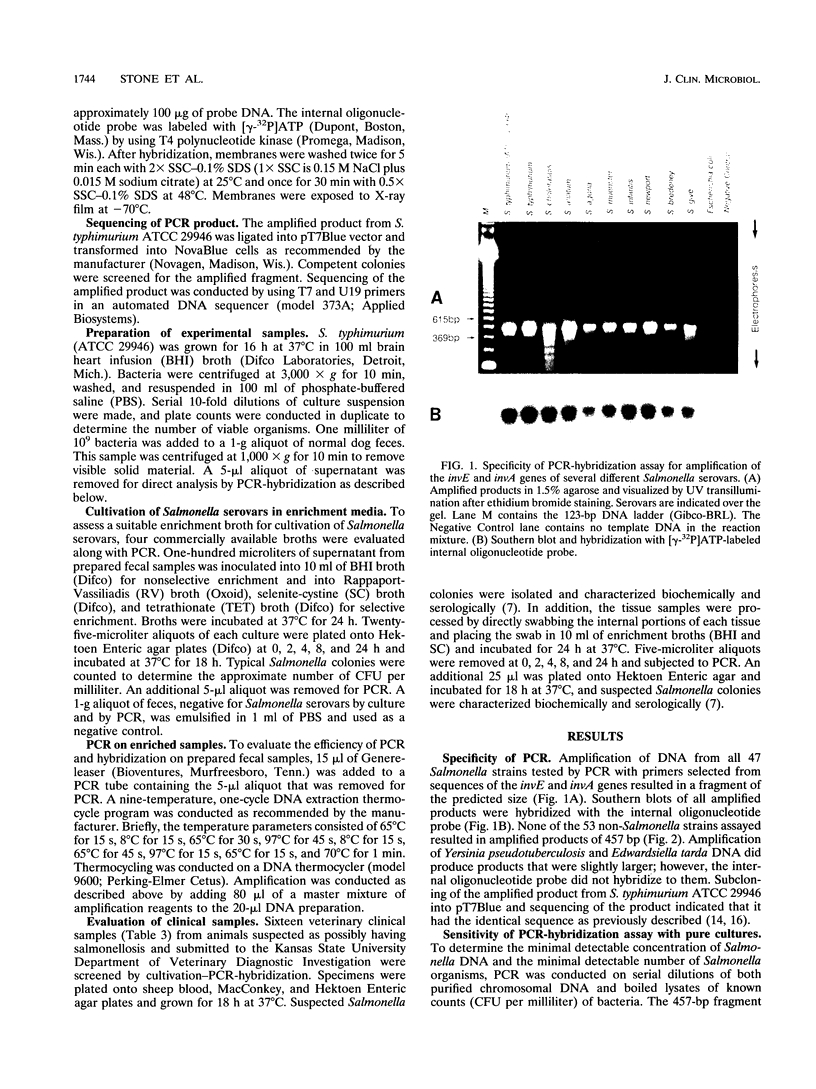
Abstract
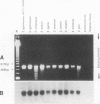
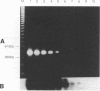
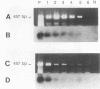
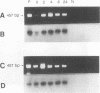
To overcome problems associated with application of PCR to clinical samples, we have combined a short cultivation procedure with a Salmonella-specific PCR-hybridization assay to specifically identify Salmonella serovars from clinical samples of various animal species. The technique was investigated by using fecal samples seeded with known numbers of Salmonella organisms and cultivated for different lengths of time in assorted selective and nonselective enrichment media. The ability of PCR to amplify a Salmonella-specific DNA product (457-bp sequence covering the Salmonella invE and invA genes) was examined in Southern hybridizations with an internal oligonucleotide probe. Forty-seven Salmonella isolates representing 32 serovars were evaluated, and all Salmonella isolates resulted in a 457-bp product that hybridized with the oligonucleotide probe, whereas no hybridizations were evident with 53 non-Salmonella organisms. The assay detected as few as 9 CFU of Salmonella organisms in pure culture and as little as 300 fg of purified chromosomal DNA. Rappaport-Vassiliadis and tetrathionate broths were inhibitory to PCR, whereas brain heart infusion and selenite-cystine broths were not. The PCR-hybridization assay coupled with a brain heart infusion enrichment culture incubated for 2 h detected as few as 80 CFU of Salmonella organisms in seeded feces. We have successfully identified Salmonella serovars in clinical samples from swine, horses, and cattle more rapidly than with conventional culture techniques. The sensitivity and specificity of this assay were both 100% compared with culture results. These results indicate that a combined cultivation-PCR-hybridization assay could be applicable and advantageous in the rapid identification of Salmonella serovars in routine diagnostic situations.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araj G. F., Chugh T. D. Detection of Salmonella spp. in clinical specimens by capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1987 Nov;25(11):2150–2153. doi: 10.1128/jcm.25.11.2150-2153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall S. T., Hindle M. A., Hutchinson D. N. Improved isolation of salmonellae from faeces using a semisolid Rappaport-Vassiliadis medium. Eur J Clin Microbiol Infect Dis. 1992 Oct;11(10):936–939. doi: 10.1007/BF01962379. [DOI] [PubMed] [Google Scholar]
- Bessesen M. T., Luo Q. A., Rotbart H. A., Blaser M. J., Ellison R. T., 3rd Detection of Listeria monocytogenes by using the polymerase chain reaction. Appl Environ Microbiol. 1990 Sep;56(9):2930–2932. doi: 10.1128/aem.56.9.2930-2932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Heijtink R., Wertheim-van Dillen P. M., van der Noordaa J. Rapid purification of hepatitis B virus DNA from serum. J Clin Microbiol. 1991 Sep;29(9):1804–1811. doi: 10.1128/jcm.29.9.1804-1811.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone G. J., Demmler G. J., Schimbor C. M., Greer J. Improved amplification of cytomegalovirus DNA from urine after purification of DNA with glass beads. Clin Chem. 1991 Nov;37(11):1945–1949. [PubMed] [Google Scholar]
- Cano R. J., Rasmussen S. R., Sánchez Fraga G., Palomares J. C. Fluorescent detection-polymerase chain reaction (FD-PCR) assay on microwell plates as a screening test for salmonellas in foods. J Appl Bacteriol. 1993 Sep;75(3):247–253. doi: 10.1111/j.1365-2672.1993.tb02773.x. [DOI] [PubMed] [Google Scholar]
- Cohen N. D., Neibergs H. L., McGruder E. D., Whitford H. W., Behle R. W., Ray P. M., Hargis B. M. Genus-specific detection of salmonellae using the polymerase chain reaction (PCR). J Vet Diagn Invest. 1993 Jul;5(3):368–371. doi: 10.1177/104063879300500311. [DOI] [PubMed] [Google Scholar]
- Curiale M. S., Klatt M. J., Mozola M. A. Colorimetric deoxyribonucleic acid hybridization assay for rapid screening of Salmonella in foods: collaborative study. J Assoc Off Anal Chem. 1990 Mar-Apr;73(2):248–256. [PubMed] [Google Scholar]
- Dyer D. W., Iandolo J. J. Rapid isolation of DNA from Staphylococcus aureus. Appl Environ Microbiol. 1983 Jul;46(1):283–285. doi: 10.1128/aem.46.1.283-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts R., Diamond M., Hamilton C., Neri M. DNA-DNA hybridization assay for detection of Salmonella spp. in foods. Appl Environ Microbiol. 1983 Nov;46(5):1146–1151. doi: 10.1128/aem.46.5.1146-1151.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G., Riley L., Giron J. A., Valmassoi J., Friedmann A., Strockbine N., Falkow S., Schoolnik G. K. Detection of Shigella in feces using DNA amplification. J Infect Dis. 1990 Jun;161(6):1252–1256. doi: 10.1093/infdis/161.6.1252. [DOI] [PubMed] [Google Scholar]
- Fricker C. R. The isolation of salmonellas and campylobacters. J Appl Bacteriol. 1987 Aug;63(2):99–116. doi: 10.1111/j.1365-2672.1987.tb02692.x. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Ginocchio C., Costeas P. Molecular and functional characterization of the Salmonella invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992 Jul;174(13):4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett C. T., Ferreira-Centeno A., Nasim S. Molecular diagnostics: issues of utilization, regulation and organization. Clin Chim Acta. 1993 Jul 30;217(1):85–103. doi: 10.1016/0009-8981(93)90240-5. [DOI] [PubMed] [Google Scholar]
- Ginocchio C., Pace J., Galán J. E. Identification and molecular characterization of a Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopo J. M., Melis R., Filipska E., Meneveri R., Filipski J. Development of a Salmonella-specific biotinylated DNA probe for rapid routine identification of Salmonella. Mol Cell Probes. 1988 Dec;2(4):271–279. doi: 10.1016/0890-8508(88)90011-4. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insalata N. F., Mahnke C. W., Dunlap W. G. Direct fluorescent-antibody technique for the microbiological examination of food and environmental swab samples for salmonellae. Appl Microbiol. 1973 Sep;26(3):268–270. doi: 10.1128/am.26.3.268-270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard D. R., Johnson W. M., Lior H., Tyler S. D., Rozee K. R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990 Mar;28(3):540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn K., De Grandis S. A., Clarke R. C., McEwen S. A., Galán J. E., Ginocchio C., Curtiss R., 3rd, Gyles C. L. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes. 1992 Aug;6(4):271–279. doi: 10.1016/0890-8508(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Ramamurthy T., Pal A., Bag P. K., Bhattacharya S. K., Nair G. B., Kurozano H., Yamasaki S., Shirai H., Takeda T., Uesaka Y. Detection of cholera toxin gene in stool specimens by polymerase chain reaction: comparison with bead enzyme-linked immunosorbent assay and culture method for laboratory diagnosis of cholera. J Clin Microbiol. 1993 Nov;31(11):3068–3070. doi: 10.1128/jcm.31.11.3068-3070.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby C. E. Enzyme-linked immunosorbent assay for detection of Salmonella lipopolysaccharide in poultry specimens. Appl Environ Microbiol. 1984 Jun;47(6):1327–1330. doi: 10.1128/aem.47.6.1327-1330.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen L., Holmstrøm K., Olsen J. E., Rasmussen O. F. A rapid polymerase chain reaction (PCR)-based assay for the identification of Listeria monocytogenes in food samples. Int J Food Microbiol. 1991 Nov;14(2):145–151. doi: 10.1016/0168-1605(91)90101-t. [DOI] [PubMed] [Google Scholar]
- Rossen L., Nørskov P., Holmstrøm K., Rasmussen O. F. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int J Food Microbiol. 1992 Sep;17(1):37–45. doi: 10.1016/0168-1605(92)90017-w. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomason B. M., Dodd D. J., Cherry W. B. Increased recovery of salmonellae from environmental samples enriched with buffered peptone water. Appl Environ Microbiol. 1977 Sep;34(3):270–273. doi: 10.1128/aem.34.3.270-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjojoatmodjo M. N., Fluit A. C., Torensma R., Keller B. H., Verhoef J. Evaluation of the magnetic immuno PCR assay for rapid detection of Salmonella. Eur J Clin Microbiol Infect Dis. 1991 Nov;10(11):935–938. doi: 10.1007/BF02005447. [DOI] [PubMed] [Google Scholar]
- Widjojoatmodjo M. N., Fluit A. C., Torensma R., Verdonk G. P., Verhoef J. The magnetic immuno polymerase chain reaction assay for direct detection of salmonellae in fecal samples. J Clin Microbiol. 1992 Dec;30(12):3195–3199. doi: 10.1128/jcm.30.12.3195-3199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Eiden J., Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990 Jun;28(6):1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren B. W., Tabaqchali S. Detection of pathogenic Yersinia enterocolitica by the polymerase chain reaction. Lancet. 1990 Sep 15;336(8716):693–693. doi: 10.1016/0140-6736(90)92191-j. [DOI] [PubMed] [Google Scholar]