Abstract
It is widely accepted that the p53 tumor suppressor restricts abnormal cells by induction of growth arrest, or by triggering apoptosis. Here we show that in addition p53 protects the genome from oxidation by reactive oxygen species (ROS), a major cause for DNA damage and genetic instability. In the absence of severe stresses relatively low levels of p53 are sufficient for up-regulation of several antioxidant genes, which is associated with a decrease in intracellular ROS. Down-regulation of p53 results in excessive oxidation of DNA, increased mutation rate, and karyotype instability, which are prevented by incubation with antioxidant N-acetylcysteine (NAC). Dietary supplementation with NAC prevents frequent lymphomas characteristic to p53 knockout mice, and slows down growth of xenografts from A549 cells with p53 inhibited by siRNA. Our results provide novel paradigm for a non-restrictive tumor suppressor function of p53 and highlight potential importance of antioxidants in prophylactics and treatment of cancer.
The major function for the p53 tumor suppressor is to restrict abnormal or stress-exposed cells before damage to DNA is converted to inherited mutation1. However, even without extended stress the DNA is exposed to endogenous damaging reactive oxygen species (ROS), which are by-products of normal respiration, and important signaling molecules2,3. Indeed, endogenous ROS is the major source of DNA damage4, and a substantial factor contributing to chromosome instability and accumulation of mutations and deletions leading to cancer4,5. As the endogenous ROS modify approximately 20,000 bases of DNA per day in a single cell6 it is unlikely that the restriction of proliferation of cells with oxidized DNA would be efficient in preventing mutations. Previously it was found that among transcriptional targets of p53 there are several potential ROS-generating genes whose action presumably contributes to p53-mediated cell death7,8. However, other p53-upregulated genes, such as glutathione peroxidase (GPX1) 9,10, Mn-superoxide dismutase (Mn-SOD)10 and aldehyde dehydrogenase 4 (ALDH4)11, would presumably act as antioxidants. In addition, the function of two p53-regulated sestrins (HI95 and PA26) is essential for regeneration of over-oxidized peroxiredoxins12, the enzymes involved in the decomposition of hydrogen peroxide3. These findings suggest that p53 might play opposite roles in ROS regulation. In this study we discriminate between pro- and antioxidant functions of p53, and establish a substantial contribution of p53-mediated antioxidant mechanisms in the control of genetic stability and cancer prevention.
Results
Down-regulation of p53 elevates intracellular ROS
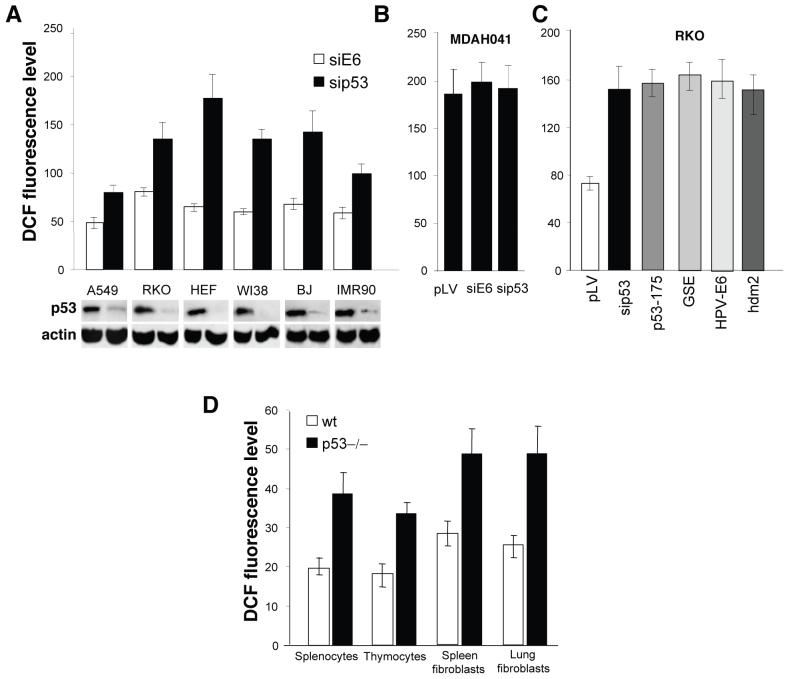
To reveal effects of p53 on ROS levels in non-stressed cells we inhibited p53 by lentiviral-mediated expression of siRNA in a set of human normal and carcinoma cell lines, which have functional p53 (Supplementary Fig. 1a,b online). Analysis of dichlorodihydrofluorescein (DCF) staining revealed approximately 2-fold increases in ROS 48 h after inhibition of p53 (Fig. 1a). The increase in ROS induced by siRNA to p53 was similar in magnitude to that observed after treatment of RKO cells with hydrogen peroxide and was completely reversed by incubation with 5mM N-acetylcysteine (NAC) (Supplementary Fig. 2a online). The expression of p53 siRNA did not change notably growth rate and cell-cycle distribution of RKO cells (Supplementary Fig. 1c,d online) and did not increase ROS in p53-negative cell lines MDAH041 and H1299, similar to empty lentivirus vector or heterologous siRNA to HPV18 E6 gene (Fig. 1b, Supplementary Fig. 2b online). In contrast, the conditional expression of wild-type p53 from tetracycline-regulated construct in MDAH041-derived TR9-7 cells13 resulted in a 50% decrease in ROS (Supplementary Fig. 2c online). The increase in ROS was observed following inhibition of p53 by other mechanisms, such as over-expression of oncogenic mutant p53 His175, dominant-negative genetic suppressor element of p53, GSE2214, or p53 inhibitors HPV16 E6, or HDM2 (Fig. 1c). A 3-5-fold increase in HDM2 transcripts was sufficient to cause a 2-fold increase in ROS, while in osteosarcoma cell line U2OS, which is known to bear amplified HDM2 gene15 the exogenous over-expression of HDM2 did not affect ROS levels (Supplementary Fig. 3a online). The pro-oxidant effect of p53 deficiency was also observed in primary lung and spleen fibroblasts and in splenocytes and thymocytes taken immediately before the test from wild-type and p53-knockout mice: the ROS levels were consistently increased in the p53-deficient cells (Fig. 1d, Supplementary Fig. 3b online).
Figure 1.
Effect of p53 status on intracellular ROS levels.
We measured ROS levels by FACS following DCF staining and expressed as the mean ± sem intensity of cell fluorescence. (a) ROS level in p53-positive carcinoma cell lines A549 and RKO, and in normal human fibroblasts HEF, WI38, BJ and IMR90 after inhibition of p53 by expression of siRNA. As control we used non-specific siRNA to human papilloma virus HPV18 E6 gene. P < 0.04 compared to the correspondent cells with control vector by the Student t test. The lower panel shows expression levels of endogenous p53 protein in control and in si-p53 expressing cells, as detected by Western analysis with the antibody to p53 DO1. (b) ROS level in p53-negative MDAH041 human cell lines expressing siRNAs to p53 or E6. P > 0.94 compared to the cells with empty vector by the Student t test. (c) Effect of p53 inhibition by over-expression of p53 siRNA, p53 mutant His175, GSE22, HPV18 E6 gene and hdm2 protein on intracellular ROS. P < 0.04 compared to the cell with empty vector by the Student t test. (d) ROS levels in mouse splenocytes, thymocytes, spleen and lung fibroblasts from wild-type and p53-/- mice. We performed DCF staining immediately after isolation of splenocytes and thymocytes. The spleen and lung fibroblasts were at passage one. P < 0.05 compared to the correspondent wild type tissue by the Student t test.
Opposite effects on ROS of different p53 target genes
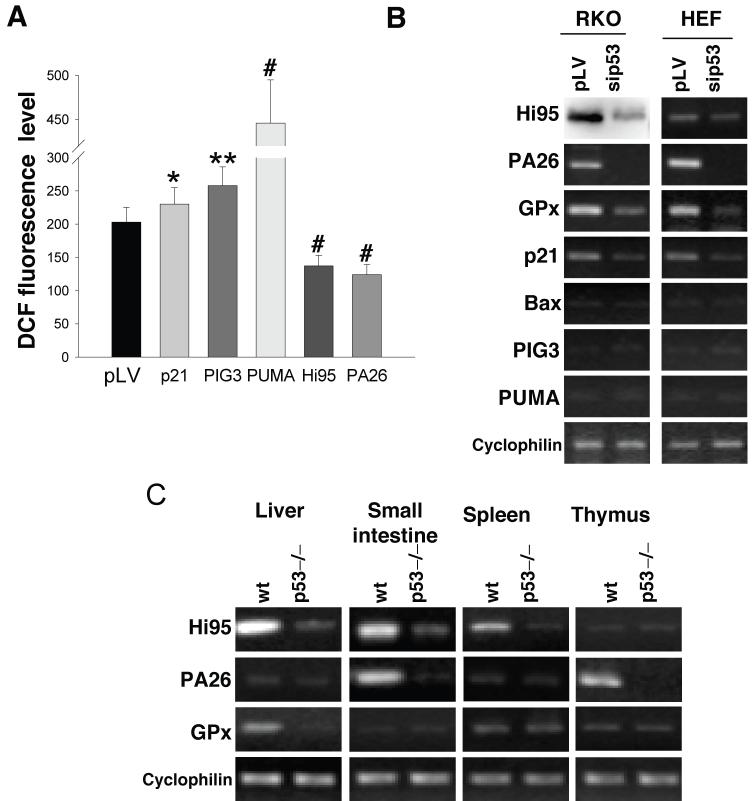
The above observations suggest that p53 may extend its protective function by participating in antioxidant defense. Such activity of p53 should be opposite to the known pro-oxidant function of some stress-induced p53-responsive genes, which supposedly contribute to p53-induced cell death7,8. To estimate the impact of pro-and anti-oxidant p53-modulated genes we over-expressed corresponding cDNAs in RKO and H1299 cells by infection with recombinant lentiviruses and compared intracellular ROS levels 48 h after infection. While the expression of CDKN1 (P21) did not have any effect, there was an increase in ROS levels after introduction of quinone oxidoreductase homolog gene PIG3 and especially of pro-apoptotic gene PUMA (2-2.5 fold), and a 50% decrease in ROS after expression of antioxidant sestrins HI95 or PA26 (Fig. 2a, Supplementary Fig. 3c online).
Figure 2.
Activity of p53 is required for maintaining functional state of several antioxidant genes. (a) Different p53 target genes produce opposite effects on intracellular ROS levels. We expressed several p53-regulated genes in H1299 cells by infection with appropriate lentiviral constructs. Forty-eight hours after infection, intracellular ROS was detected by FACS following DCF staining. The ROS levels are expressed as the mean ± sem intensity of cell fluorescence, *P = 0.84, **P = 0.07, #P < 0.03 compared to the cells with empty vector by the Student t test. (b) Expression of HI95 (western analysis with polyclonal antibody to HI95) and PA26, GPX1, P21, BAX, PIG3, PUMA and PPIA (cyclophilin A) detected by RT-PCR in control RKO cells infected with empty vector or after inhibition of p53 by siRNA-expressing lentivirus. (c) levels of transcripts from the p53-regulated antioxidant genes in different organs of control and p53-/- mice (RT-PCR).
Pro- vs. antioxidant effects depend on p53 levels
We tested how deficiency in p53 affects the basal transcription levels of p53-regulated genes. Inhibition of p53 in RKO cells and HEFs resulted in a notable decrease in HI95, and a virtual disappearance of GPX1 mRNA and p53-inducible transcript T216 of the PA26 gene, while the levels of pro-oxidant genes BAX, PIG3 and PUMA were undetectable in unstressed cells (Fig. 2b). In different organs of mice transcription levels of p53 regulated genes were diverse reflecting tissue specificity of expression. Where detectable, there was notable decrease in transcripts for the Gpx1 and sestrins in organs of p53 knockout mice (Fig. 2c) indicating that up-regulation of basal transcription of the p53 targets does not represent a cell culture artifact.
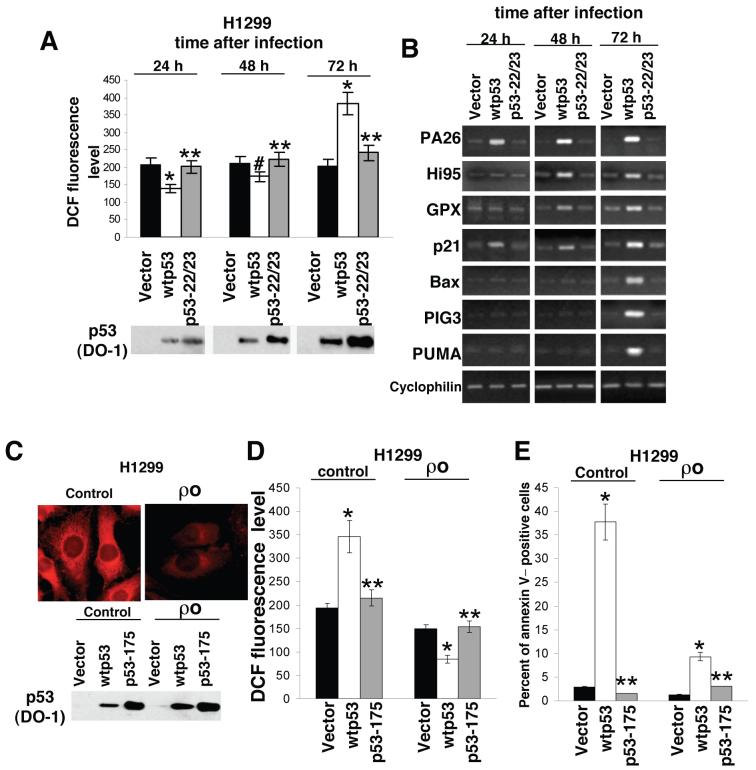
To investigate whether the transcription function of p53 is required for the ROS regulation we expressed wild-type p53 and the p53 mutant Q22L/W23S (22/23)17 with defective transcription function in p53-negative H1299 and p53-positive RKO cells. To achieve more physiological levels of the introduced p53 the expression was driven by a promoter from the p53 gene18. Transgene expression after lentiviral introduction develops slowly and reaches its maximum at 55-60 h after infection. By 24 h, the expression level of introduced p53 in H1299 cells was comparable to that in the p53-positive RKO cells; correspondingly, the cells expressing introduced wild type p53 demonstrated decreased ROS, as compared to control cells (Fig. 3a, Supplementary Figs. 4a,5a online). At this time point there was notable induction of transcription from the PA26 and P21 genes. By 48 h we also observed induction of transcripts of the antioxidant genes GPX1 and HI95. By 72 h there was a substantial increase in intracellular ROS, concomitant with apparent over-expression of wild-type p53. These late events coincided with the notable up-regulation of PIG3, BAX and PUMA, and with the induction of apoptosis (Fig. 3b, Supplementary Figs. 4b,c, 5b online). The p53 mutant 22/23 induced neither the expression of p53 target genes, nor apoptosis. Thus, the p53-dependent changes in ROS rely on transcriptional activation of p53-regulated genes.
Figure 3.
Opposite effects of p53 on ROS levels. (a,b) We infected H1299 cells with recombinant lentiviruses expressing wild-type p53 or the 22/23p53 mutant and measured ROS levels by FACS following DCF staining 24, 48 and 72 h after infection (expressed as the mean ± sem intensity of cell fluorescence; *P < 0.04, **P = 0.87, #P = 0.13 compared to the cells with empty vector by the Student t test). The lower panel shows the expression level of wild-type and mutant p53 proteins as visualized by Western analysis with the DO1 antibodies to p53. (b) RT-PCR analysis of different p53 target genes after expression of wild-type p53 or the 22/23p53 mutant in H1299 cells. (c) Mitochondria in control and π0 H1299 cells stained with MitoTracker Red. The lower panel shows the expression level of wild-type p53 and His175 p53 mutant in control and π0 H1299 cells 72 h after infection, as visualized by Western analysis with the DO1 antibody to p53. (d) ROS levels (DCF staining, *P < 0.04, **P > 0.90 compared to the cells with empty vector by the Student t test). (e) the proportion of Annexin V-labeled cells (*P < 0.02, **P > 0.83, compared to the cells with empty vector by the Student t test) were detected by FACS 72 h after infection.
Considering the role of mitochondria in the release of excessive ROS at late hours after p53 induction19 we performed similar experiments in H1299 and RKO π0 cells lacking mitochondrial DNA and defective for ROS-generating electron transport chain20 (Fig. 3c, Supplementary Fig. 6a online). While prolonged expression of p53 resulted in an approximately 2-fold increase in ROS in control cells, there was a 50% decrease in ROS in the π0 cells. Introduction of an empty vector or a transcriptionally inactive p53 mutant affected ROS level neither in control nor in π0 H1299 (Fig. 3d, Supplementary Fig. 6b online). The pattern and the magnitude of activation of different p53-dependent genes were similar in control and π0 cells (Supplementary Fig. 6c online). However, the latter were deficient in the production of ROS and demonstrated a substantially (5-fold) attenuated apoptotic response to p53 expression (Fig. 3e). These results further link the p53-induced increase in ROS with apoptosis involving the mitochondria.
p53 modulates ROS according to severity of stresses
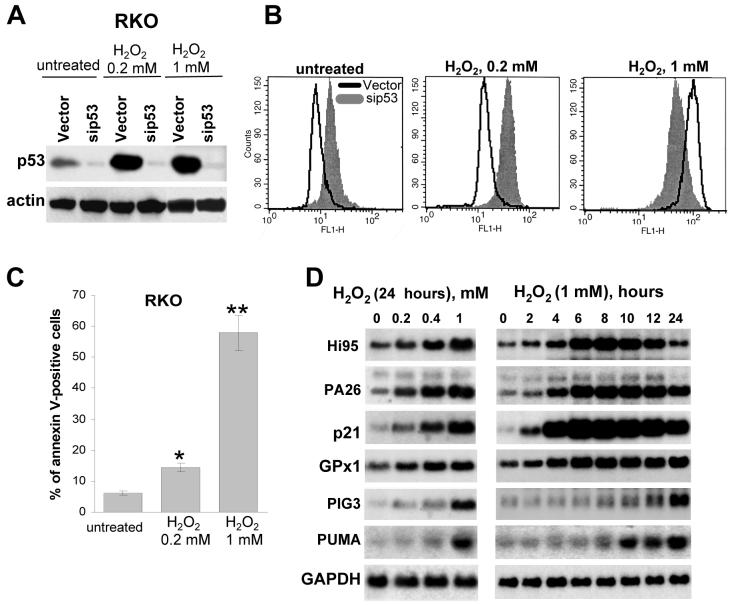
As p53 might play different roles depending on the degree of stresses we compared the tolerance of control and p53-deficient RKO cells to different concentrations of H2O2. Both untreated p53-positive RKO cells and RKO cells treated with low (non-lethal) concentration of H2O2 (0.2 mM) exhibited decreased ROS levels, as compared to p53-negative RKO/sip53 cells. However, the response was opposite at higher (lethal) concentrations of H2O2 (1 mM), where the p53-positive cells demonstrated an increase in ROS, which coincided with the induction of apoptosis (Fig. 4a,b,c). Similar results were obtained after treatment of p53-positive and p53-deficient RKO cells with different doses of UV (Supplementary Fig. 7b online).
Figure 4.
Antioxidant effect of p53 after mild stress and pro-oxidant effect of p53 after grave stress. (a) Expression of endogenous p53 in RKO cells infected with empty vector or vector bearing siRNA to p53 in control and H2O2-treated cells. (b) FACS analysis of DCF fluorescence levels in untreated and H2O2-treated (12 h) RKO and RKO/sip53 cells. (c) Apoptosis levels 24 h after treatment with 0.2 and 1mM of H2O2, as detected by FACS following Annexin V staining, *P = 0.08 and **P = 0.01 compared to untreated cell by the Student t test. (d,e) Dose-dependence (24 h after treatment) and kinetics (treatment with 1mM H2O2) of the induction of p53-responsive genes following H2O2 treatment (Northern analysis).
The kinetics of induction of different anti- and pro-oxidant p53 target genes was revealed by Northern analysis after treatment with different doses of H2O2. The anti-oxidant genes HI95, PA26 and GPX1, together with P21 were rapidly induced even at low concentrations of H2O2 in agreement with the fact that p53-positive cells are more tolerant of low doses of oxidants (Fig. 4b,d). The pro-oxidant genes PIG3 and PUMA were induced at higher concentrations of H2O2, and with a notable delay, which correlated with the lower tolerance of p53-positive cells to severe oxidative stress (Fig. 4b,d). The induction of PIG3 and PUMA was abrogated in RKO/sip53 cells (Supplementary Figs. 1b, 7a online). The results suggest that the antioxidant function of p53 is associated with highly responsive p53 target genes that are induced during physiological (non-lethal) stress, while the pro-oxidant effect of p53 in gravely-damaged cells could be associated with the delayed induction of less p53 responsive pro-apoptotic genes.
p53 deficiency promotes DNA oxidation and mutagenesis
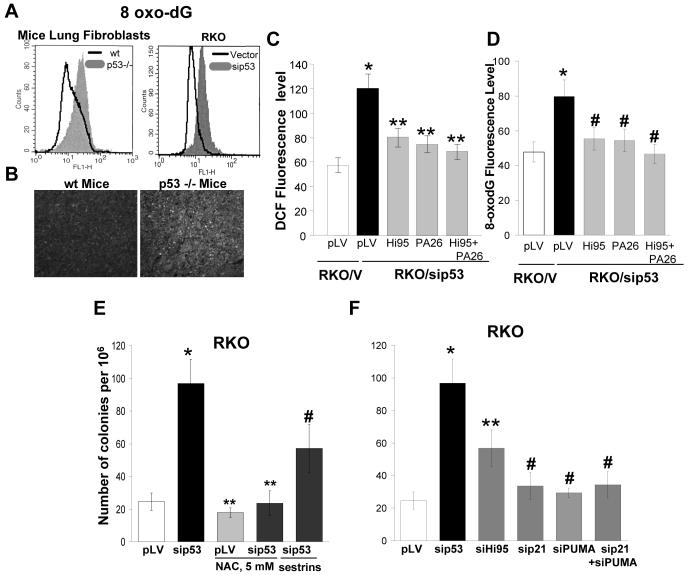
The observed increased levels of ROS could contribute to remarkable genetic instability of p53-deficient cells. To define the protective role of p53 against DNA oxidation we monitored the rate of formation of 8-oxoguanine (8-oxo-dG), the major product of oxidation in DNA, and the major source for mutations by avidin-FITC staining. RKO/sip53, A549/sip53 and p53-/- mouse primary lung fibroblasts showed an increased level of 8-oxo-dG as compared to control cells or p53+/+ mouse primary lung fibroblasts. Staining for 8-oxo-dG has also revealed a 2-fold) DNA oxidation in spleens of p53 -/- mice confirming that the effects are not related to artificial conditions in cell culture (Fig. 5a,b, Supplementary Fig. 8a,b online). Exogenous over-expression of HI95 and/or PA26 in RKO cells with inhibited p53 has only partially, though substantially reversed both the increase in ROS and the excessive DNA oxidation (Fig. 5c,d) further suggesting that sestrins are perhaps not the only mediators of p53-dependent antioxidant function.
Figure 5.
p53 decreases DNA oxidation and mutagenesis. (a) p53 deficiency in lung fibroblasts from p53-/- mouse and in RKO cells with inhibited p53 increases 8-oxo-dG staining as measured by FACS following FITC-avidin staining. (b) 8-oxo-dG levels in spleens from wild-type and p53-/- mice as detected by FITC-avidin staining. (c,d) Expression of p53-regulated sestrins inhibits the elevation of intracellular ROS (c) and DNA oxidation (d) induced by down-regulation of p53. We measured ROS and 8-oxo-dG levels by FACS following DCF (left panel), or FITC-avidin (right panel) staining and expressed as the mean ± sem intensity of fluorescence, *P < 0.03, **P > 0.5 and #P < 0.09 compared to the cells expressing empty vector, by the Student t test. (e) Mutation frequency within HPRT locus in RKO and RKO/sip53 cells in the presence of NAC (5 mM). Colony formation assay of HAT-pre-selected RKO cells in the media containing 40 μg/ml 6-TG; *P = 0.02, **P > 0.85 and #P < 0.07 compared to the untreated cell expressing empty vector by the Student t test; (f) Mutation frequency in RKO cells with knocked-down p53 or different p53 target genes. Colony formation assay of RKO cells in the media containing 40 μg/ml 6-TG. Expression of p53 and HI95, P21 or PUMA was efficiently inhibited by corresponding siRNAs (Suppl. Fig 9a) two weeks before the assay; *P = 0.02, **P = 0.05 and #P > 0.89 compared to the cell expressing empty vector by the Student t test.
Oxidative DNA damage is known to be associated with an increased incidence of mutations4. Loss of p53 function also results in a mutator phenotype21,22,23. To test the contribution of p53-modulated antioxidant mechanisms in the protection against mutations, we monitored changes in mutation frequencies within the HPRT gene locus24. In RKO cells the number of 6-thioguanine (6-TG) resistant colonies increased 5-fold following inhibition of p53 and 2.5-fold following inhibition of HI95 by appropriate siRNAs. Incubation with 5mM NAC did not affect growth of RKO cells but reversed completely the increase in 6-TG resistant colonies in RKO/sip53 cells; over-expression of HI95 and PA26 reduced the number of 6-TG colonies by a half (Fig. 5e, Supplementary Fig. 9b online). There was no significant increase in 6-TG resistant colonies following inhibition of P21 which controls the p53-dependent G1 checkpoint, or following inhibition of pro-apoptotic PUMA and combination of the latter (Fig. 5f). A similar increase (4-fold) in mutation rate in A549 cell line following inhibition of p53 was also not associated with P21 gene while inhibition of HI95 expression significantly increase the number of colonies (Supplementary Fig. 10a online). The results indicate that down-regulation of p53-modulated antioxidant genes strongly contributes to the mutator phenotype of p53-deficient cells.
p53-deficiency promotes ROS-dependent xenograft growth
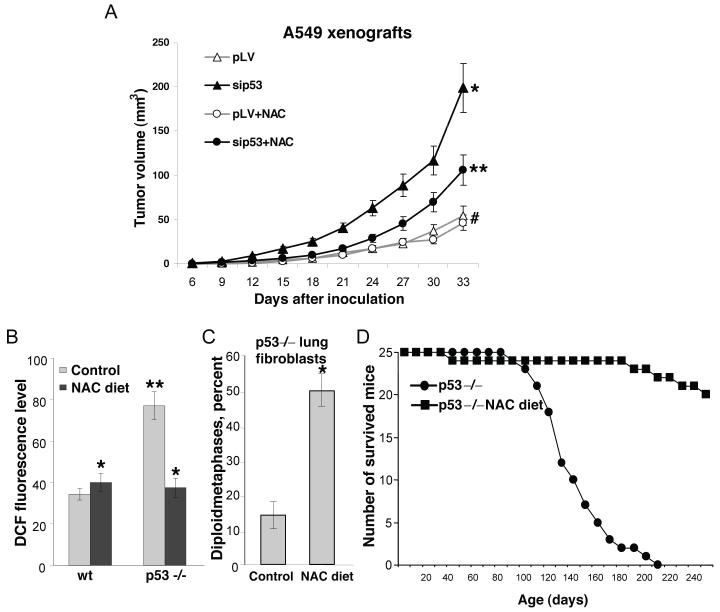
We inhibited p53 in lung carcinoma A549 cells by expression of siRNA. When tested in cell culture, there was no change in the growth rate of the cells with inhibited p53, and addition of NAC (up to 3-5 mM) did not affect cell proliferation. However, the A549 cells with inhibited p53 demonstrated increased DNA oxidation and increased mutagenesis within the HPRT locus, which was abolished by NAC (Supplementary Figs. 8b, 10b,c online). When the p53-deficient cells were injected into athymic mice they demonstrated considerable increase in tumor growth rate in comparison with the control A549 cells. Supplementation with NAC did not affected growth of xenografts from the control cells, whereas there was dramatic retardation of tumor growth with p53-deficient cells (Fig. 6a). The same effect was observed in cells with inhibited expression of HI95 gene, which also demonstrated increased intracellular ROS12 and increased oxidation to DNA (Supplementary Figs. 8b, 10d online).
Figure 6.
Elevated ROS in p53-negative tumors and in p53-/- mice contribute to accelerated tumor growth, karyotype instability and lymphomagenesis. (a) kinetics of xenografts growth with control and p53-deficient A549 cells (black lines) and the effect of NAC supplementation on tumor growth kinetics (grey lines); *P < 0.03 compared to A539/pLV cells, **P < 0.05 and #P = 0.84 compared to the cell inoculated to the correspondent control group of mice by the Student t test. (b) Intracellular ROS levels in splenocytes from p53-/- mice maintained on regular diet, and supplemented with NAC, as determined by FACS following DCF staining. Each bar represents average of the cell fluorescence intensity from three spleens; *P > 0.973 and **P = 0.05 compared to the control wild type mice by the Student t test. (c) Karyotype analysis of primary lung fibroblasts form 8-week old p53-/- mice maintained of regular and NAC-supplemented diets (average from cell cultures obtained from three mice); *P = 0.02 compared to the control p53-/- mice by the Student t test. (d) Effect of NAC supplementation on the survival of p53-/- mice. Viability of 25 animals maintained on a regular diet, and 25 animals supplemented with 40 mM NAC in drinking water was monitored over period of 250 days. The NAC-supplementation started with the pregnant female and continued through lifetime. Number of survived animals was scored with 10 days intervals. A log-rank test comparing two populations yields a two sided distributions, P value 0.005.
Antioxidant NAC prevents lymphomas in p53-/- mice
The p53-knockout mice develop normally, but succumb to neoplasia (mostly thymic lymphomas) by the age of 6 months25,26. The high tumor incidence is accompanied by a remarkable genetic instability27 that includes severe karyotype abnormalities in different organs28. We tested contribution of the elevated ROS to these phenotypes by maintaining p53-/- mice in a NAC-supplemented diet. NAC was added to drinking water starting two weeks before mating of p53+/- couples, continued though pregnancy, and through lifetime of p53-/- progeny. The dose of NAC used abolished the difference in ROS levels in splenocytes from wild-type and p53-knockout mice (Fig. 6b). Karyotype analysis in primary lung fibroblasts of 8-week old mice maintained on a regular diet revealed aneuploidy in most of the cells with only ∼15% diploid metaphases, while roughly 50% of cells from same age mice subjected to NAC diet maintained normal karyotype (Fig. 6c). By age of 6 months more than 90% of p53-/- mice have developed malignant tumors, preferentially lymphomas, which is consistent with published observations. NAC supplementation had dramatic effect on tumor incidence: out of 25 mice there was not a single case of lymphoma, though there was one case of tumor-free death, two cases of soft-tissue sarcomas, and one case of hemangioma at later ages (Fig. 6d).
DISCUSSION
The functions of the p53 tumor suppressor that restrict proliferation of abnormal cells are activated by stresses presuming that under normal conditions p53 is dormant. However, p53 might have additional non-restrictive functions addressing physiological stresses, which produce repairable injuries. One of the emerging protective functions of p53 is the enhancement of DNA repair29-31. We show that in addition p53 down-regulates intracellular ROS levels thus reducing probability of genetic alterations. The antioxidant function for p53 was not expected as p53 was known as potent pro-oxidant inducing a set of ROS-generating genes, which contribute to apoptosis8. We show that the pro-oxidant function of p53 is tightly linked to release of mitochondrial ROS during stress-induced apoptosis. However, the anti-oxidant function of p53 is mediated through a set of antioxidant genes, which are responsive to lower levels of p53 in non-stressed or physiologically-stressed cells. We propose that the antioxidant function of p53 represents an important component of its suppressor activity, which decreases probability of genetic alterations and assists the survival and repair of cells with minor injuries.
Potential contribution of p53-dependent antioxidant mechanisms in tumor suppression was demonstrated in lung carcinoma A549 cell line xenograft model. The substantial acceleration in tumor development following inhibition of p53 was reversed by NAC supplementation, although there was no effect of NAC on xenograft growth rate with the control A549 cells. The increased oxidation to DNA and increased mutation rate within the HPRT locus in p53 inhibited cells suggest that the increased tumor growth could be related to elevated genetic instability and accelerated tumor progression. Interestingly, a similar NAC-sensitive increase in xenograft growth was observed following inhibition of HI95 gene, which also results in elevated intracellular ROS and DNA oxidation. The result suggests that other genetic conditions leading to compromised antioxidant defense might also induce genetic instability and contribute to malignant progression.
We found that the increased intracellular ROS in p53-deficient mice has major contribution to the cancer-prone phenotype. Previously it was found that disruption of the p53 target genes P21, which is essential for cell-cycle arrest, or PUMA, which is an obligatory component of p53 dependent apoptosis21 produce no significant increase in tumor frequency32,33,34. In opposite, calorie restricted diet which is known to be associated with decreased ROS35 and low oxidation to DNA36 increases the latency of spontaneous tumor development in p53 -/- mice37,38. We show that dietary supplementation with NAC dramatically reduces the incidence of tumors, especially the development of thymic lymphomas, which are predominant malignancy in p53-/- mice. Certainly, the increased mutation rate associated with excessive oxidation to DNA in p53 deficient cells might contribute to the early development of lymphomas. In addition, as we found a substantial reduction in karyotype abnormalities in tissues of p53-/- mice maintained on a NAC diet the observed anti-tumor effect of NAC might be related to overall improvement of genetic stability associated with decreased intracellular ROS. Accumulation of aneuploids among p53 deficient cells was earlier shown to be a result of increased frequency of abnormal mitoses39, while reintroduction of functional p53 restricts growth of polyploids40. It suggests that p53 controls karyotype stability through both restrictive and non-restrictive mechanisms. Possibly, the increased intracellular ROS in p53-deficient cells might by itself affect adversely the coordinate chromosome segregation leading to deterioration of karyotype. Recently a mutator phenotype associated with increased genomic deletions in ATM-/- mice was found to be completely reversed by dietary supplementation with NAC41. Similar to the p53-/- mice, the ATM-/- mice demonstrate increased 8-oxodG levels, and develop early thymic lymphomas42, which are suppressed by an antioxidant43. In these mouse models the increased intracellular ROS might be common cause for frequent genetic alterations and lymphomas.
Inherited deficiency in p53, which is associated with either heterozygous germ-line mutations within the p53 gene (Li-Fraumeni syndrome44,45), or up-regulation of HDM2 from a polymorphic allele46 leads to early development of cancer. We found that similar to inhibition of p53 by genomic knockout or siRNA both introduction of mutant p53 and over-expression of HDM2 are associated with increased intracellular ROS and increased mutation rates. We suggest that genetic alterations associated with elevated ROS might substantially contribute to the earlier onset of tumors in Li-Fraumeni patients or in individuals with the polymorphous HDM2 allele. NAC or similar antioxidants could be found effective for prophylactics of cancer in individuals with these and other inherited deficiencies within the p53 pathway. Likewise, inactivation of p53 which is common for malignant cells can promote oxidation-mediated mutagenesis contributing to accelerated malignant progression. Our results on selective inhibition of p53-deficient xenograft growth by NAC supplementation suggest that the introduction of antioxidants to cancer therapy schemes could improve genetic stability of p53-deficient tumor cells and retard cancer progression.
Methods
Cell culture
Human cell lines - colon carcinoma RKO and LIM1215, lung carcinoma H1299 and A549, osteosarcoma U2OS, normal foreskin fibroblasts BJ, normal lung fibroblasts WI-38, MRC5 and IMR90, fibroblasts from a 12-week human embryo, passage 10-14 HEFs, skin fibroblasts from a Li-Fraumeni patient MDAH041 were cultured in DMEM medium supplemented with 10% fetal bovine serum (Hyclone). The MDAH041 derivatives with conditional expression of wild-type p53 (TR9-7 cells)13 were maintained in the presence of 2 μg/ml of tetracycline to suppress expression of p53. Mouse lung and spleen fibroblasts, splenocytes and thymocytes were isolated from C57Black wild-type and p53 knock-out mice47. Mitochondrial DNA-deficient (π0) H1299 and RKO cells were obtained and cultured as described20. Functional mitochondria in control and □0 cells were monitored with MitoTracker Red 589 (Molecular Probes).
Recombinant constructs
For expression of wild-type p53, His175 and Q22L/W23S p53 mutants, P21, PIG3, PUMA, HI95, and PA26 corresponding cDNAs were ligated into lentivirus vector pLV-CMV. For expression of siRNAs specific for p53 and papilloma virus E6 protein, we used lentiviral vector pLSLP as described12. The following sequences, representing 19-21 bp of the mRNAs, were present in the hairpin transcripts: p53, 5′-GACTCCAGTGGTAATCTAC-3′; E6, 5′-CTAACACTGGGTTATACAA-3′; HI95, 5′-GACCATGGCTACTCGCTGA-3′; PA26, 5′-GACATCAGTGCTCCTACTT-3′; P21, 5′-GACCATGTGGACCTGTCAC-3′; PUMA, 5′-GCAGGAAGTAACAATGAGAAA-3′. Infection with recombinant lentivirus was as described 12.
Detection of intracellular ROS and 8-oxodG levels
ROS levels were determined by incubating the cells with 10 μg/ml dichlorodihydrofluorescein diacetate (DCF-DA, Sigma-Aldrich) for 20 min at 37 °C. The cells were washed twice in PBS, trypsinized and fluorescence was measured with flow cytometer (excitation at 488 nm, emission at 515-545 nm). The data were analyzed with CELLQuest software and the mean fluorescence intensity was used to quantify the responses. A minimum of 10,000 cells was acquired for each sample. The levels of 8-oxo-dG were detected as previously described48 by avidin-FITC staining. The samples were treated with RNaseA to avoid non-specific staining. Apoptotic cells were detected by using the Annexin-V-FLUOS kit (Roche). FACScan analysis was performed with the FACSCalibur instrument (Becton Dickinson).
Spleens from wild-type and p53 knockout mice were frozen in Tissue-Tec. OCT Compound (Miles). Cryosections were fixed with 4% paraformaldehyde and permeabilizied with ice-cold methanol, then treated with RNaseA (10 μg/ml) at 37 °C for 1 h and stained with avidin-FITC.
Northern and RT-PCR analyses were performed as previously described12. RT-PCR analysis was performed with SuperScript RT-PCR System (Invitrogen) and recombinant Taq polymerase (Invitrogen). To detect the corresponding gene expression, the following primers were used:Human genes
HI95: 5′-CAAGCTCGGAATTAATGTGCC-3′ and 5′-CTCACACCATTAAGCATGGAG-3′;
PA26 (T2): 5′-CGACCAGGACGAGGAACTT-3′ and 5′-CCAATGTAGTGACGATAATGTAGG-3′;
GPX1: 5′-CAACCAGTTTGGGCATCAG-3′ and 5′-CGATGTCAATGGTCTGGAAG-3′;
P21: 5′-GACCTGTCACTGTCTTGTAC-3′ and 5′-CTCTCATTCAACCGCCTAG-3′;
BAX: 5′-GATGATTGCCGCCGTGGA-3′ and 5′-CCAACCACCCTGGTCTTG-3′;
PIG3: 5′-GCCATGTTAGCCGTGCACTTTGAC-3′ and 5′-CTGGAGACTATGTGCTAATCCATG-3′;
PUMA: 5′-TGTAGAGGAGACAGGAATCCACGG-3′ and 5′-AGGCACCTAATTGGGCTCCATATC-3′;
PPIA (cyclophilin A): 5′-CTTCACACGCCATAATGGC-3′ and 5′-GTGATCTTCTTGCTGGTCTTG-3′;
Mouse genes
Hi95: 5′-CTCACAGCTGGTCTGTGTG-3′ and 5′-CCTCCGTGTGGCAATACC-3′
Pa26 (T2): 5′-CCAGGACGAGGAACTTGG-3′ and 5′-CCAGGTAGGAACACTGATGC-3′
Gpx1: 5′-CCAAGTACATCATTTGGTCTCCG-3′ and 5′-CATTAGGTGGAAAGGCATCGG-3′
P21: 5′-GCCTCCCAGAGCATTCTATGG-3′ and 5′-CCTTCTCGTGAGACGCTTAC-3′
Ppia (cyclophilin A): 5′-GACTTTACACGCCATAATGGCAC-3′ and 5′-GTGATCTTCTTGCTGGTCTTGC-3′
Western analyses were performed as previously described12. For detection of p53, DO1 antibodies (Santa Cruz Biotech), diluted 1:2000, were used.
Mutation frequency within HPRT locus was determined by counting 6-thioguanine (6-TG) resistant colonies22. After infection with corresponding recombinant lentiviruses RKO or A549 cells were grown exponentially for two weeks to allow accumulation of mutations. The cells were seeded at 2×105 cells per plate in the medium containing 40-80 μg/ml of 6-TG and number of colonies was scored after 18 days. To study contribution of elevated ROS to mutation frequency the cells were maintained in the medium containing 5 mM NAC immediately after the infection with p53 siRNA lentivirus.
Tumorigenicity assay in athymic mice
A549 cells (control and with p53 inhibited by expression of siRNA) were injected (subcutaneously, 5×105 cells) to athymic nu/nu mice. A group of mice was supplemented with 40 mM NAC in drinking water. Tumor volume was measured with 3 days intervals.
Karyotype analysis
Primary lung fibroblasts from 8-week p53-/- mice were incubated with 0.1 μg/ml colcemid at day 6 in tissue culture and cytogenetic analysis was performed after Giemsa staining. To determine the frequency of aneuploid cells 500 metaphases per sample were karyotyped in cultures derived from three pairs of mice.
Spontaneous tumor frequency assay
Families of p53+/- mice47 (C57Black background) were maintained either on regular diet, or were supplemented with 40 mM NAC (Sigma-Aldrich) added to drinking water49 to yield an average dose of 1 g NAC per kg body weight per day. The p53-/- progeny was maintained either with or without NAC supplementation throughout lifetime. Formation of tumors was monitored by daily inspection and confirmed by histological examination.
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Kopnin for support of in vivo experiments, G. Stark, A. Levine and A. Gudkov for valuable criticism during preparation of the manuscript. The work was supported by NIH grants R01 CA10490 and R01 AG025278 to P.M.C. The authors state that they do not have competing financial interests.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Lane DP. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 4.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 5.Klungland A, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 7.Macip S, et al. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 9.Tan M, et al. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem. 1999;274:12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- 10.Hussain SP, et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 11.Yoon KA, Nakamura Y, Arakawa H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Hum Genet. 2004;49:134–140. doi: 10.1007/s10038-003-0122-3. [DOI] [PubMed] [Google Scholar]
- 12.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ossovskaya VS, et al. Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc Natl Acad Sci U S A. 1996;93:10309–10314. doi: 10.1073/pnas.93.19.10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florenes VA, et al. MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst. 1994;86:1297–1302. doi: 10.1093/jnci/86.17.1297. [DOI] [PubMed] [Google Scholar]
- 16.Velasco-Miguel S, et al. PA26, a novel target for the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Chen J, Elenbaas B, Levine AJ. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 18.Osovskaia VS, et al. Effect of on various cell lines of p53 cDNA, expressed under the control of an exogenous homologous promotor. Mol Biol (Moscow) 1995;29:61–70. [PubMed] [Google Scholar]
- 19.Kovar H, et al. Characterization of distinct consecutive phases in non-genotoxic p53-induced apoptosis of Ewing tumor cells and the rate-limiting role of caspase 8. Oncogene. 2000;19:4096–4107. doi: 10.1038/sj.onc.1203780. [DOI] [PubMed] [Google Scholar]
- 20.King MP, Attardi G. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell. 1988;52:811–819. doi: 10.1016/0092-8674(88)90423-0. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths SD, et al. Absence of p53 permits propagation of mutant cells following genotoxic damage. Oncogene. 1997;14:523–531. doi: 10.1038/sj.onc.1200871. [DOI] [PubMed] [Google Scholar]
- 22.Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res. 1995;55:4420–4424. [PubMed] [Google Scholar]
- 23.Bishop AJ, et al. Atm-, p53-, and Gadd45a-deficient mice show an increased frequency of homologous recombination at different stages during development. Cancer Res. 2003;63:5335–5343. [PubMed] [Google Scholar]
- 24.Knaap AG, Simons JW. A mutational assay system for L5178Y mouse lymphoma cells, using hypoxanthine-guanine-phosphoribosyl-transferase (HGPRT) -deficiency as marker. The occurrence of a long expression time for mutations induced by X-rays and EMS. Mutat Res. 1975;30:97–110. [PubMed] [Google Scholar]
- 25.Donehower LA, et al. Effects of genetic background on tumorigenesis in p53-deficient mice. Mol Carcinog. 1995;14:16–22. doi: 10.1002/mc.2940140105. [DOI] [PubMed] [Google Scholar]
- 26.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 27.Wahl GM, Linke SP, Paulson TG, Huang LC. Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv. 1997;29:183–219. [PubMed] [Google Scholar]
- 28.Fukasawa K, Wiener F, Vande Woude GF, Mai S. Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene. 1997;15:1295–1302. doi: 10.1038/sj.onc.1201482. [DOI] [PubMed] [Google Scholar]
- 29.Zurer I, et al. The role of p53 in base excision repair following genotoxic stress. Carcinogenesis. 2004;25:11–19. doi: 10.1093/carcin/bgg186. [DOI] [PubMed] [Google Scholar]
- 30.Seo YR, Jung HJ. The potential roles of p53 tumor suppressor in nucleotide excision repair (NER) and base excision repair (BER) Exp Mol Med. 2004;36:505–509. doi: 10.1038/emm.2004.64. [DOI] [PubMed] [Google Scholar]
- 31.Achanta G, Huang P. Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species-generating agents. Cancer Res. 2004;64:6233–6239. doi: 10.1158/0008-5472.CAN-04-0494. [DOI] [PubMed] [Google Scholar]
- 32.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 33.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 34.Jeffers JR, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, et al. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 36.Sohal RS, Agarwal S, Candas M, Forster MJ, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 37.Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A. 1994;91:7036–7040. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hursting SD, et al. Diet-Gene Interactions in p53-Deficient Mice: Insulin-like Growth Factor-1 as a Mechanistic Target. J Nutr. 2004;134:2482S–2486S. doi: 10.1093/jn/134.9.2482S. [DOI] [PubMed] [Google Scholar]
- 39.Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- 40.Agapova LS, et al. Chromosome changes caused by alterations of p53 expression. Mutat Res. 1996;354:129–138. doi: 10.1016/0027-5107(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 41.Reliene R, Fischer E, Schiestl RH. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res. 2004;64:5148–5153. doi: 10.1158/0008-5472.CAN-04-0442. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 43.Schubert R, et al. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum Mol Genet. 2004;13:1793–1802. doi: 10.1093/hmg/ddh189. [DOI] [PubMed] [Google Scholar]
- 44.Malkin D, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 45.Li FP, et al. Recommendations on predictive testing for germ line p53 mutations among cancer-prone individuals. J Natl Cancer Inst. 1992;84:1156–1160. doi: 10.1093/jnci/84.15.1156. [DOI] [PubMed] [Google Scholar]
- 46.Bond GL, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 48.Neumann CA, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 49.Balansky R, Izzotti A, Scatolini L, D’Agostini F, De Flora S. Induction by carcinogens and chemoprevention by N-acetylcysteine of adducts to mitochondrial DNA in rat organs. Cancer Res. 1996;56:1642–1647. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.