Abstract
Anxiety states and anxiety-related behaviors appear to be regulated by a distributed and highly interconnected system of brain structures including the basolateral amygdala. Our previous studies demonstrate that exposure of rats to an open-field in high- and low-light conditions results in a marked increase in c-Fos expression in the anterior part of the basolateral amygdaloid nucleus (BLA) compared to controls. The neural mechanisms underlying the anatomically specific effects of open-field exposure on c-Fos expression in the BLA are not clear, however, it is likely that this reflects activation of specific afferent input to this region of the amygdala. In order to identify candidate brain regions mediating anxiety-induced activation of the basolateral amygdaloid complex in rats, we used Cholera Toxin B subunit (CTb) as a retrograde tracer to identify neurons with direct afferent projections to this region in combination with c-Fos immunostaining to identify cells responding to exposure to an open-field arena in low-light (8–13 lux) conditions (an anxiogenic stimulus in rats). Adult male Wistar rats received a unilateral microinjection of 4% CTb in phosphate-buffered saline into the basolateral amygdaloid complex. Rats were housed individually for 11 days after CTb injections and handled for 2 min each day. On the test day rats were either, 1) exposed to an open-field in low-light conditions (8–13 lux) for 15 minutes; 2) briefly handled or 3) left undisturbed (control). We report that dual immunohistochemical staining for c-Fos and CTb revealed an increase in the percentage of c-Fos-immunopositive basolateral amygdaloid complex-projecting neurons in open-field-exposed rats compared with handled and control rats in the ipsilateral CA1 region of the ventral hippocampus, subiculum and lateral entorhinal cortex. These data are consistent with the hypothesis that exposure to the open-field arena activates an anxiety-related neuronal system with convergent input to the basolateral amygdaloid complex.
Keywords: Anxiety, basolateral amygdala, retrograde tracing, c-Fos, hippocampus
Anxiety is a complex emotional state associated with elevated autonomic and behavioral arousal and a sustained increase in avoidance behavior in environmental situations characterized by a level of uncertainty, unpredictability, or uncontrollability, including situations where a conflict between approach and avoidance exists (Gray, 1982; Lowry et al., 2005). The physiological and behavioral arousal associated with anxiety states and anxiety-related behaviors appear to be regulated by a distributed and interconnected system of forebrain and hindbrain structures including the septo-hippocampal system and entorhinal cortex (Gray, 1982), medial prefrontal cortex (Duncan et al., 1996), basolateral amygdaloid complex (Campbell and Merchant, 2003; Spiga et al., 2006) and midbrain raphe complex (Abrams et al., 2005; Singewald et al., 2003; Singewald and Sharp, 2000).
Several lines of evidence indicate that the basolateral amygdaloid complex is a key component of a distributed neuronal system regulating anxiety states. Administration of corticotropin-releasing factor (CRF), the CRF-related neuropeptide urocortin 1 (Ucn1) or the GABAA antagonist bicuculline methiodide into the basolateral amygdala increases anxiety-related behavior in the social interaction test (Sajdyk et al., 1999; Sajdyk et al., 2002; Spiga et al., 2006), while inhibition of the basolateral amygdala with blockade of NMDA and non-NMDA receptors and administration of the benzodiazepine receptor agonist, midazolam, decreases anxiety-like behavior in the social interaction (SI) test (Gonzalez et al., 1996; Sajdyk and Shekhar, 1997). In addition, repeated, daily intra-basolateral amygdala administration of sub-threshold doses of anxiogenic drugs including CRF and Ucn 1 over the course of several days, results in an increased anxiety state lasting for several weeks.
Exposure to anxiogenic stimuli induces expression of c-Fos, the protein product of the immediateearly gene, c-fos, in the basolateral amygdaloid complex (for review see: Knapska et al., 2007). Rats exposed to the elevated plus-maze (EPM), elevated T-maze or a shuttle box foot-shock avoidance test show increased c-Fos expression in the basolateral amygdaloid complex compared with controls (Duncan et al., 1996; Silveira et al., 1993; Silveira et al., 2001). We have recently reported that rats exposed to an open-field arena in low-light and high-light conditions show increased c-Fos expression in several but not all subdivisions of the basolateral amygdaloid complex compared with home cage and handled control groups, with a marked increase in c-Fos-immunoreactivity in the anterior part of the basolateral amygdaloid nucleus (BLA; Hale et al., 2006)
The neural mechanisms underlying the specific effects of open-field exposure on c-Fos expression in the basolateral amygdaloid complex are not clear, however, it is likely that this reflects activation of specific input to this region. The basolateral amygdala receives afferents from several regions thought to be part of an anxiety-related network including the prelimbic cortex (Vertes, 2004), hippocampus (Kishi et al., 2006), subiculum (Kishi et al., 2006; Naber and Witter, 1998) and dorsal raphe nucleus (Abrams et al., 2005; Ottersen, 1981; Vertes, 1991).
In this experiment we tested the hypothesis that exposure to anxiety-related stimuli activates a distributed neuronal system with convergent input to the basolateral amygdaloid complex. In order to identify candidate brain regions mediating anxiety-induced activation of the basolateral amygdaloid complex in rats, we used Cholera Toxin B subunit (CTb) as a retrograde tracer in combination with c-Fos immunostaining to identify cells responding to exposure to an open-field arena in low-light conditions.
Experimental Procedures
Animals
Adult male Wistar rats arrived from the vendor (Møllegaarden, Denmark; N = 34) weighing approximately 200g and were kept in standard laboratory conditions with ad libitum tap water and standard rat chow (Altromin, Lage, Germany) and maintained under a photoperiod of 12-h light:12-h dark with lights on at 6:00 AM. The experiment was conducted under the authority of the Animal Core Facility of the Panum Institute, Department of Neuroscience and Pharmacology, The Panum Institute, University of Copenhagen, in accordance with and approved by The Animal Experiments Inspectorate, Ministry of Justice, Denmark and the European Communities Council Directive of 24 November 1986 (86/609/EEC). Care was taken to minimize the number of animals used and their suffering.
Retrograde Tracing – Cholera Toxin B subunit (CTb)
In order to identify candidate brain regions mediating anxiety-induced activation of the basolateral amygdaloid complex in rats, we used Cholera Toxin B subunit (CTb) as a retrograde tracer to identify neurons with direct afferent projections to this region in combination with c-Fos immunostaining to identify cells responding to exposure to an open-field arena (an anxiogenic stimulus in rats). Prior to CTb injection rats were anesthetized between 4 and 6 h after light onset with Hypnorm-Dormicum mixture (3 ml/kg s.c.) (one part fentanyl citrate (0.315 mg/ml) and fluanisone (10 mg/ml): one part midazolam (5 mg/ml): and two parts sterile water) and placed in a stereotaxic frame (Kopf Instruments, Model 1404, Turjunga, CA, USA). A glass microelectrode was broken to a final tip diameter of 15–20 µm and a solution of dialysed 4% CTb (Cat. No. 104, LIST Biological Laboratories, Campbell, CA) in PBS was applied iontophoretically using positive current pulses of 10 µA (7 s on; 7 s off) for 10 min. The coordinates used for the basolateral amygdaloid complex were: posterior −2.8 mm; lateral 4.8 mm; ventral −8.5 mm with reference to Bregma according to a standard rat brain stereotaxic atlas (Paxinos and Watson, 1998). Rats were housed individually (cage size: 45 × 25 × 20 cm) for 7–11 days after CTb injections and handled for 2 min each day. On the test day rats were either; 1) exposed to open-field in low-light conditions (8–13 lux) for 15 min 2) briefly handled in the testing room or 3) left undisturbed (control). Following open-field exposure or handling rats were immediately returned to the housing room.
Behavior in the open-field arena
The open-field arena (90 cm width × 90 cm length × 40 cm height) was divided into a 6 × 6 grid of equally-sized squares using black tape. The outer section of the box was defined as the sum of all squares adjacent to a wall, not including the 4 corner squares (i.e. 16 out of 36 squares). The central region of the box (4 × 4 = 16 squares) was subdivided into a large center and a small center of 12 and 4 squares respectively. The test started by placing the rat in the same side of the outer section (halfway along one of the four walls of the box, facing the center) such that the rat could visit the center area first or move to one of the corners. The behavior of each rat in the open-field box was recorded on video and scored afterwards using The Observer® 5.0 software (Noldus Information Technologies BV, Wageningen, The Netherlands, supplied by Tracksys Ltd. Nottingham, UK). For behavior, the data were collapsed for the 4 identical quarters of the box (with each quarter of the box containing 3 × 3 squares, consisting of 1 corner, 4 outer side, 3 large center and 1 small center square(s)). Time spent in each category of square was recorded. Total locomotor activity was scored as the number of line crossings (all four paws crossing a line) during each of 3 blocks of 5 min during the fifteen min open-field test. In addition, the frequency of the following behaviors was recorded: rearing (standing on hind legs, with or without contact with the sides of the arena), grooming (using paws or tongue to clean/scratch body), stretched-attend posture (stretching forward with the forelimbs extended, often with the back arched in order to maintain a low profile) and corner-facing (i.e. standing or sitting with the face directed toward the corner of the box).
Tissue collection and preparation
Two hours after the start of the open-field test or handling, rats were anesthetized with Hypnorm and midazolam and perfused transcardially with PBS for 3 min followed by a solution of 10% formalin (Merck, Germany) in 0.1 M phosphate buffer (pH 7.4) for 15 min. The brains were post-fixed in 10% formalin and 0.1M phosphate buffer for 24 h, and stored in PBS + 0.1% sodium-azide. Brains were cryoprotected for 3 days in 30% sucrose in PBS, frozen in dry ice and 40 µm sections were cut on a cryostat. Sections were divided into six series, and stored in PBS +/0.1% sodium-azide until immunohistochemical procedures were performed.
Immunohistochemistry for Cholera Toxin b-subunit and c-Fos
One set of slices was used for double immunostaining using primary antibodies directed against Cholera Toxin b-subunit (goat anti-CTb, Cat No. 703, 1:3000 List Biological Laboratories, Campbell, CA, USA) and c-Fos (rabbit anti-c-Fos polyclonal antibody, Cat No. PC38 (Ab-5), 1:3000; Oncogene Research Products, San Diego, CA, USA).
Immunohistochemistry for CTb and c-Fos was conducted on free-floating tissue in 30ml tubes and gently shaken on an orbital shaker throughout double immunostaining. Tissue was rinsed in 3% hydrogen peroxide (H2O2) in PBS for 30 min, followed by washing in PBS containing 0.1% Triton X-100 (PBST) for 3 × 10 min; tissue was then blocked in 1% HSA (human serum albumin) in 0.1% PBST for 30 min. Sections were incubated overnight at 4 °C with 1:3000 goat anti-CTb in 1% HSA/PBST. After 15 h, tissue was washed three times (for 10 min each) in 0.1% PBST followed by incubation with a biotinylated donkey anti-goat polyclonal antibody (Cat#705-096-147; 1:2000; Jackson Immuno Research Laboratories, PA, USA) in 1% HSA/PBST for 60 min. Tissue was washed in 0.1% PBST for 3 × 10 min followed by incubation with an avidin-biotin complex (Elite ABC reagent; Cat. No. PK-6100, 1:200; Vector Laboratories, Peterborough, UK) in 0.1% PBST for 60 min. Tissue was washed in 0.1% PBST for 3 × 10 min then incubated in 0.05% 3-3’diaminobenzidine tetrahydrochloride (DAB) in PBS and 0.0066% H2O2 for 15 min. After the chromogen reaction, tissue was immediately washed in PBS (2 × 10 min), 3% H2O2 in PBS (30 min), 0.1% PBST respectively (3 × 10 min) and preincubated in 1% HSA in 0.1% PBST for 30 min. Slices were then incubated with a rabbit anti-c-Fos antibody (Cat. No. PC38 (Ab-5), 1:3000; Oncogene Research Products, San Diego, CA, USA) in 0.1% PBST overnight at room temperature. Tissue was then washed twice in 0.3% PBST for 15 min followed by incubation in biotinylated swine anti-rabbit secondary antibody (Cat. No. E0353, 1:200; DakoCytomation Ltd, Cambridgeshire, UK) in 0.1% PBST for 90 min. Following incubation with the secondary antibody, tissue was washed twice in 0.3% PBST for 15 min and incubated with Elite ABC reagent (as above). Tissue was washed in 0.3% PBST for 15 min and rinsed in 0.05 M PBS. Tissue was then placed in SG substrate (Vector Laboratories; Cat. No. SK4700; diluted as recommended by the vendor) in PBS for 15 min. Finally, sections were washed twice in PBS to stop the reaction. Brain sections were rinsed briefly in distilled water then mounted on SuperFrost Plus microscope slides (Fisher Scientific UK, Leicestershire, UK), dehydrated through an alcohol series and cleared with xylene. Slides were then coverslipped using DPX mounting medium (RA Lamb, London, UK). The color reaction of the c-Fos immunostaining was blue-black and localized to the nucleus while CTb immunostaining was orange-brown and localized to the cytoplasm.
Data quantification
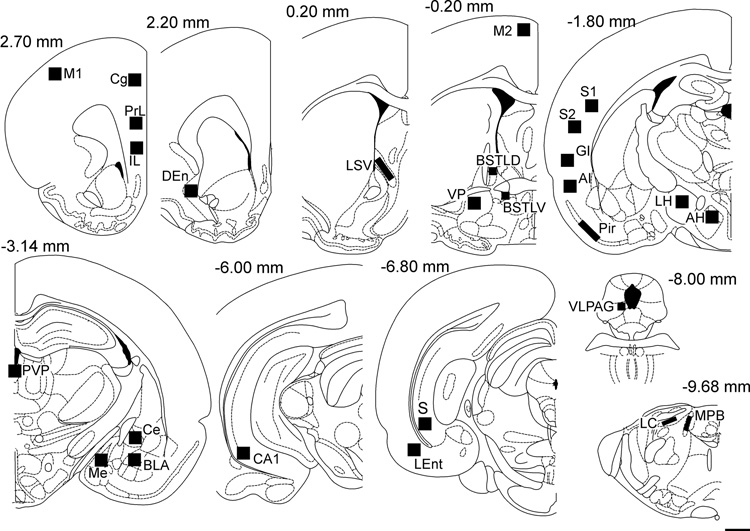
Quantitative analysis of c-Fos-immunoreactive (c-Fos-ir) nuclei and CTb-immunoreactive (CTb-ir) neurons was conducted in 27 forebrain and hindbrain regions thought to be part of a distributed but interconnected system regulating anxiety-states and anxiety-related behaviors. The regions were selected based on three criteria; 1) if they showed increased c-Fos expression following treatment with at least one of a range of pharmacologically diverse anxiogenic drugs (FG-7142, yohimbine, mCPP, and caffeine; (Singewald et al., 2003; Singewald and Sharp, 2000)), 2) if the region was identified as having efferent projections to the basolateral amygdaloid complex in Ottersen’s survey of afferent projections of the amygdaloid complex (Ottersen, 1980; Ottersen, 1981; Ottersen, 1982; Ottersen and Ben-Ari, 1979) and 3) if they showed at least 1 CTb-ir neuron from a representative BLA injection (T2818) in the present study. The regions selected for analysis were; AP 2.60mm: primary motor cortex (M1), cingulate cortex (Cg1), infralimbic cortex (IL), prelimbic cortex (PrL), AP 2.20mm: dorsal endopiriform cortex (DEn); AP 0.20mm: lateral septal nucleus, ventral part (LSV); AP −0.20mm: secondary motor cortex (M2), bed nucleus of the stria terminalis, lateral division, dorsal part (BSTLD), bed nucleus of the stria terminalis, lateral division, ventral part (BSTLV), ventral pallidum (VP); AP −1.80mm: primary somatosensory cortex (S1), secondary somatosensory cortex (S2), agranular insular cortex (AI), granular insular cortex (GI), piriform cortex (Pir), anterior hypothalamic area, posterior part (AH), lateral hypothalamic area (LH); AP −3.14mm: central amygdaloid nucleus (Ce), medial amygdaloid nucleus (Me), basolateral amygdaloid nucleus, anterior part (BLA) contralateral to the injection site only, paraventricular thalamic nucleus, posterior part (PVP); AP −6.00mm: CA1 field of the hippocampus (CA1); AP −6.80mm: subiculum (S), lateral entorhinal cortex (LEnt); AP −8.00mm: ventrolateral periaqueductal gray (VLPAG); AP −9.68mm: locus coeruleus (LC), medial parabrachial nucleus (MPB). All cell counts were conducted in the hemisphere ipsilateral to injection site except for the Ce, BLA and Me as the injection site precluded analysis on the ipsilateral side. Singewald and Sharp (2000) examined c-Fos expression following administration of anxiogenic drugs in the dorsal rather than the ventral hippocampus. As the dorsal hippocampus does not send projections to the basolateral amygdala (Ottersen, 1982), two subregions of the ventral hippocampus, CA1 and subiculum, and the lateral entorhinal cortex were chosen for the quantitative analysis.
Round or oval-shaped nuclei with blue-black immunostaining darker than background were counted as c-Fos-ir nuclei. Cells with orange-brown staining throughout the cytoplasm were counted as CTb-ir cells. Cells with orange-brown staining of the cytoplasm and blue-black staining of nuclei were counted as c-Fos/CTb double-immunostained cells. The numbers of c-Fos-ir/CTb-ir neurons, the numbers of c-Fos-ir/CTb-immunonegative cells, and the total numbers of CTb-ir neurons were counted in a tissue area of 0.3 or 0.5 mm2 using brightfield microscopy using a 10x objective lens (c-Fos-ir/CTb-ir cells were confirmed using a 40x objective lens) by an investigator blind to the assignment of treatment groups.
Data analysis
Data were analyzed using analysis of variance (ANOVA) with repeated measures. ANOVA was followed, when appropriate, by post hoc analysis using Bonferroni pairwise comparisons using SPSS (Version 14 for Windows, SPSS Inc., Chicago, IL, USA). A Greenhouse-Geisser correction epsilon (ε) was used for repeated measures analysis to correct for potential violation of the sphericity assumption (Vasey and Thayer, 1987); this correction multiplies both the numerator and the denominator degrees of freedom by epsilon and the significance of the F-ratio is evaluated with the new degrees of freedom, resulting in a more conservative statistical test.
Locomotor activity was analyzed using time (3 levels: 0–5 min, 5–10 min, 10–15 min) as a within-subjects factor. The time spent in each square type (corner, outer side, large center and small center) are reported as percentage of total time in the open-field arena.
Cell counts for the numbers of c-Fos-ir/CTb-ir (basolateral amygdaloid complex-projecting) neurons, the total number of c-Fos-ir nuclei, total CTb-ir neurons and the percentage of c-Fos-ir/CTb-ir neurons (i.e. c-Fos-ir/CTb-ir / total CTb-ir × 100) were analyzed separately using treatment group (3 levels: control, handled and open-field) as a between-subjects factor and brain region (27 levels) as a within-subjects factor.
Outliers (3.0% of the total data) were identified by Grubbs test (Grubbs, 1969) and excluded. Replacement data for the repeated measures ANOVAs were calculated using the Petersen method (Petersen, 1985). Replacement data were not included in post hoc analyses and are not represented in graphical representation of the data. Significance was accepted for the ANOVAs and post hoc pairwise Bonferroni comparisons when p < 0.05.
Correlational analysis was performed using Pearson’s correlation. Within rats exposed to the open-field arena, potential correlations were determined between the percentage of c-Fos-ir/CTbir neurons and measures of anxiety-related behavior, including time spent in the center of the arena and habituation (calculated as the number of line crossings in the first five minutes minus the number of line crossings in the last five minutes of the open-field test). Correlations between the percentage of c-Fos-ir/CTb-ir neurons and total locomotion were also performed. Bonferroni correction was used to allow for multiple comparisons.
Results
Behavior in the open-field
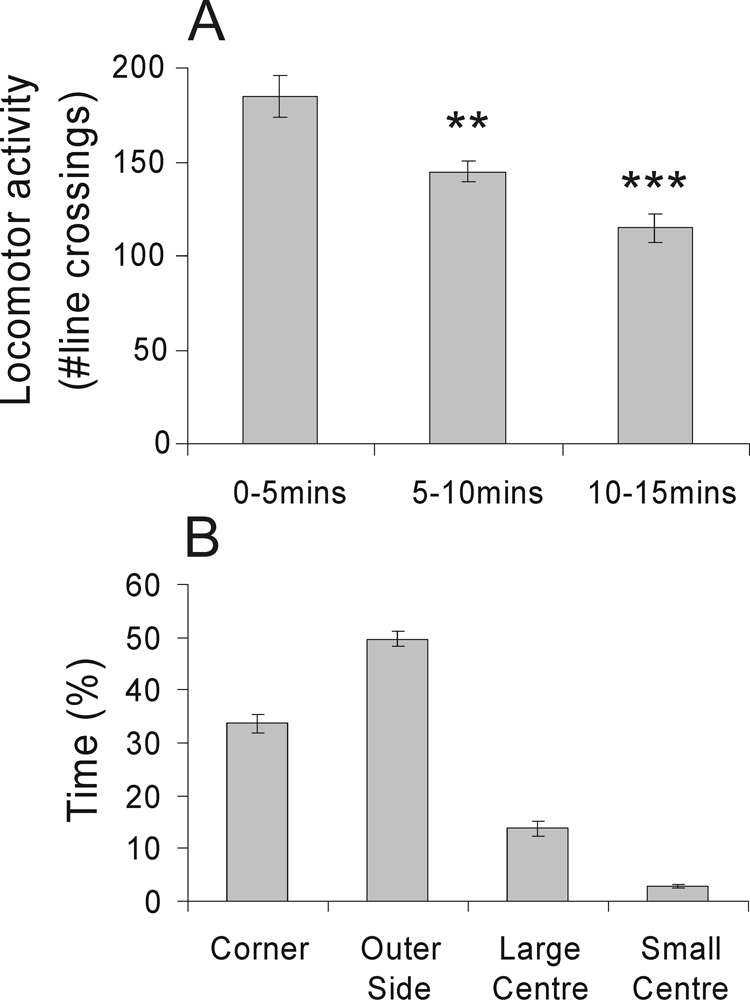
There was a reduction in locomotor activity during the 15 min open-field test (F(2,20) = 27.92, p < 0.001, ε = 0.77; Fig 1A). Rats made fewer line crossings during the 5 – 10 min and 10 – 15 min time periods compared with the 0 – 5 min time period and spent the most time exploring the outer side (49.7 ± 1.5%) and corners of the open-field arena (33.8 ± 1.7%). Rats spent less time in the large center and small center of the arena (13.8 ± 1.4% and 2.8 ± 0.3% respectively). The percentage of time spent in the different sectors of the open-field arena is illustrated in Fig. 1B.
Figure 1.
Graphs illustrating the effects of open-field exposure in low-light conditions (8–13 lux) on behavior (mean ± SEM), including A) locomotor activity, scored as the number of square entries during three 5 min periods of the 15 min open-field test, **p < 0.01, ***p < 0.001 versus the 0–5 min time period; post hoc Bonferroni comparisons, and B) percentage of time spent in each square type.
Distribution of CTb injection sites
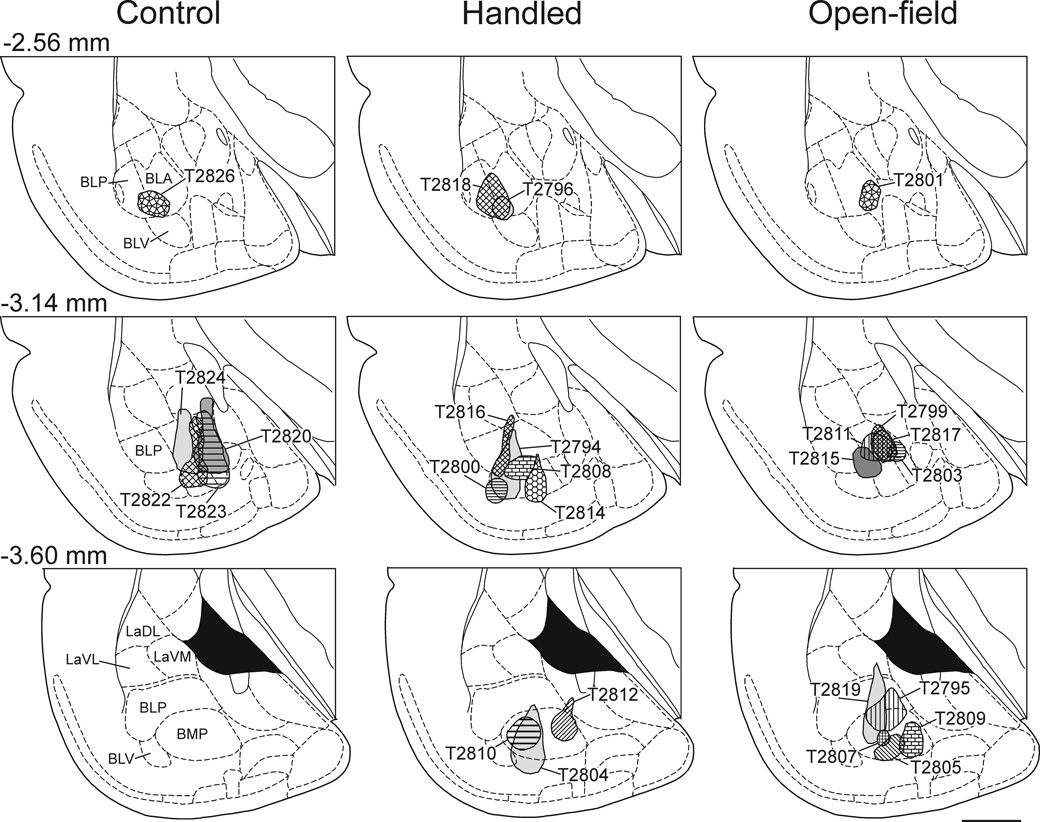
The distribution of CTb injection sites in the control rats, handled rats, and rats exposed to the open-field are illustrated in Fig 2. Photomicrographs of representative injections sites are presented in Fig. 3. The target of the injection was the basolateral amygdaloid complex and injection sites were often centered in two or more subdivisions of the basolateral amygdaloid complex (i.e. BLA, BLP, BLV or BMP). In 5 rats (16%) the injections sites were located outside the basolateral amygdaloid complex and therefore these rats were excluded from the study. The data from 1 rat were excluded due to poor tissue quality.
Figure 2.
Line drawings of frontal sections arranged rostrocaudally (top row, −2.56 mm; middle row, −3.14 mm; bottom row, −3.60 mm posterior to Bregma; Paxinos & Watson, 1998) through the amygdala showing the CTb injection sites for control, handled and open-field-exposed rats (left-, middle-and right-columns respectively). The center of each CTb injection is illustrated by shading pattern and case number (e.g. T2826). Abbreviations; BLA, basolateral amygdaloid nucleus, anterior part; BLP, basolateral amygdaloid nucleus, posterior part; BLV, basolateral amygdaloid nucleus, ventral part; BMP, basomedial amygdaloid nucleus, posterior part; LaDL, lateral amygdaloid nucleus, dorsolateral part; LaVL, lateral amygdaloid nucleus, ventrolateral part; LaVM, lateral amygdaloid nucleus, ventromedial part. Scale bar, 1mm.
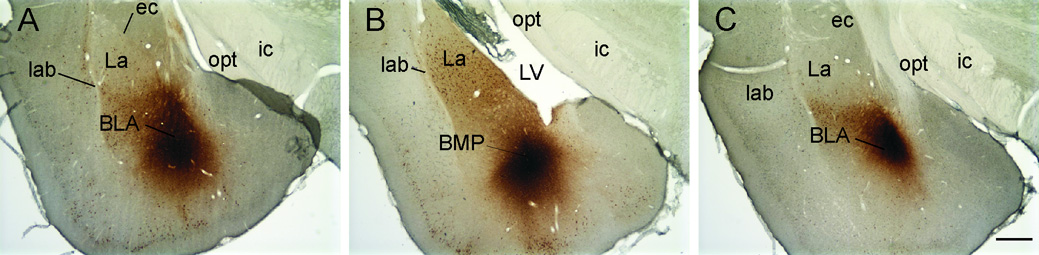
Figure 3.
Photomicrographs of the basolateral amygdaloid complex (approximately −3.14 — −3.60 mm Bregma (Paxinos and Watson, 1998)) showing CTb-injections from representative A) control (T2824), B) handled (T2810) and C) open-field exposed (T2799) rats. Abbreviations: BLA, basolateral amygdaloid nucleus, anterior part; BMP, basomedial amygdaloid nucleus, posterior part; ec, external capsule; ic, internal capsule; La, lateral amygdaloid nucleus; lab, longitudinal association bundle; LV, lateral ventricle; opt, optic tract. Scale bar 500 µm.
Total CTb-ir neurons
The regions selected for the cell counts are illustrated in Figure 4. As expected, different brain regions had different total numbers of CTb-ir neurons (F(26,598) = 48.82, p < 0.001, ε = 0.128; Table 1). There were also differences among the treatment groups (treatment: F(2,23) = 10.389, p = 0.001). Although in the majority of cases, the number of retrogradely labeled cells did not differ among treatment groups, post hoc Bonferroni pairwise comparisons detected a greater number of CTb-ir neurons in CO compared with HA and open-field exposed (OF) rats in the DEn, BLA (contralateral to injection site) and the LC, and in CO compared with OF rats in the LEnt, VP and IL. As we are not primarily concerned with differences between CO and HA rats, these differences have little impact in the interpretation of results. There were also a greater number of CTb-ir neurons in HA compared with OF rats in the piriform cortex.
Figure 4.
Line drawings (adapted from Paxinos & Watson, 1998) showing the 27 brain regions where CTb-ir neurons and c-Fos-ir nuclei were quantified. The squares indicate the placement of the grids for counting c-Fos-ir and CTb-ir cells. Numbers above each diagram indicate the distance (in mm) from Bregma. Cell counts for the Ce, Me and BLA were conducted in the hemisphere contralateral to the injection site. Abbreviations; AH, anterior hypothalamic area; AI, agranular insular cortex; BLA, basolateral amygdaloid nucleus, anterior part; BSTLD, bed nucleus of the stria terminalis, lateral division, dorsal part; BSTLV, bed nucleus of the stria terminalis, lateral division, ventral part; CA1, CA1 field of the ventral hippocampus; Ce, central amygdaloid nucleus; Cg1, cingulate cortex; DEn, dorsal endopiriform cortex; GI, granular insular cortex; IL, infralimbic cortex; LC, locus coeruleus; LH, lateral hypothalamic area; LEnt, lateral entorhinal cortex; LSV, lateral septal nucleus, ventral part; M1, primary motor cortex; M2, secondary motor cortex; Me, medial amygdaloid nucleus; MPB, medial parabrachial nucleus; PrL, prelimbic cortex; Pir, piriform cortex; PVP, paraventricular thalamic nucleus, posterior part; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; S, subiculum; VLPAG, ventrolateral periaqueduatal grey. Scale bar, 1mm.
Table 1.
Table showing the total numbers of CTb-ir neurons (mean ± SEM) in 27 regions of the rat brain
| Region | Rostrocaudal Level (mm Bregma) | All treatments | Control | Handled | Open-field | Grid size |
|---|---|---|---|---|---|---|
| PrL | 2.60 | 6.8 ± 1.6 | 11.4 ± 2.4 | 8.9 ± 3.4 | 2.7 ± 1.1 | 0.5mm2 |
| IL | 2.60 | 13.4 ± 2.4 | 23.6 ± 6.5 | 14.9 ± 4.3 | 7.3 ± 1.5* | 0.5mm2 |
| Cg1 | 2.60 | 0.4 ± 0.1 | 1.0 ± 0.5 | 0.5 ± 0.2 | 0 ± 0* | 0.5mm2 |
| M1 | 2.60 | 0.4 ± 0.2 | 0 ± 0 | 0.9 ± 0.5 | 0 ± 0 | 0.5mm2 |
| DEn | 2.20 | 12.0 ± 1.4 | 21.8 ± 2.3 | 12.1 ± 1.6** | 7.4 ± 1.1*** | 0.5mm2 |
| LSV | 0.20 | 0.4 ± 0.2 | 0.8 ± 0.6 | 0.5 ± 0.2 | 0.1 ± 0.1 | 0.5mm2 |
| M2 | −0.20 | 0.1 ± 0.1 | 0 ± 0 | 0.1 ± 0.1 | 0 ± 0 | 0.5mm2 |
| BSTLD | −0.20 | 0.4 ± 0.2 | 0 ± 0 | 0.3 ± 0.2 | 0.7 ± 0.3 | 0.3mm2 |
| BSTLV | −0.20 | 2.2 ± 0.5 | 3.8 ± 1.8 | 2.5 ± 0.2 | 1.2 ± 0.4 | 0.3mm2 |
| VP | −0.20 | 3.9 ± 0.7 | 7.0 ± 1.1 | 4.2 ± 1.5 | 2.0 ± 0.6* | 0.5mm2 |
| S1 | −1.80 | 0.5 ± 0.3 | 0.4 ± 0.2 | 1.2 ± 0.6 | 0 ± 0 | 0.5mm2 |
| S2 | −1.80 | 0.8 ± 0.3 | 1.4 ± 0.7 | 1.3 ± 0.6 | 0.2 ± 0.1 | 0.5mm2 |
| GI | −1.80 | 0.8 ± 0.2 | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.8 ± 0.3 | 0.5mm2 |
| AI | −1.80 | 4.8 ± 1.5 | 4.0 ± 2.1 | 7.6 ± 3.3 | 2.6 ± 1.0 | 0.5mm2 |
| AH | −1.80 | 1.1 ± 0.3 | 2.0 ± 1.3 | 0.6 ± 0.3 | 1.2 ± 0.4 | 0.5mm2 |
| LH | −1.80 | 1.2 ± 0.3 | 1.6 ± 0.8 | 1.6 ± 0.3 | 0.7 ± 0.3 | 0.5mm2 |
| Pir | −1.80 | 31.8 ± 5.8 | 29.6 ± 4.2 | 48.5 ± 12.0 | 17.6 ± 5.5++ | 0.5mm2 |
| BLA | −3.14 | 4.4 ± 2.0 | 18.8 ± 6.4 | 1.0 ± 0.4*** | 0.3 ± 0.2*** | 0.5mm2 |
| Ce | −3.14 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.5mm2 |
| Me | −3.14 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.1 | 0.5mm2 |
| PVP | −3.14 | 20.9 ± 2.8 | 31.2 ± 4.5 | 21.9 ± 5.6 | 15.4 ± 3.3 | 0.5mm2 |
| CA1 | −6.00 | 54.3 ± 8.4 | 72.0 ± 28.6 | 61.2 ± 11.0 | 35.0 ± 5.4 | 0.5mm2 |
| Subiculum | −6.80 | 54.6 ± 6.7 | 71.0 ± 12.1 | 60.9 ± 11.9 | 38.2 ± 9.7 | 0.5mm2 |
| LEnt | −6.80 | 34.6 ± 4.1 | 49.2 ± 8.6 | 39.8 ± 6.3 | 20.9 ± 3.5* | 0.5mm2 |
| VLPAG | −8.00 | 0.8 ± 0.2 | 0.8 ± 0.4 | 1.0 ± 0.4 | 0.6 ± 0.2 | 0.3mm2 |
| LC | −9.68 | 1.5 ± 0.4 | 3.8 ± 1.9 | 1.0 ± 0.3* | 1.0 ± 0.3* | 0.3mm2 |
| MPB | −9.68 | 2.9 ± 0.6 | 5.8 ± 2.5 | 1.9 ± 0.4 | 2.6 ± 0.7 | 0.3mm2 |
P < 0.05
P < 0.01
P < 0.001 versus control
P < 0.01 versus handled; post hoc Bonferroni comparisons.
There were few CTb-ir neurons in Cg1, M1, LSV, M2, BSTLD, S1, S2, GI, Me and VLPAG. CTb-ir neurons were never observed in the Ce contralateral to the injection site. There were many CTb-ir neurons in the PrL, IL, DEn, BSTLV, AI, Pir, BLA (contralateral to injection site), PVP, CA1, subiculum and the LEnt. Of the 27 regions examined the greatest numbers of basolateral amygdaloid complex-projecting neurons were observed in the mPFC (PrL and IL), DEn, Pir, CA1, S, LEnt and PVP.
Total c-Fos-ir cells
Exposure to the open-field arena induced widespread increases in c-Fos expression (treatment × region: F(52,598) = 6.23, p < 0.001, ε = 0.268; treatment: F(2,23) = 27.23, p < 0.001; region: F(26,598) = 28.38, p < 0.001, ε = 0.268; Table 2). Post hoc Bonferroni pairwise comparisons revealed an increase in c-Fos expression in OF compared with HA groups in all sampled brain regions except M1, VLPAG, MPB, VP and the Ce (contralateral to the injection site). The increase in c-Fos expression in OF compared with HA groups in the BSTLD and GI approached statistical significance (p = 0.075 and p = 0.053 respectively). Consistent with our previous studies (Hale et al., 2006), exposure to the open-field arena increased c-Fos expression in the anterior part of the basolateral amygdaloid complex contralateral to the injection site (Fig. 5). There was no difference in c-Fos expression between CO and HA groups in any of the sampled regions.
Table 2.
Table showing the total numbers of c-Fos-ir nuclei (mean ± SEM) in 27 regions of the rat brain
| Region | Rostrocaudal Level (mm Bregma) | Control | Handled | Open-field | Grid size |
|---|---|---|---|---|---|
| PrL | 2.60 | 7.6 ± 4.1 | 9.9 ± 3.4 | 53.0 ± 8.6**+++ | 0.5mm2 |
| IL | 2.60 | 8.8 ± 2.9 | 13.0 ± 3.2 | 32.4 ± 4.2**++ | 0.5mm2 |
| Cg1 | 2.60 | 5.0 ± 2.6 | 2.0 ± 1.2 | 35.7 ± 6.3**+++ | 0.5mm2 |
| M1 | 2.60 | 0 ± 0 | 0 ± 0 | 1.0 ± 0.7 | 0.5mm2 |
| DEn | 2.20 | 9.8 ± 3.5 | 5.2 ± 1.5 | 29.2 ± 4.3**+++ | 0.5mm2 |
| LSV | 0.20 | 34.4 ± 18.3 | 12.6 ± 3.2 | 55.6 ± 7.2++ | 0.5mm2 |
| M2 | −0.20 | 1.8 ± 0.9 | 0.3 ± 0.2 | 19.9 ± 4.6*++ | 0.5mm2 |
| BSTLD | −0.20 | 1.0 ± 0.0 | 1.6 ± 0.9 | 10.4 ± 3.3 | 0.3mm2 |
| BSTLV | −0.20 | 3.0 ± 1.5 | 5.0 ± 1.1 | 18.7 ± 4.3*++ | 0.3mm2 |
| VP | −0.20 | 2.2 ± 0.9 | 1.1 ± 0.5 | 4.4 ± 1.6 | 0.5mm2 |
| S1 | −1.80 | 2.2 ± 0.9 | 0.1 ± 0.1 | 16.3 ± 4.3*++ | 0.5mm2 |
| S2 | −1.80 | 2.4 ± 1.1 | 1.3 ± 0.8 | 14.4 ± 3.6*++ | 0.5mm2 |
| GI | −1.80 | 0 ± 0 | 0.2 ± 0.2 | 6.5 ± 2.4 | 0.5mm2 |
| AI | −1.80 | 0 ± 0 | 0.3 ± 0.3 | 3.6 ± 1.1+ | 0.5mm2 |
| AH | −1.80 | 12.6 ± 2.0 | 21.8 ± 3.8 | 47.7 ± 4.9***++ | 0.5mm2 |
| LH | −1.80 | 4.4 ± 1.8 | 9.7 ± 1.9 | 34.6 ± 4.0***+++ | 0.5mm2 |
| Pir | −1.80 | 8.4 ± 3.1 | 8.2 ± 1.7 | 22.4 ± 3.8*++ | 0.5mm2 |
| BLA | −3.14 | 6.2 ± 3.3 | 14.9 ± 4.4 | 29.8 ± 2.3**+ | 0.5mm2 |
| Ce | −3.14 | 6.2 ± 1.8 | 8.3 ± 1.9 | 8.8 ± 1.5 | 0.5mm2 |
| Me | −3.14 | 8.4 ± 4.4 | 8.7 ± 1.4 | 37.7 ± 7.0**++ | 0.5mm2 |
| PVP | −3.14 | 20.6 ± 7.0 | 35.7 ± 4.3 | 81.3 ± 8.3***+++ | 0.5mm2 |
| CA1 | −6.00 | 10.6 ± 2.2 | 9.0 ± 2.9 | 36.9 ± 6.9**++ | 0.5mm2 |
| Subiculum | −6.80 | 7.8 ± 3.4 | 7.9 ± 1.5 | 37.9 ± 5.1***+++ | 0.5mm2 |
| LEnt | −6.80 | 4.0 ± 2.1 | 10.9 ± 3.1 | 27.8 ± 3.2***++ | 0.5mm2 |
| VLPAG | −8.00 | 5.4 ± 1.8 | 5.1 ± 1.2 | 7.8 ± 1.1 | 0.3mm2 |
| LC | −9.68 | 2.5 ± 1.6 | 1.1 ± 0.5 | 10.7 ± 1.6**+++ | 0.3mm2 |
| MPB | −9.68 | 0 ± 0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.3mm2 |
P < 0.05
P < 0.01
P < 0.001 versus control
P < 0.05
P < 0.01
P < 0.001 versus handled; post hoc Bonferroni comparisons.
Figure 5.
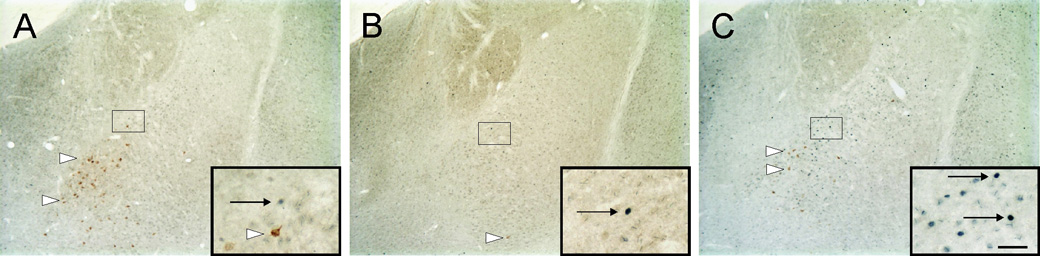
Photomicrographs illustrating c-Fos-ir and CTb-ir neurons in the basolateral amygdaloid complex contralateral to injection site (−3.14 mm Bregma) of rats exposed to A) control (T2824), B) handling (T2814) and C) open-field conditions (T2811). Black boxes in A, B and C, indicate regions shown at higher magnification in insets in the lower right-hand corner of these panels. Arrows indicate examples of c-Fos-ir nuclei (blue/black nuclear staining); white arrowheads indicate c-Fos-immunonegative/CTb-ir (basolateral amygdaloid complex-projecting) neurons (brown/orange cytoplasmic staining). Scale bar, 200µm; inset 50µm.
Percentage of c-Fos-ir/CTb-ir neurons
Open-field exposure increased the percentage of CTb-ir neurons expressing c-Fos (treatment × region: F(52,598) = 2.27, p = 0.016, ε = 0.203; treatment: F(2,23) = 16.66, p < 0.001; region: F(26,598) = 4.94, p < 0.001, ε = 0.203: Table 3). Post hoc Bonferroni comparisons detected increases in OF compared with HA and CO rats in the CA1, subiculum and LEnt (Table 3; Figure 6 & Figure 7). There were no differences between CO and HA groups in any of the sampled regions.
Table 3.
Table showing the percentage of c-Fos-ir/CTb-ir neurons (i.e. c-Fos-ir/CTb-ir / total CTb-ir × 100; mean ± SEM) in 27 regions of the rat brain.
| Region | Rostrocaudal Level (mm Bregma) | Control | Handled | Open-field | Grid size |
|---|---|---|---|---|---|
| PrL | 2.60 | 0 ± 0% | 0 ± 0% | 1.0 ± 1.0% | 0.5mm2 |
| IL | 2.60 | 1.6 ± 1.2% | 0 ± 0% | 10.6 ± 4.5% | 0.5mm2 |
| Cg1 | 2.60 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| M1 | 2.60 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| DEn | 2.20 | 2.6 ± 1.6 % | 3.5 ± 1.8% | 14.0 ± 4.0% | 0.5mm2 |
| LSV | 0.20 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| M2 | −0.20 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| BSTLD | −0.20 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.3mm2 |
| BSTLV | −0.20 | 0 ± 0% | 3.7 ± 3.7% | 0 ± 0% | 0.3mm2 |
| VP | −0.20 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| S1 | −1.80 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| S2 | −1.80 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| GI | −1.80 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| AI | −1.80 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| AH | −1.80 | 0 ± 0% | 0 ± 0% | 10.0 ± 6.7% | 0.5mm2 |
| LH | −1.80 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| Pir | −1.80 | 0 ± 0% | 0.4 ± 0.2% | 0 ± 0% | 0.5mm2 |
| BLA | −3.14 | 3.4 ± 2.5% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| Ce | −3.14 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| Me | −3.14 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.5mm2 |
| PVP | −3.14 | 0.8 ± 0.8% | 4.8 ± 1.8% | 10.9 ± 3.1% | 0.5mm2 |
| CA1 | −6.00 | 1.2 ± 0.5% | 2.6 ± 1.0% | 11.0 ± 2.7%**++ | 0.5mm2 |
| Subiculum | −6.80 | 2.6 ± 1.3% | 2.0 ± 0.5% | 12.4 ± 2.1**+++ | 0.5mm2 |
| LEnt | −6.80 | 0.6 ± 0.4% | 5.0 ± 2.5% | 10.8 ± 1.9%* | 0.5mm2 |
| VLPAG | −8.00 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.3mm2 |
| LC | −9.68 | 0 ± 0% | 0 ± 0% | 5.0 ± 5.0% | 0.3mm2 |
| MPB | −9.68 | 0 ± 0% | 0 ± 0% | 0 ± 0% | 0.3mm2 |
P < 0.05
P < 0.01 versus control
P < 0.01
P < 0.001 versus handled; post hoc Bonferroni comparisons.
Figure 6.
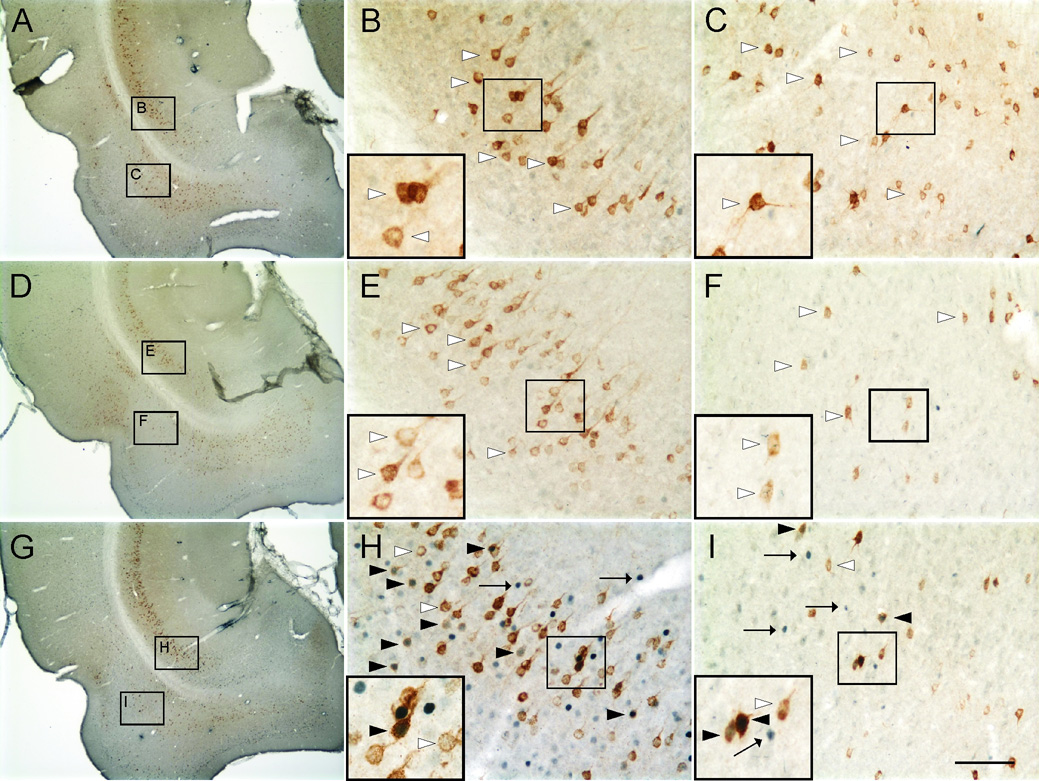
Photomicrographs illustrating c-Fos-ir nuclei and CTb-ir neurons in the CA1 region of the ventral hippocampus (−6.00 mm Bregma) of rats exposed to A,B) Control (T2823), C,D) Handling (T2812), and E,F) Open-field conditions (T2795). Black boxes in A, C and E indicate regions shown at higher magnification in B, D and F respectively. Black boxes in B, D and F indicate regions shown at higher magnification in insets in the lower left-hand corner of these panels. Arrows indicate examples of c-Fos-ir nuclei (blue/black nuclear staining); white arrowheads indicate c-Fos-immunonegative/CTb-ir (basolateral amygdaloid complex-projecting) neurons (brown/orange cytoplasmic staining). Black arrowheads indicate c-Fos-ir/CTb-ir neurons. Scale bar, A, C and E, 800µm; B, D and F, 100µm; inset, 50µm.
Figure 7.
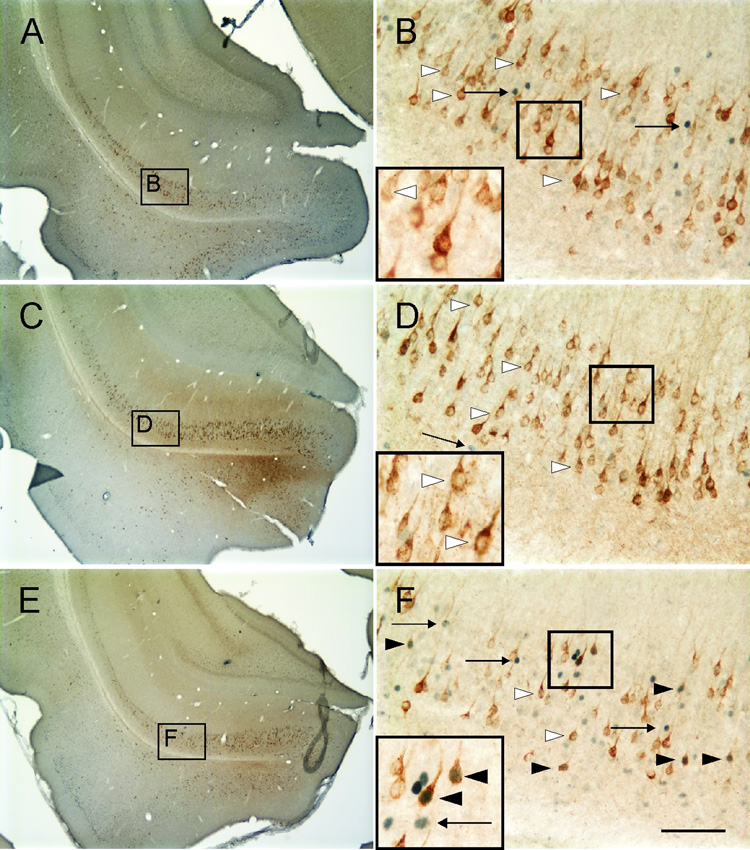
Photomicrographs illustrating c-Fos-ir nuclei and CTb-ir neurons in the subiculum and LEnt (−7.00 mm Bregma) of rats exposed to A,B,C) control (T2824), D,E,F) handling (T2816), and G,H,I) open-field conditions (T2819). Black boxes in A, D and G indicate regions shown at higher magnification in B, C, E, F, I and I respectively. Black boxes in B, C, E, F, H and I indicate regions shown at higher magnification in insets in the lower left-hand corner of these panels. Arrows indicate examples of c-Fos-ir nuclei (blue/black nuclear staining); white arrowheads indicate c-Fos-immunonegative/CTb-ir (basolateral amygdaloid complex-projecting) neurons (brown/orange cytoplasmic staining). Black arrowheads indicate c-Fos-ir/CTb-ir neurons. Scale bar, A, D and G, 800µm; C, E and G, 100µm; inset, 50µm.
Correlations
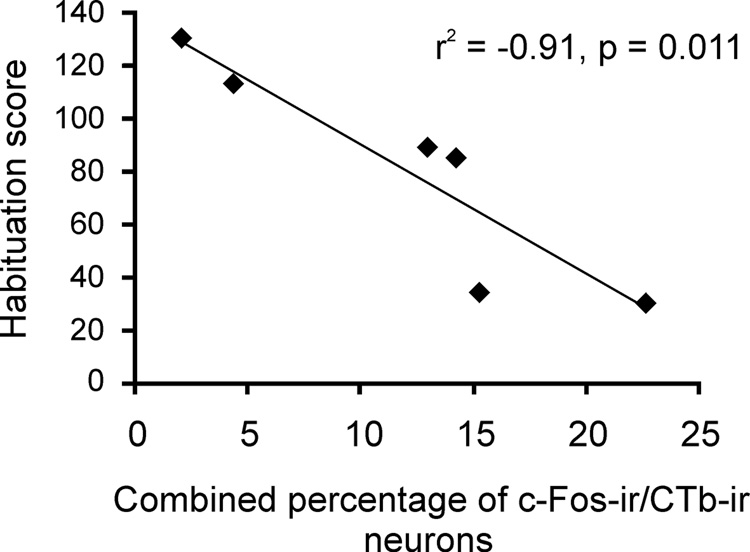
The level of activation of basolateral amygdala-projecting regions with significant increases in c-Fos expression following exposure to the open-field (CA1, subiculum, and LEnt) was correlated with measures of anxiety-related behavior, such as habituation, defined here as the progressive decline in locomotion during the open-field exposure. The combined percentages of c-Fos-ir/CTb-ir neurons in the CA1, subiculum and LEnt were negatively correlated with the degree to which the rat habituated to the open-field environment (r2 = −0.91, p = 0.011). In other words, rats with greater activation of the basolateral amygdala-projecting circuit habituated to the open-field to a lesser extent compared to rats with less activation of the basolateral amygdala. The time spent in the center of the arena was negatively correlated (r2 = −0.87, p = 0.026) with the combined percentages of c-Fos-ir/CTb-ir neurons; however with the Bonferroni-adjusted alpha (p = 0.017) this correlation did not reach statistical significance. The total locomotion in the open-field arena was not correlated with the combined percentages of c-Fos-ir/CTb-ir cells (r2 = −0.11, p = 0.833), indicating that these correlations were not due to differences in motor activity.
Discussion
Exposure to a mild anxiogenic stimulus, placement in an open-field arena under low light conditions for 15 min, compared to home cage and handling control conditions, activated components of a previously defined anxiety-related neuronal system with convergent input to the basolateral amygdaloid complex. Open-field exposure increased c-Fos expression in the anterior part of the basolateral amygdaloid nucleus contralateral to the injection site consistent with a role for this structure in the regulation of anxiety states and anxiety-related behavior. Retrogradely labeled neurons were observed in several forebrain and hindbrain regions. Of the 27 regions examined the regions that had the greatest numbers of retrogradely labeled neurons were the mPFC (PrL and IL), DEn, Pir, CA1 region of the ventral hippocampus, subiculum, LEnt and PVP. Open-field exposure increased the percentage of basolateral amygdala-projecting neurons that were c-Fos-ir in the CA1 region of the ventral hippocampus, subiculum, and LEnt. These data are consistent with the hypothesis that mild anxiogenic stimuli engage a distributed neuronal system, involved in the regulation of anxiety states and anxiety-related behavior, with convergent projections to the basolateral amygdala.
Rats exposed to the open-field spent most of the time exploring the outer sides and corners of the arena, and much less time exploring the small and large center regions. Consistent with our previous findings, rats showed a progressive decline in locomotor activity during the 15 min open-field test, suggesting habituation to the open-field during the test (Bouwknecht et al., 2007). Consequently, c-Fos expression in the anxiety-related regions sampled may be associated with the initial anxiety state or anxiety-related behaviors observed in the open-field, with the habituation to the test, or with recovery from the test following return to the home cage. Correlation analysis indicated that greater activation of basolateral amygdala-projecting neurons in the CA1, subiculum and LEnt was correlated with less habituation to the open-field environment, suggesting that c-Fos expression in the basolateral amygdala-projecting circuit is correlated with a prolonged anxiety state.
Open-field exposure induced robust and widespread increases in c-Fos expression in 20 of the 27 sampled forebrain and hindbrain regions that are thought to be part of a distributed but interconnected system regulating anxiety states and anxiety-related behaviors. Increases in c-Fos expression were observed in cortical (mPFC, cingulate, motor, somatosensory, insular, piriform and entorhinal cortices), septal (LSV), striatal (BSTLV), thalamic (PVP), hypothalamic (AH, LH), amygdalar (BLA, Me), hippocampal (CA1, subiculum) and hindbrain (LC) regions. These widespread increases in c-Fos expression within an anxiety-related network correspond with previous reports examining expression of c-Fos protein (Emmert and Herman, 1999; Salome et al., 2004) and c-fos mRNA (Emmert and Herman, 1999; Nagahara and Handa, 1997) following exposure to the open-field test. Wistar rats bred for either high (HAB) or low (LAB) anxiety-related behavior show increases in c-Fos expression following both open-field test (conducted under moderately high light conditions; 150 lux) and exposure to the open-arms of an elevated plus-maze (Salome et al., 2004). Interestingly, increased c-Fos expression in the PrL and IL were observed only in the LAB rats and these effects were associated with an increase the number of entries to, and percentage of time spent in the center of the open-field arena. In our own study, the open-field test was conducted under low-light (8–11 lux) conditions and resulted in marked increases in c-Fos expression in the PrL and IL cortices. Together with previous studies (Maier and Watkins, 2005), increased expression in the PrL and IL may be associated with inhibition of amygdala circuits mediating anxiety responses, and reduction or termination of anxiety-related responses (Amat et al., 2005; Quirk and Beer, 2006).
Consistent with our previous research, exposure to the open field arena increased c-Fos expression in the anterior part of the basolateral amygdaloid complex contralateral to the injection site (Hale et al., 2006). We have previously reported that exposure to an open-field arena in both low- and high-light conditions results in increases in c-Fos expression in some but not all subdivisions of the basolateral amygdaloid complex, with a marked increase in the anterior part of the basolateral amygdala.
Open-field exposure did not increase c-Fos expression in 6 of the 27 sampled regions including the M1, BSTLD, VLPAG, MPB, and the Ce (contralateral to the injection site). There was no difference in c-Fos expression between CO and HA groups in any of the sampled regions.
Retrogradely labeled neurons were observed in several forebrain and hindbrain regions associated with anxiety-states and anxiety-related behavior. Of the 27 regions examined the greatest numbers of neurons that projected to the basolateral amygdaloid nucleus were observed in the mPFC (PrL and IL), PVP, CA1 region of the ventral hippocampus, subiculum, DEn, LEnt and Pir. The distribution of basolateral amygdala-projecting neurons in this study corresponds with previous reports using retrograde (Ottersen, 1980; Ottersen, 1981; Ottersen, 1982; Ottersen and Ben-Ari, 1979) and anterograde (Canteras and Swanson, 1992; Kishi et al., 2006) tracing methods.
Open-field exposure increased the percentage of basolateral amygdala-projecting neurons that expressed c-Fos in the CA1 region of the ventral hippocampus compared with handled and control rats. Among the neurons within the CA1 region of the ventral hippocampus that project to the basolateral amygdala, 11.0% (± 2.7) expressed c-Fos protein following open-field exposure compared with 2.6% (±1.0) and 1.2% (±0.5) in HA and CO rats respectively. Converging lines of evidence suggest that the hippocampus is an important structure in the regulation of anxiety states and anxiety-related behavior, with a dissociation of function along its septotemporal extent (Bannerman et al., 2004; Engin and Treit, 2007). Lesions to the ventral, but not dorsal, hippocampus reduce anxiety-like behavior in the EPM (Kjelstrup et al., 2002) successive alleys test (a modified version of the EPM) and social interaction test (McHugh et al., 2004). Ventral, but not dorsal, hippocampal lesions also reduced the latency to begin eating in a test of food hyponeophagia (McHugh et al., 2004). Temporary inactivation of the ventral hippocampus using tetrodotoxin (TTX) increased open arm-exploration in the EPM and reduced burying behavior in a shock probe-burying test, but had no effect on the number of probe shocks received during the test. Conversely, TTX inactivation of the dorsal hippocampus was without effect on open arm exploration in the EPM or burying behavior in the shock probe-burying test, but increased the number of shocks received during the test (Degroot and Treit, 2004).
Unlike lesion studies, the evidence for a dissociation of function of the dorsal and ventral divisions of the hippocampus using intra-hippocampal infusion of drugs is less clear (for review see (Engin and Treit, 2007)). Infusion of the NMDA-receptor antagonist, (±)-2-amino-5-phosphopentanoic acid (AP-5) or 2-diethyl-N-[2,6-diethylphenyl]-acetamide HCl (lidocaine), into the ventral hippocampus increases open-arm exploration in the EPM, while infusion into the dorsal hippocampus is without effect (Bertoglio et al., 2006; Hackl and Carobrez, 2007). In contrast, infusion of the benzodiazepine agonist, midazolam, into the dorsal hippocampus increases open arm exploration in the EPM (Menard and Treit, 2001).
In addition to the CA1 region of the ventral hippocampus, we also observed an increase in c-Fos expression in basolateral amygdala-projecting neurons in the subiculum and LEnt. In the subiculum, 12.4% (±2.1) of basolateral amygdala-projecting neurons expressed c-Fos compared with 2.0% (±0.5) and 2.6 (±1.3) in HA and CO rats. The subiculum is an important region in the regulation of anxiety-related behavior and in the hypothalamic-pituitary-adrenal (HPA) axis response to stress-related or anxiogenic stimuli (Herman et al., 1998; O'Mara, 2005). Ibotenic acid lesions to the ventral subiculum increase time spent in the open arms of the EPM and increase the plasma corticosterone (CORT) response to EPM exposure (Mueller et al., 2004) while excitotoxic lesions to the ventral subiculum using quinolinic acid result in decreases in food neophobia (Burns et al., 1996).
The neural mechanisms underlying the increased activity in the BLA and BLA-projecting cells in the CA1, subiculum, and LEnt are unclear. The increase in activity may be due to a circuit-driven activation of a distributed anxiety-related network (for discussion, see Singewald et al., 2003; Singewald and Sharp, 2000). Alternatively, the increase in activity may be due to altered input from global neuromodulatory systems with collateral projections to these regions, such as brainstem serotonergic or noradrenergic systems (Abrams et al., 2005; Lowry et al., 2005). Notably, serotonergic systems within the caudal dorsal raphe nucleus and median raphe nucleus give rise to collateral projections to the CA1 region of the hippocampus and entorhinal cortex (Kohler and Steinbusch, 1982). Finally, it is possible that the increase in activity is due to input from hormonal signals, such as glucocorticoid hormones, which have been implicated in the regulation of anxiety-related behavior and anxiety states (File et al., 1979).
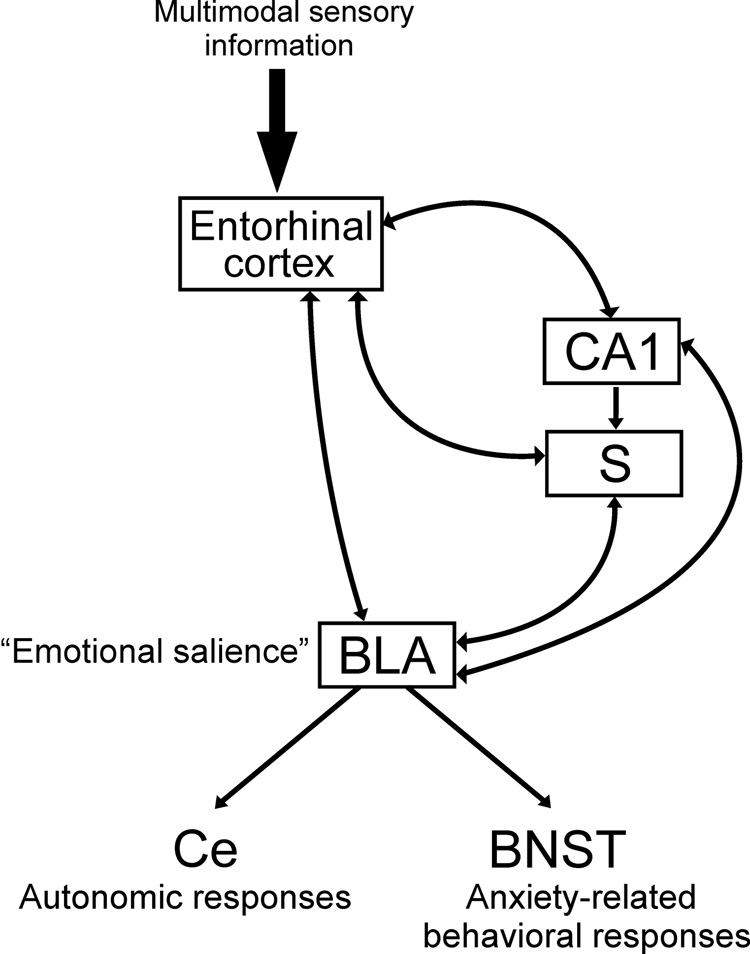
Our data suggest that the ventral hippocampus, entorhinal cortex and BLA are part of an integrated circuit regulating responses to mild anxiogenic stimuli. The CA1 region of the ventral hippocampus (Pikkarainen et al., 1999; Pitkanen et al., 2000), subiculum (French et al., 2003; Kishi et al., 2006) and entorhinal cortex (Krettek and Price, 1977; McDonald and Mascagni, 1997; Ottersen, 1982) have reciprocal connections to the basolateral amygdaloid complex and may act in concert to regulate the behavioral response to mild anxiogenic stimuli. A hypothetical model of the neural circuits mediating anxiety responses in the open-field is presented in Figure 9. In this model the hippocampal structures, ventral CA1 and subiculum, act as a comparator (Gray, 1982) comparing expected outcomes versus actual outcomes. In a conflict model of anxiety-related behavior, expected outcomes include rewarding outcomes (including safety), and aversive outcomes (Gray, 1982; Gray and McNaughton, 2003). The ventral CA1 and subiculum sample multimodal sensory information via connections with the entorhinal cortex (Tamamaki and Nojyo, 1995; Van Groen and Wyss, 1990; Vinogradova, 2001; Witter et al., 1988) in order to detect mismatch between expected and actual outcomes. In the case of a mismatch between the expected and actual outcomes (unpredictability), as in exposure to a novel open-field, the ventral CA1 and subiculum send input to the basolateral amygdala which is itself a critical structure in the regulation of anxiety-related behavior following unpredictable stimuli (Herry et al., 2007). The basolateral amygdala attaches emotional salience to the novel stimulus and regulates the autonomic (via connections with the central nucleus of the amygdala) and behavioral (via connections with the bed nucleus of the stria terminalis) responses to the anxiety-related stimulus. The precise interactions of these structures, especially in the relation to anxiety-related behavior during mild anxiogenic stimulation, require further investigation.
Figure 9.
Hypothetical model of the neural circuits mediating anxiety-related responses in the open-field. In this model the subiculum and CA1 region of the ventral hippocampus act as a comparator (Gray, 1982) comparing expected outcomes versus actual outcomes. These regions sample multimodal sensory information via connections with the entorhinal cortex in order to detect mismatch between expected and actual outcomes. In the case of a mismatch between the expected and actual outcome (unpredictability), as in exposure to a novel open-field, the ventral CA1 and subiculum send input to the basolateral amygdala which attaches emotional salience to the novel stimulus and regulates the autonomic (via the central nucleus of the amygdala) and behavioral (via the bed nucleus of the stria terminalis) responses to the anxiety-related stimulus.
The open-field task is an anxiogenic task with spatial and motor components. Therefore activation of hippocampal neurons projecting to the basolateral amygdala may represent the spatial and/or motor demands of the task. However, most studies suggest that the dorsal hippocampus plays a dominant role in spatial navigation tasks, while the ventral hippocampus has been associated with anxiety- or stress-related responses, including inhibition of the hypothalamic-pituitary-adrenal (HPA) axis (Herman et al., 1998). Indeed an investigation of the firing patterns of complex spike (CS) cells in the dorsal and ventral hippocampus indicated that only 18% of ventral hippocampal CS cells had place fields while 45% of dorsal hippocampal CS cells had place fields. In addition, ventral hippocampal CS cells had both lower spatial specificity and larger place field size when compared with dorsal hippocampal CS cells (Jung et al., 1994).
Conclusions
The results from this study indicate that exposure to a mild anxiogenic stimulus activated an interconnected network of structures projecting to the basolateral amygdaloid complex, and support the hypothesis that anxiety-related behavior and anxiety states are regulated by a distributed and interconnected anxiety-related network with convergent input to the basolateral amygdaloid complex.
Figure 8.
Graph illustrating the correlation between habituation and activation of BLA-projecting neurons in the CA1, subiculum and LEnt. Habituation (calculated as the number of line crossings in the first five minutes minus the number of line crossings in the last five minutes of the open-field test) was negatively correlated with the combined percentages of c-Fos-ir/CTb-ir neurons in the CA1, subiculum and LEnt.
Abbreviations
- AH
anterior hypothalamic area, posterior part
- AI
agranular insular cortex
- BLA
basolateral amygdaloid nucleus, anterior part
- BLP
basolateral amygdaloid nucleus, posterior part
- BLV
basolateral amygdaloid nucleus, ventral part
- BMP
basomedial amygdaloid nucleus, posterior part
- BSTLD
bed nucleus of the stria terminalis, lateral division, dorsal part
- BSTLV
bed nucleus of the stria terminalis, lateral division, ventral part
- CA1
CA1 field of the ventral hippocampus
- Ce
central amygdaloid nucleus
- Cg1
cingulate cortex
- CO
control
- CTb
Cholera toxin, B subunit
- DEn
dorsal endopiriform cortex
- GI
granular insular cortex
- HA
handled
- IL
infralimbic cortex
- LaDL
lateral amygdaloid nucleus, dorsolateral part
- LaVL
lateral amygdaloid nucleus, ventrolateral part
- LaVM
lateral amygdaloid nucleus, ventromedial part
- LC
locus coeruleus
- LEnt
lateral entorhinal cortex
- LH
lateral hypothalamic area
- LSV
lateral septal nucleus, ventral part
- M1
primary motor cortex
- M2
secondary motor cortex
- Me
medial amygdaloid nucleus
- MPB
medial parabrachial nucleus
- OF
open-field exposed
- Pir
piriform cortex
- PrL
prelimbic cortex
- PVP
paraventricular thalamic nucleus, posterior part
- S1
primary somatosensory cortex
- S2
secondary somatosensory cortex
- S
subiculum
- VLPAG
ventrolateral periaqueductal gray
- VP
ventral pallidum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav. Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Joca SRL, Guimaraes FS. Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav. Brain Res. 2006;175:183–188. doi: 10.1016/j.bbr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Research Bulletin. 2007;72:32–43. doi: 10.1016/j.brainresbull.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav. Neurosci. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J. Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Research. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Research. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav. Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- File SE, Vellucci SV, Wendlandt S. Corticosterone -- an anxiogenic or an anxiolytic agent? J. Pharm. Pharmacol. 1979;31:300–305. doi: 10.1111/j.2042-7158.1979.tb13505.x. [DOI] [PubMed] [Google Scholar]
- French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res. 1996;732:145–153. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Clarendon Press; 1982. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. New York: Oxford University Press; 2003. [Google Scholar]
- Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Hackl LPN, Carobrez AP. Distinct ventral and dorsal hippocampus AP5 anxiolytic effects revealed in the elevated plus-maze task in rats. Neurobiology of Learning and Memory. 2007;88:177–185. doi: 10.1016/j.nlm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Hale MW, Bouwknecht JA, Spiga F, Shekhar A, Lowry CA. Exposure to high- and low-light conditions in an open-field test of anxiety increases c-Fos expression in specific subdivisions of the rat basolateral amygdaloid complex. Brain Res. Bull. 2006;71:174–182. doi: 10.1016/j.brainresbull.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J. Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J. Comp Neurol. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav. Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal L-glutamate. Brain Research. 2001;888:163–166. doi: 10.1016/s0006-8993(00)03046-8. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145:3763–3768. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J. Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Age-related changes in c-fos mRNA induction after open-field exposure in the rat brain. Neurobiol. Aging. 1997;18:45–55. doi: 10.1016/s0197-4580(96)00166-2. [DOI] [PubMed] [Google Scholar]
- O'Mara S. The subiculum: what it does, what it might do, and what neuroanatomy has yet to tell us. Journal of Anatomy. 2005;207:271–282. doi: 10.1111/j.1469-7580.2005.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J. Comp Neurol. 1980;194:267–289. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. J. Comp Neurol. 1981;202:335–356. doi: 10.1002/cne.902020304. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J. Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Ben-Ari Y. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J. Comp Neurol. 1979;187:401–424. doi: 10.1002/cne.901870209. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press, Inc.; 1998. [Google Scholar]
- Petersen RG. In: Design and analysis of experiments. Dekker M, editor. New York: 1985. [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr. Opin. Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology. 2002;43:1165–1172. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav. Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Salome N, Salchner P, Viltart O, Sequeira H, Wigger A, Landgraf R, Singewald N. Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol. Psychiatry. 2004;55:715–723. doi: 10.1016/j.biopsych.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav. Brain Res. 1993;56:115–118. doi: 10.1016/0166-4328(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Silveira MC, Zangrossi H, de BV, Silveira R, Graeff FG. Differential expression of Fos protein in the rat brain induced by performance of avoidance or escape in the elevated T-maze. Behav. Brain Res. 2001;126:13–21. doi: 10.1016/s0166-4328(01)00233-9. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol. Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Singewald N, Sharp T. Neuroanatomical targets of anxiogenic drugs in the hindbrain as revealed by Fos immunocytochemistry. Neuroscience. 2000;98:759–770. doi: 10.1016/s0306-4522(00)00177-9. [DOI] [PubMed] [Google Scholar]
- Spiga F, Lightman SL, Shekhar A, Lowry CA. Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience. 2006;138:1265–1276. doi: 10.1016/j.neuroscience.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J. Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J. Comp Neurol. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Witter MP, Griffioen AW, Jorritsma-Byham B, Krijnen JL. Entorhinal projections to the hippocampal CA1 region in the rat: an underestimated pathway. Neurosci. Lett. 1988;85:193–198. doi: 10.1016/0304-3940(88)90350-3. [DOI] [PubMed] [Google Scholar]