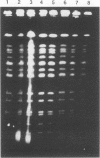
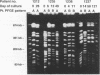
Abstract
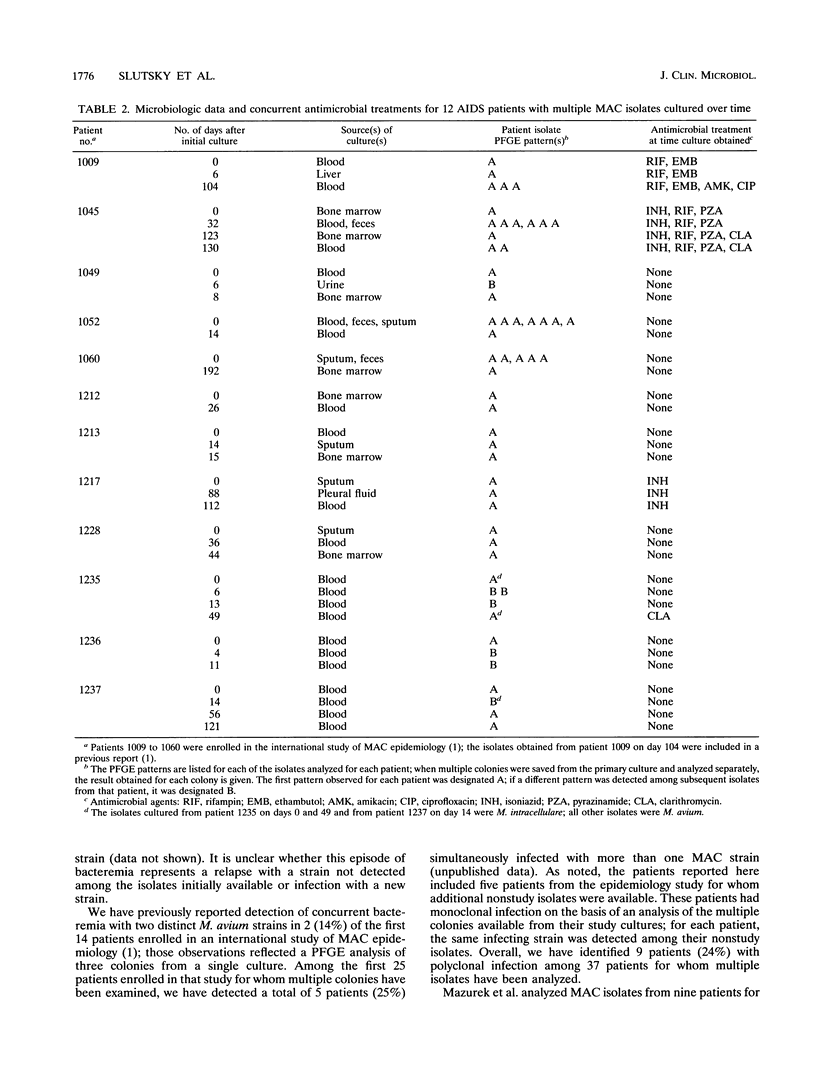
Invasive infection with organisms of the Mycobacterium avium complex (MAC) is common among patients with advanced human immunodeficiency virus infection. In previous studies, we analyzed multiple individual colonies of MAC isolated from specimens obtained at the same time and observed that 14 to 20% of patients are simultaneously infected with more than one strain. In this study, we examined sequential isolates from 12 patients with AIDS who had two or more MAC isolates available from clinical specimens collected more than 1 week apart; the intervals between the first and last specimens ranged from 8 to 192 (median, 46) days. For each isolate, restriction digests of genomic DNA were analyzed by pulsed-field gel electrophoresis; DNA was prepared by using a protocol, described here in detail, which had been optimized for conditions of bacterial growth and lysis. The pulsed-field gel electrophoresis analysis identified four patients (33%) infected with two different MAC strains. Both M. avium and M. intracellulare were cultured from blood specimens from two patients. In each of the four patients, the second strain was identified from a culture taken within 14 days of the initial study isolate, and in three of these patients, the first strain was detected again in a subsequent culture. These observations suggest that the presence of two different strains among isolates from sequential cultures may reflect ongoing polyclonal infection. We conclude that polyclonal infection with MAC is common among patients with AIDS. The identification of such infections may be critical in the development of effective treatments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Slutsky A., Barber T. W., Maslow J. N., Niemczyk S., Falkinham J. O., 3rd, O'Connor G. T., von Reyn C. F. Genetic diversity among strains of Mycobacterium avium causing monoclonal and polyclonal bacteremia in patients with AIDS. J Infect Dis. 1993 Jun;167(6):1384–1390. doi: 10.1093/infdis/167.6.1384. [DOI] [PubMed] [Google Scholar]
- Burns D. N., Wallace R. J., Jr, Schultz M. E., Zhang Y. S., Zubairi S. Q., Pang Y. J., Gibert C. L., Brown B. A., Noel E. S., Gordin F. M. Nosocomial outbreak of respiratory tract colonization with Mycobacterium fortuitum: demonstration of the usefulness of pulsed-field gel electrophoresis in an epidemiologic investigation. Am Rev Respir Dis. 1991 Nov;144(5):1153–1159. doi: 10.1164/ajrccm/144.5.1153. [DOI] [PubMed] [Google Scholar]
- Conville P. S., Keiser J. F., Witebsky F. G. Mycobacteremia caused by simultaneous infection with Mycobacterium avium and Mycobacterium intracellulare detected by analysis of a BACTEC 13A bottle with the Gen-Probe kit. Diagn Microbiol Infect Dis. 1989 May-Jun;12(3):217–219. doi: 10.1016/0732-8893(89)90018-7. [DOI] [PubMed] [Google Scholar]
- Hector J. S., Pang Y., Mazurek G. H., Zhang Y., Brown B. A., Wallace R. J., Jr Large restriction fragment patterns of genomic Mycobacterium fortuitum DNA as strain-specific markers and their use in epidemiologic investigation of four nosocomial outbreaks. J Clin Microbiol. 1992 May;30(5):1250–1255. doi: 10.1128/jcm.30.5.1250-1255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson M., Green P., Manning H. L., Slutsky A. M., Brecher S. M., von Reyn C. F., Arbeit R. D., Maslow J. N. Molecular confirmation of bacillus Calmette-Guérin as the cause of pulmonary infection following urinary tract instillation. Clin Infect Dis. 1993 Aug;17(2):228–230. doi: 10.1093/clinids/17.2.228. [DOI] [PubMed] [Google Scholar]
- Lombardo P. C., Weitzman I. Isolation of Mycobacterium tuberculosis and M. avium complex from the same skin lesions in AIDS. N Engl J Med. 1990 Sep 27;323(13):916–917. doi: 10.1056/NEJM199009273231313. [DOI] [PubMed] [Google Scholar]
- Lévy-Frébault V. V., Thorel M. F., Varnerot A., Gicquel B. DNA polymorphism in Mycobacterium paratuberculosis, "wood pigeon mycobacteria," and related mycobacteria analyzed by field inversion gel electrophoresis. J Clin Microbiol. 1989 Dec;27(12):2823–2826. doi: 10.1128/jcm.27.12.2823-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy-Frébault V., Pangon B., Buré A., Katlama C., Marche C., David H. L. Mycobacterium simiae and Mycobacterium avium-M. intracellulare mixed infection in acquired immune deficiency syndrome. J Clin Microbiol. 1987 Jan;25(1):154–157. doi: 10.1128/jcm.25.1.154-157.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow J. N., Mulligan M. E., Arbeit R. D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993 Aug;17(2):153–164. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- Massenkeil G., Opravil M., Salfinger M., von Graevenitz A., Lüthy R. Disseminated coinfection with Mycobacterium avium complex and Mycobacterium kansasii in a patient with AIDS and liver abscess. Clin Infect Dis. 1992 Feb;14(2):618–619. doi: 10.1093/clinids/14.2.618-a. [DOI] [PubMed] [Google Scholar]
- Mazurek G. H., Hartman S., Zhang Y., Brown B. A., Hector J. S., Murphy D., Wallace R. J., Jr Large DNA restriction fragment polymorphism in the Mycobacterium avium-M. intracellulare complex: a potential epidemiologic tool. J Clin Microbiol. 1993 Feb;31(2):390–394. doi: 10.1128/jcm.31.2.390-394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Daffe M., Brennan P. J. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem. 1990 Oct 25;265(30):18200–18206. [PubMed] [Google Scholar]
- Nightingale S. D., Byrd L. T., Southern P. M., Jockusch J. D., Cal S. X., Wynne B. A. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992 Jun;165(6):1082–1085. doi: 10.1093/infdis/165.6.1082. [DOI] [PubMed] [Google Scholar]
- Stepanov A. S., Puzanova O. B., Dityatkin SYa, Loginova O. G., Ilyashenko B. N. Glycine-induced cryotransformation of plasmids into Bacillus anthracis. J Gen Microbiol. 1990 Jul;136(7):1217–1221. doi: 10.1099/00221287-136-7-1217. [DOI] [PubMed] [Google Scholar]
- Torres R. A., Nord J., Feldman R., LaBombardi V., Barr M. Disseminated mixed Mycobacterium simiae-Mycobacterium avium complex infection in acquired immunodeficiency syndrome. J Infect Dis. 1991 Aug;164(2):432–433. doi: 10.1093/infdis/164.2.432. [DOI] [PubMed] [Google Scholar]
- Whipple D. L., Le Febvre R. B., Andrews R. E., Jr, Thiermann A. B. Isolation and analysis of restriction endonuclease digestive patterns of chromosomal DNA from Mycobacterium paratuberculosis and other Mycobacterium species. J Clin Microbiol. 1987 Aug;25(8):1511–1515. doi: 10.1128/jcm.25.8.1511-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakrus M. A., Good R. C. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990 May;28(5):926–929. doi: 10.1128/jcm.28.5.926-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Mazurek G. H., Cave M. D., Eisenach K. D., Pang Y., Murphy D. T., Wallace R. J., Jr DNA polymorphisms in strains of Mycobacterium tuberculosis analyzed by pulsed-field gel electrophoresis: a tool for epidemiology. J Clin Microbiol. 1992 Jun;30(6):1551–1556. doi: 10.1128/jcm.30.6.1551-1556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Reyn C. F., Maslow J. N., Barber T. W., Falkinham J. O., 3rd, Arbeit R. D. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994 May 7;343(8906):1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]