Abstract
Proteins harbor a number of cavities of relatively small volume. Although these packing defects are associated with the thermodynamic instability of the proteins, the cavities also play specific roles in controlling protein functions, e.g., ligand migration and binding. This issue has been extensively studied in a well-known protein, myoglobin (Mb). Mb reversibly binds gas ligands at the heme site buried in the protein matrix and possesses several internal cavities in which ligand molecules can reside. It is still an open question as to how a ligand finds its migration pathways between the internal cavities. Here, we report on the dynamic and sequential structural deformation of internal cavities during the ligand migration process in Mb. Our method, the continuous illumination of native carbonmonoxy Mb crystals with pulsed laser at cryogenic temperatures, has revealed that the migration of the CO molecule into each cavity induces structural changes of the amino acid residues around the cavity, which results in the expansion of the cavity with a breathing motion. The sequential motion of the ligand and the cavity suggests a self-opening mechanism of the ligand migration channel arising by induced fit, which is further supported by computational geometry analysis by the Delaunay tessellation method. This result suggests a crucial role of the breathing motion of internal cavities as a general mechanism of ligand migration in a protein matrix.
Keywords: hydrophobic cavity, molecular movie, protein dynamics, time-resolved crystallography
Localized electronic excitation by photons often induces large-scale structural modulations and novel physical properties in condensed matter (1, 2). Myoglobin (Mb), often referred to as the hydrogen atom of biology and a paradigm of complexity (3), has played a central role in research on the photo-induced response of proteins and migration of gases, solvents, and ligands in the protein matrix (3, 4). Despite the large number of details known about Mb dynamics, it remains unclear how a ligand molecule escapes from the protein matrix to the solvent and how the protein matrix responds to the ligand migration at the atomic level. A number of time-resolved spectroscopic measurements of Mb photoproducts have revealed a complex ligand-binding reaction with multiple kinetic intermediates (4–8). After dissociation from the heme iron atom, ligand gas molecules either rebind internally from the distal pocket (DP) (Fig. 1) or escape into the solvent. It has been deduced that the escape of the ligand is assisted by the thermal fluctuations that transiently open exit channels. Lowering the temperature slows down the thermal fluctuations, and the internal binding process becomes dominant (4, 5).
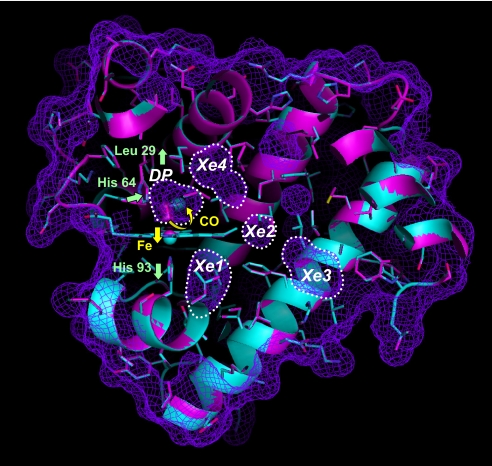
Fig. 1.
The crystal structures of MbCO before and after photodissociation of CO at 40 K are superimposed and shown in magenta and cyan, respectively. The molecular surface of MbCO and the surface of internal cavities are shown by the mesh in purple. The internal cavities (DP, Xe1, Xe2, Xe3, and Xe4) are also indicated by dotted lines. The electron densities of bound and photodissociated CO molecules in the DP are represented in magenta and cyan, respectively, by using a 2Fo − Fc map (contoured at 0.7 e/Å3). The movement of CO, heme iron atom, His-64, Leu-29, and His-93 after photodissociation is shown by yellow and green arrows.
The multiple kinetic intermediates scheme of Mb has motivated researchers to characterize the structural features of the the intermediates by using both time-resolved (9–14) and cryogenic crystallographic measurements (15–22). A general picture emerging from these experiments is that Mb has several internal cavities, identified as Xe-binding sites (23) (Fig. 1), which are favorable locations for gas molecules, and that the cavities are involved in ligand migration dynamics. Cryogenic crystallography of native and mutant carbonmonoxy Mb (MbCO) photoproducts revealed intermediate structures in which the photodissociated CO molecule is trapped in the DP, Xe1, and Xe4 cavities. When photolysis of MbCO was followed by thermal cycling above the Mb glass transition temperature, the CO molecule hopped between the cavities (20, 21). Furthermore, time-resolved crystallographic studies of native and mutant MbCO provided molecular movies showing that the photolyzed CO molecule migrates from the DP to the Xe4 and/or Xe1 sites accompanying the structural relaxation of the protein (9–14). Picosecond time-resolved crystallography of mutant L29F MbCO, where Leu-29 is replaced by phenylalanine, revealed a highly strained intermediate structure at 100 ps after the photolysis and enabled visualization of how the photolyzed CO molecule is quickly swept out from the primary docking site to the Xe4 cavity by the correlated side-chain motions of His-64 and Phe-29 (11). These time-resolved methods have revealed the fast dynamics of the MbCO photoproduct on the picosecond time scale. However, migration of the CO molecule in the protein matrix proceeds on a relatively longer time scale, and the overall ligand migration process between the cavities remains unclear.
Theoretical analyses of Mb dynamics examine this issue, and considerable new ground has been covered recently (24–27). Long time-scale molecular dynamics simulations (>80 ns) of the migration of CO or NO inside Mb reproduced some of the previous crystallographic results (26). More recently, the gas migration pathways inside Mb were studied by an implicit ligand sampling method (27), which enabled the location of gas migration pathways to be inferred on the basis of a free-energy perturbation approach and provided complete 3D maps of the potential of the mean force. These theoretical studies stimulated us to search for the overall ligand-binding pathways in Mb from an experimental perspective. We developed a cryogenic crystallographic method by using continuous illumination of crystals with a pulsed laser, as described in Results and Discussion.
Results and Discussion
Photodissociation of CO in Mb at 40 K.
In our cryogenic crystallographic experiments the cryogenic temperature was set at 40–140 K, and native MbCO crystals were continuously illuminated by a nanosecond pulsed YAG laser with a 15-kHz repetition rate and 4.6 mW/mm2 average power. Molecular dynamics simulations have shown that absorption of a photon by a heme heats up the heme itself by 500–700 K under conditions far from equilibrium and the heat is dissipated from the heme to the surrounding protein matrix in a picosecond time regime, which causes transient local melting (28). The crystal is also cooled repeatedly by the cold gas flow. Thus, this method allows thermally driven conformational fluctuations to take place even at cryogenic temperatures and enables the photolyzed CO molecule to migrate in the protein matrix over several hundred minutes.
First, we analyzed the crystal structure of the Mb photoproduct at 40 K as the starting structure for CO migration. As is consistent with the previous cryogenic and time-resolved crystallographic studies, we observed similar structural features of initial photoproduct but at a higher resolution, as shown in Fig. 1 (15–17). The CO molecule moves from the heme-binding site to the primary docking site in the heme cavity 3.74 Å apart from the heme iron atom, and the iron atom shifts by 0.26 Å out of the mean heme plane. The displacement is transmitted through His-93 to the F-helix region. In the distal pocket, concerted motions of His-64 and Leu-29 side chains are evident upon photodissociation of the CO molecule. The data collection and crystallographic analysis statistics at 40 K are shown in Table 1.
Table 1.
Data collection and crystallographic analysis statistics at 40 K
| Statistic | Laser off | Laser on |
|---|---|---|
| Space group | P 1 21 1 | P 1 21 1 |
| Cell dimensions a, Å | 34.312 | 34.310 |
| b, Å | 30.618 | 30.630 |
| c, Å | 63.714 | 63.729 |
| β, ° | 105.742 | 105.761 |
| Wavelength, Å | 0.827 | 0.827 |
| Resolution, Å | 50.0–1.21 | 50.0–1.21 |
| Rmerge | 0.033 (0.205) | 0.033 (0.196) |
| I/sigma | 15.4 (3.1) | 15.1 (3.1) |
| Completeness | 0.947 (0.807) | 0.955 (0.713) |
| Redundancy | 3.7 (3.1) | 3.7 (3.0) |
| No. reflections | 37292 | 37319 |
| R factor | 0.142 | 0.143 |
| Rfree | 0.182 | 0.179 |
| No. atoms | 1,457 | 1,446 |
| Mean B values | 13.496 | 13.301 |
| rmsd bond length, Å | 0.019 | 0.020 |
| rmsd angle,° | 1.862 | 1.933 |
Values in parentheses are for the highest-resolution shell (1.25–1.21 Å). A summary of the whole data collection and refinement statistics at 100–140 K is available in Tables S1–S3.
Slow Dynamics of CO Migration in Mb at 100–140 K.
When the cryogenic temperature was raised from 40 K to 120 K, most of the photodissociated CO molecule at the primary docking site bound to the heme iron because the internal rebinding rate of CO to the heme iron atom overcomes the rate of photodissociation at 120 K. However, at the same time, CO starts to migrate slowly into the internal cavities, as shown in Fig. 2 and Movie S1. Because the crystal structures shown in Fig. 2 are the average structure of a large number of Mb molecules in the crystal, the occupancy of the CO molecule in each cavity corresponds to the population of Mb molecules in a particular intermediate structure. Fig. 2 A and D shows that CO migrates into the cavities in the lower part of the heme plane (Xe1 cavity) and in the back of the DP (Xe4 cavity) after 300-min laser irradiation. It is worth noting that this migration does not take place upon continuous-wave laser illumination at the same average laser power (4.6 mW/mm2). This result is consistent with the previous results reported by Teng et al. (18) and clearly shows that the repeated heat-and-cool cycle by pulsed-laser illumination is essential if the ligand migration dynamics are to take place. Other features emerge in the Xe2 cavities after 450-min laser irradiation. This study provides direct evidence of the time-dependent evolution of the electron density of the CO molecule in the Xe2 cavity, which clearly indicates that the Xe2 cavity is also involved in the CO migration pathway of Mb. The Xe3 cavity is originally occupied by a water molecule (Fig. 2C), and it is difficult to estimate precisely whether the increased electron density in Xe3 corresponds to the photodissociated CO molecule or to a water molecule from the external solvent.
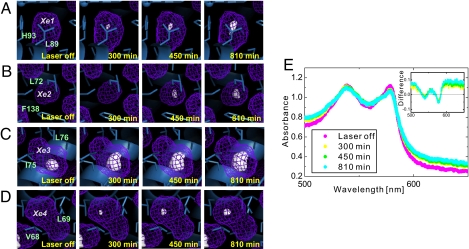
Fig. 2.
Photodissociation and migration of the CO molecule in Mb at 120 K. (A–D) Internal cavities and time-dependent evolution of the CO electron density in the Xe1 (A), Xe2 (B), Xe3 (C), and Xe4 (D) cavities at 120 K. The surfaces of the internal cavities are shown by the mesh in purple. The electron densities of the CO molecules in the cavities are presented by using the 2Fo − Fc map (contoured at 0.3 e/Å3). (E) Visible absorption spectra of MbCO crystal measured by microspectrophotometry at 120 K. (Inset) The differential absorption spectra against the initial MbCO spectrum.
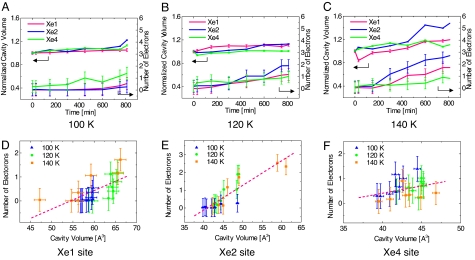
Photodissociation of the CO molecule from the heme iron was confirmed by microspectroscopic measurement of the MbCO crystal under the same pulsed-laser illumination conditions at 120 K, as shown in Fig. 2E. The optical absorption spectral change around the visible region (500–700 nm) clearly indicates that the CO molecule is photodissociated from the heme iron atom and the MbCO form is partly converted to the deoxy Mb form. The differential absorbance at 580 nm corresponds to the photolyzed population of ≈25% after 810-min laser irradiation at 120 K. The photolyzed population estimated by microspectrophotometry is nearly equal to the fraction of the integrated number of electrons in the Xe1, Xe2, and Xe4 cavities within the experimental error. The temporal evolution of the number of integrated electrons in each cavity and the cavity volume at 100–140 K are plotted in Fig. 3 and shown in Movies S1–S3. Migration of CO to the Xe4 site is dominant at 100 K (Fig. 3A), and CO starts to migrate further at 120 K. Migration to the Xe1 and Xe2 cavities becomes dominant at 140 K (Fig. 3 B and C). The number of integrated electrons correlates with the volume of each cavity, which indicates that each cavity in Mb becomes slightly enlarged to accommodate a CO molecule (Fig. 3 D–F).
Fig. 3.
Temporal evolution and correlation of the occupancy of CO and the cavity volume. (A–C)The number of electrons integrated in each cavity and normalized volumes of the Xe1 (magenta), Xe2 (blue), and Xe4 (green) cavities at 100 K (A), 120 K (B), and 140 K (C) are shown. The number of electrons was integrated by using the CCP4 program suite (31). The volume of each cavity was calculated with the program CASTp (33) and normalized by the initial volume at time 0. (D–F) The correlation between the number of electrons and the volume of cavities Xe1 (D), Xe2 (E), and Xe4 (F) is shown.
Ligand Migration Pathways in Mb.
A number of kinetic studies have suggested putative ligand migration pathways through the distal pathway (directly escaping from the DP to the solvent outside of the Mb molecule gated by His-64), and theoretical simulations have proposed several ligand migration pathways, including small internal cavities other than the Xe cavities. Our result also supports the idea of the ligand migration pathway, which is suggested by previous time-resolved and cryogenic X-ray crystallography (9–21) and is also consistent with one of the putative migration pathways predicted by the implicit ligand sampling method (27). Our result suggests that the pathway from the DP to the Xe3 cavity involving the Xe4, Xe2, and Xe1 cavities is a major ligand migration pathway in Mb at cryogenic temperatures. The consistency between the present results and those of time-resolved crystallography at room temperature strongly suggests the importance of this pathway at both room and cryogenic temperatures (9–14).
Breathing Motion of Internal Cavities in Concert with the Ligand Migration.
Our structural analysis clearly shows that the time dependence of the electron density of the CO molecule in the cavities correlates with the change in the estimated volume of each cavity, as shown in Fig. 3. This result suggests that the CO migration in the cavity causes expansion of the cavity itself, resulting in self-opening of the channel. We examined the structural changes induced by ligand migration in more detail and conducted computational geometry analysis along the pathway by using the Delaunay tessellation method (29, 30). It should be noted that the fact that none of the curves in Fig. 3 level off at longer time points shows that a steady state is not reached by this method, which makes it difficult to provide kinetic model and detailed theoretical fitting to the results.
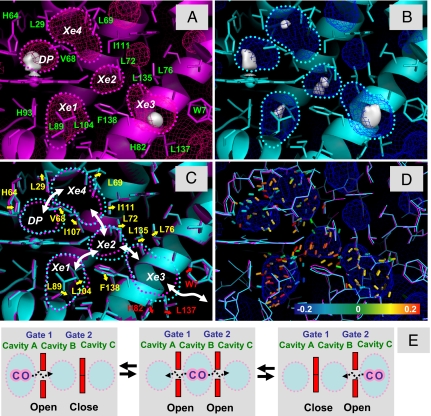
Fig. 4 shows the structural changes around the ligand migration pathway observed at 140 K, which reveals expansion of cavities and putative gating motions of surrounding amino acid residues. The residues involved are: Leu-29, His-64, and Val-68 (between the DP and the Xe4 cavity), Leu-69, Leu-72, Ile-107, and Ile-111 (between the Xe4 and Xe2 cavities), Leu-76, Leu-135, and Phe-138 (between the Xe2 and Xe3 cavities), Leu-89, Leu-104, and Phe-138 (between the Xe2 and Xe1 cavities), and Trp-7, His-82, and Leu-137 (between the Xe3 cavity and the solvent). A further quantitative depiction of the structural change along the ligand migration pathway is obtained by a computational geometry analysis. The tensor field of the structural change (principal axes and eigenvalues) is represented by colored 0.5-Å wires in Fig. 4D and Movie S4. Expansion tensors (color-coded in red in Fig. 4) are evident at the channel regions between the DP, Xe4, Xe2, Xe1, and Xe3 cavities and the center regions of the cavities, supporting the correlation between the ligand migration and the cavity volume change.
Fig. 4.
Correlated breathing motion of the internal cavities in Mb. (A and B) Structure of MbCO at 140 K before laser illumination (magenta) (A) and after 750-min laser illumination (cyan) (B). The electron densities of the CO molecules in the Xe cavities are presented by using the 2Fo − Fc map (contoured at 0.3 e/Å3). The surfaces of the internal cavities are shown by the mesh. The cavities are also outlined by dotted lines. (C) Amino acid residues lining the DP, Xe4, Xe2, Xe1, and Xe3 cavities. The color scheme is the same as that in A and B. The outlines of the cavities are also superimposed. The movements of amino acid residues between the cavities are shown by yellow arrows, and those between the Xe3 cavity and solvent area are shown by red arrows. The white arrows represent the ligand migration pathway between the cavities. (D) Strain tensors calculated by using 2 coordinates without laser illumination and after 750-min laser illumination. The strain tensors are shown with the maximum absolute eigenvalue, and the color of the segment shows the magnitude of the eigenvalue (blue, −0.20; green, 0, red, +0.20). The blue segments represent contraction, and the red segments show expansion. (E) Schematic drawing of the correlated ligand migration in a protein.
Our method proposes another view of the multiple-kinetic model, as described in the following. The migration of the CO molecule in a cavity induces the correlated expansion of the cavity itself with a breathing motion, which promotes the self-opening of the CO migration channel by an induced fit. The breathing motion facilitates further ligand migration, as shown in the scheme in Fig. 4E. Thus, in this picture, the kinetic potential barriers between the cavities are no longer defined statically but rather they are tuned dynamically by the ligand migration itself (3, 4). This picture should be further examined by mapping the potential of the mean force (27).
Implications of a Role of the Internal Cavities in Proteins.
Finally, we will attempt to answer the question of why such a complex multistep ligand migration is engineered and evolutionally conserved in Mb. The amino acids lining the Xe cavities are much more highly conserved than other amino acids in mammalian Mb and are, thus, likely to be important for function. The answer lies in the ligand migration mechanism and the discrimination of small gas ligand molecules from the outer water molecules by using the internal cavities. As shown in the crystal structure of Mb, the hydrophobic nature of the internal cavities does not favor water molecules residing inside the cavities but allows in small ligand molecules, such as O2, CO, and NO. Because the concentration of water is much higher than that of O2 in solution, Mb must discriminate between O2 and the external solvent. Our result provides a picture of the ligand migration that the ligand migration itself opens the gate. It proposes a general mechanism of the correlated migration of gases and ligands in protein matrix, which might explain why the amino acids lining the cavities are highly conserved. It is reasonable to assume that these features of internal cavities in proteins have been acquired during the long history of evolution.
Materials and Methods
Sample Preparation.
Native sperm whale MbCO crystals were crystallized as reported (9). The MbCO crystals were transferred from their mother liquor to a cryoprotectant solution consisting of the mother liquor with 15% glycerol. Subsequently, the crystals were flash-cooled by cold nitrogen gas and stored in liquid nitrogen until the experiment.
Cryogenic Crystallography Using Repeated Pulsed-Laser Illumination.
The crystals were cooled to 40 K by cold helium gas or 100–140 K by cold nitrogen gas. Photolysis was done using the second harmonic of a Nd-YAG pulse laser at 532 nm (Elforlight SPOT250–1064/532) with a 15-kHz repetition rate and an average power of 4.6 mW/mm2. Each MbCO crystal was illuminated from both sides of the crystal. The pulse duration and the pulse energy were 2 ns and 0.97 μJ/pulse, respectively, corresponding to the steeple head power density of 485 W/mm2. Microspectrophotometry of the MbCO crystal was performed by using a microscope and a spectrometer as reported (31).
Data Collection, Structure Determination, Refinement, and Analysis.
The diffraction data were collected at beamline NW14A at PF-AR, High Energy Accelerator Research Organization, using the undulator U20 and marDTB stage with the marCCD165 detector (32). The datasets were processed and scaled with the HKL2000 program (33). The structures were refined with the CCP4 program suite by using Protein Data Bank entry 1MBC as the starting model (34). The electrons in each cavity were calculated with the CCP4 program suite (mainly, fft, mapmask, and mapdump). The error bars of integrated electrons were plotted by using the rmsd of each 2Fo − Fc map in e/Å3. Molecular graphics were created with PyMOL (35). The cavity volumes were estimated by CASTp (36). For all structures, the size of the probe sphere radius was set at 1.4 Å. The position of the corresponding cavities was assigned with the program CASTpyMOL (the PyMOL plug-in for the CASTp). A summary of all data collection and refinement statistics is available in Tables S1–S3.
Computational Geometry Analysis.
Delaunay tessellation was applied to the X-ray coordinates of all atoms along the putative ligand migration pathway at 140 K. The pathway and the cavities were represented as an assembly of Delaunay tetrahedra, where each tetrahedron consists of 4 atoms adjacent to each other. After 750 min from the start of the photodissociation of CO, each tetrahedron changes its geometry; the structural changes of the tetrahedra are illustrated by means of a strain tensor analysis with a colored segment at the center of gravity of each tetrahedron (29, 30). The orientation of the segment represents the principal axis of the strain tensor with the maximum absolute eigenvalue, and the color of the segment shows the magnitude of the eigenvalue (blue: −0.20, green: 0, red: +0.20). The blue segments represent contraction, and the red segments show expansion.
Supplementary Material
Acknowledgments.
We thank T. Koda, H. Ihee, and K. Moffat for comments on the preliminary version of the manuscript. This work was partly supported by the Global Center of Excellence Program, Japan Society for the Promotion of Science (to S.-y.K.) and grants from the Ministry of Education, Culture, Sports, Science, and Technology (to S.-i.A.), and it was performed under the approval of Photon Factory Program Advisory Committee (PF-PAC 2004S1-001).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3E4N, 3ECL, 3E55, 3ECX, 3EDA, 2ZSN, 2ZSO, 2ZSZ, 2ZT0, 2ZT1, 3E5I, 3ECZ, 3EDB, 2ZSP, 2ZSR, 2ZT2, 2ZT3, 2ZT4, 3E5O, 3ED9, 2ZSQ, 2ZSS, 2ZST, 2ZSX, and 2ZSY).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807774106/DCSupplemental.
References
- 1.Nasu K, et al. In: Photoinduced Phase Transition. Nasu K, editor. Singapore: World Scientific; 2004. pp. 1–342. [Google Scholar]
- 2.Koshihara S, Adachi S. Photo-induced phase transition in an electron-lattice correlated system: Future role of a time-resolved X-ray measurement for materials science. J Phys Soc Jpn. 2006;75 011005-1-10. [Google Scholar]
- 3.Fenimore PW, Frauenfelder H, McMahon BH, Parak FG. Slaving: Solvent fluctuations dominate protein dynamics and functions. Proc Natl Acad Sci USA. 2002;99:16047–16051. doi: 10.1073/pnas.212637899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin RH, Beeson KW, Eisenstein L, Frauenfelder H, Gunsalus IC. Dynamics of ligand binding to myoglobin. Biochemistry. 1975;14:5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- 5.Alben JO, et al. Infrared spectroscopy of photodissociated carbonmonoxymyoglobin at low temperatures. Proc Natl Acad Sci USA. 1982;79:3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry ER, Sommer JH, Hofrichter J, Eaton WA. Geminate recombination of carbon monoxide to myoglobin. J Mol Biol. 1983;166:443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- 7.Olson JS, Phillips GN., Jr Kinetic pathways and barriers for ligand binding to myoglobin. J Biol Chem. 1996;271:17593–17596. doi: 10.1074/jbc.271.30.17593. [DOI] [PubMed] [Google Scholar]
- 8.Scott EE, Gibson QH. Ligand migration in sperm whale myoglobin. Biochemistry. 1997;36:11909–11917. doi: 10.1021/bi970719s. [DOI] [PubMed] [Google Scholar]
- 9.Srajer V, et al. Photolysis of the carbon monoxide complex of myoglobin: Nanosecond time-resolved crystallography. Science. 1996;274:1726–1729. doi: 10.1126/science.274.5293.1726. [DOI] [PubMed] [Google Scholar]
- 10.Srajer V, et al. Protein conformational relaxation and ligand migration in myoglobin: A nanosecond to millisecond molecular movie from time-resolved Laue X-ray diffraction. Biochemistry. 2001;40:13802–13815. doi: 10.1021/bi010715u. [DOI] [PubMed] [Google Scholar]
- 11.Schotte F, et al. Watching a protein as it functions with 150-ps time-resolved X-ray crystallography. Science. 2003;300:1944–1947. doi: 10.1126/science.1078797. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeois D, et al. Complex landscape of protein structural dynamics unveiled by nanosecond Laue crystallography. Proc Natl Acad Sci USA. 2003;100:8704–8709. doi: 10.1073/pnas.1430900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schotte F, Soman J, Olson JS, Wulff M, Anfinrud PA. Picosecond time-resolved X-ray crystallography: Probing protein function in real time. J Struct Biol. 2004;147:235–246. doi: 10.1016/j.jsb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, et al. Ligand migration pathway and protein dynamics in myoglobin: A time-resolved crystallographic study on L29W MbCO. Proc Natl Acad Sci USA. 2005;102:11704–11709. doi: 10.1073/pnas.0504932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng T-Y, Srajer V, Moffat K. Photolysis-induced structural changes in single crystals of carbonmonoxymyoglobin at 40 K. Nat Struct Biol. 1994;1:701–705. doi: 10.1038/nsb1094-701. [DOI] [PubMed] [Google Scholar]
- 16.Schlichting I, Berendzen J, Phillips GN, Jr, Sweet RM. Crystal structure of photolysed myoglobin. Nature. 1994;371:808–812. doi: 10.1038/371808a0. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann H, et al. X-ray structure determination of a metastable state of carbonmonoxy myoglobin after photodissociation. Proc Natl Acad Sci USA. 1996;93:7013–7016. doi: 10.1073/pnas.93.14.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng T-Y, Srajer V, Moffat K. Initial trajectory of carbon monoxide after photodissociation from myoglobin at cryogenic temperatures. Biochemistry. 1997;36:12087–12100. doi: 10.1021/bi971140x. [DOI] [PubMed] [Google Scholar]
- 19.Vitkup D, Petsko GA, Karplus MA. A comparison between molecular dynamics and X-ray results for dissociated CO in myoglobin. Nat Struct Biol. 1997;4:202–208. doi: 10.1038/nsb0397-202. [DOI] [PubMed] [Google Scholar]
- 20.Chu K, et al. Structure of a ligand-binding intermediate in wild-type carbonmonoxy myoglobin. Nature. 2000;403:921–923. doi: 10.1038/35002641. [DOI] [PubMed] [Google Scholar]
- 21.Ostermann A, Waschipky R, Parak FG, Nienhaus GU. Ligand binding and conformational motions in myoglobin. Nature. 2000;404:205–208. doi: 10.1038/35004622. [DOI] [PubMed] [Google Scholar]
- 22.Adachi S, Park S-Y, Tame JRH, Shiro Y, Shibayama N. Direct observation of photolysis-induced tertiary structural changes in hemoglobin. Proc Natl Acad Sci USA. 2003;100:7039–7044. doi: 10.1073/pnas.1230629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilton RF, Jr, Kuntz ID, Jr, Petsko GA. Cavities in proteins: Structure of a metmyoglobin-xenon complex solved to 1.9 Å. Biochemistry. 1984;23:2849–2857. doi: 10.1021/bi00308a002. [DOI] [PubMed] [Google Scholar]
- 24.Case DA, Karplus M. Dynamics of ligand binding to heme proteins. J Mol Biol. 1979;132:343–368. doi: 10.1016/0022-2836(79)90265-1. [DOI] [PubMed] [Google Scholar]
- 25.Elber R, Karplus M. Multiple conformational states of proteins: A molecular dynamics analysis of myoglobin. Science. 1987;235:318–321. doi: 10.1126/science.3798113. [DOI] [PubMed] [Google Scholar]
- 26.Bossa C, et al. Extended molecular dynamics simulation of the carbon monoxide migration in sperm whale myoglobin. Biophys J. 2004;86:3855–3862. doi: 10.1529/biophysj.103.037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J, Arkhipov A, Braun R, Schulten K. Imaging the migration pathways for O2, CO, NO, and Xe inside myoglobin. Biophys J. 2006;91:1844–1857. doi: 10.1529/biophysj.106.085746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry ER, Eaton WA, Hochstrasser RM. Molecular dynamics simulations of cooling in laser-excited heme proteins. Proc Natl Acad Sci USA. 1986;83:8982–8986. doi: 10.1073/pnas.83.23.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamato T. Strain tensor field in proteins. J Mol Graphics. 1996;14:105–107. doi: 10.1016/0263-7855(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 30.Yamato T, Higo J, Seno Y, Go N. Conformational deformation in deoxymyoglobin by hydrostatic pressure. Proteins Struct Funct Genet. 1993;16:327–340. doi: 10.1002/prot.340160403. [DOI] [PubMed] [Google Scholar]
- 31.Sakai K, Matsui Y, Kouyama T, Shiro Y, Adachi S. Optical monitoring of freeze-trapped reaction intermediates in protein crystals: A microspectrophotometer for cryogenic protein crystallography. J Appl Crystallogr. 2002;35:270–273. [Google Scholar]
- 32.Nozawa S, et al. Developing 100-ps-resolved X-ray structural analysis capabilities on beamline NW14A at the Photon Factory Advanced Ring. J Synchrotron Rad. 2007;14:313–319. doi: 10.1107/S0909049507025496. [DOI] [PubMed] [Google Scholar]
- 33.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 34.Collaborative Computational Project 4. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 35.Delano WL. The PyMOL Molecular Graphics System. San Carlos, CA: Delano Scientific; 2002. [Google Scholar]
- 36.Dundas J, et al. CASTp: Computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acid Res. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.