Abstract
We document major declines of many species of salamanders at several sites in Central America and Mexico, with emphasis on the San Marcos region of Guatemala, one of the best studied and most diverse salamander communities in the Neotropics. Profound declines of several formerly abundant species, including 2 apparent extinctions, are revealed. Terrestrial microhabitat specialists at mid- to high elevations have declined more than microhabitat generalists. These terrestrial microhabitat specialists have largely disappeared from multiple sites in western Guatemala, including in well-protected areas, suggesting that the phenomenon cannot be explained solely by localized habitat destruction. Major declines in southern Mexican plethodontid salamanders occurred in the late 1970s to early 1980s, concurrent with or preceding many reported frog declines. The species in decline comprise several major evolutionary lineages of tropical salamanders, underscoring that significant portions of the phylogenetic diversity of Neotropical salamanders are at risk. Our results highlight the urgent need to document and understand Neotropical salamander declines as part of the larger effort to conserve global amphibian diversity.
Keywords: climate change, elevational transect, Guatemala, Mexico, Plethodontidae
It is estimated that one-third of all amphibian species worldwide are endangered or threatened with extinction (1). Efforts to understand the causes of this alarming decline, known as the global amphibian crisis, have focused primarily on frogs; comparatively little attention has been paid to salamanders (2–5). Reasons for this bias include the fact that most salamanders are secretive in nature, so population trends may not be apparent as in frogs. At well-studied sites in Central America where salamanders are present, they are often relatively uncommon compared with frogs (6). Nonetheless, given the precipitous declines and recently documented extinctions (2, 6, 7) in an array of tropical frog species, tropical salamanders demand attention as well.
The lungless salamanders (family Plethodontidae) include the only tropical salamanders, which have radiated dramatically in the Neotropics and now account for 40% of all salamanders (www.amphibiaweb.org). Nuclear Central America (between the Isthmus of Tehuantepec and the Nicaraguan lowlands) and southern Mexico contain a high diversity of plethodontid species (8). The salamander fauna has long been of interest to herpetologists; past studies and associated museum collections provide a rich historical database of abundance and distribution. One of the richest datasets available for tropical salamanders comes from the studies of Wake and colleagues (9, 10), who sampled along an elevation gradient on the southern slopes of Volcán Tajumulco in the Department of San Marcos, Guatemala. This dataset provides the best opportunity to examine changes in salamander populations over time. Here, we assess salamander population status by using historical (1970s) and recent (2005–2007) survey data from sites in Guatemala and Mexico across multiple elevations. Our sampling focuses on high elevation and cloud forest communities, which harbor the highest diversity of salamander species (9, 10). Our data include a broad phylogenetic sample of the Middle American salamanders across a wide geographic area (Fig. 1). According to this dataset, salamander species exhibit differences in elevational distribution, habitat, and microhabitat preference, allowing us to identify factors associated with declines or changes in population status and differentiate among hypotheses to explain these changes.
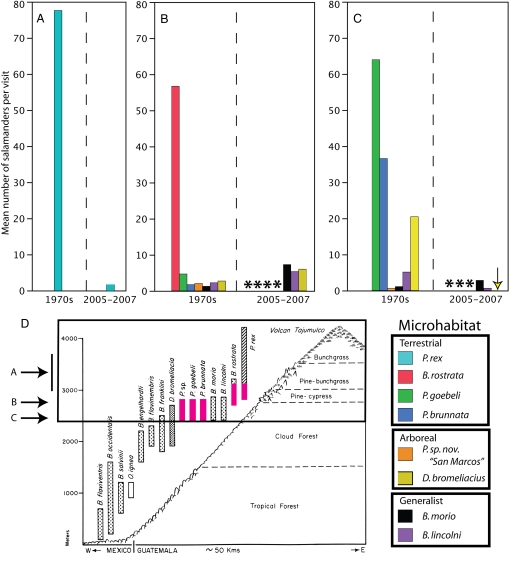
Fig. 1.
Survey sites in Guatemala and Mexico. San Marcos elevational gradient: A) Upper slopes of Volcán Tajumulco, B) El Rincon, C) Buena Vista, D) Finca Insula and E) Finca Santa Julia, F) Volcán Chicabal. Guatemalan sites: 1) High point on Panamerican Highway; 2) Cerro Tecpan; 3) Rancho de Tejo; 4) Sierra de los Cuchumatanes. Mexican sites: 5) Cerro Mozotal; 6) San Cristobal de las Casas area; 7) Cerro San Felipe; 8) Puerto del Aire; 9) Cerro Chicahuaxtla; 10) Parque Nacional El Chico. Volcán Tajumulco is visible near the center of the Landsat image (Inset); the Guatemalan Plateau is at the upper right, and the Pacific coastal plains are in the lower left.
Results
Dramatic declines in abundance were seen for most species from the upper cloud forest and high elevation salamander assemblages on the San Marcos transect (Table 1). Two of the most common salamander species in the 1970s, Pseudoeurycea brunnata and Pseudoeurycea goebeli (10), were not found on any of our recent visits (see Methods) at either of the 2 sites where they previously occurred in great abundance (Fig. 2). The third, undescribed species of Pseudoeurycea (P. sp. nov.“San Marcos”) was also not found on any of our recent surveys, despite being found on 15 of 25 visits to the El Rincon site in the 1970s. At El Rincon, Bolitoglossa rostrata was found on 22 of 25 visits in the past, generally in high abundance (mean = 64.4 salamanders per visit, n = 22 visits), but not a single specimen was seen on our recent surveys. This site represents the lower elevational limit of B. rostrata, and its absence provides evidence of an elevational range contraction on the transect. The species found at the highest elevations on the transect, Pseudoeurycea rex, was only found on 2 of our 5 visits, at a significantly lower encounter rate [0.58 salamanders found per person-hour vs. 8.92 in the 1970s (Student's t test: t = −8.338, P = 0.023)] and lower abundance (mean = 1.8 individuals per visit vs. 77.7 in the past). The species was absent on our 2 surveys at a site in the high elevation zone where 174 specimens were found on a single visit in 1972. Three of the 4 species of Pseudoeurycea found on the upper part of the San Marcos transect (P. rex, P. brunnata, and P. goebeli) were also known from nearby Volcán Chicabal. Despite 2 intensive searches in 2006 and 2007, we found only a single specimen of P. goebeli and no P. rex or P. brunnata. This finding represents a precipitous decline in encounter rate for all 3 of these species (Table 1 and Fig. 2), and raises the possibility that P. brunnata may be extinct.
Table 1.
Encounter rate (salamanders per person per hour) for selected species from San Marcos upper cloud forest sites
| Site | Time period | No. of visits | P. rex | P. brunnata | P. goebeli | P. sp. nov San Marcos | B. rostrata | B. lincolni | B. morio | D. bromeliacius |
|---|---|---|---|---|---|---|---|---|---|---|
| El Rincon | 1970s | 25 | ** | 0.18 (13/25) | 0.43 (13/25) | 0.17 (15/25) | 4.87 (22/25) | 0.20 (12/25) | 0.12 (13/25) | 0.23 (5/25) |
| (2,700 m) | 2005–2007 | 3 | ** | 0 (0/3) | 0 (0/3) | 0 (0/3) | 0 (0/3) | 0.28 (3/3) | 0.47 (3/3) | 0.52 (2/3) |
| Buena Vista | 1970s | 36 (27) | ** | 1.77 (23/27) | 1.51 (21/27) | 0.02 (15) | ** | 0.45 (17/36) | 0.07 (12/36) | 1.30 (26/36) |
| (2,400 m) | 2005–2007 | 8 (5) | ** | 0 (0/5) | 0 (0/5) | 0 (0/5) | ** | 0.0625 (3/8) | 0.92 (6/8) | 0.007 (1/8) |
| Volcán Chicabal (2,700 m) | 1970s | 4 | 1.15 (3/4) | 1.69 (3/4) | 3.07 (4/4) | ** | ** | ** | ** | ** |
| (2,700 m) | 2005–2007 | 2 | 0 (0/2) | 0 (0/2) | 0.04 (1/2) | ** | ** | ** | ** | ** |
** indicates that a species did not occur at a site or was not considered at that site for analysis. Number of visits encountered per total visits is shown in parentheses.
Fig. 2.
Mean number of salamanders per visit in the 1970s and from 2005 to 2007 at upper cloud forest and high-elevation sites on the San Marcos transect and microhabitat associations of species. (A) Slopes of Volcán Tajumulco. (B) El Rincon. (C) Buena Vista. Asterisk indicates that species was not detected in time period. Arrow in C indicates bar for D. bromeliacius (mean = 0.017 salamanders per visit). Microhabitat classifications of upper cloud forest and high-elevation species from ref. 10 are shown at bottom right. (D) Location of 3 sites on San Marcos transect, with elevational extirpations shown in pink. Upper cloud forest and high-elevation sites are outlined in black, with arrows indicating elevation of sites A–C. Transect diagram has been adapted from ref. 9.
Surveys of other sites in the region reveal that the decline in salamander abundance seen for upland species is not unique to San Marcos. Either no P. rex or only a single individual was found at other sites in western Guatemala and Chiapas (Table S1). Populations of this species were denser (usually 10 to >100 salamanders per visit) than any other salamander in Guatemala in the past (11), and large areas of suitable habitat still exist. Although B. rostrata remains fairly common in some areas, we did not find it at several sites (Table S1) where it was common in the 1970s (210 collected in 6 visits at the high point on the Panamerican Highway, contrasted with none on 3 recent visits).
Two species from the San Marcos upper cloud forest assemblage displayed no evidence of population declines. Bolitoglossa morio was found on each of our 3 recent visits to El Rincon vs. about half of the visits in the 1970s and was also found significantly more often than in the past at Buena Vista (Wilcoxon test: Z = 3.0228, P = 0.0025). Bolitoglossa lincolni was also found on all recent visits to El Rincon vs. approximately half of the visits in the 1970s, and the species displayed similar abundance at Buena Vista compared with past surveys (Wilcoxon test: Z = −0.8645, P = 0.387) (Table 1). In addition to being more abundant than in the past at San Marcos, B. morio was collected above its previous elevational limit at the high point on the Panamerican Highway, where only B. rostrata and P. rex occurred previously. Although Dendrotriton bromeliacius was not found at Finca Insula in 2005–2007, it was found at both El Rincon and Buena Vista. All 4 species occupying the lower cloud forest at San Marcos were found less often on recent visits and in much lower abundance than in the 1970s (Table 1), although the limited amount of search time on recent surveys makes it difficult to draw strong conclusions about population status. At the lowest elevation site, Finca Santa Julia, Bolitoglossa occidentalis was found on 1 of 2 visits with relatively little search effort.
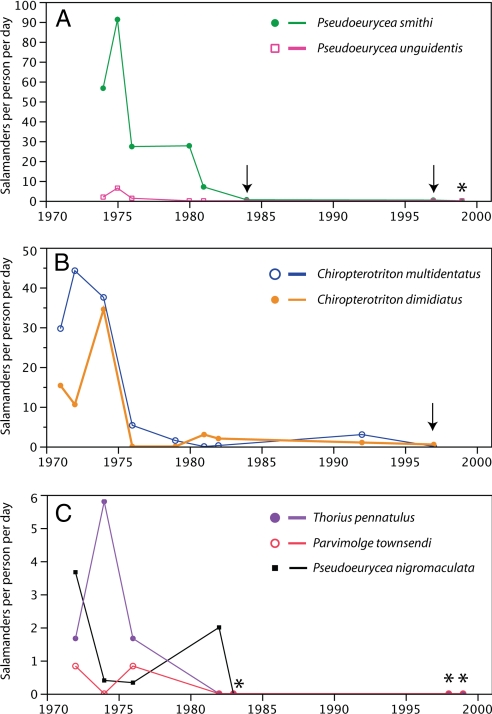
The 4 sites in Mexico north of the Isthmus of Tehuantepec demonstrate similar patterns to the declines in Guatemala, despite having no species in common. Both high-elevation species of Pseudoeurycea from Cerro San Felipe, Oaxaca, show steep declines in density beginning in the early 1980s, and neither has been seen at the site in 10 years despite recent search efforts (Fig. 3). The same trend is evident at El Chico National Park, where a sharp decline in encounter rate of 2 species of Chiropterotriton, C. multidentatus and C. dimidiatus, occurred between 1974 and 1976 (Fig. 3). On Cerro Chicahuaxtla, surveys in the 1980s found decreasing numbers of salamanders, and no salamanders at all were encountered in 8 person-days of fieldwork in 1999. The number of Thorius found at Puerto del Aire declined dramatically between 1977 and 1982, and few or no salamanders were found on subsequent visits through 2001, a pattern consistent with the other sites (Table S2). These results suggest a decline in abundance of salamanders at this site in the late 1970s to early 1980s.
Fig. 3.
Salamander encounter rate of selected species at 3 sites in Mexico north of the Isthmus of Tehuantepec. (A) Cerro San Felipe, Oaxaca. (B) Parque Nacional El Chico, Hidalgo. (C) Cerro Chicahuaxtla, Veracruz. All sites show a major decline in salamander abundance between the late 1970s and mid-1980s, with very few (if any) salamanders found in recent years. Arrows indicate visits with nonzero encounter rates. Asterisks indicate visits when no salamander of any species was found.
Discussion
The results of our surveys indicate a collapse of the upper cloud forest and high elevation salamander assemblages at most sites and demonstrate changes in abundance at most sites surveyed. At our focal survey sites in San Marcos, the complete disappearance of 3 species of Pseudoeurycea from the upper cloud forest is especially striking, given the remarkable abundance of both P. brunnata and P. goebeli in the past. P. goebeli, P. brunnata, P. rex, and B. rostrata are terrestrial microhabitat specialists (10) that make little or no use of arboreal bromeliads. B. morio and B. lincolni, which were encountered with equal or greater frequency than in the past, are microhabitat generalists, found not only in terrestrial habitats but also in bromeliads, logs, and under bark (10). D. bromeliacius, an arboreal bromeliad specialist, persists Buena Vista and El Rincon, where appropriate forest habitat still exists. Thus, there appears to be a strong correspondence between terrestrial microhabitat preference and declines in encounter rate. This pattern holds when considering the Mexican salamander communities; the mid- to high elevation species of Pseudoeurycea, Chiropterotriton, and Thorius showing major declines are all terrestrial microhabitat specialists. The continued presence of 3 species at Finca Insula and the abundance of B. occidentalis at lower elevations suggest that there is an elevational pattern to the declines as well, with the most severely affected species inhabiting the highest 2 elevational zones. The lower-elevation species of Bolitoglossa also make use of arboreal microhabitats (e.g., bromeliads and leaf axils), much like B. morio and B. lincolni (10).
The decline and disappearance of Pseudoeurycea in the upper cloud forest of San Marcos cannot be attributed to habitat loss alone. Both P. goebeli and P. brunnata were found in disturbed areas near forest in the past, and some cloud forest remains on the San Marcos transect. Similarly, Volcán Chicabal is within a protected reserve and contains primary cloud forest where we surveyed. The marked decrease in encounter rate of P. rex and B. rostrata at many sites throughout the western highlands of Guatemala and adjacent areas of Chiapas suggests that the factors causing these changes in populations are regional in scope. At least some suitable habitat remains at all of the sites, and at the sites in southern Mexico, but salamanders are absent or occur at dramatically lower densities than in the past.
In addition to an association between high elevation, terrestrial microhabitat preference and population declines, there appears to be a phylogenetic pattern. Of the salamanders on the San Marcos transect, all of the Bolitoglossa involved in our analysis except B. occidentalis are part of the subgenus Magnadigita (12); we found conclusive evidence for the decline of only one of these species (B. rostrata). All of the Pseudoeurycea from San Marcos are close relatives (13, 14). At the broader regional scale, several major clades (Pseudoeurycea, Thorius, Chiropterotriton) seem to be most severely affected (Fig. S1). This strong phylogenetic clustering of decline indicates that large portions of the evolutionary history of the tropical salamanders are at risk of being lost. Furthermore, the recently released results of the 2008 Global Amphibian Assessment (15) show that, in addition to the declines that we report here, a high percentage of species of tropical salamanders is threatened with extinction (Fig. S1); indeed, more than half of the species in all but 1 genus (Oedipina) are in the 3 highest International Union for Conservation of Nature threat categories.
We cannot determine the exact timing of salamander population declines in San Marcos from our dataset, given the sampling gap between 1979 and 2005 caused by political conditions in Guatemala. The Mexican sites, however, have more continuous sampling through the early 1980s and provide a better estimate of the time of decline. Salamander declines at these 4 sites seem to have occurred between the mid-1970s and early 1980s (Fig. 3). At Puerto del Aire, Thorius were consistently found in moderate to high numbers until 1977 (Table S2). These declines appear to have taken place before or at the same time as many of the reported frog population declines in Central America (2, 6, 7)
The fungal disease chytridiomycosis, caused by the pathogen Batrachochytrium dendrobatidis (Bd) (16), has been implicated in the decline of multiple species of Middle American amphibians (4, 17). Analysis of skin swabs from adult salamanders collected in 2006 and 2007 found only 7 of 62 individual salamanders to be positive for Bd (Table S3). Four of the 7 positive salamanders from the San Marcos transect were from the abundant low-elevation species B. occidentalis; the other 3 were from upper-cloud forest species that show no declines in abundance. The negative samples include some of the upper-cloud forest and high-elevation species that have undergone major declines (P. rex, B. rostrata). No direct evidence currently links Guatemalan salamander declines to Bd, although its importance in other Neotropical amphibian declines suggests that it may potentially have played a role.
The most drastic declines in encounter rate of salamanders on the San Marcos transect occurred in terrestrial species, which do not use arboreal bromeliads as refuges. These species would be expected to respond more directly to changes in climate, particularly changes in humidity and precipitation, because they lack the moist, buffered microenvironment that bromeliads provide. Cloud water provides an important source of moisture in tropical montane cloud forests during the dry season (18, 19), when salamanders would be expected to be under the highest physiological stress; most cloud forest habitats contain little or no standing water, and plethodontid salamanders depend on adequate moisture for surface activity because of their permeable skins (20). Lawton et al. (21) and Nair et al. (22) showed that deforestation in lowland and premontane regions leads to an elevational increase of the cloud base, and Ray et al. (23) used a modeling approach to demonstrate that deforestation in the lowlands of Costa Rica has led to an increase in mist-free conditions in the cloud forest at Monteverde. The Pacific lowlands and premontane areas of Guatemala have been extensively deforested; although the Pacific plain deforestation occurred before the salamander sampling in the 1970s, much of the premontane forest disappeared during and after this time (24). The results of the studies cited above indicate that lowland and premontane deforestation could have had a substantial impact on moisture conditions in the areas where the greatest declines occurred. Pounds et al. (25) used modeling to show that large-scale warming led to a greater decrease in relative humidity at Monteverde compared to that caused by deforestation. Species of cloud forest salamanders that can still be found rely at least in part on bromeliads. Bromeliads depend on cloud water deposition and are predicted to be particularly vulnerable to climate change (26, 27). Therefore, if climate change is in part responsible for the declines we observed, arboreal salamander species that are presently not in decline may soon suffer the same fate as the fully terrestrial species.
The results of this study point to widespread and severe declines of upland salamanders at multiple sites in Guatemala and Mexico, including the most intensively-studied salamander transect in the neotropics. Although the causes of these declines are not yet well understood, the drastic reductions in salamander numbers and changes in community composition in this region indicate that the salamander populations of many upland species are in need of protection. Until the forces causing these declines are identified, however, an effective conservation strategy cannot be devised. Protecting habitat, although important, is insufficient to conserve populations of many of these species. Furthermore, other recent studies have also provided evidence of declining salamander populations in the neotropics (4, 5). The global amphibian crisis, usually discussed in terms of frogs, clearly involves Middle American salamanders as well.
Methods
Most salamanders analyzed in this study were collected as voucher specimens and deposited in the Museum of Vertebrate Zoology (MVZ). Detailed field notes and specimen catalogues were recorded and are stored at the MVZ, including data on numbers of salamanders seen but not collected. We made 3 visits to the San Marcos transect in November 2005, September 2006, and August 2007 during the wet season when salamanders, especially terrestrial species, were found in large numbers in the past. We revisited 5 of the same sites along the San Marcos transect that were worked by D. Wake and colleagues in the 1970s, summarized in ref. 10 (Fig. 1 and Table S4). In addition to the San Marcos sites, we surveyed a nearby Volcáno with primary upper cloud forest in a protected reserve, Volcán Chicabal, in an attempt to distinguish between habitat alteration and other potential reasons for decline. Several of the same collectors who worked in the 1970s (D.B.W., T.J.P., locals) were involved in the more recent surveys, allowing us to visit the exact sites that were surveyed in the past and to use similar methods.
We collected all salamanders seen and quantified collecting effort by recording the number of collectors and search time. Each of the 5 sites was visited at least twice, and efforts were made to search at each site under favorable weather conditions to maximize the chance of finding salamanders. We attempted to survey the best habitat available, which included areas of primary forest, well-established secondary forest with extensive epiphyte growth, and more disturbed areas such as road banks and cleared areas.
We used the field notes of the collectors who worked most extensively on the San Marcos transect in the 1970s (D.B.W., J. Lynch, T.J.P., and L. Houck) and several other MVZ collectors to quantify the encounter rate of salamanders at the 5 focal sites on the transect (see SI Text for a detailed description of methods for field note data). Catalogues and field journal entries were used to record the number of salamanders of each species collected or observed during each collecting event. The search time and number of collectors were also recorded in the journal entries in most cases. Collecting events were assigned to the 5 focal sites (listed in Table S4), and only species known to be present at a site based on the detailed surveys of the 1970s were considered at each site (Table S4). For each collecting event, the number of individuals found was divided by the number of people searching and the number of hours spent searching to give a measure of salamanders encountered per person per hour for each species. The mean encounter rate and proportion of visits on which a species was found were calculated for each one of the sites. The same procedure was repeated for the 2005–2007 visits.
Although our systematic survey efforts concentrated on the San Marcos area, records were also kept of species detection in other areas of western Guatemala and Chiapas, Mexico that share species in common with San Marcos (Fig. 1 and Table S1). P. rex, B. rostrata, and P. goebeli were found at these sites in the 1970s. Adult salamanders collected in 2006 and 2007 were swabbed and tested for B. dendrobatidis by using the real-time PCR assay described by Boyle et al. (28).
To compare population trends in a broader geographic and phylogenetic scope, data from 4 sites in Mexico north of the Isthmus of Tehuantepec were analyzed: Cerro San Felipe in Oaxaca, Parque Nacional El Chico in Hidalgo, Cerro Chicahuaxtla in Veracruz, and Puerto del Aire on the Puebla/Veracruz border (Fig. 1). These sites, similar in elevation, habitat type, species diversity, and patterns of elevational zonation (10) to the Guatemalan sites, provide independent communities in which to analyze salamander population trends. In addition to Pseudoeurycea, they contain salamanders of the genera Thorius and Chiropterotriton not found in Guatemala. The 4 sites were visited on multiple occasions by researchers (D.B.W., T.J.P., J. Lynch, and G.P.-O.) who kept detailed field notes. For the first 3 sites, the number of salamanders of each species found was recorded and totaled by year, and the number of collectors and field days were also recorded. Encounter rate (salamanders per person per day) for each species was estimated by dividing the number of salamanders found each year by the number of people searching and the number of days spent searching. For the Puerto del Aire site, the number of Thorius found per visit was obtained from 5 museums (American Museum of Natural History, Natural History Museum of Los Angeles County, MVZ, National Museum of Natural History, University of Michigan Museum of Zoology) dating back to 1970, with no quantification of collection effort. Although 3 species are present at that site (Thorius dubitus, Thorius magnipes, Thorius troglodytes), identification to species level was not always possible, so only the total number was used. All 3 of these species use terrestrial microhabitats, and 2 (T. dubitus and T. troglodytes) are exclusively terrestrial (29).
Supplementary Material
Acknowledgments.
We thank C. Moritz, M. H. Wake, J. Patton, V. Vredenburg, T. Devitt, M. Fujita, G. Goldsmith, S. Schoville, S. Singhal, and C. Moritz's and D.B.W.'s laboratory groups for comments and discussion; A. Muñoz Alonso and E. Recuero for valuable assistance in the field; El Consejo Nacional de Areas Protegidas for providing Guatemalan research and collecting permits; R. Perez for logistical assistance and permission to do fieldwork at Finca Insula; L. D. Houck and J. F. Lynch, whose work and detailed field notes from San Marcos made this study possible; and L. D. Houck and E. D. Brodie, Jr. for reviewing the manuscript. S.M.R. was supported by a National Science Foundation Graduate Research Fellowship and a Berkeley graduate fellowship, and G.P.O. was supported by a University of California Institute for Mexico and the United States visiting scholar fellowship. Secretaria de Educacion Publica/Consejo Nacional de Ciencia y Tecnologia No. 50563 and Programa de Apoyo a Proyectos de Investigacion e Innovacion Tecnologica/Universidad Nacional Autonoma de Mexico IN211808. Fieldwork was supported by University of California Institute for Mexico and the United States Research Grant 022043, the AmphibiaTree Project (National Science Foundation Grant EF-0334939) and the Museum of Vertebrate Zoology. Funding for analysis of swabs for chytrid fungus was provided by George Rabb and National Science Foundation Grant DEB-0139273.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813051106/DCSupplemental.
References
- 1.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 2.Lips KR. Decline of a tropical montane amphibian fauna. Conserv Biol. 1998;12:106–117. [Google Scholar]
- 3.Parra-Olea G, García-París M, Wake DB. Status of some populations of Mexican salamanders (Amphibia: Plethodontidae) Rev Biol Trop. 1999;47:217–223. [Google Scholar]
- 4.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitfield SM, et al. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proc Natl Acad Sci USA. 2007;104:8352–8356. doi: 10.1073/pnas.0611256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young BE, et al. Population declines and priorities for amphibian conservation in Latin America. Conserv Biol. 2001;15:1213–1223. [Google Scholar]
- 7.Pounds JA, Crump ML. Amphibian declines and climatic disturbance: The case of the Golden Toad and the Harlequin Frog. Conserv Biol. 1994;8:72–85. [Google Scholar]
- 8.Wake DB. Adaptive radiation of salamanders in Middle American cloud forests. Ann Mo Bot Gard. 1987;74:242–264. [Google Scholar]
- 9.Wake DB, Papenfuss TJ, Lynch JF. Distribution of salamanders along elevational transects in Mexico and Guatemala. Tulane Publ Zool Bot Suppl Publ. 1992;1:303–319. [Google Scholar]
- 10.Wake DB, Lynch JF. The distribution, ecology, and evolutionary history of plethodontid salamanders in tropical America. Sci Bull Mus Nat Hist Los Angeles Co. 1976;25:1–65. [Google Scholar]
- 11.Elias P. Salamanders of the northwestern highlands of Guatemala. Contrib Sci Nat Hist Mus Los Angeles Co. 1984;348:1–20. [Google Scholar]
- 12.Parra-Olea G, García-París M, Wake DB. Molecular diversification of salamanders of the tropical American genus Bolitoglossa (Caudata: Plethodontidae) and its evolutionary and biogeographic implications. Biol J Linn Soc. 2004;81:325–346. [Google Scholar]
- 13.Parra-Olea G. Molecular phylogenetic relationships of neotropical salamanders of the genus Pseudoeurycea. Mol Phylogenet Evol. 2002;22:234–246. doi: 10.1006/mpev.2001.1048. [DOI] [PubMed] [Google Scholar]
- 14.Wiens JJ, Parra-Olea G, García-París M, Wake DB. Phylogenetic history underlies elevational diversity patterns in tropical salamanders. Proc R Soc London Ser B. 2007;274:919–928. doi: 10.1098/rspb.2006.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Union for Conservation of Nature and Natural Resources, Conservation International, and NatureServe. [Accessed November 14, 2008];Global Amphibian Assessment. 2006 Available at www.globalamphibians.org.
- 16.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- 17.Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holder CD. The hydrological significance of cloud forests in the Sierra de las Minas Biosphere Reserve, Guatemala. Geoforum. 2006;37:82–93. [Google Scholar]
- 19.Bruijnzeel LA. Hydrology of tropical montane cloud forests: A reassessment. Land Use Water Resour Res. 2001;1:1–18. [Google Scholar]
- 20.Welsh HH, Droege S. A case for using plethodontid salamanders for monitoring biodiversity and ecosystem integrity of North American forests. Conserv Biol. 2001;15:558–569. [Google Scholar]
- 21.Lawton RO, Nair US, Pielke RA, Welch RM. Climatic impact of tropical lowland deforestation on nearby montane cloud forests. Science. 2001;294:584–587. doi: 10.1126/science.1062459. [DOI] [PubMed] [Google Scholar]
- 22.Nair US, Lawton RO, Welch RM, Pielke RA. Impact of land use on Costa Rican tropical montane cloud forests: Sensitivity of cumulus cloud field characteristics to lowland deforestation. J Geophys Res D. 2003 10.1029/2001JD001135. [Google Scholar]
- 23.Ray DK, Nair US, Lawton RO, Welch RM, Pielke RA. Impact of land use on Costa Rican tropical montane cloud forests: Sensitivity of orographic cloud formation to deforestation in the plains. J Geophys Res D. 2006 111:10.1029/2005JD006096. [Google Scholar]
- 24.Leonard HJ. Natural Resources and Economic Development in Central America: A Regional Environmental Profile. New Brunswick, NJ: Transaction Books; 1987. pp. 117–127. [Google Scholar]
- 25.Pounds AL, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 26.Benzing DH. Vulnerabilities of tropical forests to climate change: The significance of resident epiphytes. Clim Change. 1998;39:519–540. [Google Scholar]
- 27.Nadkarni NM, Solano R. Potential effects of climate change on canopy communities in a tropical cloud forest: An experimental approah. Oecologia. 2002;131:580–586. doi: 10.1007/s00442-002-0899-3. [DOI] [PubMed] [Google Scholar]
- 28.Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 29.Hanken J, Wake DB. Biology of tiny animals: Systematics of the minute salamanders (Thorius: Plethodontidae) from Veracruz and Puebla, Mexico, with descriptions of five new species. Copeia. 1998;1998:312–345. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.