Abstract
Organotypic cultures of mouse and rat magnocellular neurons (MCNs) in the hypothalamo-neurohypophysial system (HNS) have served as important experimental models for the molecular and physiological study of this neuronal phenotype. However, it has been difficult to maintain significant numbers of the MCNs, particularly vasopressin MCNs, in these cultures for long periods. In this paper, we describe the use of the neurotrophic factors, leukemia inhibiting factor (LIF) and ciliary neurotrophic factor (CNTF) to rescue rat vasopressin (Avp)- and oxytocin (Oxt) – MCNs from axotomy-induced, programmed cell death in vitro. Quantitative data are presented for the efficacy of the LIF family of neurotrophic factors on the survival of MCNs in three nuclei, the paraventricular (PVN), supraoptic (SON), and accessory (ACC) nuclei in the mouse and rat hypothalamus.
Keywords: hypothalamus, neurotrophic, organotypic, CNTF, LIF, magnocellular neurons, oxytocin, vasopressin
INTRODUCTION
Organotypic cultures of specific regions of the rat brain have served as important models for the study of central nervous system functions and metabolism (Gahwiler, 1981; Gahwiler et al., 1997; Gahwiler and Hefti, 1985). These cultures have the virtues of being derived from postnatal animals, and therefore in most cases fully differentiated neurons, for long-term culture. Neurons in organotypic culture also maintain more appropriate cell-to-cell (e.g., glial-neuronal, and interneuronal) associations than in other in vitro models. However, an important caveat about organotypic cultures to keep in mind is that during preparation of the slices for culture the long afferent fibers which normally innervate the neurons in vivo are often cut in the slice-explants. This often leads to the formation of abnormal synaptic connections during the long culture periods. Nevertheless, despite the latter drawback, organotypic cultures provide the best experimental models to study the physiological and molecular properties of a wide variety of specific neuronal phenotypes. Among these phenotypes are neurons found in the hippocampus (Bergold and Casaccia-Bonnefil, 1997; Bruce et al., 1995; Gahwiler and Hefti, 1985), cortex (Baratta et al., 1996; Dammerman et al., 2000; Snyder-Keller, 2004), cerebellum (Birgbauer et al., 2004; Davids et al., 2002; Dupont et al., 2006), spinal cord (Bonnici and Kapfhammer, 2008; Eustache and Gueritaud, 1995; Li et al., 2008), brain stem (Rusnak and Gainer, 2005), hypothalamus (House et al., 1998; Israel et al., 2008; Shimizu et al., 2008; Wray et al., 1988), Mesencephalon-(Franke et al., 2003; Holmes et al., 1995; Larsen et al., 2008; Lyng et al., 2007; Plenz and Kitai, 1998), retina (Kaempf et al., 2008) and cochlear nucleus (Kesser et al., 2007; Khan et al., 2007; Qi et al., 2008). One important technical advance that has made the organotypic culture method accessible to many laboratories has been the development of the stationary explant (Stoppini et al., 1991)as an alternative to the roller-tube approach which was originally developed by Gahwiler and co-workers (Gahwiler, 1981; Gahwiler et al., 1997).
Hypothalamic organotypic cultures have been used for many types of neurobiological studies. These range from electrophysiological analyses of bursting neurons (Gahwiler and Dreifuss, 1979; Israel et al., 2008), metabolic studies of hypophysiotrophic CRH neurons (Arima et al., 2001; Bali et al., 2008; Bertini et al., 1993), and deletion analyses of oxytocin and vasopressin gene promoters (Fields et al., 2003). Certain neuronal systems in the hypothalamus such as LHRH neurons in the OVLT-MPOA region (Wray et al., 1988)and the suprachiasmatic nuclei (SCN) (Belenky et al., 1996)survive exceptionally well in organotypic cultures. As a result, the SCN in organotypic culture has been an outstanding and reliable model for the study of the generation of circadian rhythms in this nucleus (Maywood et al., 2007; Maywood et al., 2006; O’Neill et al., 2008; Rusnak et al., 2007; Tominaga et al., 1994). For other neuronal phenotypes, however, such as the magnocellular neurons (MCNs) in the hypothalamo-neurohypophysial system (HNS) it has been found that while the topography of the MCNs is maintained in organotypic culture, the survival of these neurons particularly the Avp-MCNs, the PVN and SON is very poor and highly variable (House et al., 1998; Wray et al., 1991). In some studies, this has been an advantage. For studies of Avp gene expression in parvocellular CRH neurons in the PVN, the relative absence of Avp-MCNs in this nucleus allowed for an analysis of the regulation of Avp-gene expression in the CRH neurons by cAMP (Arima et al., 2001). Similarly, the relative paucity of the Avp-MCNs in the organotypic cultures facilitated the study of the electrophysiology of identified Oxt-MCNs (Israel et al., 2008; Jourdain et al., 1998).
The vulnerability of the MCNs to axotomy-induced programmed cell death that occurs in organotypic cultures is analogous to the extensive retrograde degeneration of these neurons that occur in vivo after axonal damage (Alonso et al., 1996; Dohanics et al., 1992; Hare, 1937; Shahar et al., 2004). This led to the idea that this vulnerability of the MCNs is related to the loss of retrograde trophic factors from the posterior pituitary after axonal injury. Vitskits and colleagues were the first to systematically examine this issue, and they reported that members of the LIF family of neurotrophic factors (e.g., CNTF and LIF) could rescue Avp-MCNs in PVNs in organotypic culture from programmed cell death (presumed to be due to apoptosis) (Vutskits et al., 1998). Subsequent studies showed that both Oxt-and Avp-MCNs in the SON in organotypic culture could also be rescued from cell death by CNTF (Rusnak et al., 2002; Rusnak et al., 2003). Since the application of Bc1-XL and caspase inhibition was also found to increase the survival of the Oxt-and Avp-MCNs in the SON in organotypic culture (House et al., 2006) this lent further support for the proposal that the mechanism of axotomy-induced cell death was apoptosis (Vutskits et al., 1998). CNTF and the CNTF receptor have found to be present in the HNS system in vivo (Lo et al., 2008; Watt et al., 2006; Watt, et al, 2008) consistent with the view that this neurotrophic factor normally plays a role to sustain the MCNs in vivo.
To date, studies of the impact of the LIF family of neurotrophic factors on MCN survival have been restricted to either the rat PVN or SON in organotypic culture. In this paper, we describe the effects of both CNTF and LIF on the rescue of the MCNs in all three of the principal nuclei that constitute the rat HNS, the PVN, SON and the ACC, and different responses of the neurons in these nuclei to the neurotrophic factors were found. In addition, we compare the responses of the MCNs in rat versus mouse organotypic cultures, and report here significant differences between these rodent species.
MATERIALS AND METHODS
Slice-explant culture procedures
Sprague-Dawley pups, 6–7 days old (Charles River Laboratories, Wilmington, MA) and 7–10 day old Swiss-Webster mouse pups (Charles River, Wilmington, MA) were decapitated, and their brains were quickly removed. All procedures were carried out in accordance with the NIH guidelines on the Care and Use of Animal study protocol approved by the NINDS Animal Care and Use Committee at NIH. Organotypic hypothalamic cultures were made as previously described (House et al., 1998). Briefly, hypothalamic tissue blocks were sectioned (at 300 μm for mice, and 350 μm for rats) on a McIlwain tissue slicer. In rats four (in mice five) coronal slices containing the specific areas to be investigated, were separated in 4°C Gey’s Balanced Salt Solution (Gibco, Grand Island, NY, USA) enriched with glucose (5 mg/ml). Selected sections were trimmed dorsally above the top of the third ventricle and laterally from SON and placed on Millicell-CM filter inserts (pore size 0.4 μm, diameter 30 mm, Millipore Products Division, Bedford, MA). Each filter insert was placed in a Petri dish (35 mm), containing 1.2 ml of culture medium wetting the exposed surfaces of the explants, but not submerging them. Incubation of the cultures was stationary in 5% CO2 enriched air at 35°C for rat hypothalamic sections, whereas mouse slices were cultured at 32°C. These temperatures were found to be optimal for the particular type of culture in our experimental model. Cultures were routinely maintained for 14 days in serum containing medium (SCM in the presence or absence of either rat CNTF (10ng/ml; Sigma-Aldrich, St Louis, MO, USA) or mouse LIF (10ng/ml; Sigma-Aldrich, St Louis, MO, USA). Medium was replaced 3 times a week.
Media and Incubations
The serum containing medium (SCM) was composed of Eagle’s Basal Medium with Earle’s salts (50 %), heat inactivated horse serum (25%), Hanks balanced salt solution (25%), glucose (0.5%), 1mM glutamine, and penicillin/streptomycin 25 units/ml. The osmotic pressure of the medium was 314 mOsm/l. All ingredients the media were obtained from Gibco, BRL (Grand Island, N.Y. USA). Osmolarity of all media was measured by using a vapor pressure osmometer (Wescor, Buffalo, N.Y.). The explants were cultured for 14 days in SCM. In these experiments, slices were cultured either with CNTF or LIF as well as without any of these neurotrophic factors.
Immunohistochemistry
After culturing, the explants on the filters were fixed in 4 % formaldehyde in PBS for 1hour; rinsed 3 × 10 min in PBS, and then placed into cryoprotectant medium (Watson et al., 1986) where they were stored at 4°C until used for immunohistochemistry. For immunostaining, filters containing the fixed slices were excised from the inserts using a scalpel and then placed in Netwell carriers (Costar, Mass). Filters were then thoroughly rinsed in PBS, and blocked in 10 % normal goat serum (NGS)/0.6 % triton X-100, for 1.5 hours at room temperature to prevent non-specific binding.
For peroxidase-DAB immunohistochemistry, the filters were incubated in primary antiserum overnight at 4 ° C. The polyclonal antibody against Avp-neurophysin, THR (prepared by Dr. Alan Robinson, UCLA School of Medicine, and supplied by the Pituitary Hormones and Antisera Center, Torrance, California) was used at dilution of 1: 20,000, overnight at 4°C, and a mouse monoclonal antibody against Oxt-neurophysin, PS 38 (Ben-Barak et al., 1985), was used at a dilution of 1: 25. On day 2, the filters were rinsed in PBS 3 times and then incubated in biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) at 1:500 dilution for 1.5 h, rinsed in PBS 3 times then followed by avidin-biotinylated horseradish peroxidase (1:600) for 1h, (Vectastain Elite ABC Kit, Vector Labs). Following this incubation, the filters were rinsed three times in PBS and the reaction product was visualized using the chromogen 3,3′ diaminobenzidine tetrahydrochloride (DAB, 5mg/10 ml, Sigma). The DAB containing solution, consisted of 85 ml of PBS, 40 mg NH4 Cl, 136 mg imidazole, and 400 mg glucose. This solution was combined with 10 ml DAB and filtered immediately before adding 500 μl of glucose oxidase (100 units/ml, Calbiochem.), with or without nickel sulfate (70–80 mg/100 ml). Gentle rocking was implemented in the final stage of staining. Tissue was eased off the filters using a # 1 fine sable brush and placed on double subbed slides and allowed to dry overnight before counterstaining. Tissue too fragile to remove from their filters were counterstained in the netwells. Tissue on slides were cleared with xylene and mounted with Permount (Fisher, Pittsburgh, PA). Fragile tissue on the filters were dehydrated up to 100 % ethanol and cleared using Americlear Histology Clearing Solvent (Baxter Healthcare, Mcgraw Park, Il), and mounted in Permount, and photographed using an Olympus IX70 microscope (Olympus Instrument Co, Valley Central, PA).
For double immunofluorescence the rabbit polyclonal antibody against Avp-neurophysin, THR, was used at dilution of 1: 2000, overnight at 4°C, and was detected by Alexa 488 conjugated goat anti-rabbit (Molecular Probe Inc., Oregon, USA) second antibody at 1: 500 dilution. The mouse monoclonal antibody against Oxt-neurophysin, PS 38 (Ben-Barak et al., 1985; Whitnall et al., 1985)was used at a dilution of 1: 25 and followed by Alexa 594 conjugated goat anti-mouse second antibody (Molecular Probe Inc., Oregon, USA) staining at 1: 1000 dilution. The fluorescent slides were viewed under epi- fluorescence using a Nikon Labophot fluorescence microscope (Nikon Instrument Co, Melville, NY).
Counts of the PS 41- and THR-immunoreactive neurons in the cultures were made using the Nikon microscope as described elsewhere (House et al., 1998; Rusnak et al., 2002; Rusnak et al., 2003), and each measurement (e.g, the n values in Tables 1 and 2) represents all the identified cells found in the hypothalamic nuclei (i.e, from the 4 rat slices and 5 mouse slices on a given filter) which were derived from a single animal. The data were statistically evaluated by one-way analysis of variance (ANOVA). Fischer’s Protected LSD test was performed to determine differences between all investigated groups. Data are expressed as means ± S.E.M.s and statistical significance was when p < 0.01.
Table 1.
Effects of CNTF and LIF on survival of rat neurons
| A. Rat Oxt neurons
| ||||
|---|---|---|---|---|
| N | ACC | PVN | SON | |
| Control | 20 | 86±23 | 201±21 | 213±42 |
| CNTF | 18 | 492±44* | 594±67* | 445±56* |
| LIF | 18 | 526±41* | 518±47* | 462±58* |
| B. Rat Avp neurons
| ||||
|---|---|---|---|---|
| N | ACC | PVN | SON | |
| Control | 20 | 11±3 | 365±34 | 58±12 |
| CNTF | 18 | 84±22* | 402±36 | 211±37* |
| LIF | 18 | 45±7 | 453±41 | 216±21* |
Data expressed as as means ± SEMs per nucleus per rat (N equals number of animals).
Abbreviations: ACC, accessory nuclei; PVN, paraventricular nuclei; SON, supraoptic nuclei
Statistically significant difference from control, p<0.05
Table 2.
Effects of CNTF and LIF on survival of mouse neurons
| A. Mouse Oxt neurons
| ||||
|---|---|---|---|---|
| N | ACC | PVN | SON | |
| Control | 17 | 35±9 | 300±30 | 234±29 |
| CNTF | 15 | 41±9 | 308±37 | 179±25 |
| LIF | 16 | 67±9* | 379±39 | 266±39 |
| B Mouse Avp neurons
| ||||
|---|---|---|---|---|
| N | ACC | PVN | SON | |
| Control | 17 | 19±6 | 121±21 | 209±31 |
| CNTF | 14 | 25±9 | 112±19 | 307±45 |
| LIF | 16 | 25±4 | 290±43* | 275±51 |
Data expressed as as means ± SEMs per nucleus per mouse (N equals number of animals).
Abbreviations: ACC, accessory nuclei; PVN, paraventricular nuclei; SON, supraoptic nuclei
Statistically significant difference from control, p<0.05
RESULTS
Figure 1 illustrates the principal nuclei of the HNS system present in the rat hypothalamic organotypic cultures after 14 days in vitro. A similar distribution of MCNs is seen in organotypic cultures of mouse hypothalamus (not illustrated). A detailed description of the MCNs in both rat and mouse hypothalamic cultures after long-term culture have been described previously (House et al., 2006). The peroxidase-DAB-labeled MCNs shown in Fig 1 were visualized by immunohistochemistry using either a mouse monoclonal antibody, PS38, directed at Oxt-neurophysin (top) or a rabbit polyclonal antibody, THR (bottom), directed at Avp-neurophysin, highly specific markers of the Oxt- and Avp-MCNs, respectively. MCNs are found in the SON, PVN, and a dispersed population of MCNs called the ACC nucleus. The Avp immunopositive SCN neurons, which contain only small Avp-expressing neurons, are also found in these organotypic cultures (Rusnak et al., 2007; Wray et al., 1993). We find that most of the Oxt and Avp MCNs are typically located in one slice (as shown in Fig 1), but smaller numbers of these cells from each nucleus can be found in the three other slices from a single animal. In this study, the immunopositive cells from all 4 slices are counted using Oxt and Avp fluorescent immunohistochemical assays (see Methods), and are presented separately as numbers of cells/nucleus/animal in Tables 1 and 2.
Figure 1.
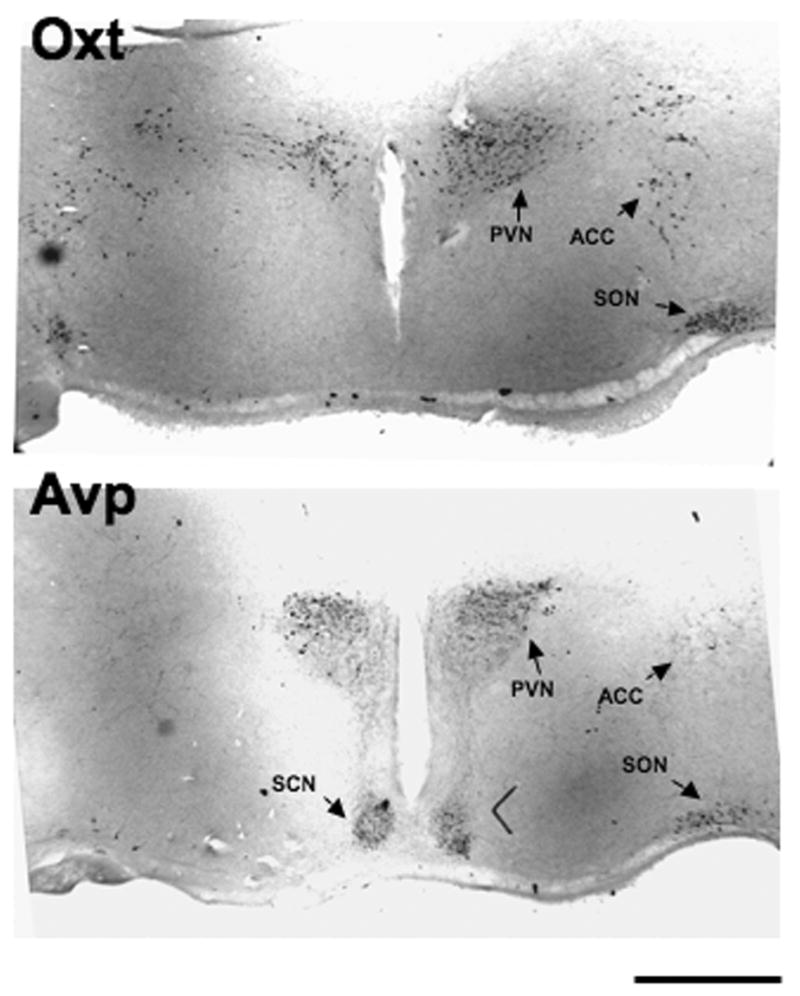
Representative low power views of rat hypothalamic organotypic culures after 14 days in vitro. The Oxt and Avp expressing neurons are identified by immunohistochemistry using antibodies against Oxt- (top panel) or Avp- (bottom panel) associated neurophysins (see Methods). All three hypothalamic nuclei containing the magnocellular neurons (MCNs) are present in these slice-explants, and are identified here as ACC (accessory nucleus), paraventricular nucleus (PVN) and the supraoptic nucleus (SON). Another nucleus in the explant that is shown is the suprachiasmatic nucleus (SCN) which expresses Avp but not Oxt, and in neurons that do not belong to the MCN phenotype. The scale line shown represents 1 mm in both panels.
Effects of CNTF and LIF on the survival of rat MCNs in organotypic cultures
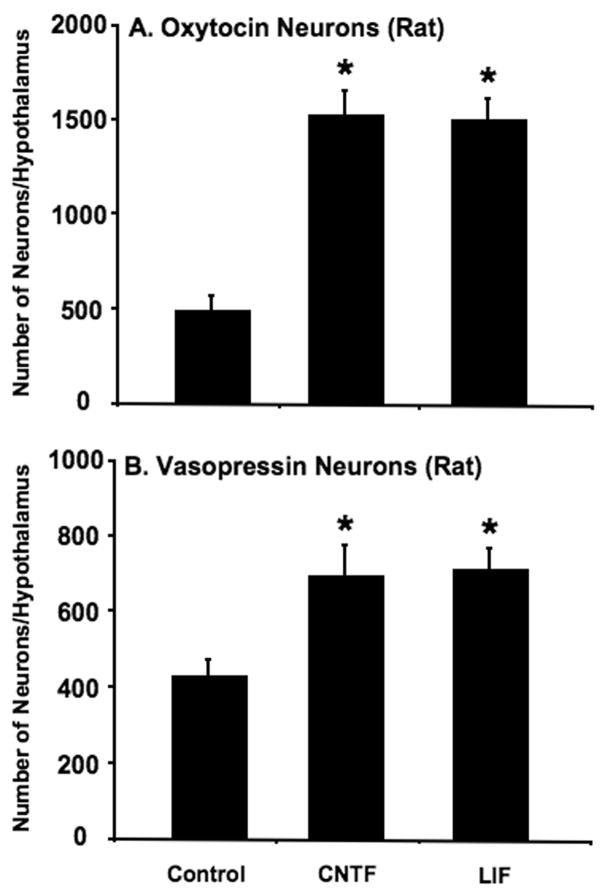
Figure 2 shows the effects of 10ng/ml CNTF or 10ng/ml LIF on the total numbers of Oxt (A) and Avp (B) MCNs surviving in organotypic culture in the absence or presence of the neurotrophic factors, CNTF or LIF. In control (untreated) cultures there were an average of 500 Oxt MCNs and 434 Avp MNCs that were present in the hypothalamic cultures obtained from a single rat pup. Culturing the slice-explants in CNTF or LIF for 14 days in vitro produced a 3.3-fold increase to about 1531 Oxt neurons and a 1.6-fold increase to about 697 Avp neurons in CNTF, and a 3.2-fold increase to about 1506 Oxt neurons, and a 1.66-fold increase to 714 Avp neurons in LIF containing media. In the rat hypothalamus, the CNTF and LIF appear to be equivalent in their abilities to rescue the Oxt and Avp MCNs from cell death during organotypic culture.
Figure 2.
Effect of culturing rat hypothalamic slices in the absence (control) and presence of either 10ng/ml CNTF or 10ng/ml LIF in the culture medium for 14 days, on the total number of immunohistochemically identified Oxt and Avp neurons in the organotypic cultures (see Table 1 for the numbers of neurons in the individual nuclei under these conditions). Data are expressed as means ± SEM, with n=20 for controls and 18 each for CNTF- and LIF-treated cultures. Statistical differences, shown over columns by asterisks represent p values < 0.01 (compared to control), were calculated by ANOVA, followed by Fischer’s protected LSD test.
The MCNs in the three nuclei differ in their responses to the neurotrophic factors. This is shown in Table 1 where the cell numbers in each nucleus are evaluated for their responses to CNTF and LIF. Under control conditions 80% of the Oxt MCNs are equally divided between the PVN and SON, and the cell numbers increase about 2-fold in both of these nuclei after either CNTF or LIF treatment. In contrast, the ACC nuclei averages only 86 Oxt MCNs/animal under control conditions, but increase about 6-fold to 492 after CNTF and 526 after LIF treatments, and reaches a total survival equivalent to the PVN and SON. The situation for the rat Avp MCNs differs somewhat from the Oxt MCNs in that there is no significant effect of the neurotrophic factors for the Avp MCNs in the PVN, but a 3.7-fold increase in Avp MCNs in the SON as a result of either treatment. The Avp MCNs in the ACC are very sparse, but do increase significantly after CNTF and also apparently after LIF treatment (but not statistically significant in the LIF).
Effects of CNTF and LIF on the survival of mouse MCNs in organotypic culture
Figure 3 shows the effects of 10ng/ml CNTF or 10ng/ml LIF on the total numbers of Oxt (A) and Avp (B) MCNs present in organotypic cultures of mouse hypothalamus after 14 days in vitro in the absence or presence of the neurotrophic factors. Unlike the rat MCNs (Fig 2), there was either no statistically significant change in survival of the mouse MCNs or a small significant increase of Avp MCNs, about 1.7-fold, in the presence of LIF only (Fig 3B). Virtually, all of this significant increase in LIF occurred in the mouse PVN (Table 2B), which is in contrast to the situation with the rat PVN which was the one nucleus that was unresponsive to the neurotrophic factor (Table 1B). Taken together, these data emphasize the selectivity in the responses of the MCNs to the neurotrophic factors, both with regard to their specific nuclei and the rodent species.
Figure 3.
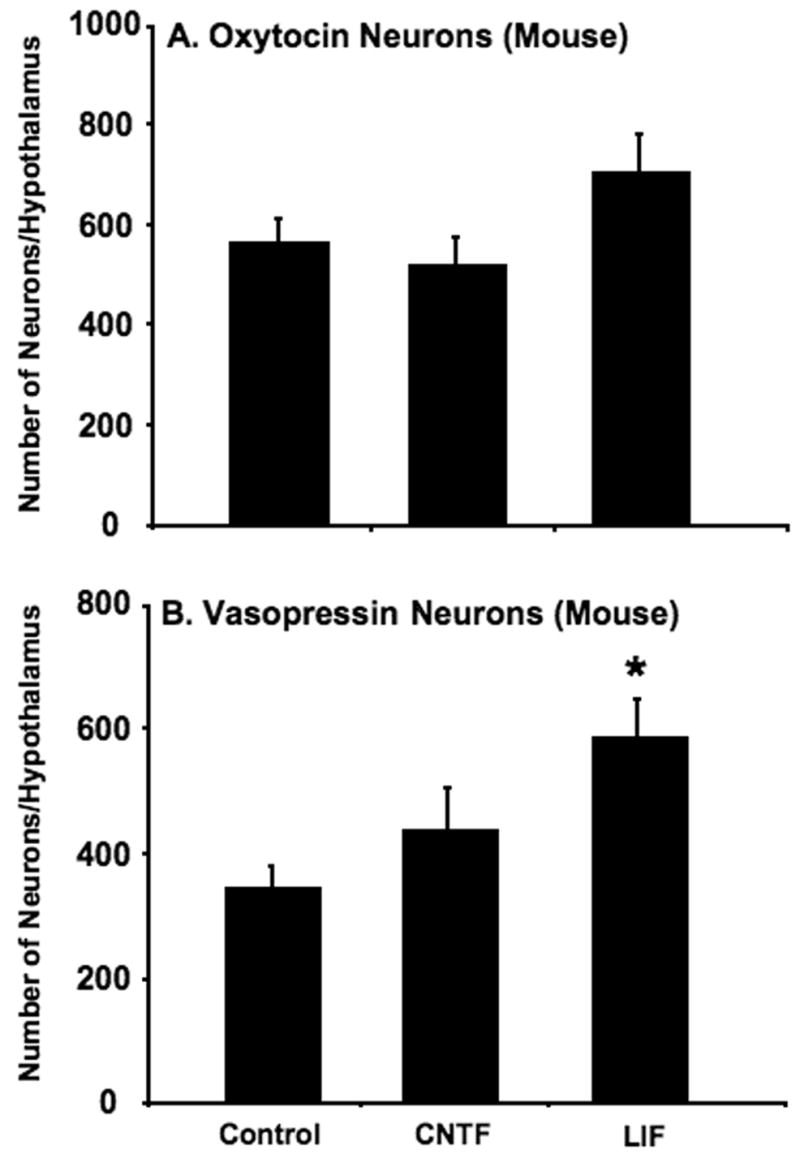
Effect of culturing mouse hypothalamic slices in the absence (control) and presence of either 10ng/ml CNTF or 10ng/ml LIF in the culture medium for 14 days, on the total number of immunohistochemically identified Oxt and Avp neurons in the organotypic cultures (see Table 2 for the numbers of neurons in the individual nuclei under these conditions). Data are expressed as means ± SEM, with n=20 for controls, 15 for CNTF- and 16 for LIF-treated cultures. Statistical differences, shown over columns by asterisks represent p values < 0.01 (compared to control), were calculated by ANOVA, followed by Fischer’s protected LSD test.
DISCUSSION
In this paper, we examine the effects of two LIF-family neurotrophic factors, CNTF and LIF, on their abilities to rescue MCNs in the HNS from axotomy-induced, programmed cell death. We also compare the responses of the MCNs in several different nuclei in the HNS, the PVN, SON, and ACC in two rodent species, mice and rats. We have made this effort to do this systematic, quantitative analysis, since the MCNs are very intensively studied in vivo as models and prototypes of neuroendocrine cells in the CNS, and these organotypic cultures are the only in vitro experimental models for the fully differentiated phenotypes that can be studied. Optimizing the survival of the Oxt- and Avp-MCNs in these cultures should greatly facilitate future cell-biological and physiologic studies of these important neuronal systems.
In this study, we have, for the first time, considered the responses of the MCNs in the ACC nucleus, in addition to the MCNs in the PVN and SON to the neurotrophic factors. Although most work on the HNS system has been focused on the PVN and SON nuclei because of their obvious and compact cell groupings, it has been known for many years that the rat hypothalamus has other MCNs in less conspicuous but consistent cellular groups (Armstrong et al., 1980; Fisher et al., 1979; Herman et al., 1987; Ju et al., 1986; Kelly and Swanson, 1980; Peterson, 1966; Sherlock et al., 1975). Many investigators have referred to these various cell clusters as the “accessory nucleus” (ACC) and we have used this nomenclature here. The ACC is much more prominent in the rat versus the mouse hypothalamus, and very little if any literature can be found for the mouse ACC. Several papers in the literature have counted the numbers of Oxt- and Avp MCNs in the rat PVN and SON, but only one paper included the ACC in the analysis (Rhodes et al., 1981). Unlike the SON where there are twice as many Avp-MCNs than Oxt MCNs, and the PVN where there were equivalent numbers of each phenotype, in contrast the ACC the Oxt-MCNs were two-fold greater in number than the Avp-MCNs (Rhodes et al., 1981). Interestingly, in the rat hypothalamic organotypic cultures the Oxt-MCNs were similarly the predominant phenotype in the ACC (see Table 1).
Comparison of the rat MCN cell numbers found in vivo (Rhodes et al., 1981)versus those found after 14 days in organotypic culture (Table 1) shows that there are a total of 500 Oxt- and 434 Avp MCNs surviving in the control cultured slice explants, or 6.4% and 4.6% of the total Oxt- and Avp-MCNs, respectively, found in vivo. After treatment with the neurotrophic factors the cultured rat MCN numbers rose to about 1600 for Oxt and 700 for Avp (Fig 2) representing a value of about 21% and 7% respectively, of these phenotypes found in vivo. Even though the neurotrophic factors produced a substantial improvement in survival in vitro, the total numbers are considerably lower than the in vivo MCN numbers. What is most striking about the data in Table 1 are the different degrees of responsiveness to the neurotrophic factors among the different nuclei and between the peptide neuronal phenotypes. For the Oxt-MCNs, the CNTF and LIF produced a two-fold increase in cell number in both the SON and PVN, whereas in the ACC the increased survival was nearly six-fold greater (Table 1A). For the Avp-MCNs (Table 1B), there was nearly four-fold increase in the SON, but in contrast there was no significant increase in the PVN. The total number of Avp-MCNs in the ACC is very small, but the increase after neurotrophic factor treatment is nearly eight-fold for CNTF, and four-fold for LIF. The failure to see an increase in survival of the Avp-MCN phenotype in the PVN, as opposed to the robust effect in the SON (Table 1B) is intriguing. Whether this is due to a real difference between the MCNs in these nuclei, or whether the parvocellular Avp-neurons known to be present in the in the PVN in organotypic culture (Arima et al., 2001; Bertini et al., 1993) predominate, remains to be determined.
Similar data were gathered for the mouse hypothalamo organotypic cultures (Table 2). The total numbers of control MCNs are comparable between the mouse and rat cultures. While this suggests a better survival rate for the mouse under control conditions, there was surprisingly no effect at all for most of the nuclei. Only the Avp-MCNs in the PVN showed about a two-fold increase in survival (Table 2B), but this was statistically significant only after the LIF treatment. These differences between the mouse and rat organotypic cultures were unexpected and will require further study.
Acknowledgments
This research was supported by the intramural research program of the NIH, NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso G, Bribes E, Chauvet N. Survival and regeneration of neurons of the supraoptic nucleus following surgical transection of neurohypophysial axons depend on the existence of collateral projections of these neurons to the dorsolateral hypothalamus. Brain Res. 1996;711:34–43. doi: 10.1016/0006-8993(95)01350-4. [DOI] [PubMed] [Google Scholar]
- Arima H, House SB, Gainer H, Aguilera G. Direct stimulation of arginine vasopressin gene transcription by cAMP in parvocellular neurons of the paraventricular nucleus in organotypic cultures. Endocrinology. 2001;142:5027–30. doi: 10.1210/endo.142.11.8595. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–58. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Bali B, Ferenczi S, Kovacs KJ. Direct inhibitory effect of glucocorticoids on corticotrophin-releasing hormone gene expression in neurones of the paraventricular nucleus in rat hypothalamic organotypic cultures. J Neuroendocrinol. 2008;20:1045–51. doi: 10.1111/j.1365-2826.2008.01759.x. [DOI] [PubMed] [Google Scholar]
- Baratta J, Marienhagen JW, Ha D, Yu J, Robertson RT. Cholinergic innervation of cerebral cortex in organotypic slice cultures: sustained basal forebrain and transient striatal cholinergic projections. Neuroscience. 1996;72:1117–32. doi: 10.1016/0306-4522(95)00603-6. [DOI] [PubMed] [Google Scholar]
- Belenky M, Wagner S, Yarom Y, Matzner H, Cohen S, Castel M. The suprachiasmatic nucleus in stationary organotypic culture. Neuroscience. 1996;70:127–43. doi: 10.1016/0306-4522(95)00327-f. [DOI] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergold PJ, Casaccia-Bonnefil P. Preparation of organotypic hippocampal slice cultures using the membrane filter method. Methods Mol Biol. 1997;72:15–22. doi: 10.1385/0-89603-394-5:15. [DOI] [PubMed] [Google Scholar]
- Bertini LT, Kursner C, Gaillard RC, Corder R, Kiss JZ. A tissue culture model of the hypophysiotrophic CRF producing neuronal system. Neuroendocrinology. 1993;57:716–28. doi: 10.1159/000126430. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res. 2004;78:157–66. doi: 10.1002/jnr.20248. [DOI] [PubMed] [Google Scholar]
- Bonnici B, Kapfhammer JP. Spontaneous regeneration of intrinsic spinal cord axons in a novel spinal cord slice culture model. Eur J Neurosci. 2008;27:2483–92. doi: 10.1111/j.1460-9568.2008.06227.x. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Sakhi S, Schreiber SS, Baudry M. Development of kainic acid and N-methyl-D-aspartic acid toxicity in organotypic hippocampal cultures. Exp Neurol. 1995;132:209–19. doi: 10.1016/0014-4886(95)90026-8. [DOI] [PubMed] [Google Scholar]
- Dammerman RS, Noctor SC, Kriegstein AR. Extrinsic GABAergic innervation of developing neocortical layer 1 in organotypic slice co-cultures. J Comp Neurol. 2000;423:112–20. doi: 10.1002/1096-9861(20000717)423:1<112::aid-cne9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Davids E, Hevers W, Damgen K, Zhang K, Tarazi FI, Luddens H. Organotypic rat cerebellar slice culture as a model to analyze the molecular pharmacology of GABAA receptors. Eur Neuropsychopharmacol. 2002;12:201–8. doi: 10.1016/s0924-977x(02)00024-x. [DOI] [PubMed] [Google Scholar]
- Dohanics J, Hoffman GE, Smith MS, Verbalis JG. Functional neurolobectomy induced by controlled compression of the pituitary stalk. Brain Res. 1992;575:215–22. doi: 10.1016/0006-8993(92)90082-k. [DOI] [PubMed] [Google Scholar]
- Dupont JL, Fourcaudot E, Beekenkamp H, Poulain B, Bossu JL. Synaptic organization of the mouse cerebellar cortex in organotypic slice cultures. Cerebellum. 2006;5:243–56. doi: 10.1080/14734220600905317. [DOI] [PubMed] [Google Scholar]
- Eustache I, Gueritaud JP. Electrical properties of embryonic rat brainstem motoneurones in organotypic slice culture. Brain Res Dev Brain Res. 1995;86:187–202. doi: 10.1016/0165-3806(95)00031-8. [DOI] [PubMed] [Google Scholar]
- Fields RL, House SB, Gainer H. Regulatory domains in the intergenic region of the oxytocin and vasopressin genes that control their hypothalamus-specific expression in vitro. J Neurosci. 2003;23:7801–9. doi: 10.1523/JNEUROSCI.23-21-07801.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AW, Price PG, Burford GD, Lederis K. A 3-dimensional reconstruction of the hypothalamo-neurohypophysial system of the rat. The neurons projecting to the neuro/intermediate lobe and those containing vasopressin and somatostatin. Cell Tissue Res. 1979;204:343–54. doi: 10.1007/BF00233647. [DOI] [PubMed] [Google Scholar]
- Franke H, Schelhorn N, Illes P. Dopaminergic neurons develop axonal projections to their target areas in organotypic co-cultures of the ventral mesencephalon and the striatum/prefrontal cortex. Neurochem Int. 2003;42:431–9. doi: 10.1016/s0197-0186(02)00134-1. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–42. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–7. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Dreifuss JJ. Phasically firing neurons in long-term cultures of the rat hypothalamic supraoptic area: pacemaker and follower cells. Brain Res. 1979;177:95–103. doi: 10.1016/0006-8993(79)90920-x. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Hefti F. Striatal acetylcholinesterase-containing interneurons innervate hippocampal tissue in co-cultured slices. Brain Res. 1985;350:311–4. doi: 10.1016/0165-3806(85)90276-7. [DOI] [PubMed] [Google Scholar]
- Hare K. Degeneration of the supra-optic nucleus following hypophysectomy in the dog. Am J Physiol. 1937;119:326. [Google Scholar]
- Herman JP, Marciano FF, Wiegand SJ, Gash DM. Selective cell death of magnocellular vasopressin neurons in neurohypophysectomized rats following chronic administration of vasopressin. J Neurosci. 1987;7:2564–75. [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Jones SA, Greenfield SA. The influence of target and non-target brain regions on the development of mid-brain dopaminergic neurons in organotypic slice culture. Brain Res Dev Brain Res. 1995;88:212–9. doi: 10.1016/0165-3806(95)00112-q. [DOI] [PubMed] [Google Scholar]
- House SB, Rusnak M, Liu XH, Youle RJ, Gainer H. Bcl-xL and caspase inhibition increase the survival of rat oxytocin and vasopressin magnocellular neurons in organotypic culture. Exp Neurol. 2006;200:267–71. doi: 10.1016/j.expneurol.2006.02.009. [DOI] [PubMed] [Google Scholar]
- House SB, Thomas A, Kusano K, Gainer H. Stationary organotypic cultures of oxytocin and vasopressin magnocellular neurones from rat and mouse hypothalamus. J Neuroendocrinol. 1998;10:849–61. doi: 10.1046/j.1365-2826.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- Israel JM, Poulain DA, Oliet SH. Oxytocin-induced postinhibitory rebound firing facilitates bursting activity in oxytocin neurons. J Neurosci. 2008;28:385–94. doi: 10.1523/JNEUROSCI.5198-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Dupouy B, Bonhomme R, Theodosis DT, Poulain DA, Israel JM. Electrophysiological studies of oxytocin neurons in organotypic slice cultures. Adv Exp Med Biol. 1998;449:135–45. doi: 10.1007/978-1-4615-4871-3_16. [DOI] [PubMed] [Google Scholar]
- Ju G, Liu S, Tao J. Projections from the hypothalamus and its adjacent areas to the posterior pituitary in the rat. Neuroscience. 1986;19:803–28. doi: 10.1016/0306-4522(86)90300-3. [DOI] [PubMed] [Google Scholar]
- Kaempf S, Walter P, Salz AK, Thumann G. Novel organotypic culture model of adult mammalian neurosensory retina in co-culture with retinal pigment epithelium. J Neurosci Methods. 2008;173:47–58. doi: 10.1016/j.jneumeth.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Kelly J, Swanson LW. Additional forebrain regions projecting to the posterior pituitary: preoptic region, bed nucleus of the stria terminalis, and zona incerta. Brain Res. 1980;197:1–9. doi: 10.1016/0006-8993(80)90430-8. [DOI] [PubMed] [Google Scholar]
- Kesser BW, Hashisaki GT, Fletcher K, Eppard H, Holt JR. An in vitro model system to study gene therapy in the human inner ear. Gene Ther. 2007;14:1121–31. doi: 10.1038/sj.gt.3302980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Amarjargal N, Gross J, Haupt H, Scherer H, Mazurek B. Coenzyme Q10 does not protect cochlear hair cells from death in the ischemic organotypic culture. Otolaryngol Head Neck Surg. 2007;137:950–2. doi: 10.1016/j.otohns.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Larsen TR, Rossen S, Gramsbergen JB. Dopamine release in organotypic cultures of foetal mouse mesencephalon: effects of depolarizing agents, pargyline, nomifensine, tetrodotoxin and calcium. Eur J Neurosci. 2008;28:569–76. doi: 10.1111/j.1460-9568.2008.06354.x. [DOI] [PubMed] [Google Scholar]
- Li B, Liu XY, Li Z, Bu H, Sun MM, Guo YS, Li CY. Effect of ALS IgG on motor neurons in organotypic spinal cord cultures. Can J Neurol Sci. 2008;35:220–5. doi: 10.1017/s0317167100008672. [DOI] [PubMed] [Google Scholar]
- Lo D, Sunrhodes N, Watt JA. Perivascular cells increase expression of ciliary neurotrophic factor following partial denervation of the rat neurohypophysis. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng GD, Snyder-Keller A, Seegal RF. Dopaminergic development of prenatal ventral mesencephalon and striatum in organotypic co-cultures. Brain Res. 2007;1133:1–9. doi: 10.1016/j.brainres.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, O’Neill JS, Chesham JE, Hastings MH. Minireview: The circadian clockwork of the suprachiasmatic nuclei--analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–34. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–53. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RP. Magnocellular neurosecretory centers in the rat hypothalamus. J Comp Neurol. 1966;128:181–90. doi: 10.1002/cne.901280205. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Regulation of the nigrostriatal pathway by metabotropic glutamate receptors during development. J Neurosci. 1998;18:4133–44. doi: 10.1523/JNEUROSCI.18-11-04133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Gainer H. Differential effects of forskolin on tyrosine hydroxylase gene transcription in identified brainstem catecholaminergic neuronal subtypes in organotypic culture. Eur J Neurosci. 2005;21:889–98. doi: 10.1111/j.1460-9568.2005.03913.x. [DOI] [PubMed] [Google Scholar]
- Rusnak M, House SB, Arima H, Gainer H. Ciliary neurotrophic factor increases the survival of magnocellular vasopressin and oxytocin neurons in rat supraoptic nucleus in organotypic cultures. Microsc Res Tech. 2002;56:101–12. doi: 10.1002/jemt.10015. [DOI] [PubMed] [Google Scholar]
- Rusnak M, House SB, Gainer H. Long-term effects of ciliary neurotrophic factor on the survival of vasopressin magnocellular neurones in the rat supraoptic nucleus in vitro. J Neuroendocrinol. 2003;15:933–9. doi: 10.1046/j.1365-2826.2003.01080.x. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Toth ZE, House SB, Gainer H. Depolarization and neurotransmitter regulation of vasopressin gene expression in the rat suprachiasmatic nucleus in vitro. J Neurosci. 2007;27:141–51. doi: 10.1523/JNEUROSCI.3739-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar T, House SB, Gainer H. Neural activity protects hypothalamic magnocellular neurons against axotomy-induced programmed cell death. J Neurosci. 2004;24:6553–62. doi: 10.1523/JNEUROSCI.0886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock DA, Field PM, Raisman G. Retrograde transport of horseradish peroxidase in the magnocellular neurosecretory system of the rat. Brain Res. 1975;88:403–14. doi: 10.1016/0006-8993(75)90653-8. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology. 2008;149:4544–53. doi: 10.1210/en.2008-0229. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller A. Pattern of corticostriatal innervation in organotypic cocultures is dependent on the age of the cortical tissue. Exp Neurol. 2004;185:262–71. doi: 10.1016/j.expneurol.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Tominaga K, Inouye SI, Okamura H. Organotypic slice culture of the rat suprachiasmatic nucleus: sustenance of cellular architecture and circadian rhythm. Neuroscience. 1994;59:1025–42. doi: 10.1016/0306-4522(94)90303-4. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Bartanusz V, Schulz MF, Kiss JZ. Magnocellular vasopressinergic neurons in explant cultures are rescued from cell death by ciliary neurotrophic factor and leukemia inhibiting factor. Neuroscience. 1998;87:571–82. doi: 10.1016/s0306-4522(98)00177-8. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–9. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Watt JA, Bone S, Pressler M, Cranston HJ, Paden CM. Ciliary neurotrophic factor is expressed in the magnocellular neurosecretory system of the rat in vivo: evidence for injury- and activity-induced upregulation. Exp Neurol. 2006;197:206–14. doi: 10.1016/j.expneurol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Watt JAL, Cranston D, Paden HJ. CNTF Receptor Alpha is expressed by Magnocellular neurons and expression is upregulated in the rat supraoptic nucleus during axonal sprouting. Experimental Neurology. 2008 doi: 10.1016/j.expneurol.2008.09.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnall MH, Key S, Ben-Barak Y, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. II. Immunocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J Neurosci. 1985;5:98–109. doi: 10.1523/JNEUROSCI.05-01-00098.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Castel M, Gainer H. Characterization of the suprachiasmatic nucleus in organotypic slice explant cultures. Microsc Res Tech. 1993;25:46–60. doi: 10.1002/jemt.1070250108. [DOI] [PubMed] [Google Scholar]
- Wray S, Gahwiler BH, Gainer H. Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides. 1988;9:1151–75. doi: 10.1016/0196-9781(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Wray S, Kusano K, Gainer H. Maintenance of LHRH and oxytocin neurons in slice explants cultured in serum-free media: effects of tetrodotoxin on gene expression. Neuroendocrinology. 1991;54:327–39. doi: 10.1159/000125910. [DOI] [PubMed] [Google Scholar]