Abstract
Rationale
This study was designed to determine safety, to provide preliminary data regarding potential efficacy and to investigate the effects on autoimmunity and fibrosis of rituximab in patients with diffuse cutaneous systemic sclerosis (dcSSc).
Methods
Fifteen patients with dcSSc, having their first non-Raynaud's associated disease manifestation within 18 months of trial entry, were recruited to receive two intravenous doses of 1000 mg rituximab two weeks apart. Safety, clinical, and exploratory outcomes were evaluated at baseline and at 6 months. The primary outcome was the change in modified Rodnan skin score (mRSS) at 6 months compared to baseline.
Results
Adverse events included frequent infusion reactions and rare infections (urinary tract infection and dental abscess, each in one patient). Mean mRSS change was not significantly different between baseline (20.6) and 6-months (20.2). Pulmonary function tests and other measures of major organ involvement were stable. Modest B cell infiltrates present in most skin biopsies at baseline were completely depleted at 6-months in most subjects. Autoantibody titers showed only modest and variable changes after treatment.
Conclusions
In this pilot study, treatment with rituximab appeared to be safe and well-tolerated among patients with dcSSc. Rituximab resulted in both depletion of circulating B cells and depletion of dermal B cells but had little effect on levels of SSc-associated autoantibodies. Rituximab did not appear to result in a significant beneficial effect on skin disease; the potential efficacy in other organs such as the lung could not be clearly evaluated in this small open label trial.
No underlying pathogenic mediator or pathway has clearly emerged to guide targeting therapy in SSc. Despite the clinical overlap with systemic lupus erythematosus (SLE) and the presence of autoantibodies to nuclear antigens as seen in SLE, the importance of autoimmunity in SSc remains uncertain. A recent study highlighted the potential role of autoantibodies to platelet derived growth factor (PDGF) receptors in SSc (1). Other studies revealed highly upregulated immunoglobulin genes and B cells in SSc skin (2), and suggested that B cells are important in the tight skin, murine model of SSc skin disease (3, 4). In addition, we have recently reported that B cells are prominent in lymphocytic infiltrates seen in SSc-associated interstitial lung disease (5). Although intriguing, these observations do not directly implicate B cells or SSc-specific autoantibodies in SSc.
We describe here an open-label study of treatment with the B cell depleting agent, rituximab, in patients with diffuse cutaneous systemic sclerosis (dcSSc). We conducted this open label trial to assess the potential efficacy of this medication for dcSSc, to examine potential mechanisms of action; skin B cell and autoantibody depletion, and also to further investigate the utility of supplementary outcome measures for trials of SSc: durometry and the degree of dermal myofibroblast infiltration.
Patients and Methods
Patient selection and treatment
All patients recruited into the study had early dcSSc (6) with first non-Raynaud's disease manifestation within 18 months of trial entry. Initially, patients were excluded if taking other immunosuppressive medication or greater than 10 mg/day of prednisone (or other equivalent corticosteroid). Later in the study one patient on a stable dose of methotrexate was permitted to enter the trial. Patients with a forced vital capacity or diffusion capacity less than 50% predicted, or with significant cardiac arrythymia or an ejection fraction less than 40% were excluded from the study.
All patients received two doses of rituximab 1000 mg intravenously two weeks apart. No premedication was given. Infusion reactionwere treated with corticosteroids, acetaminophen and/or diphenhydramine as clinically indicated.
Outcome measures
Safety was assessed by history and physical exam, complete blood counts, metabolic panels, and urinalyses at baseline, 2 weeks and 1, 2, 4, 6, 9 and 12 months after treatment. Serial electrocardiograms and echocardiograms assessed cardiac safety.
The primary efficacy outcome was the modified Rodnan skin score (mRSS) at baseline, and 6 and 12 months after treatment, obtained by two trained physician scorers. Additional outcome measures included high-resolution computerized chest tomography (HRCT) and pulmonary function testing (PFT) obtained within 2 months of trial entry and 6 months after treatment. The scleroderma modification of the health assessment questionnaire (SHAQ) was administered at baseline, and 6 and 12 months after treatment.
Durometer Measurements
In 12 of the subjects, skin hardness was measured using a hand held digital durometer (Rex Gauge type OO, Buffalo Grove, IL). Measurements expressed in standardized durometer units (DU) were made at predetermined “landmark” sites at the forearms, upper arms, abdomen, thighs, and legs and combined into a 9-site durometry score (7).
Skin biopsies and immunohistochemistry
Skin biopsies were obtained in all except one patient at a uniform site at the midpoint between wrist and elbow on the dorsal side of the forearm. Fixed sections were stained for CD20 (L26, DakoCytomation) or α-smooth muscle actin (1D4, DakoCytomation). B cell infiltration in the specimens was assessed by counting the total number of CD20+ B cells in the section. Myofibroblast score was assessed with blinding to disease, treatment, and order of serial biopsy as described (8).
Autoantibody analyses
Autoantibodies to chromatin, Sm, topoisomerase I (topo-I), U1 ribonucleoprotein, ribosomal P protein, Jo-1 (histidyl tRNA synthetase), SS-A/Ro60, Ro52 and SS-B/La were detected by an addressable laser bead immunoassay (ALBIA) utilizing a commercially available kit (QuantaPlex ENA8, INOVA Diagnostics, San Diego, CA) as previously described (9). Antibodies to RNA polymerase III (RNAP-III were detected by ELISA using a commercial kit (INOVA Diagnostics Inc) as described previously (9).
Data analysis
Pearson correlations, paired t-tests and descriptive statistics, including 95% confidence intervals were calculated using the Excel statistical package (Microsoft).
Results
Study subject and safety outcomes
Baseline characteristics of 15 patients with dcSSc recruited into this study are found in Table I. Infusion reactions were common (46.7% of patients), but patients were not pre-medicated and all patients were able to complete treatment. Mild hypotension developed in two patients, and flushing, fatigue, nausea/abdominal cramping, rigors and hand tingling each developed in one patient. One patient developed a urinary tract infection and another a dental abscess thought possibly related to study medication. The only serious adverse event was the development of prostate cancer in one patient, thought to be unrelated to the study medication.
Table I.
Baseline characteristic of study patients (n=15)
| Gender M:F | 2:13 |
| Age, years* | 45.8 (32−57) |
| Disease duration (months) | 14.5 (9−18) |
| Baseline modified Rodnan skin score | 20.6 (9−31) |
| Baseline autoantibodies: (number of patients with each) | |
| Topo-I (Scl-70) | 3 |
| RNAP-III | 5 |
| PM/Scl | 1 |
| CENP-B | 1 |
| U1-RNP | 1 |
| Ro60 | 1 |
Values represent the average and (range).
Clinical outcomes in rituximab-treated patients
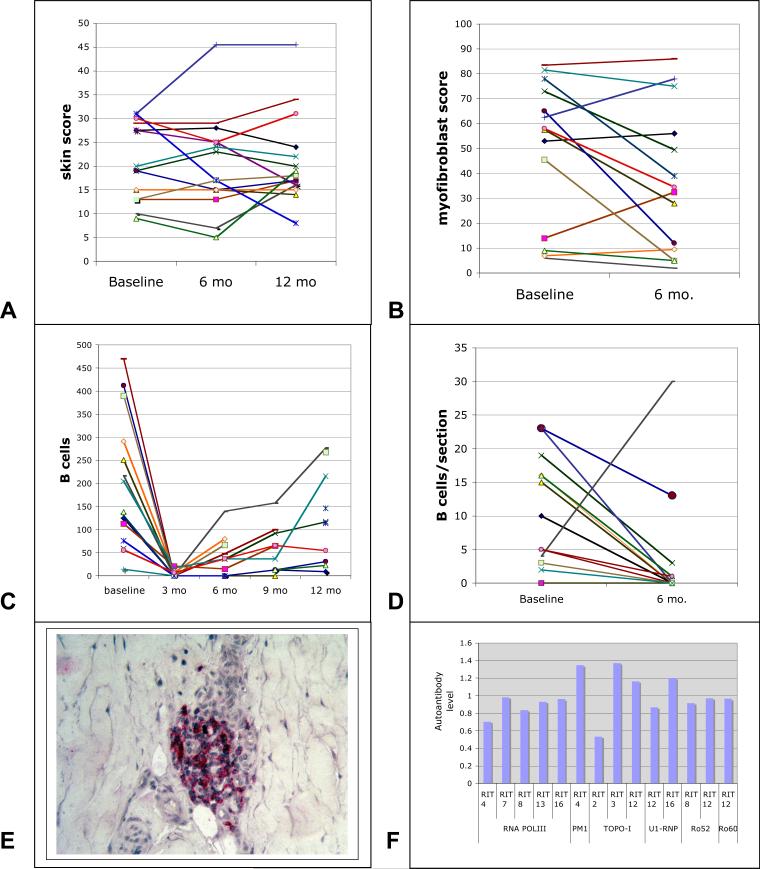
The mRSS did not change significantly between baseline and 6-months (mean change = −0.37, p=0.82; range of change: −14.5 (improvement) to +14.0 (progression) (Figure 1A). The average change in mRSS at 6-months compared to baseline showed a 95% confidence interval of −3.8 to 3.0. The average change in mRSS at 12 months was +0.9, p=0.83.
Figure 1.
Clinical and pathological outcomes in dcSSc patients treated with rituximab. Modified Rodnan skin score (A), myofibroblast score (B), circulating CD20+ B cell count (C) and B cells in skin sections (D) are shown at baseline, and 6 and 12 months (A) at baseline and 6 months (B and D), or at baseline and 3, 6, 9 and 12 months after rituximab treatments. The same color line represents the same patient in each of the graphs. A typical example of perivascular B cell staining in one of the patients is shown in panel E. Autoantibodies in patients with dcSSc at baseline and 6-months after rituximab treatment. (F) Autoantibodies to chromatin, Sm, topoisomerase I (topo-I), U1 ribonucleoprotein, ribosomal P protein, Jo-1 (histidyl tRNA synthetase), SS-A/Ro60, Ro52 and SS-B/La or RNA polymerase III (RNAP-III) detected by immunoassay or ELISA at 6 months were normalized to values at baseline. Patients not shown did not have detectable autoantibodies to any of the specificities tested.
The average FVC and DLCO showed no significant differences at 6-months (respectively, 92.7% and 77.9 % predicted) compared to the baseline (respectively, 89.2% and 79.7 % predicted). None of the patients showed new or progressive pulmonary disease by HRCT, or any signs of progressive cardiac disease with stable ejection fractions, and EKGs. None of the patients developed renal crisis or symptoms suggesting progressive gastrointestinal disease. Thus, none of the patients showed evidence of progressive major end-organ involvement.
Skin durometry in study patients
There was very strong correlation between the 9-site durometry score and the modified Rodnan skin score at baseline (R2=0.81), at 6 months (R2=0.97), and for change in measurement value over the 6 months (R2=0.57). There was also a correlation between durometry score and myofibroblast score at baseline (R2=0.19), and 6 months (R2=0.58). There was no significant change in the 9-site durometry score at 6 months (291 DU) compared to baseline score (289 DU, p=0.86).
Evaluation of Peripheral B Cells
Circulating B cells were depleted almost completely in all study patients (n=14) at 3 months (Figure 1C), recovering between 6 and 12 months in most patients.
Skin B Cell Quantification
B cells in skin biopsies from patients with SSc were generally clustered around blood vessels (Figure 1E). In baseline biopsies, the number of B cells in the entire biopsy (average 10.4/biopsy, n=15 was strikingly higher than control, healthy skin biopsies, which uniformly showed no B cells (n=8, p<.0005). Most patients showed complete or nearly complete depletion of dermal B cells 6 months after administration of rituximab (average B cell number: 3.4/biopsy, Figure 1D and Table II). One patient showing increased numbers of B cells in the 6-month biopsy (30 B cells/biopsy compared to 13 at baseline) had significant recovery of peripheral B cells at 6 months (compare Figure 1C brown line with Figure 1D brown line). CD4+, CD8+ and sialoadhesin+ cells did not change significantly at 6 months compared to baseline (average cells/section, baseline/6 month: CD4+= 43.4/36.4; CD8+ = 15.5/14.2; sialoadhesin 35.5/28.7).
Table II.
Clinical and laboratory outcomes
| Baseline | 6 months | 12 months | |
|---|---|---|---|
| Modified Rodnan skin score* | 20.6 ± 4.4 (9−31/19) | 20.2 ± 5.5 (5−45.5/17) | 21.1 ± 5.2 (8−45.5/17) |
| Pulmonary Function testing | |||
| Forced vital capacity (% predicted) | 89.2 ± 10.8 (62−119) | 92.7 ± 10.3 (53−120) | |
| DLCO (% predicted) | 79.7 ± 8.3 (61−107) | 77.8 ± 7.5 (52−95) | |
| Health Assessment Questionnaire | |||
| Disability Index | 0.67 ± 0.32 (0−2.0) | 0.64 ± 0.36 (0−2.25) | 0.55 ± 0.33 (0−2.25) |
| VAS | 0.73 ± 0.26 (0−1.875) | 0.53 ± 0.21 (0−1.5) | 0.56 ± 0.21 (0.03−1.5) |
| Immunoglobulins | |||
| IgM | 107 ± 25 (15−172) | 86 ± 24 (9−172) | 86 ± 24 (11−172) |
| IgG | 1106 ± 197 (756−2210) | 1066 ± 173 (696−1970) | 1021 ± 181 (588−1970) |
| IgA | 270 ± 100 (61−539) | 288 ± 105 (58−546) | 274 ± 94 (58−532) |
| Sedimentation Rate | 27.9 ± 11.7 (2−69) | 20.5 ± 7.8 (6−40) | 17.0 ± 6.1 (6−38) |
| Exploratory Outcomes | |||
| B cell score | 10.4 ± 4.6 (0−23) | 3.4 ± 4.8 (0−30) | |
| SMA score | 49.5 ± 16.6 (6−81.5) | 36.6 ± 16.6 (2−78) |
Values represent the average ± 95% confidence limit (range)
Skin Myofibroblasts
Myofibroblasts in the deep dermis in both baseline and 6-month biopsies correlated highly with the mRSS (n=28, R2=0.54, p=0.00001, compare Figure 1, A and B). The average myofibroblast score improved in rituximab-treated patients from 49.5 at baseline to 36.6 (p<0.05, Table II).
Autoantibody Levels
Of autoantibodies commonly seen in dcSSc, five patients had anti-RNAP-III and three patients anti-topo-I. The autoantibody levels in these patients did not show consistent changes during the course of the study. Anti-RNAP-III levels declined modestly in all patients (0.70−0.98-fold change, Fig. 2), while anti-topo-I levels varied between modest decline and modest increase (0.53−1.3-fold change, Fig. 1F). Autoantibodies to U1RNP, PM/Scl and Ro60 showed only modest declines in titer as well (0.87−1.3-fold change).
Discussion
This pilot study of dcSSc patients with rituximab shows that standard doses of rituximab depletes B cells in both peripheral blood and skin, and is well-tolerated. Rituximab appears safe in patients with dcSSc. Infusion reactions were common but mild. Infectious complications were rare and easily treated.
Treatment efficacy is particularly difficult to access in open label trials in patients with SSc due to the wide variability in the natural history of disease progression. Even in untreated patients with early diffuse SSc, past studies have suggested that the average modified Rodnan skin score changes modestly at 6 and 12 months compared to baseline (10-13). On average rituximab-treated patients showed little change in skin score at 6 and 12 months compared to baseline. The 95% confidence interval suggests that rituximab might promote at most a modest improvement in skin score.
Several features of the clinical course of these treated patients provide some motivation to consider further evaluation of efficacy. Patients entered into this study showed no major end organ progression. This includes a lack of progressive pulmonary or renal disease in a population that is at relatively high risk for these complications. Although scleroderma renal crisis (SRC) is more common in patients with anti-RNAP-III (5/15 study patients), the frequency of this complication is still too low to strongly suggest a treatment effect.
Progressive pulmonary disease is relatively common. Although patients with advanced pulmonary disease were excluded from this study, 7/15 patients at study entry showed changes on HRCT indicating mild pulmonary disease. For comparison, the FVC of patients in the penicillamine trial showed modest improvements in both low dose- (increase FVC of 2.6% predicted) and high dose- (increase FVC of 1.9% predicted) penicillamine treated groups at 12 months (13). Rituximab-treated patients showed an average increase in FVC of 3.5% predicted at 6-months compared to baseline, but with a wide 95% confidence limit, emphasizing the difficulty in interpreting changes in a study of this size. Indeed, the landmark trial of cyclophosphamide, showing a benefit for SSc-associated pulmonary fibrosis (change in predicted FVC of −1.0 % in cyclophosphamide treated compared to −2.6% for placebo treated patients), required data from 145 patients (10). Thus, the current study provides no clear picture regarding potential rituximab efficacy for SSc-associated interstitial lung disease.
This study confirms prior work, showing an excellent correlation between the Rodnan skin score and durometry score in a clinical therapeutic trial. As for the skin score we observed no significant change in patients treated with rituximab.
This study also explored the potential utility of the myofibroblast score as a supplemental measure for skin disease in SSc trials. Confirming our recently published observations (8), we again found in this new patient cohort that the myofibroblast score correlates highly with the skin score. Several patients had marked reduction in the myofibroblast score after treatment with rituximab. If a decrease in myofibroblasts is an early, pre-clinical indicator of improvement in scleroderma then these data suggest that B cell depletion may be efficacious for SSc.
Despite the clear effect of rituximab to deplete circulating B cells, the mechanism behind its action in connective tissue diseases remains uncertain. Rituximab treatment led to variable, but always modest, changes in autoantibody levels. Thus, this mechanism could not appear to provide rationale for a therapeutic benefit in patients with dcSSc. In recent studies, rituximab depleted B cells in most (but not all) patient synovial tissues in patients with rheumatoid arthritis (14, 15). Although local B cell functions, i.e. antigen presenting cell function or cytokine secretion, have been considered as alternative mechanisms for rituximab activity, the clinical response in patients with RA did not correlate with the degree of pretreatment B cell infiltration in synovial tissues. To the extent that local B cell infiltration is an important component of rituximab's mode of action, this therapy might prove more efficacious when targeting tissues showing dense B cell infiltrates, such as in SSc-associated pulmonary fibrosis.
At baseline, B cells in skin biopsies were generally perivascular as is the case for most of the mononuclear cell infiltrates seen in SSc skin. As no B cells were identified in skin from eight healthy controls (data not shown), perivascular B cell infiltrates are clearly a pathologic feature of SSc skin.
In summary, rituxumab was found to deplete peripheral and dermal B cells, but was not found to provide any clear benefit on scleroderma skin disease. However, the safety of the medication and the potential efficacy related to B cell infiltration of other organs such as the lungs suggest that rituximab might be worth further study for SSc-associated pulmonary fibrosis, perhaps in combination with cyclophosphamide.
Acknowledgements
This study was supported by NIH grants for the Boston University Medical Center General Clinical Research Center Grant: M01 RR00533 and U01AR055063 (RL), and by Genentech and BiogenIdec.
REFERENCES
- 1.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354(25):2667–76. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 2.Whitfield ML, Finlay DR, Murray JI, Troyanskaya OG, Chi JT, Pergamenschikov A, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci U S A. 2003;100(21):12319–24. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita T, Fujimoto M, Hasegawa M, Matsushita Y, Komura K, Ogawa F, et al. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J Invest Dermatol. 2007;127(12):2772–80. doi: 10.1038/sj.jid.5700919. [DOI] [PubMed] [Google Scholar]
- 4.Saito E, Fujimoto M, Hasegawa M, Komura K, Hamaguchi Y, Kaburagi Y, et al. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J Clin Invest. 2002;109(11):1453–62. doi: 10.1172/JCI15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafyatis R, O'Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56(9):3167–8. doi: 10.1002/art.22847. [DOI] [PubMed] [Google Scholar]
- 6.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 7.Kissin EY, Schiller AM, Gelbard RB, Anderson JJ, Falanga V, Simms RW, et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 2006;55(4):603–9. doi: 10.1002/art.22093. [DOI] [PubMed] [Google Scholar]
- 8.Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54(11):3655–60. doi: 10.1002/art.22186. [DOI] [PubMed] [Google Scholar]
- 9.Santiago M, Baron M, Hudson M, Burlingame RW, Fritzler MJ. Antibodies to RNA polymerase III in systemic sclerosis detected by ELISA. J Rheumatol. 2007;34(7):1528–34. [PubMed] [Google Scholar]
- 10.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 11.Seibold JR, Korn JH, Simms R, Clements PJ, Moreland LW, Mayes MD, et al. Recombinant human relaxin in the treatment of scleroderma. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;132(11):871–9. doi: 10.7326/0003-4819-132-11-200006060-00004. [DOI] [PubMed] [Google Scholar]
- 12.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56(1):323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 13.Clements PJ, Furst DE, Wong WK, Mayes M, White B, Wigley F, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42(6):1194–203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Teng YK, Levarht EW, Hashemi M, Bajema IM, Toes RE, Huizinga TW, et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum. 2007;56(12):3909–18. doi: 10.1002/art.22967. [DOI] [PubMed] [Google Scholar]
- 15.Vos K, Thurlings RM, Wijbrandts CA, van Schaardenburg D, Gerlag DM, Tak PP. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(3):772–8. doi: 10.1002/art.22400. [DOI] [PubMed] [Google Scholar]