Abstract
Recent experiments have studied the development of orientation selectivity in normal animals, visually deprived animals, and animals where patterns of neuronal activity have been altered. Results of these experiments indicate that orientation tuning appears very early in development, and that normal patterns of activity are necessary for its normal development. Visual experience is not needed for early development of orientation, but is crucial for maintaining orientation selectivity. Neuronal activity and vision thus seem to play similar roles in the development of orientation selectivity as they do in the development of eyespecific segregation in the visual system.
Keywords: visual cortex, orientation selectivity, ocular dominance, activity maps, ferret, development
One of the most conspicuous functional properties of neurons in the adult visual cortex is their orientation selectivity. How orientation selectivity develops during early postnatal life, and what role visual experience plays in this process are questions of considerable interest. Experience-dependent development of another response property of cortical cells, ocular dominance, has been widely studied, but it is only recently that a substantial amount of new information about the development of orientation preference has been gathered. Electrophysiological and optical imaging studies have elucidated the timing of the development of orientation selectivity at the single-cell level and in orientation preference maps. Pharmacological manipulations and visual deprivation studies have examined the roles of spontaneous and visually driven neuronal activity. These studies show that orientation selectivity and its orderly representation in orientation preference maps appear early in development. Furthermore, they show that normal neuronal activity patterns are vital for the development of proper orientation selectivity. In contrast, visually driven activity is not needed for the initial development of orientation selectivity or orientation maps. Vision does, however, become important for normal maturation and maintenance of cortical orientation selectivity.
EXPERIMENTAL RESULTS
Early Experiments
Single-Cell Approaches
Efforts to determine when cells in cat visual cortex first show signs of orientation selectivity have proved difficult, yielding somewhat conflicting results. The reported percentages of orientation-selective cells in 1-week-old kittens has varied from zero (Barlow and Pettigrew, 1971; Pettigrew, 1974), through 20–30% (Blakemore and Van Sluyters, 1975; Buisseret and Imbert, 1976; Fregnac and Imbert, 1978; Albus and Wolf, 1984), to 100% (Hubel and Wiesel, 1963). Some of this discrepancy may be due to differences in the definitions of “orientation-selective” responses used by the different authors. Moreover, cortical cells in young animals are poorly responsive and habituate rapidly (Hubel and Wiesel, 1963), making a precise assessment of neuronal response characteristics rather difficult.
Functional Organization
In their initial studies on the tangential organization of the cat visual cortex Hubel and Wiesel (1962) described the orderly arrangement of cells into orientation preference maps. Studying how and when these maps develop in the young animal has proven to be an even greater technical challenge than studying the development of orientation selectivity at the single-cell level. Single-unit electrophysiological mapping experiments provide very limited information about orientation preference maps even in adult animals, and are impractical in very young animals. 2-Deoxyglucose labeling techniques have provided some information about early orientation maps (LeVay et al., 1978, 1980; Des Rosier et al., 1978; Thompson et al., 1983), but the fact that such studies cannot be performed chronically, together with the individual differences in the layouts of the maps between animals, makes these results difficult to interpret.
Newer Experimental Approaches
Recent advances, including the use of the ferret as an experimental animal and the use of the optical imaging technique, have helped to mitigate the technical difficulties of studying the development of orientation preference in visual cortex and have led to new insights in this field.
Ferrets have a visual system which is quite similar to that of the cat (Law et al., 1988), but they are born developmentally approximately 3 weeks younger (Linden et al., 1981). Therefore, ferrets provide a relatively hardy electrophysiological preparation during the time when orientation-specific responses are developing, and are thus an ideal animal to study the development of orientation preference in young animals.
Optical imaging of intrinsic signals (Grinvald et al., 1986; Bonhoeffer and Grinvald, 1996) is a technique used to study the functional tangential organization of the cerebral cortex. Because of its relative noninvasiveness [demonstrated early on by Frostig et al. (1990) by imaging through the closed skull], it is ideally suited for studying cortical maps in young animals. Most important, chronic optical imaging for the first time has allowed the development of orientation preference maps to be followed in individual animals.
In addition to those major improvements, a number of other new techniques have become available to pharmacologically and electrically influence activity of cells in the developing visual system. The combination of these methodological advances has over the past 5–10 years allowed more precise experimental assessment of the factors controlling the emergence of orientation preference in the visual cortex.
Normal Development of Orientation Selectivity Studied at the Single-Cell Level
Early postnatal development of orientation selectivity was studied in normal pigmented ferrets (Chapman and Stryker, 1993). The earliest age at which visually driven activity was observed in ferret visual cortex was postnatal day (P)23, comparable to P2 in the cat. This developmental stage is considerably earlier than the time when it was possible to record neuronal activity in kitten cortex. Nevertheless, at this age good orientation selectivity was found in some ferret cortical cells. Most neurons at this age, however, showed unoriented responses. The percentage of oriented cells remained constant until the end of postnatal week 5 (corresponding to week 2 in kitten), when more oriented cells could be observed. The number of oriented cells continued to increase until postnatal week 7, at which time the proportion of orientation-selective neurons was indistinguishable from that seen in the adult.
Normal Development of Orientation Maps
The timing of the development of orientation preference maps does not necessarily follow that of the appearance of the first oriented responses in visual cortex. For an orientation map to be seen with optical imaging, a substantial number of oriented cells is required. In addition, it is conceivable that neurons first acquire their orientation selectivity in a spatially unorganized fashion, and that the orderly representation of the orientation preference map is only achieved in a second developmental step.
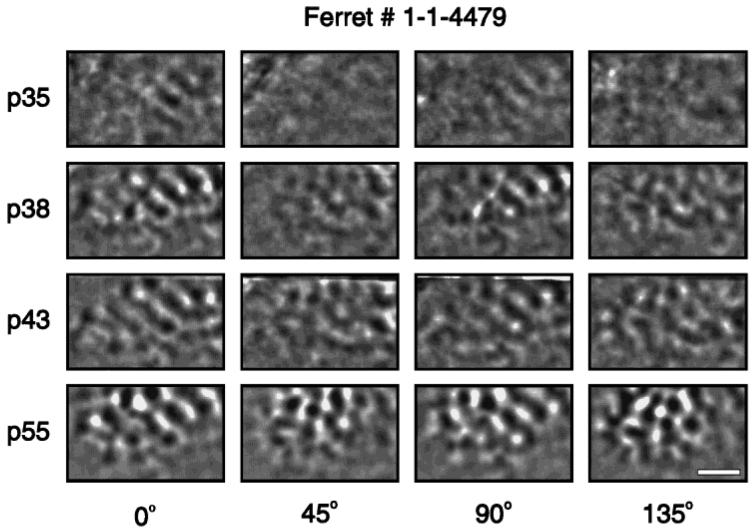
The emergence of orientation maps in the visual cortex was first studied in ferrets (Chapman et al., 1996). Figure 1 shows an example of a chronic optical imaging experiment following the development of orientation maps in an individual animal. In this representative example (for others, see Chapman et al., 1996), it is apparent that while the maps get stronger over time, their general structure (i.e., size, shape, location of the iso-orientation domains) changes very little during the course of development.
Figure 1.
Development of orientation maps revealed by chronic optical imaging. Single-condition orientation maps at four different ages in one animal. Each row of the figure shows orientation maps recorded in the left primary visual cortex of one ferret at the age [postnatal day (p)] indicated at the left of the row. Each column of single-condition maps shows orientation maps recorded in response to a particular orientation of a moving square wave grating (0° = horizontal). For each map, caudal is up and medial is to the left. The curve in the upper left corner of each map indicates the location of the caudal pole of the cortex behind which the skull remained intact over the cerebellum. In this example, the first clear orientation maps can be seen at postnatal day 38. Individual iso-orientation domains remain stable over time and do not change their position, shape, or size. Scale bar = 2 mm.
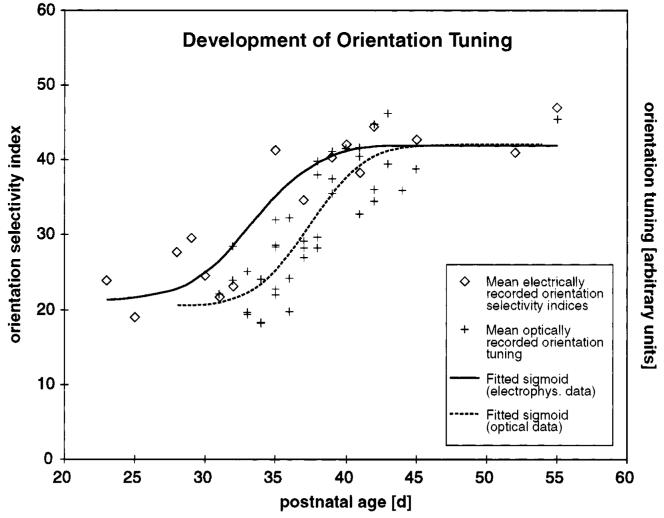
Later studies in kittens (Gödecke et al., 1997) yielded similar results. In both ferret and cat, the timing of the map development lagged behind the development of orientation selectivity at the single cell level (Fig. 2).
Figure 2.
Comparison of the development of orientation tuning assessed by optical imaging and electrophysiology. Orientation tuning assessed electrophysiologically from single-unit recordings compared with optical imaging of the development of orientation tuning. Optical imaging data from Chapman et al. (1996) (crosses); single-unit data from Chapman and Stryker (1993) (diamonds). The orientation selectivity index for electrophysiological data is calculated from the Fourier transform of the orientation tuning histogram recorded for each neuron. It equals the amplitude of the second harmonic component normalized by dividing by the sum of the DC level and the amplitude of the second harmonic component and multiplying by 100. The orientation tuning for the optical imaging data is the median length of the vectors in the polar maps. The solid curve indicates the best-sigmoid-fit curve through electrophysiological data, while the dashed curve is the best-sigmoid fit from the optical imaging data. The mean of the best-fit sigmoid for the electrophysiological data is 4 days earlier (P33.4) than the mean for the optical imaging data (P37.4).
Effects of Altered Patterns of Neuronal Activity
In addition to studies of normal development, manipulations designed to disrupt spontaneous or visually driven neuronal activity have proved useful for understanding how orientation selectivity develops in the visual cortex.
The critical dependence of orientation selectivity development on neuronal activity has been demonstrated by experiments in which neuronal activity in the cortex was completely abolished. This was achieved by intracortical infusion of the sodium channel blocker tetrodotoxin (TTX) during the time when cortical orientation selectivity normally develops. This activity blockade prevented the development of cortical orientation selectivity (Chapman and Stryker, 1993). Ferrets treated with TTX during the period of orientation maturation (from postnatal week 4 through postnatal week 7) maintained the immature distribution of orientation selectivities typical of postnatal week 4 animals.
A more subtle pharmacological manipulation of activity consists of disrupting the normal equilibrium between on-center and off-center responses in the visual system. This can be achieved by silencing on-center retinal ganglion cells during development with daily intravitreal injections of the glutamate analog 2-amino-4-phosphonobutyrate (APB). This procedure, like cortical application of TTX, “freezes” orientation selectivity in an immature state (Gödecke and Chapman, 1998). Interestingly, when ferrets were treated with an APB dosage which blocked on-center activity only half the time, normally organized (though weak) orientation maps developed Gödecke and Chapman, 1998). This indicates that discontinuous periods of “normal” activity are sufficient to produce a normal orientation map.
Normal patterns of activity have also been disrupted by chronic electrical stimulation of the optic nerve during development (Weliky and Katz, 1997). This overrides intrinsic patterned neuronal activity originating from the retina (Meister et al. 1991; Wong and Oakley, 1996) by artificially increasing the correlation between inputs to the cortex. The stimulation protocol, which activated the visual pathway for 10% of the time while leaving activity normal for 90% of the time, radically reduced the orientation selectivity of individual cells, while leaving the overall pattern of orientation maps in the cortex intact (Weliky and Katz, 1997).
Effects of Altered Visual Experience
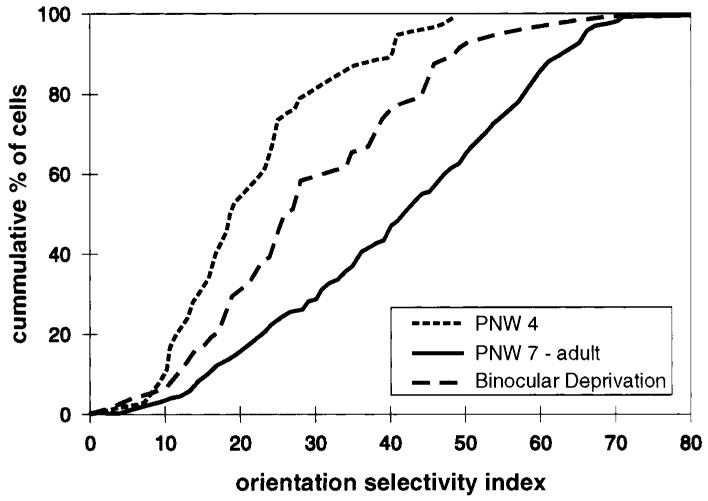
It must have been surprising to see that a newborn monkey without any visual experience shows well-developed orientation selectivity (Hubel and Wiesel, 1974). This study demonstrated that in the monkey, visual experience is not needed for the initial development of orientation selectivity. Indeed, later studies in cat (Blakemore and Van Sluyters, 1975; Buisseret and Imbert, 1976) and ferret (Chapman and Stryker, 1993) showed that even during binocular visual deprivation some degree of single-cell orientation selectivity does develop. Figure 3 demonstrates that ferrets binocularly deprived from before the time of natural eye opening through the 8th to 13th postnatal week showed significantly better orientation tuning than immature animals. However, these animals showed significantly poorer orientation tuning than age-matched controls. In this study, no short-term binocular deprivations were performed, so it is not clear to what extent these results were due to effects of deprivation on the initial emergence of orientation and to what extent they were due to effects on the maintenance of orientation selectivity.
Figure 3.
Cumulative histogram showing orientation selectivity index distributions for normal immature ferrets (postnatal week 4; n = 4 animals), normal mature ferrets (postnatal week 7 through adult; n = 16 animals), and binocularly deprived ferrets (lid sutured from before the time of natural eye opening through recording sessions on P55, P56, P66, P87, P88, and P90; n = 6 animals). The three distributions are statistically distinct; Mann–Whitney U test, p < .001). Data are from Chapman and Stryker (1993).
Visual experience is also not needed for the initial development of cortical orientation maps. Early maps are seen in ferret visual cortex before the time of natural eye opening (Chapman et al., 1996), and normal orientation maps develop in kittens binocularly deprived for the first 3 weeks of life (Gödecke et al., 1997; Crair et al., 1998). However, longer periods of binocular deprivation cause degradation of orientation preference maps (Crair et al., 1998), indicating that visual experience is needed for their maintenance, consistent with prior electrophysiological results (Blakemore and Van Sluyters, 1975; Buisseret and Imbert, 1976).
These studies show that the initial development of orientation selectivity does not require visual experience. However, visual experience is needed for maintenance and maturation of both orientation selectivity at the single-cell level (Chapman and Stryker, 1993) and orientation preference maps (Crair et al., 1998).
It has also become clear that visual experience is not responsible for the alignment of orientation maps between the two eyes. Kittens raised under a reverse-suture protocol such that the two eyes never had visual experience at the same time nevertheless developed nearly identical orientation preference maps for the two eyes (Gödecke and Bonhoeffer, 1996).
DISCUSSION
The detailed analysis of the development of orientation selectivity at the single-cell level which has been possible in the ferret has raised new questions about how cortical orientation develops. The presence of a few orientation selective cells very early in development, along with the fact that the percentages of tuned cells remain constant for more than a week after the cortex first becomes responsive, suggests that there may be two different mechanisms underlying orientation selectivity in the young cortex. Nothing is known so far about the development of the earliest oriented cells in the cortex. Further experimentation is needed to determine whether the development of this early orientation selectivity is activity dependent, to study the earliest organization of orientation preference across the cortex, and to discover what neuronal connections may underlie the early orientation-tuned responses.
Later development during the fifth postnatal week, when orientation selectivity starts to mature in the ferret, is known to be dependent on neuronal activity, and seems to proceed normally only when there are natural patterns of on- and off-center input activity. These results are consistent with computational models of cortical orientation selectivity development which rely on correlations and anticorrelations in the activities of on- and off- center inputs to cortex to produce oriented cortical cell receptive fields (Miller, 1992, 1994; Miyashita and Tanaka, 1992).
Because there is little maturation of orientation in ferret layer IV cells (Chapman and Stryker, 1993), it is possible that the inputs from the lateral geniculate nucleus afferents are already arranged with receptive fields aligned in space (Chapman et al., 1991; Reid and Alonso, 1995; Ferster et al., 1996) creating orientation selectivity in layer IV as soon as the cortex is visually responsive. The maturation of orientation selectivity which occurs later in layer II/III and then in layer VI could be due first to the refinement of patchy connections in the supragranular layers (Luhman et al., 1986; Callaway and Katz, 1990; Ruthazer and Stryker, 1996) and then to the maturation of connections between supra- and infragranular layers.
Chronic imaging studies show that orientation maps develop normally in the absence of vision, in the same way that single-cell studies show that visual experience is not needed for the initial appearance of orientation preference. The functional layout of the orientation maps, including the typical map structure with iso-orientation domains forming “pinwheels” (Bonhoeffer and Grinvald, 1991), also develops without any visual experience. Proper maintenance of both individual cell orientation selectivity and orientation preference maps, however, only occurs if visual input is present.
Once development has proceeded to the point where orientation preference maps can be seen using optical imaging, the tangential arrangement of orientation preferences across cortex is remarkably stable. In both ferrets and kittens, orientation maps remain stable both during normal development (Chapman et al., 1996) and under conditions of altered visual experience (Gödecke and Bonhoeffer, 1996). Therefore, the layout of orientation preference maps is unaffected by the massive rearrangements of geniculocortical afferents which occur during development or plasticity of ocular dominance columns. Since geniculocortical afferents are thought to substantially contribute to the determination of orientation selectivity (Chapman et al., 1991; Reid and Alonso, 1995; Ferster et al., 1996), this map stability might appear surprising. However, there is no reason to expect that the loss of one eye's inputs to a given cortical domain (either during normal segregation of afferents into ocular dominance columns or during monocular deprivation) should affect the arrangement of the other eye's geniculate inputs to that domain. In addition, the patchy intracortical connections already established during this time may help further to stabilize the orientation maps.
The development of orientation selectivity in the cortex does not require visual experience, but is critically dependent on normal patterns of spontaneous neuronal activity. In this regard, development of orientation selectivity is similar to development of ocular dominance in the cortex where normal development of columns is seen in binocularly deprived animals (Hubel and Wiesel, 1965; Wiesel and Hubel, 1974), while development is prevented by neuronal activity blockade (Stryker and Harris, 1986) or synchronized activity from the two eyes (Stryker and Strickland, 1984). As in the case of ocular dominance (Blakemore and Van Sluyters, 1975; Fregnac and Imbert, 1978), visual experience is necessary for the maintenance of orientation selectivity in the cortex.
From the experiments discussed above, it is apparent that proper neuronal activity is needed for the establishment of orientation and ocular dominance maps. However, it is unclear whether activity patterns control the individual layout of these maps or whether intrinsic or even genetic factors are the main determinants of the functional layout. One attractive and feasible approach to distinguish between these possibilities is to record from genetically identical individuals and assess the similarity of their orientation and ocular dominance maps.
Acknowledgments
Contract grant sponsor: NIH; contract grant number: EY11369 Contract grant sponsor: Max-Planck Gesellschafr
Financial support was provided by NIH Grant EY11369, to BC, and the Max-Planck Gesellschaft, to TB.
REFERENCES
- Albus K, Wolf K. Early postnatal development of neuronal function in the kitten's visual cortex: a laminar analysis. J Physiol (Lond) 1984;348:153–185. doi: 10.1113/jphysiol.1984.sp015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Pettigrew JD. Lack of specificity of neurones in the visual cortex of young kittens. J Physiol (Lond) 1971;218:98–100. [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol (Lond) 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T, Grinvald A. Optical imaging based on intrinsic signals: the methodology. In: Toga A, Mazziotta JC, editors. Brain mapping, the methods. Academic Press; San Diego, CA: 1996. pp. 55–97. [Google Scholar]
- Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark-reared kittens. J Physiol (Lond) 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Emergence and refinement of clustered horizontal connections in cat striate cortex. J Neurosci. 1990;10:1134–1153. doi: 10.1523/JNEUROSCI.10-04-01134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Zahs KR, Stryker MP. Relationship of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J Neurosci. 1991;11:1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Rosiers MH, Sakurada O, Jehle J, Shinohara M, Kennedy C, Sokoloff L. Demonstration of functional plasticity in the immature striate cortex of the monkey by means of 14C-deoxyglucose method. Science. 1978;200:447–449. doi: 10.1126/science.417397. [DOI] [PubMed] [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature. 1996;380:249–252. doi: 10.1038/380249a0. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: the relationship between orientation selectivity and ocular dominance. J Physiol (Lond) 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and microstimulation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gödecke I, Bonhoeffer T. Development of identical orientation maps for two eyes without common visual experience. Nature. 1996;379:251–254. doi: 10.1038/379251a0. [DOI] [PubMed] [Google Scholar]
- Gödecke I, Chapman B. Effects of on-center retinal ganglion cell blockade on development of visual cortical responses. Soc Neurosci Abstr. 1989;24:1051. [Google Scholar]
- Gödecke I, Kim D-S, Bonhoeffer T. Development of orientation preference maps in area 18 of kitten visual cortex. Eur J Neurosci. 1997;9:1754–1762. doi: 10.1111/j.1460-9568.1997.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Lieke EE, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol (Lond) 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965;28:1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J Comp Neurol. 1988;278:157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ. Ocular dominance columns and their development in Layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978;179:223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Linden DC, Guillery RW, Cucciaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Martinez Millen L, Singer W. Development of horizontal intrinsic connections in cat striate cortex. Exp Brain Res. 1986;63:443–448. doi: 10.1007/BF00236865. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong ROL, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;17:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol (Lond) 1974;237:49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD. Development of orientation columns via competition between ON- and OFF-center inputs. Neuroreport. 1992;3:73–76. doi: 10.1097/00001756-199201000-00019. [DOI] [PubMed] [Google Scholar]
- Miller KD. A model for the development of simple cell receptive fields and the ordered arrangement of orientation columns through activity-dependent competition between on- and off-center inputs. J Neurosci. 1994;14:409–441. doi: 10.1523/JNEUROSCI.14-01-00409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita M, Tanaka S. A mathematical model for the self-organization of orientation columns in visual cortex. Neuroreport. 1992;3:69–72. doi: 10.1097/00001756-199201000-00018. [DOI] [PubMed] [Google Scholar]
- Reid RC, Alonso JM. Specificity of monosynaptic connections from thalamus to visual cortex. Nature. 1995;378:281–284. doi: 10.1038/378281a0. [DOI] [PubMed] [Google Scholar]
- Ruthazer E, Stryker MP. The role of activity in the development of long-range horizontal connections in area 17 of the ferret. J Neurosci. 1996;16:7253–7269. doi: 10.1523/JNEUROSCI.16-22-07253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents formation of ocular dominance columns in the cat's visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Strickland SL. Physiological segregation of ocular dominance columns depends on the pattern of afferent electrical activity. Invest Opthalmol Vis Sci. 1984;25(Suppl):278. [Google Scholar]
- Thompson ID, Kossut M, Blakemore C. Development of orientation columns in cat striate cortex revealed by 2-deoxyglucose autoradiography. Nature. 1983;301:712–715. doi: 10.1038/301712a0. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature. 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol. 1974;158:307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- Wong ROL, Oakley DM. Changing patterns of spontaneous bursting activity of on and off retinal ganglion cells during development. Neuron. 1996;16:1087–1095. doi: 10.1016/s0896-6273(00)80135-x. [DOI] [PubMed] [Google Scholar]