Abstract
ADP-ribosylation factor 6 (ARF6) is a small GTP-binding protein that regulates peripheral vesicular trafficking and actin cytoskeletal dynamics, and it has been implicated as critical to regulated secretion. Expression of a dominant-inhibitory ARF6 mutant, ARF6(T27N), impaired glucose-, depolarization-, and γ-thio-GTP-stimulated insulin secretion in the pancreatic β cell line, MIN6. In response to depolarization, MIN6 cells expressing ARF6(T27N) displayed an unaltered initial fast phase but an impaired subsequent slow phase of insulin secretion. Actin cytoskeletal disassembly with latrunculin A enhanced insulin secretion, whereas stabilization with jasplakinolide inhibited secretion, consistent with the actin cytoskeleton serving as a barrier to exocytosis in these cells. ARF6(T27N) led to a depolarization-dependent reduction in the levels of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] with a time course that paralleled the inhibition of secretion. Moreover, blockade of PI(4,5)P2-dependent events by expression of a lipid-binding protein resulted in inhibition of depolarization-induced secretion in a manner identical to ARF6(T27N). These results indicate that ARF6 is required to sustain adequate levels of PI(4,5)P2 during periods of increased PI(4,5)P2 metabolism such as regulated secretion.
ADP-ribosylation factor 6 (ARF6) protein represents a Ras-related small GTP-binding protein superfamily member that regulates intracellular trafficking events (1, 2). ARF6 has been implicated in vesicular transport and actin cytoskeleton reorganization and may also play a role in the secretion of preformed vesicles. In chromaffin cells, ARF6 was localized to secretory granules in the resting state and translocated to the plasma membrane on stimulation, and ARF6 peptides inhibited the regulated secretion of constituents of large, dense core granules (3, 4). In addition, expression of a catalytically inactive version of one of the putative exchange factors for ARF6, ADP ribosylation factor nucleotide exchange factor, inhibited secretion (5).
ARF6 activates phospholipase D (PLD) (6) and, because the product of PLD, phosphatidic acid, is required for many vesicular trafficking events, much attention has been focused on the ability of ARF6 to regulate PLD activity as a mechanism to explain the effects of ARF6 on secretion (7-10). However, ARF6 also influences other cellular processes that could contribute to the requirement for ARF6 for optimal regulated secretion. In particular, ARF6 has a direct role in mediating actin cytoskeletal rearrangements (11-13), which may be important for access of secretory vesicles to the plasma membrane (14-18), and evidence is accumulating that ARF proteins can stimulate the production of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] (19), which itself regulates the polymerization state of the actin cytoskeleton and primes secretory vesicles (20).
The secretion of granules containing insulin from pancreatic β cells represents an example of regulated secretion that has a major influence on whole-body glucose homeostasis. The best characterized pathway for the stimulation of secretion in β cells involves the metabolism of glucose, an increase in cytoplasmic ATP, depolarization of the plasma membrane, and an influx of Ca2+ (21). In this study, we confirm the results reported with synthetic peptides by using dominant-inhibitory ARF6 constructs. We then show that ARF6 is required for the maintenance of PI(4,5)P2 production during periods of rapid secretion and present data that this regulation of phosphoinositide metabolism is sufficient to explain the role of ARF6 in regulated secretion.
Materials and Methods
Virus and General Reagents. The recombinant adenovirus expressing Cre recombinase (AdCre) was a gift from F. L. Graham of McMaster University (Hamilton, ON, Canada) (22), and recombinant adenovirus expressing a PLCδPH-GFP fusion protein (AdPLCδPH-GFP) was generated by E. Whiteman (University of Pennsylvania) using a construct provided by T. Balla (National Institutes of Health, Bethesda) (23). Affinity-purified rabbit anti-hemagglutinin, mouse anti-ARF6, and all horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology. Latrunculin A and jasplakinolide were from Biomol (Plymouth Meeting, PA) and Molecular Probes, respectively. BSA and all other chemicals were purchased from Sigma. Construction of the inducible retroviral vector used in this study, pLPNPX1, has been described (24). Human hemagglutinin-tagged ARF6 mutant constructs, a gift from J. G. Donaldson (National Institutes of Health), and enhanced GFP from the plasmid pEGFP-N1 (Clontech) were cloned into pLPNPX1 by using the unique XhoI-BglII sites and the XhoI-NotI sites, respectively.
Cell Culture and Viral Gene Transfer. The MIN6 cells (passage 27-40) were a gift from K. S. Polonsky (Washington University, St. Louis). MIN6 cells were maintained at 37°C in a humidified atmosphere containing 7.5% CO2/92.5% air. To stably transduce pools of MIN6 cells, recombinant retrovirus was packaged in 293T cells transfected with the two pantropic retroviral packaging constructs, pVSV G and pCgp (gifts from M. H. Malim, Guy's Hospital, London), and a pLPNPX1 derivative containing either enhanced GFP or ARF6. Stably infected populations were selected in media containing 600 μg/ml G418. MIN6 cells were either mock-infected or infected with AdCre at an multiplicity of infection (moi) of ≈100 for 1 h at 37°C in a minimal volume of normal growth medium containing 1.5% FBS and experiments were performed 40-48 h later. For experiments examining the subcellular localization of PLCδPH-GFP, cells were infected as above but at an moi of ≈10 and imaged 24 h after infection. For experiments using AdPLCδPH-GFP to block PIP(4,5)P2 signaling, cells were infected with either AdGFP or AdPLCδPH-GFP at an moi of ≈100 and assayed 36-40 h after infection.
Insulin Secretion Assays. Cells were preincubated for 20 min in Hanks' balanced salt solution (HBSS) plus 0.25% RIA-grade BSA and 0.1 mM 3-isobutyl-1-methylxanthine at 37°C in room air. For experiments in which depolarization was used to stimulate secretion, the extracellular potassium concentration was raised to 59 mM with a concomitant drop in extracellular sodium to maintain osmolarity. Insulin content in media or cells was analyzed by RIA by the RIA Core of Diabetes Research Center at the University of Pennsylvania. For experiments in which actin-modifying drugs or alcohols were added to cells, agents were added for an hour before initiation of secretion and were present throughout the duration of the experiment. Experiments were repeated a minimum of four times in duplicate and expressed as the mean ± SEM.
Intracellular Ca2+ Measurements by Flow Cytometry. Cells were loaded in 2 μg/ml indo-1 AM (Molecular Probes) in the presence of 4 mM probenecid for 30 min at 37°C. Fifty-five minutes after depolarization, acquisition of the nondepolarized cells was initiated. After 1 min, these cells were depolarized and data were acquired for an additional 4 min, at which time the cells that had been depolarized an hour earlier were analyzed. At the conclusion of the experiment, ionomycin was added. Data were collected on an LSR flow cytometer (Becton Dickinson) and analyzed by using FLOWJO software (Tree Star, Ashland, OR).
Live Cell Image Acquisition and Analysis. Images were acquired on an inverted Nikon Eclipse TE300 with a ×100/1.4 Plan Apo long-working-distance objective by using an UltraVIEW LCI spinning-disk confocal imaging system (Perkin-Elmer Life Sciences) and quantified by using the METAMORPH suite (Universal Imaging, Downingtown, PA). For each frame, eight measurements of the maximum plasma membrane and five measurements of cytosol intensity were averaged and divided to give a maximum plasma membrane to average cytosol ratio. The values presented represent the means from five cells for each condition.
32P-Labeling, Lipid Extraction, and TLC Analysis. Cells were incubated for 4 h in 5 ml of HBSS plus 0.25% RIA-grade BSA, 0.1 mM 3-isobutyl-1-methylxanthine, and 2 mM glucose with 100 μCi/ml (1 Ci = 37 GBq) [32P]orthophosphate. Fifteen minutes after depolarization in the continued presence of [32P]orthophosphate, the cells were scraped from the dish into ice-cold 1 M HCl and added to 3.2 ml of chloroform/methanol (1:1) on ice, and the organic phase was collected. Samples were applied to 1% potassium oxalate-coated silica gel H TLC plates that had been preactivated at 90°C for 45 min, allowed to dry for 20 min, and developed in chloroform/methanol/4 M NH4OH (9:7:2).
Results
Expression of ARF6 Mutants by Using a Cre-Inducible Retroviral Vector. An inducible retroviral expression system was used to study the effect of ARF6 on secretion of insulin from the pancreatic cell line MIN6. As a control, MIN6 cells were infected with retrovirus encoding GFP, and pools of G418-resistant colonies were selected (24). Induction of GFP was detected 12 h after infection of with AdCre and reached a uniformly high level of expression in ≈85% of the cells between 36 and 48 h (see Fig. 8 A and B, which is published as supporting information on the PNAS web site). MIN6 cells were infected with retrovirus encoding WT and mutants of hemagglutinin-tagged ARF6. Western immunoblot analysis revealed no expression of the gene product in uninfected cells but robust expression of the ARF6 constructs 40 h after infection with AdCre (Fig. 8C, published as supporting information on the PNAS web site). The hemagglutinin epitope tag attached to the exogenously expressed ARF6 protein retarded electrophoretic mobility compared with the endogenous protein, allowing an estimate of the degree of overexpression. The WT ARF6 and the mutants, except ARF6(T27N), were expressed at levels at least 10 times greater than the endogenous ARF6, whereas expression of the dominant-interfering ARF6(T27N) mutant was somewhat variable but in the range of 2-fold above endogenous levels (Fig. 8C, which is published as supporting information on the PNAS web site).
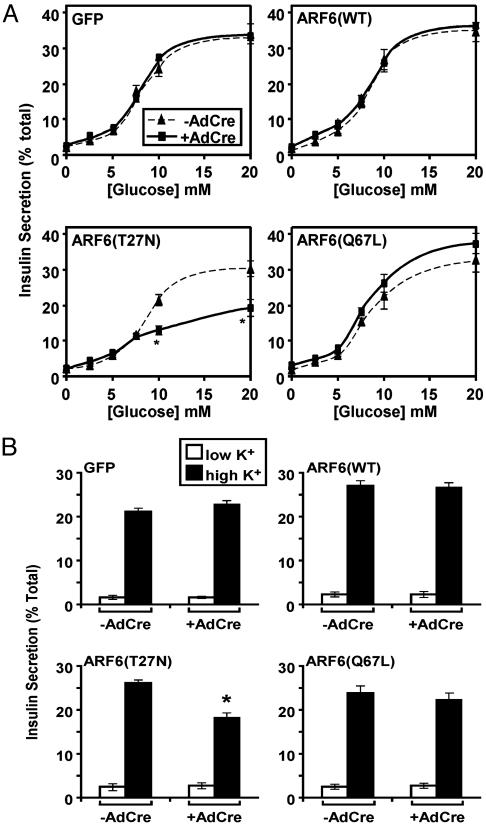
Expression of a Dominant-Inhibitory ARF6 Protein Inhibits Insulin Secretion. Cells infected with retroviruses encoding the indicated gene products were either mock-infected or exposed to AdCre, and 40 h later the secretion of insulin was determined in response to glucose. As shown in Fig. 1A, infection with AdCre and expression of GFP had no effect on insulin secretion in response to glucose. Expression of WT ARF6, ARF6(WT), or constitutively active ARF6, ARF6(Q67L), did not alter glucose-dependent insulin secretion. However, ARF6(T27N) reduced glucose-stimulated insulin secretion without changing the half-maximal dose of glucose required to elicit secretion (Fig. 1 A). As with glucose-induced insulin secretion, depolarization-induced secretion was not affected by expression of GFP or any of the ARF6 mutants, except ARF6(T27N), which attenuated insulin secretion over 1 h (Fig. 1B). Although the reduction in this experiment appears modest, the slow phase of insulin release was completely suppressed and is presented below. Expression of ARF6(T27N) also did not affect the level of intracellular Ca2+ during 1 h of depolarization (data not shown).
Fig. 1.
Role of ARF6 in glucose- and depolarization-stimulated insulin secretion. Pools of MIN6 cells that express the indicated proteins upon infection with AdCre were either mock-infected (-AdCre) or infected with AdCre (+AdCre). (A) Cells were stimulated with the indicated concentration of glucose, and the amount of insulin secreted after 1 h, as a percentage of the total, was determined. *, P < 0.05, statistically significant difference between the -AdCre and +AdCre samples. (B) Cells were either left unstimulated (low K+) or were depolarized in medium containing a high concentration of potassium (high K+) for 1 h, and the amount of insulin secreted was determined. Data are the means ± SEM of four independent experiments. *, P < 0.05, statistically significant difference between the -AdCre and +AdCre.
In streptolysin O-permeabilized cells the nonhydrolyzable GTP analogue guanosine 5′-[γ-thio]triphosphate (GTPγS) stimulates insulin secretion through a poorly defined mechanism. Expression of GFP, ARF6(WT), and ARF6(Q67L) had no effect on GTPγS-dependent insulin secretion; however, ARF6(T27N) reduced GTPγS-stimulated secretion of insulin (Fig. 2). These results indicate that ARF6 is necessary, but not rate-determining, for maximal insulin secretion, and that the site of action of ARF6 is downstream of Ca2+ influx and common to both the nutrient- and GTPγS-stimulated pathways.
Fig. 2.
Role of ARF6 in GTPγS-induced insulin secretion. After either mock infection (-AdCre) or infection with AdCre (+AdCre), MIN6 cells were permeabilized with streptolysin O (SLO) and incubated in the absence (-GTPγS) or presence (+GTPγS) of GTPγS. The amount of insulin secreted after 1 h was determined. *, P < 0.05, statistically significant difference between the -AdCre and +AdCre samples.
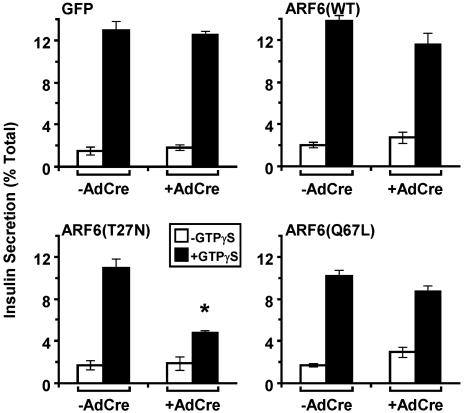
Like many neuroendocrine cells, MIN6 cells displayed an initial fast phase of insulin secretion lasting ≈10 min followed by a second phase of slower, sustained secretion (Fig. 3). ARF6(T27N) abrogated the slow phase of insulin secretion without altering the initial fast phase of insulin release (Fig. 3).
Fig. 3.
Effect of ARF6 on the time course of insulin secretion after depolarization. MIN6 cell lines, either mock-infected (-AdCre) or infected with AdCre (+AdCre), were depolarized, and the total amount of insulin released over time was determined.
ARF6 Targets as Potential Mediators of the ARF6(T27N)-Dependent Inhibition of Insulin Secretion. The production of phosphatidic acid by PLD is inhibited by primary alcohols, such as 1-butanol but is unaffected by secondary or tertiary alcohols, such as 2-propanol and tert-butyl alcohol; for this reason, alcohols have been used as relatively crude but effective probes for the role of PLD in a given biological process (25). Whereas 2-propanol or tert-butyl alcohol had no effect on the secretion of insulin, 1-butanol reduced insulin secretion with an ID50 that corresponds well with prior reports of the inhibition of PLD activity (Fig. 4A) (25). Inhibition of insulin secretion by even submaximal concentrations of primary alcohol primarily reduced the rapid phase of insulin secretion (Fig. 4B). This pattern differed markedly from that observed with ARF6(T27N) (Fig. 3) and thus, although consistent with a role for PLD in secretion, suggests that inhibition of PLD alone is unlikely to mediate the ARF6(T27N) phenotype.
Fig. 4.
Effect of primary alcohols on depolarization-induced insulin secretion. (A) MIN6 cells were preincubated with the indicated concentration of the primary alcohols 1-butanol (1-BuOH) and ethanol (EtOH) or the control secondary alcohols 2-butanol (2-BuOH) and 2-propanol (2-Prop) for 1 h. The amount of insulin secreted in 1 h in response to depolarization in the continued presence of the alcohols was then determined. (B) The pattern of insulin secretion in response to depolarization after treatment, as in A, with the indicated concentrations of 1-butanol and 2-propanol, was determined.
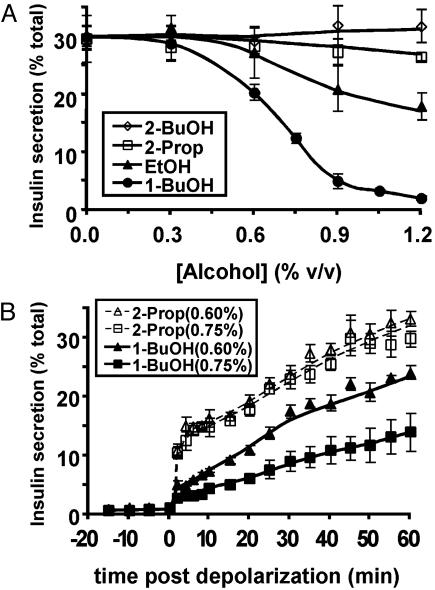
We next evaluated the role of cortical actin cytoskeleton. Treatment of WT MIN6 cells with the actin-depolymerizing drug latrunculin A markedly potentiated insulin secretion, whereas treatment with jasplakinolide, which prevents depolymerization, inhibited insulin secretion (Fig. 5A). Examination of the pattern of inhibition by jasplakinolide revealed that the initial rapid secretion response was relatively intact, but that, after the first few minutes, very little insulin was released (Fig. 5B). This finding is similar to the pattern of inhibition observed in the presence of ARF6(T27N), although the overall amount of hormone released was reduced to a greater extent by jasplakinolide. These data are consistent with the idea that the cortical actin cytoskeleton functions as a barrier that, when unable to be disassembled locally, allows only those secretory vesicles that are already outside of the barrier to fuse with the plasma membrane (14-18, 26, 27).
Fig. 5.
Role of the actin cytoskeleton in mediating the dominant-negative ARF6(T27N) inhibition of secretion. (A) MIN6 cells were preincubated with vehicle (DMSO) or cytochalasin D (CytoD), latrunculin A (LatA), or jasplakinolide (Jas) for 1 h. The amount of insulin secreted after 1 h under both basal (low K+) and depolarizing (high K+) conditions, in the continued presence of the actin-modifying agents, was determined. (B) The pattern of insulin secretion in response to depolarization after treatment with jasplakinolide (Jas), as in A, compared with vehicle (DMSO) was determined.
The PH domain of phospholipase Cδ (PLCδ) specifically binds PI(4,5)P2 with high affinity, and a GFP fusion of this domain (PLCδPH-GFP) has been used to assay both PI(4,5)P2 localization and levels in vivo (23). PLCδPH-GFP was expressed in MIN6 cells in which ARF6(T27N) had or had not been induced, and confocal images were acquired through the center of the cell every 30 sec for 5 min. High K+ medium was added, and images were collected for an additional 55 min. In cells uninfected by AdCre, PLCδPH-GFP was localized peripherally, and depolarization produced no discernable effect on this distribution. In contrast, MIN6 cells that expressed ARF6(T27N) were unable to maintain the plasma membrane localization of the PLCδPHGFP throughout the duration of the experiment. In these cells, PLCδPH-GFP quickly redistributed to a diffuse cytosolic pattern after depolarization (Fig. 6A). In some cells, aggregation of PLCδPH-GFP was apparent in the cell interior, although this finding was inconsistent (Fig. 6A). Quantitation of the plasma membrane-to-cytosol intensity ratio revealed that expression of ARF6(T27N) resulted in a significant decrease in plasma membrane PLCδPH-GFP immediately after depolarization, a time course that parallels the inhibition of secretion by ARF6(T27N) (Fig. 6B). As a complementary approach to assess the effects of ARF6(T27N) on PI(4,5)P2, phosphoinositides were measured directly by in vivo labeling. Consistent with the estimates of PI(4,5)P2 levels when the PLCδPH-GFP fusion was used, expression of dominant-interfering ARF6 mutant dramatically reduced the levels of PI(4,5)P2 after depolarization (Fig. 6C).
Fig. 6.
Role of ARF6 in maintenance of plasma membrane PI(4,5)P2 after depolarization-induced secretion. MIN6 cells that express ARF6(T27N) on infection with AdCre were either mock-infected (-AdCre) or infected with AdCre (+AdCre). All cells were then infected with AdPLCδPH-GFP at low titer. (A) The localization of the PI(4,5)P2, as indicated by the fluorescence from the expressed PLCδPH-GFP, was assessed in live cells by confocal microscopy through the center of the cell every 30 sec. A composite photomicrograph at the indicated times (minutes) relative to depolarization at time 0 is presented. The full time-lapse recording can be found in Movie 1, which is published as supporting information on the PNAS web site. (B) From five time-lapse recordings for each condition, an average maximum plasma membrane (PM) to average cytosol ratio ± SD was determined; this ratio is presented for each frame captured over time relative to the initiation of depolarization. (C) Cells were 32Pi-labeled and depolarized for 15 min in the continued presence of label. The extracted lipids from duplicate samples were resolved by TLC together with the indicated phosphoinositide standards and detected by autoradiogram. The locations of phosphoinositide standards are indicated.
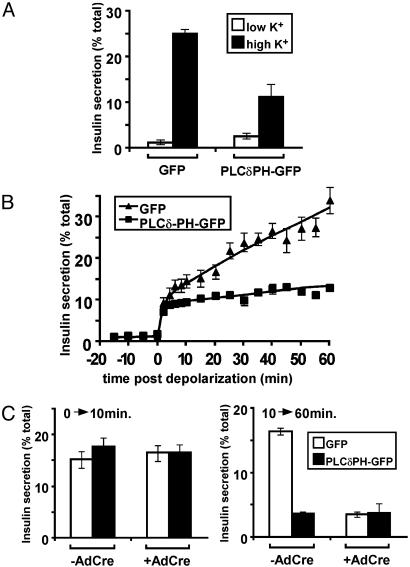
If indeed plasma membrane PI(4,5)P2 were critical to a cellular action, one might predict that expression of a PI(4,5)P2-binding protein at high levels would sequester the phospholipid and block downstream signaling (28). To test this idea, MIN6 cells were infected by an adenovirus expressing PLCδPH-GFP at substantially higher multiplicities of infection than those used in the experiments shown in Fig. 6, such that greater levels of expression were achieved. Sequestration of the PI(4,5)P2 by high levels of PLCδPH-GFP significantly blocked the secretion of insulin in response to depolarization (Fig. 7A). The phospholipid-binding protein only reduced the second, slow phase of insulin secretion, in a manner indistinguishable from that produced by ARF6(T27N) (Fig. 7B). If ARF6(T27N) and PLCδPHGFP were blocking insulin secretion by the same mechanism, one would expect their effects not to be additive. In fact, expression of PLCδPH-GFP in cells also expressing ARF6(T27N) had no additional effect on insulin secretion (Fig. 7C).
Fig. 7.
Role of PI(4,5)P2 in the secretion of insulin. (A) MIN6 cells were infected with either a high titer of AdPLCδPH-GFP or an equivalent titer of the control virus AdGFP. The amount of insulin secreted after 1 h under both basal (low K+) and depolarizing (high K+) conditions was determined. (B) The pattern of insulin secretion in response to depolarization after expression of PLCδPH-GFP or control GFP as in A was determined. (C) MIN6 cells that express ARF6(T27N) on infection with AdCre were either mock-infected (-AdCre) or infected with AdCre (+AdCre). High titers of either AdPLCδPH-GFP or AdGFP were then used to initiate expression of PLCδPH-GFP and GFP, respectively. The amount of insulin secreted after 10 and 60 min in response to depolarization was determined.
Discussion
In this study we have described a role for ARF6 in the secretion of insulin in the pancreatic β cell line MIN6. More importantly, we have localized the site of requirement for ARF6 to the slow, second phase of stimulus-dependent exocytosis. Two experiments strongly support the idea that the mechanism by which ARF6 allows sustained secretion is by the maintenance of PI(4,5)P2 during prolonged exposure to secretagogue. First, whereas WT insulinoma cells display little change in the level or distribution of PI(4,5)P2 when depolarized, cells expressing a dominant-inhibitory mutant of ARF6 rapidly lose plasma membrane and total PI(4,5)P2 after depolarization. Second, blockade of PI(4,5)P2 signaling by overexpression of a specific, high-affinity lipid-binding protein also inhibits insulin secretion and, most critically, does so with a time course precisely mimicking that of dominant-inhibitory ARF6. Taken together, these data present a compelling argument that a major role of ARF6 in regulated secretion is in the regeneration of PI(4,5)P2.
The ability of ARF6(T27N) to reduce the levels of PI(4,5)P2 in stimulated MIN6 cells provides additional support to a growing body of literature describing the complex relationship between ARFs and PI(4,5)P2. ARF1 or ARF6 directly activates a type I phosphatidylinositol 4-phosphate [PI(4)P] 5-kinase on Golgi complex membranes and in plasma membrane ruffles, respectively (19, 29). In addition, phosphatidic acid, a product of ARF-stimulated PLD activity, also activates PI(4)P 5-kinase (30). ARF and phosphatidylinositol transfer protein reconstitute secretion in cytosol-depleted HL90 cells by stimulating PI(4,5)P2 synthesis (31). In HeLa cells, ARF6 colocalizes with PI(4,5)P2-containing regions of the plasma membrane and tubular endosomes, and expression of a dominant-active ARF6 mutant induces the formation of PI(4,5)P2-positive membranes (32). Nonetheless, up until now, it has proved difficult to demonstrate any requirement for ARF6 in the maintenance of PI(4,5)P2 levels in vivo.
Based on substantial evidence supporting a role for PI(4,5)P2 during the phase of secretion in which vesicles become competent for fusion, we suggest that a major mechanism by which ARF6(T27N) inhibits is through the lack of sufficient plasma membrane PI(4,5)P2 to sustain secretion. In pancreatic β cells, the turnover of phosphoinositides is dramatically increased by secretagogue, such that as much as 30% of the total cellular pools of PI(4)P and PI(4,5)P2 turn over in the first minute after depolarization (33-35). Despite this finding, total levels of the phospholipid are well preserved, indicating a significant, concomitant acceleration in phosphoinositide synthesis. Presumably, both the breakdown and the synthesis of PI(4,5)P2 must be coordinately stimulated during secretion. It is likely that the initial influx of Ca2+ after membrane depolarization initiates a positive-feedback loop, activating phospholipase C-dependent cleavage of PI(4,5)P2 in addition to stimulating exocytosis. Although the generation of inositol 1,4,5-trisphosphate is important for the release of Ca2+ from internal stores, it also creates the need for increased PI(4,5)P2 synthesis to prevent a depletion of this phosphoinositide. We propose that ARF6 is required for this increased synthesis of PI(4,5)P2. One attractive aspect of this model is that it explains the lack of effect of a constitutively active mutant of ARF6; even if this mutant were capable of elevating PI(4,5)P2, it is unlikely that this alone would have any appreciable effect on secretion. Even more compelling is the obvious predicted consequences on secretion, i.e., a loss of slow, delayed secretion.
The requirement for PI(4,5)P2 during both Ca2+- and GTPγS-stimulated secretion has been demonstrated in several systems. At least two possibly interrelated explanations can be advanced to explain this: (i) a need for PI(4,5)P2 in vesicle priming, i.e., somehow making the vesicle competent for Ca2+-dependent fusion, and (ii) disassembly of the cortical actin barrier. It is likely that both targets might well contribute to the lack of slow-phase secretion when PI(4,5)P2 is depleted in cells lacking ARF6 activity. Under resting conditions, the basal activity of the PI(4)P 5-kinase is sufficient to keep a pool of vesicles primed and ready for release during the fast initial phase of secretion. However, to rapidly translocate and prepare new secretory vesicles for sustained secretion, PI(4)P 5-kinase must be activated by ARF6. Thus, during low secretory states, enough PI(4,5)P2 is available to maintain a pool of readily releasable granules even in the presence of either ARF6(T27N) or high concentrations of the PLCδ PH domain, whereas during periods of increased demand for secretion, such as after depolarization, either depletion or sequestration of PI(4,5)P2 selectively eliminates the slow phase of secretion.
Experiments in permeabilized chromaffin cells by using ARF6-inhibitory peptides, and experiments in PC12 cells using ARF6 mutants unable to activate PLD have implicated a role for ARF6 in the regulation of large, dense core granule secretion through the regulation of PLD activity (3, 4, 36). In our investigation of PLD as a potential mechanism for the inhibition, we have presented evidence that PLD activity is required for insulin secretion, but, because 1-butanol blocks predominantly the first phase of insulin secretion, we think it is unlikely that inhibition of PLD activity by expression of dominant-inhibitory ARF6 represents the sole mechanism by which ARF6 regulates secretion. This likelihood is consistent with prior suggestions that PLD is required in a late stage of secretion, possibly fusion itself (36). The requirement for ARF6 in the maintenance of PI(4,5)P2 as demonstrated in this study results from the activation of phospholipase C by the secretagogue. Thus, in cell types exposed to agonists that do not increase phosphoinositide turnover, it is likely that levels of PI(4,5)P2 will not depend on ARF6 function, and the role of the G protein in regulating PLD might predominate.
In summary, we have presented evidence that ARF6 is required for optimal regulated secretion by supporting the maintenance of PI(4,5)P2 levels, thus allowing cortical actin breakdown and priming of secretory granules during the slower, secondary phase of exocytosis.
Supplementary Material
Acknowledgments
We thank Martha Jordan for help in the calcium measurements and Sung-Ro Jo for the critical reading of the manuscript. We acknowledge services provided by the RIA Core of the Penn Diabetes Center (National Institutes of Health Grant DK19525). This work was supported by National Institutes of Health Grant P01-DK49210 (to M.J.B.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ARF6, ADP-ribosylation factor 6; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; AdCre, adenovirus expressing Cre recombinase; PLD, phospholipase D; PLCδ, phospholipase Cδ; PI(4)P, phosphatidylinositol 4-phosphate; GTPγS, guanosine 5′-[γ-thio]triphosphate.
References
- 1.Donaldson, J. G. & Jackson, C. L. (2000) Curr. Opin. Cell Biol. 12, 475-482. [DOI] [PubMed] [Google Scholar]
- 2.Moss, J. & Vaughan, M. (1998) J. Biol. Chem. 273, 21431-21434. [DOI] [PubMed] [Google Scholar]
- 3.Caumont, A. S., Galas, M. C., Vitale, N., Aunis, D. & Bader, M. F. (1998) J. Biol. Chem. 273, 1373-1379. [DOI] [PubMed] [Google Scholar]
- 4.Galas, M. C., Helms, J. B., Vitale, N., Thierse, D., Aunis, D. & Bader, M. F. (1997) J. Biol. Chem. 272, 2788-2793. [DOI] [PubMed] [Google Scholar]
- 5.Caumont, A. S., Vitale, N., Gensse, M., Galas, M. C., Casanova, J. E. & Bader, M. F. (2000) J. Biol. Chem. 275, 15637-15644. [DOI] [PubMed] [Google Scholar]
- 6.Massenburg, D., Han, J. S., Liyanage, M., Patton, W. A., Rhee, S. G., Moss, J. & Vaughan, M. (1994) Proc. Natl. Acad. Sci. USA 91, 11718-11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackwood, R. A., Smolen, J. E., Transue, A., Hessler, R. J., Harsh, D. M., Brower, R. C. & French, S. (1997) Am. J. Physiol. 272, C1279-C1285. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y. G., Siddhanta, A., Austin, C. D., Hammond, S. M., Sung, T. C., Frohman, M. A., Morris, A. J. & Shields, D. (1997) J. Cell Biol. 138, 495-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humeau, Y., Vitale, N., Chasserot-Golaz, S., Dupont, J. L., Du, G., Frohman, M. A., Bader, M. F. & Poulain, B. (2001) Proc. Natl. Acad. Sci. USA 98, 15300-15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale, N., Caumont, A. S., Chasserot-Golaz, S., Du, G., Wu, S., Sciorra, V. A., Morris, A. J., Frohman, M. A. & Bader, M. F. (2001) EMBO J. 20, 2424-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boshans, R. L., Szanto, S., van Aelst, L. & D'Souza-Schorey, C. (2000) Mol. Cell. Biol. 20, 3685-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, Q., Calafat, J., Janssen, H. & Greenberg, S. (1999) Mol. Cell. Biol. 19, 8158-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radhakrishna, H., Al-Awar, O., Khachikian, Z. & Donaldson, J. G. (1999) J. Cell Sci. 112, 855-866. [DOI] [PubMed] [Google Scholar]
- 14.Burgoyne, R. D. & Cheek, T. R. (1987) Biosci. Rep. 7, 281-288. [DOI] [PubMed] [Google Scholar]
- 15.Cheek, T. R. & Burgoyne, R. D. (1986) FEBS Lett. 207, 110-114. [DOI] [PubMed] [Google Scholar]
- 16.Sontag, J. M., Aunis, D. & Bader, M. F. (1988) Eur. J. Cell Biol. 46, 316-326. [PubMed] [Google Scholar]
- 17.Trifaro, J. M., Rodriguez del Castillo, A. & Vitale, M. L. (1992) Mol. Neurobiol. 6, 339-358. [DOI] [PubMed] [Google Scholar]
- 18.Vitale, M. L., Seward, E. P. & Trifaro, J. M. (1995) Neuron 14, 353-363. [DOI] [PubMed] [Google Scholar]
- 19.Honda, A., Nogami, M., Yokozeki, T., Yamazaki, M., Nakamura, H., Watanabe, H., Kawamoto, K., Nakayama, K., Morris, A. J., Frohman, M. A. & Kanaho, Y. (1999) Cell 99, 521-532. [DOI] [PubMed] [Google Scholar]
- 20.Hay, J. C., Fisette, P. L., Jenkins, G. H., Fukami, K., Takenawa, T., Anderson, R. A. & Martin, T. F. (1995) Nature 374, 173-177. [DOI] [PubMed] [Google Scholar]
- 21.Matschinsky, F. M. & Collins, H. W. (1997) Chem. Biol. 4, 249-257. [DOI] [PubMed] [Google Scholar]
- 22.Anton, M. & Graham, F. L. (1995) J. Virol. 69, 4600-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varnai, P. & Balla, T. (1998) J. Cell Biol. 143, 501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence, J. T. & Birnbaum, M. J. (2001) Mol. Cell. Biol. 21, 5276-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exton, J. H. (1999) Biochim. Biophys. Acta 1439, 121-133. [DOI] [PubMed] [Google Scholar]
- 26.Burgoyne, R. D., Morgan, A. & O'Sullivan, A. J. (1989) Cell. Signalling 1, 323-334. [DOI] [PubMed] [Google Scholar]
- 27.Burgoyne, R. D., Morgan, A., Robinson, I., Pender, N. & Cheek, T. R. (1993) J. Anat. 183, 309-314. [PMC free article] [PubMed] [Google Scholar]
- 28.Holz, R. W., Hlubek, M. D., Sorensen, S. D., Fisher, S. K., Balla, T., Ozaki, S., Prestwich, G. D., Stuenkel, E. L. & Bittner, M. A. (2000) J. Biol. Chem. 275, 17878-17885. [DOI] [PubMed] [Google Scholar]
- 29.Godi, A., Pertile, P., Meyers, R., Marra, P., Di Tullio, G., Iurisci, C., Luini, A., Corda, D. & De Matteis, M. A. (1999) Nat. Cell Biol. 1, 280-287. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara, H., Shibasaki, Y., Kizuki, N., Katagiri, H., Yazaki, Y., Asano, T. & Oka, Y. (1996) J. Biol. Chem. 271, 23611-23614. [DOI] [PubMed] [Google Scholar]
- 31.Fensome, A., Cunningham, E., Prosser, S., Tan, S. K., Swigart, P., Thomas, G., Hsuan, J. & Cockcroft, S. (1996) Curr. Biol. 6, 730-738. [DOI] [PubMed] [Google Scholar]
- 32.Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T. & Donaldson, J. G. (2001) J. Cell Biol. 154, 1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clements, R. S., Jr., & Rhoten, W. B. (1976) J. Clin. Invest. 57, 684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laychock, S. G. (1983) Biochem. J. 216, 101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prentki, M. & Matschinsky, F. M. (1987) Physiol. Rev. 67, 1185-1248. [DOI] [PubMed] [Google Scholar]
- 36.Vitale, N., Chasserot-Golaz, S., Bailly, Y., Morinaga, N., Frohman, M. A. & Bader, M. F. (2002) J. Cell Biol. 159, 79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.