Abstract
One therapeutic approach to treating Parkinson’s disease is to convert endogenous striatal cells into levo-3,4-dihydroxyphenylalanine (l-dopa)–producing cells. A defective herpes simplex virus type 1 vector expressing human tyrosine hydroxylase was delivered into the partially denervated striatum of 6-hydroxydopamine–lesioned rats, used as a model of Parkinson’s disease. Efficient behavioral and biochemical recovery was maintained for 1 year after gene transfer. Biochemical recovery included increases in both striatal tyrosine hydroxylase enzyme activity and in extracellular dopamine concentrations. Persistence of human tyrosine hydroxylase was revealed by expression of RNA and immunoreactivity.
Parkinson’s disease (PD), a neurodegenerative disorder, is characterized by the progressive loss of the dopaminergic neurons in the substantia nigra pars compacta that project to the corpus striatum (1). The principal therapy for PD is the oral administration of l-dopa (2), which is converted to dopamine (DA) by endogenous striatal aromatic amino acid decarboxylase (AADC) (3). Although it is initially effective, l-dopa therapy loses efficacy over a period of several years (1). Transplantation of cells that produce l-dopa or DA into the striatum can correct animal models of PD (4) but has not been a viable therapy in most human trials (5). Peripheral cell types that are genetically modified to express tyrosine hydroxylase (TH) and produce l-dopa have supported only short-term improvement (less than 2 months) in animal models of PD (6, 7). Genetically modified muscle cells support longer improvements (6 months) (8), but the viability of a muscle cell graft in the human striatum is not yet clear. An alternative therapeutic strategy is to convert a fraction of the striatal cells into l-dopa–producing cells by expression of TH in striatal cells (9) from a defective herpes simplex virus type 1 (HSV-1) vector (10). Potential advantages of this approach include production of l-dopa at the required site of action, so that diffusion over substantial distances is not necessary, and alleviation of potential problems caused by graft rejection or tumor formation. To test this strategy, a human TH complementary DNA (cDNA) (form II) (11, 12) was inserted into an HSV-1 vector (pHSVth). Infection of cultured striatal cells with pHSVth resulted in expression of human TH RNA, TH immunoreactivity, and the release of l-dopa into the culture medium (13). The amounts of l-dopa released per infected cell suggested that pHSVth might be evaluated in the 6-hydroxydopamine (6-OHDA)–lesioned rat, a model of PD.
pHSVth virus or pHSVlac virus or vehicle alone [phosphate-buffered saline (PBS)], was delivered by stereotactic injection into the partially denervated striatum of unilaterally 6-OHDA–lesioned rats (14). The apomorphine-induced rotation rate was measured as an index of behavioral recovery. The average decrease in the rotation rate caused by pHSVth was 64 ± 6% at 2 weeks after gene transfer. This value remained relatively constant over a 1-year period, and the decrease remained statistically significant at both 6 months (P < 0.01) and 1 year (P < 0.05) after gene transfer as compared with the control groups (Fig. 1A and Table 1). The rotation rate of each rat in the pHSVth group remained relatively constant and was similar to the rotation rate in the final test (Table 1).
Fig. 1.
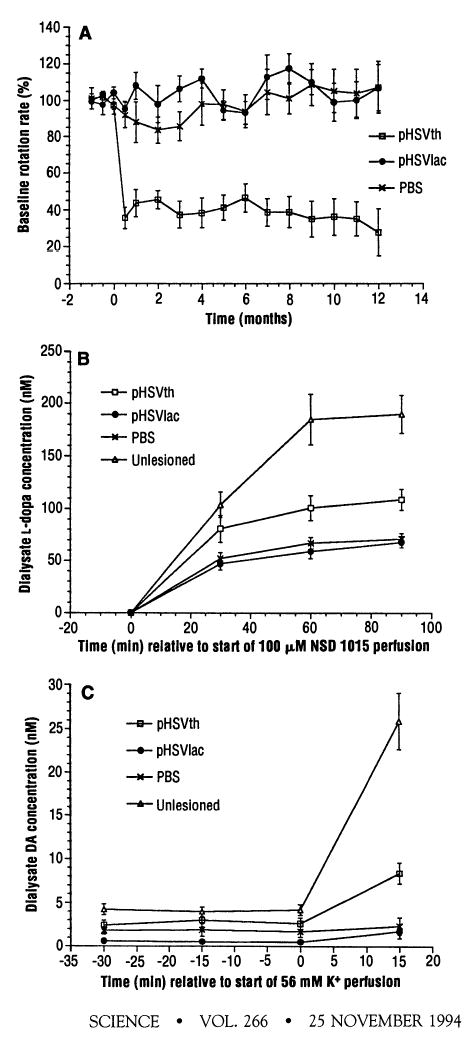
Rotation rates and striatal l-dopa or DA concentrations after stereotactic injection of pHSVth, pSHVlac, or PBS into the partially denervated striatum (14). (A) The rats were tested at various times (14) for the apomorphine-induced rotation rate, and the values shown are the average percent of the baseline rotation rate for each group. (B) Striatal l-dopa concentrations were measured by microdialysis (15) after perfusion with NSD 1015 as an indication of striatal TH activity (16). (C) Striatal DA levels were measured by microdialysis in low K+ (3 mM) and after perfusion with high K+ (56 mM) (15).
Table 1.
Summary of rotation rates and histological analysis of rats receiving pHSVth, pHSVlac, or PBS.
| Histological analysis
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decrease in baseline rotation rate* (%)
|
TH-IR§
|
X-Gal
|
TH PCR|| |
|||||||||
| Rat | Injection | Average | Final | Time of autopsy† | Microdialysis‡ | S | C | GP | S | RS | LS | CB |
| 2 | pHSVth | 57 | 56 | 16 | N | + | + + | + | + | − | − | |
| 3 | pHSVth | 93 | 97 | 12 | N | + | − | + | ND | |||
| 4 | pHSVth | 46 | 29 | 11 | N | + | + | − | + | + | ND | |
| 9 | pHSVth | 62 | 62 | 15.5 | N | + | − | − | + | − | − | |
| 26 | pHSVth | 39 | 56 | 5.5 | Y | PP¶ | + | − | ND | |||
| 27 | pHSVth | 34 | 26 | 5.5 | Y | + + | + + | + + | + | − | ND | |
| 30 | pHSVth | 26 | 16 | 6 | N | − | + + | − | ND | |||
| 31 | pHSVth | 41 | 46 | 8 | Y | + + | + | + | − | − | ND | |
| 33 | pHSVth | 66 | 70 | 6 | Y | + | − | − | + | − | ND | |
| 1 | pHSVlac | −7 | −31 | 12 | Y | + | ||||||
| 6 | pHSVlac | −11 | 0 | 12 | N | − | − | − | + + | |||
| 8 | pHSVlac | −2 | −14 | 10 | Y | ND | ||||||
| 20 | pHSVlac | 11 | 21 | 13 | Y | − | − | − | + | − | − | ND |
| 32 | pHSVlac | −10 | 13 | 11 | Y | ND | ||||||
| 12 | PBS | 14 | 20 | 13 | Y | − | − | − | − | |||
| 14 | PBS | 8 | 0 | 6 | N | |||||||
| 17 | PBS | 29 | 32 | 12 | Y | − | − | − | − | |||
| 19 | PBS | −13 | 0 | 12 | Y | − | − | − | − | |||
| 25 | PBS | −7 | −9 | 8 | Y | PP/ND | ||||||
| 38 | PBS | −5 | 3 | 12 | Y | − | − | − | − | |||
The two tests from each month of the first 3 months were averaged; those three values and the other monthly values (14) were used to calculate the average.
Months after gene transfer.
Microdialysis was done (Y, yes; N, no).
−, 0; +, 1 to 3; or + +, 4 to 20 cells contained TH immunoreactivity (TH-IR) in one or more sections (18). S, striatum; C, cortex; GP, globus pallidus; PP, poor perfusion; ND or no entry, not done.
pHSVth DNA was detected with PCR (23); RS, injected right striatum; LS, uninjected left striatum; Cb, cerebellum.
Presumably because of poor tissue preservation, attempts to detect cells with TH immunoreactivity were unsuccessful, although pHSVth DNA was detected with PCR.
TH enzyme activity and extracellular DA concentrations in the injected striatum were evaluated in selected rats 4 to 6 months after gene transfer by means of in vivo microdialysis (15) (Table 1). An inhibitor of AADC [100 μM NSD 1015 (3-hydroxybenzylhydrazine)] was added to the microdialysis perfusate, and accumulation of l-dopa was measured (15) as an indication of TH enzyme activity (16) (Fig. 1B). Ninety minutes after addition of NSD 1015, pHSVth directed a 60% average increase in striatal l-dopa concentrations as compared with those of the control groups (pHSVlac or PBS; P < 0.05), whereas normal (unlesioned and uninjected) rats showed 180% higher striatal l-dopa concentrations as compared with those of the control groups (P < 0.01).
To determine whether or not pHSVth directed an increase in DA production, extracellular DA levels were measured (15). In the basal state (3 mM K+), pHSVth mediated a 120% (P < 0.05) increase in striatal DA concentrations as compared with those of the control groups, whereas normal rats showed DA concentrations that were 250% (P < 0.01) above those of the control groups (Fig. 1C). After depolarization of neurons with high K+ (56 mM K+ in microdialysis perfusate), pHSVth mediated a 310% (P < 0.05) increase in DA concentrations as compared with those of the control groups, whereas normal rats showed an 1150% (P < 0.005) increase in DA concentrations. Concentrations of γ-aminobutyric acid and acetylcholine, the predominant neurotransmitters of striatal neurons, were unaltered in the pHSVth group as compared with concentrations in the control groups.
Histological analysis was done 6 to 16 months after gene transfer. Because these defective HSV-1 vectors should not replicate in vivo (17), gene transfer is most likely to occur in striatal neurons and glia that are proximal to the injection site. However, HSV-1 particles can also diffuse through the extracellular space or be retrogradely transported through processes to the cell bodies of striatal projection neurons. TH immunoreactivity was detected with an antibody to TH (18). The normal rat striatum lacks cells with TH immunoreactivity (19). Striata injected with pHSVth, but not pHSVlac or PBS, contained immunoreactive cells, frequently in clusters spread over 200 to 300 μm (Fig. 2, A through C, and Table 1), and many of these cells displayed neuronal morphology (Fig. 2C). Again, only in the pHSVth group, two striatal projection areas that normally lack cells with TH immunoreactivity, namely the pallidum (20) and the medial agranular cortex (bilateral, layers 3 and 5) (21), contained cells with TH immunoreactivity (Fig. 3, D and E, respectively, and Table 1). One rat (pHSVth no. 30) lacked immunoreactive striatal cells, although cortical cells with immunoreactivity were detected, and this was the only rat in the group that did not show a decrease in the rotation rate.
Fig. 2.
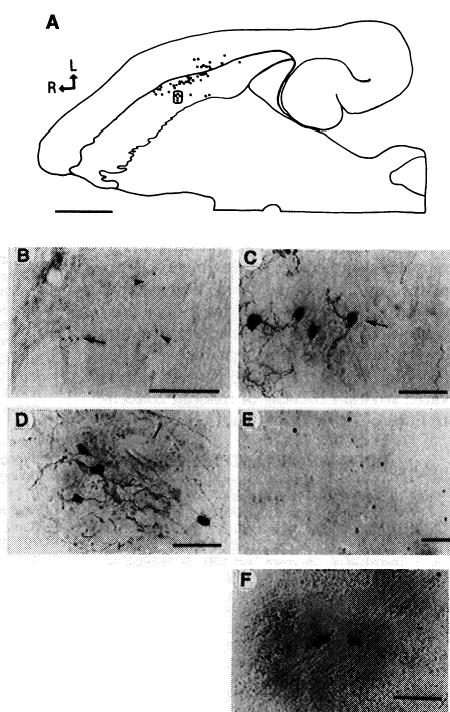
TH immunoreactivity was detected with an antibody to TH (78), and β-Gal was detected with X-Gal (22). (A) through (C) show rat pHSVth no. 27. (A) Composite drawing of charted sections, showing the positions of 48 cells containing TH immunoreactivity in the striatum and neocortex. Every third section was analyzed. L, lateral; R, rostral; scale bar, 2 mm. (B) Low-magnification photomicrograph of clusters of striatal cells containing TH immunoreactivity. Arrowheads point to two clusters, and the arrow indicates a third cluster [boxed in (A)]; scale bar, 500 μm. (C) High-magnification view of a cluster of striatal cells containing TH immunoreactivity with neuronal morphology [boxed (A)]; scale bar, 50 μm. (D) and (E) show rat pHSVth no. 31. (D) A cluster of pallidal neurons containing TH immunoreactivity; scale bar, 50 μm (E) A cluster of cortical neurons (agranular frontal cortex, layers 3 and 5) containing TH immunoreactivity; scale bar, 100 μm (F) High-magnification view of X-Gal–positive striatal cells from rat pHSVlac no. 1; scale bar, 50 μm.
Fig. 3.
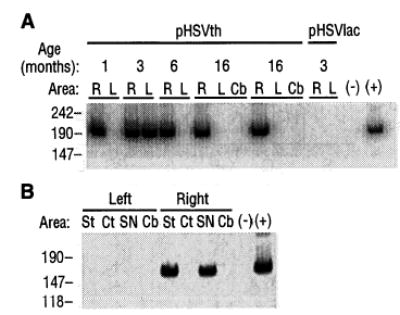
Persistence of pHSVth DMA and expression of human TH RNA. (A) DMA was extracted from sections and subjected to PCR with the use of primers specific to the human TH gene, and the products were electrophoresed (23). Age is the time after gene transfer a rat was analyzed (6 months, rat pHSVth no. 27; 16 months, rats pHSVth no. 2, and no. 9). Brain areas: R, right injected striatum; L, left uninjected striatum; Cb, cerebellum. Minus sign indicates no DMA; plus sign indicates pHSVth DMA isolated from Escherichia coli, which should direct production of a 186-bp fragment (number of base pairs is shown at left). (B) RT-PCR analysis of RNA isolated from specific brain areas 1 month after injection of pHSVth (14) into the right striatum. Brain areas: St, striatum; Ct, cortex; SN, midbrain (substantia nigra); Cb, cerebellum. Minus sign indicates no RNA; plus sign indicates pHSVth DNA isolated from E. coli; the methods used (24) should generate a 160-bp fragment (number of base pairs is shown at left).
In the pHSVth group, the total number of cells containing TH immunoreactivity (18) ranged from approximately 5 to 10 to 200 to 300, the majority of which were striatal cells. The number of immunoreactive cells did not correlate with the extent of behavioral recovery, possibly because multiple cell types contained TH immunoreactivity and the amount of l-dopa produced by recombinant TH is cell-type–dependent (6–8). Cells expressing β-galactosidase (β-Gal) were detected with X-Gal (22) in striata injected with pHSVlac but not with pHSVth or PBS (Fig. 2F and Table 1).
pHSVth DNA was detected by means of polymerase chain reaction (PCR) (23) in pHSVth-injected striata for up to 16 months after gene transfer (Fig. 3A and Table 1). pHSVth DNA was detected in the uninjected contralateral striatum in two rats (pHSVth no. 4 and the rat analyzed 3 months after gene transfer in Fig. 3A). This could be due to pHSVth virus rising up a needle track to infect axons projecting from the contralateral striatum or neocortex [some samples contained small amounts of neocortex (23)] or to a projection from the contralateral neocortex to the injected striatum (21). pHSVth DNA was not detected in the cerebellum, which does not project to the striatum, or in a striatum that received pHSVlac.
Because the brains of the rats used for rotation rate analysis were fixed with 4% paraformaldehyde, this tissue was not suitable for reverse transcriptase–PCR (RT-PCR) analysis of human TH RNA. To investigate long-term expression of human TH RNA, pHSVth was injected into the striatum (14) of normal rats, and 1 month later human TH RNA was detected (24) in specific brain areas in 3 of 10 rats (Fig. 3B). The negative rats may be due to the limitations of the assay or to other possibilities, including inefficient gene transfer or loss of expression. Typically, 1 week is required before HSV-1 particles are absent from the brain and a persistent infection is established (25). Thus, expression of human TH RNA 1 month after gene transfer indicates that the IE 4/5 promoter can direct persistent expression in this HSV-1 vector (26).
The present configuration of the vector system has limitations. Virus prepared with this packaging system contains wild-type HSV-1 (frequency ~10−5) (17), and HSV-1 particles contain specific HSV-1–encoded proteins that mediate acute cytopathic effects. These factors may have contributed to a number of rat deaths (<10%) that occurred within 2 weeks after gene transfer (27); however, the majority of the rats (>90%) were healthy and gained weight until deliberately killed. The brains from the pHSVth and pHSVlac groups were of normal size and showed normal morphology except for a small zone of necrosis around the injection sites (pHSVth group, approximately ≤1 × 104 to 5 × 105 μm2; PBS group, approximately 1 × 105 μm2). No brain tumors were observed, and no cells contained HSV-1 particle immunoreactivity (28). Expression of TH in striatal projection neurons could potentially interfere with other brain functions, although ingestive and gross motor behaviors appeared unaltered (29). In an initial comparison of short- and long-term expression, significantly more positive cells were observed at 4 days than at 1 to 3 months after gene transfer (14) (pHSVth or pHSVlac), although expression occurred in the same cell types. The decrease in expression could be due to the acute cytopathic effects associated with gene transfer, to downregulation of the IE 4/5 promoter, or to other properties of the vector system. Thus, whereas improvements in the vector system are needed, these studies demonstrate the feasibility of this approach.
An alternative explanation for the results is that trauma to the partially denervated rat striatum induced trophic factor–mediated growth of remaining TH axons, resulting in behavioral recovery (30). This is unlikely, because the pHSVlac group did not show behavioral recovery, and because the number of axons with TH immunoreactivity in the injected striatum was similar in the pHSVth, pHSVlac, and PBS groups and was low. It is also unlikely that the vector system induced expression of endogenous TH, because the pHSVlac group lacked striatal, pallidal, or cortical cells with TH immunoreactivity, and because human TH RNA was expressed.
The pHSVth group showed persistence of vector DNA, long-lasting expression of TH, increased striatal TH activity and extracellular DA concentrations, and long-term behavioral recovery. The capability of a limited number of cells expressing TH to support sustained responses might be explained by the relatively wide dispersion of the cells, potentially elevating l-dopa concentrations over an extended area, and by observations suggesting that increased diffusion of DA occurs in the partially denervated striatum because of reduced concentrations of DA transporters (31).
References
- 1.M. D. Yahr and K. J. Bergmann, Eds., Parkinson’s Disease (Raven, New York, 1987).
- 2.Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM. Arch Neurol. 1969;21:343. doi: 10.1001/archneur.1969.00480160015001. [DOI] [PubMed] [Google Scholar]
- 3.Tashiro Y, et al. Neurosci Lett. 1989;100:29. doi: 10.1016/0304-3940(89)90655-1. [DOI] [PubMed] [Google Scholar]; Li XM, et al. J Neurochem. 1992;59:1172. doi: 10.1111/j.1471-4159.1992.tb08363.x. [DOI] [PubMed] [Google Scholar]
- 4.Redmond DE, et al. Lancet. 1986;i:1125. doi: 10.1016/s0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]; Freed WJ, et al. Ann Neurol. 1987;8:510. doi: 10.1002/ana.410080508. [DOI] [PubMed] [Google Scholar]; Bakay RAE, et al. Ann N Y Acad Sci. 1987;495:623. doi: 10.1111/j.1749-6632.1987.tb23705.x. [DOI] [PubMed] [Google Scholar]; Sladek JR, et al. Prog Brain Res. 1988;18:497. doi: 10.1016/s0079-6123(08)60323-4. [DOI] [PubMed] [Google Scholar]; Yurek DM, Sladek JR. Annu Rev Neurosci. 1990;13:415. doi: 10.1146/annurev.ne.13.030190.002215. [DOI] [PubMed] [Google Scholar]
- 5.Lindvall O, et al. Science. 1990;247:574. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]; Spencer DD, et al. N Engl J Med. 1992;327:1541. doi: 10.1056/NEJM199211263272201. [DOI] [PubMed] [Google Scholar]; C. R. Freed et al., ibid., p. 1549 [DOI] [PubMed]; H. Widner et al., ibid., p. 1556.
- 6.Wolff JA, et al. Proc Natl Acad Sci USA. 1989;86:9011. [Google Scholar]
- 7.Horrelou P, Brundin P, Kalen P, Mallet J, Bjorklund A. Neuron. 1990;5:393. doi: 10.1016/0896-6273(90)90078-t. [DOI] [PubMed] [Google Scholar]; Gage FH, Kawaja MD, Fisher LJ. Trends Neurosci. 1991;14:328. doi: 10.1016/0166-2236(91)90156-o. [DOI] [PubMed] [Google Scholar]
- 8.Jiao S, Gurevich V, Wolff JA. Nature. 1993;262:450. doi: 10.1038/362450a0. [DOI] [PubMed] [Google Scholar]
- 9.L. Vecsei et al., in Neurological Disorders, K. O’Malley, and A. I. Geller, Eds. (Simon and Schuster, New York, 1992), pp. 223–248.
- 10.Geller AI, Breakefield XO. Science. 1988;241:1667. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]; Geller AI, Freese A. Proc Natl Acad Sci USA. 1990;87:1149. doi: 10.1073/pnas.87.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]; Freese A, Geller AI. Nucleic Acids Res. 1991;19:7219. doi: 10.1093/nar/19.25.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Malley K, et al. Biochemistry. 1987;26:6910. doi: 10.1021/bi00396a007. [DOI] [PubMed] [Google Scholar]
- 12.Grima B, et al. Nature. 1987;326:707. doi: 10.1038/326707a0. [DOI] [PubMed] [Google Scholar]
- 13.A. I. Geller, M. J. During, Y. J. Oh, A. Freese, K. L. O’Malley, J. Neurochem., in press. [DOI] [PMC free article] [PubMed]
- 14.Adult male Sprague-Dawley rats (280 to 300 g) were tested for apomorphine-induced [1 mg per kilogram of body weight, administered intraperitoneally (IP)] asymmetrical rotations to ensure no preexisting rotational bias (fewer than two turns every 5 min) and then lesioned with 6-OHDA [Perese DA, Ulman J, Viola J, Ewing SE, Bankiewicz KS.Brain Res 494285.1989]. Starting at least 10 days later, rats were tested at least three times at 2-week intervals with apomorphine, and rats that showed six or more rotations per minute for the interval 15 to 20 min after drug administration were used. This rotation rate is consistent with a ≥90% reduction in striatal TH activity and dopamine concentration [F. Hefti, E. Melamed, B. J. Sahakian, R. J. Wurtman, Pharmacol. Biochem. Behav. 12, 185 1980]. pHSVth and pHSVlac were packaged into HSV-1 particles 17, and virus was purified and concentrated [ [DOI] [PubMed] [Google Scholar]; Federoff HJ, Geschwind MD, Geller AI, Kessler JA. Proc Natl Acad Sci USA. 1992;89:1636. doi: 10.1073/pnas.89.5.1636. ]. The titers of the virus stocks were as follows: 5 × 104 infectious particles of pHSVth per microliter the ratio of pHSVth to HSV-1 strain 17 D30EBA was 0.9, and the ratio of wild-type HSV-1 to D30EBA was 1 × 10−5, and 5 × 104 infectious particles of pHSVlac per microliter the ratio of pHSVlac to D30EBA was 1.0, and the ratio of wild-type HSV-1 to D30EBA was 4 × 10−5. Rats were randomly placed into three groups that received by stereotactic injection 2 μl of pHSVth, pHSVlac, or PBS at each of three sites in the right striatum {AP 0.6, ML 2.0, and DV 5.0; AP 0.6, ML 3.2, and DV 5.0; AP 1.8, ML 2.6, and DV 5.0 numbers indicate millimeters relative to Bregma and the Bregma-Lambda plane [G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates Academic Press, San Diego, CA, ed. 2, 1986]}. Starting 2 weeks after injection, the rotation rate was tested every 2 weeks for the first 3 months and subsequently at monthly intervals. Statistical analysis of rotation rate and neurochemical data 15 was done by means of repeated analyses of variance with posthoc tests Systat, Evanston, IL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A probe was implanted through a guide cannula (AP 0.6 and ML 2.6; proximal to the three injection sites), and the exposed 4-mm membrane spanned the dorso-ventral coordinates of the striatum. One day later, microdialysis was done and catecholamines were quantitated by high-performance liquid chromatography with electrochemical detection [During MJ, et al. Exp Neurol 115193.1992. 1735466 [Google Scholar]
- 16.Westerink BHC, De Vries JB, Duran R. J Neurochem. 1990;54:381. doi: 10.1111/j.1471-4159.1990.tb01884.x. [DOI] [PubMed] [Google Scholar]; Robert F, Lanbas-Sena L, Ortemann C, Pujol JF, Renaud B. ibid. 1993;60:721. doi: 10.1111/j.1471-4159.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 17.Geller AI, Keyomarski K, Bryan J, Pardee AB. Proc Natl Acad Sci USA. 1990;87:8950. doi: 10.1073/pnas.87.22.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rats were deeply anesthetized with pentobarbital (60 mg/kg, IP), perfused with 50 ml of PBS, followed by 200 ml of 4% paraformaldehyde in PBS, and cryoprotected. Horizontal brain sections (30 μm) were cut on a cryostat, and free-floating sections were incubated overnight with an antibody to TH (1:100 to 1:200 dilution); Rohrer H, Acheson AL, Thibault J, Thoenen H. J Neurosci. 1986;6:2616. doi: 10.1523/JNEUROSCI.06-09-02616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]; followed by a biotinylated horse antibody to mouse immunoglobulin G IgG 1:220 dilution and an avidin-biotin-peroxidase complex Vector, Burlingame, CA [; Lombroso PJ, et al. ibid. 1993;13:3064. doi: 10.1523/JNEUROSCI.13-07-03064.1993. ]. Every sixth section was analyzed for TH immunoreactivity, and 10 to 20 of these sections contained the structures of interest striatum, globus pallidus, and medial agranular cortex. In one case, sections were charted with the aid of a drawing tube attachment to a microscope, and a composite drawing was reconstructed to show the positions of the cells containing TH immunoreactivity. Attempts to use a type II antibody to human TH. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lewis DA, Melchitzky DS, Haycock JW. Neurosci. 1993;54:477. doi: 10.1016/0306-4522(93)90267-j. Failed to produce consistent results, presumably because of the low sensitivity of this antibody J. W. Haycock, personal communication. [DOI] [PubMed] [Google Scholar]
- 19.Dubach M, et al. Neurosci Lett. 1987;75:205. doi: 10.1016/0304-3940(87)90298-9. There is one report, in which a different antibody to TH was used, of TH immunoreactivity in a limited number of rat striatal cells [ [DOI] [PubMed] [Google Scholar]; Tashiro Y, et al. ibid. 1989;97:6. doi: 10.1016/0304-3940(89)90130-4. [DOI] [PubMed] [Google Scholar]
- 20.Staines WA, Atmadja S, Fibiger HC. Brain Res. 1981;206:446. doi: 10.1016/0006-8993(81)90545-x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson CJ. J Comp Neurol. 1987;263:567. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]; McGeorge AJ, Faull RLM. Brain Res. 1987;423:318. doi: 10.1016/0006-8993(87)90855-9. [DOI] [PubMed] [Google Scholar]; ———, Neuroscience 29503.1989Some cortical cells contain TH immunoreactivity [ [DOI] [PubMed] [Google Scholar]; Kosaka T, Hama K, Nagatsu I. Exp Brain Res. 1987 but TH immunoreactivity was not found in the pHSVlac or PBS groups in the agranular frontal cortex;68:393. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- 22.Emson PC, et al. Exp Brain Res. 1990;79:427. doi: 10.1007/BF00608254. [DOI] [PubMed] [Google Scholar]
- 23.The entire striatum (with fragments of neocortex) or the cerebellum, in sections adjacent to those used for immunohistochemistry, was extracted (0.5 mg of tissue per microliter) in proteinase K (10 mg/ml) buffer [R. Higuchi, Amplifications 2, 1 (1989)]. Recovered DNA was normalized with radiolabeled primers derived from the single-copy rat gene encoding TH [E. R. Brown, G. T. Coker, K. L. O’Malley, Biochemistry 26, 5208 (1987)], followed by analysis with a phosphorimager (Molecular Dynamics, Sunnyvale, CA). Samples were subjected to nested PCR with the use of primers (5 pmol) derived from the cDNA sequence encoding human TH [note 11 and figure 1A in (72)]. The first reaction used 100 ng of normalized DNA in a total volume of 50 μl; primers were nucleotides 771 to 789 (5′-GCTGGAGCGCTTCAGCGGC-3′) and complementary to nucleotides 1105 to 1122 (5′-CACCGTGAACCATGACAG-3′). DNA was processed for 45 cycles at 94°C for 1 min, at 52°C for 1 min, and at 72°C for 1 min. The second reaction used 2 μl of the first reaction in a total volume of 50 μl; primers were nucleotides 914 to 931 (5′-TCCGCGTGTTCCAGTGCA-3′) and complementary to nucleotides 1081 to 1100 (5′-GACAGCTTCTCAATTTCCTC-3′). DNA was processed for 20 cycles at 94°C for 0.5 min, at 52°C for 0.5 min, and at 72°C for 0.5 min. All analyses were conducted without knowledge of the injection conditions of the animal.
- 24. One month after pHSVth was injected into the right striatum (14), RNA was isolated.; Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]; from brain areas and normalized with the use of 18 S ribosomal RNA probes.; Chan YL, et al. J Biol Chem. 1984;259:224. [PubMed] [Google Scholar]; After reverse transcription.; Krug MS, Berger SL. Methods Enzymol. 1987;152:316. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]; with a human TH-specific primer {complementary to nucleotides 156 to 184 5′-AGGGGACTGCAGCGGCCGCTGCTGCCACC-3′ [; Coker GT, Studelska D, Harmon S, Burke W, O’Malley KL. Mol Brain Res. 1993;8:93. doi: 10.1016/0169-328x(90)90052-f. [DOI] [PubMed] [Google Scholar]; cDNA was amplified by nested PCR across the intron in the HSV-1 IE 4/5 5′ untranslated region.; McGeoch DJ, et al. Nucleic Acids Res. 1986;14:1727. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first reaction used 250 ng of cDNA in a total volume of 50 μl; primers 5 pmol were complementary to nucleotides 435 to 464 HSV-1 primer: 5′-ACGAACGACGGGAGCGGCTGCGGAGCACGC-3′ and complementary to nucleotides 156 to 184 TH primer. The second reaction used 4 μl of the first reaction in a total volume of 50 μl; primers 5 pmol were complementary to nucleotides 252 50 281 HSV-1 primer; 5′-GGGCCTCCGACGACAGAAACCCACCGGTCC-3′ and complementary to nucleotides 91 to 120 TH primer: 5′-GCTCTGCCTGCGCCCAATGAACCGCGGGGA-3′. For both reactions, cDNA was processed for 35 cycles at 94°C for 1.5 min, at 68°C for 0.5 min, and at 72°C for 1.5 min. To further ensure specificity, the products were digested with Hind III [producing a 160–base pair bp fragment containing the TH sequences] and subjected to Southern DMA analysis with a radiolabeled nucleotide TH nucleotides 36 to 65: 5′-GGGCTTCCGCAGGGCCGTGTCTGAGCTGGA-3′. All analyses were conducted without knowledge of the injection conditions of the animal.
- 25.Watson K, Stevens JG, Cook ML, Subak-Sharpe JH. J Gen Virol. 1980;49:149. doi: 10.1099/0022-1317-49-1-149. [DOI] [PubMed] [Google Scholar]
- 26.In a defective HSV-1 vector, three IE promoters (IE1, IE3, and IE 4/5) support expression of the LacZ gene for up to 3 months in cultured rat sensory neurons (R. Smith, A. I. Geller, C. Wilcox, in preparation). Also, in stable transformants of fibroblast cell lines, the IE3 promoter is stably expressed at a low level; Mosca JD, Reyes GR, Pitha PM, Hayward GS. J Virol. 1985;56:867. doi: 10.1128/jvi.56.3.867-878.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]; In contrast, in a latent infection of wild-type HSV-1, IE promoters are not transcribed.; Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. Science. 1987;235:1056. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]; IE promoters may be regulated by other sequences in the HSV-1 genome or by transcripts from wild-type HSV-1. Similarly, although the murine leukemia virus long terminal repeat promoter can be stably expressed in retrovirus vectors or in transgenic mice, this promoter is not stably expressed in the HSV-1 genome.; Dobson AT, Margolis TP, Sedaerati F, Stevens JG, Feldman LT. Neuron. 1990;5:353. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]; Understanding the mechanism or mechanisms underlying these paradoxes may provide information about HSV-1 latency but is not required in order to use IE promoters in this defective HSV-1 vector
- 27.These rats were not analyzed; however, HSV-1 vectors prepared with this packaging system (17) were injected into the midbrain, and the rats that died within 2 weeks after gene transfer contained HSV-1 particle immunoreactivity (28) in multiple brain areas (S. Song, Y. Wang, A. I. Geller, unpublished results).
- 28.Adam RL, Springall DR, Levene MN, Bushell TE. J Pathol. 1984;143:241. doi: 10.1002/path.1711430403. Cells containing HSV-1 particle immunoreactivity were detected 4 days after gene transfer. [DOI] [PubMed] [Google Scholar]
- 29.Cortical or pallidal cells expressing TH may contribute to striatal TH concentrations by axonal transport of TH. Injection of pHSVth into other brain areas was not evaluated; however, data from rat pHSVth no. 30 suggest that expression of TH in cortical cells is not effective. Cell transplantation approaches to treatment of PD use striatal delivery (4–8), and the location of the site in the striatum can be critical for obtaining behavioral recovery (6).
- 30.Wang J, Bankiewicz KS, Plunkett RJ, Oldfield EH. J Neurosurg. 1994;80:484. doi: 10.3171/jns.1994.80.3.0484. [DOI] [PubMed] [Google Scholar]
- 31.van Horne C, Hoffer BJ, Stromberg I, Gerhardt GA. J Pharmacol Exp Ther. 1992;263:1285. [PubMed] [Google Scholar]; Cass WA, Zahniser NR, Flach KA, Gerhardt GA. J Neurochem. 1993;61:2269. doi: 10.1111/j.1471-4159.1993.tb07469.x. [DOI] [PubMed] [Google Scholar]; Schneider JS, Rothblat DS, DiStefano L. Brain Res. 1994;643:86. doi: 10.1016/0006-8993(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 32.We thank K. Burns, K. Davis, S. Harmon, M. Kake, P. Leone, E. Lukacsi, G. Mirchandani, and D. Ullrey for technical assistance. We thank J. Haycock and R. Roth for critical readings of the manuscript. Supported by NIH grants NS28227 and NS06208 and the Parkinson’s Disease Foundation (M.J.D.); by NIH grants EY09749 and MH49351 and the Tourette Association (J.R.N.); by the American Parkinson’s Disease Association and NIH grant AG10827 (A.I.G. and K.L.O’M.); by NIH grant 50081 (K.L.O’M.) and by Alkermes Inc., the American Health Assistance Foundation, the Burroughs Wellcome Fund, and the National Parkinson Foundation (A.I.G.).


