Abstract
The persistence of fetal stem cells with multilineage potential in women who have been pregnant, a phenomenon known as fetal microchimerism, is emerging as a potential contributing factor in certain diseases including cancer. For example, fetal microchimerism has been implicated in autoimmune disease, wound healing, and cancer. Studies of this phenomenon may provide a novel perspective on cancer in women, including in breast, ovarian, and lung cancers.
Background
It has been known for some time that, following pregnancy, a small number of fetal cells can persist in the mother’s body for long periods of time, even decades. This phenomenon is known as fetal microchimerism. The percentage of parous women that are microchimeric is not known with certainty. Assaying for the presence of the Y chromosome in women who had prior male pregnancies is a convenient way to assess microchimerism. Using this method, male cells of presumed fetal origin have been detected in 30-50% of women in a number of studies (1, 2). However, this number is likely to be an underestimate and is likely to increase as the sensitivity of detection methods improves and larger blood samples are tested.
Fetal cells are present in the maternal circulation and can infiltrate all tissues in the body. The role that fetal microchimerism may play in the health of parous women is only recently coming to be appreciated. In some instances, it appears that fetal cells are the root cause, or at least contribute, to disease. For example, there is now considerable evidence implicating a role for fetal cells in autoimmune diseases in women, particularly in systemic sclerosis (3, 4). On the other hand, there is also evidence that fetal cells may participate in the repair of injured tissues. In humans, fetal cells having the characteristics of hematopoietic, epithelial, and hepatic cells have been identified in a number of maternal tissues having various pathologies, suggesting that these cells have a stem cell-like multilineage potential that responds to tissue damage and/or malfunction (5).
The role that fetal microchimerism may play in tumor development in parous women has only recently been considered and is the focus of this mini-review. A handful of studies now suggest that fetal cells have a protective function and help combat cancer in parous women. However, given their proposed dichotomous participation as causative agents in non-cancerous diseases and as curative agents due to their observed stem cell properties, it seems plausible, but as yet unproven, that when placed in the right microenvironment, fetal cells may also be the cells in which tumorigenesis initiates.
Fetal Microchimeric Cells Suppress Cancer
The protective role of fetal microchimerism in suppressing tumor development in women that have been pregnant is best documented for breast cancer. In a pilot study with 82 patients, Nelson and collaborators detected fetal microchimerism in significantly fewer women who had breast cancer than in healthy women (6). In a follow-up case-control study with 99 patients, these investigators confirmed the finding of the pilot study and showed additionally that the frequency of fetal cells in those breast cancer patients that tested positive for fetal cells was significantly lower than in general population control women (7). These authors suggest that allogeneic fetal microchimeric cells might provide immune surveillance for breast cancer in parous women, and further that parous women that do develop breast cancer may have a reduced source of acquired allogeneic immunity. A recent study in which patients with advanced-stage chemotherapy-refractory solid tumors were treated with activated haplo-identical peripheral blood stem cells (haplo-PBSC) donated by parents or children lends support to this idea. Using HLA markers, the fetal or maternal (maternal cells in children) microchimerism status (+ or -) of patients was determined prior to treatment with the haplo-PBSC derived from the donor. Both the survival time and therapeutic response rate of microchimerism-positive patients was higher than in microchimerism-negative patients (8). In yet another study, the percentage of parous patients with malignancies that were positive for fetal microchimeric cells was significantly less than for normal parous donors (9). Taken together, these studies suggest that new therapies based on immune surveillance by allogeneic fetal cells may hold promise for the treatment of cancer.
The inverse association between fetal microchimerism and breast cancer suggests that fetal cells might protect parous women from other cancers as well. To date, however, there has been very little work done to investigate this possibility. It has been known for some time that nulliparous women are at greater risk for developing bladder cancer than are parous women. The results of a recent study using a transgenic mouse model for bladder cancer suggest that pregnancy, parity, lactation, or a combination of these may play a protective role in bladder cancer by inhibiting tumor growth (10). The authors of this study suggest that changes in estrogen levels during pregnancy and lactation may be responsible for this protective role. Given what we now know about the protective role of fetal microchimerism in breast cancer, a study of fetal microchimerism and bladder cancer seems warranted.
In a similar vein, pregnancy at older ages has been associated with a reduced risk for ovarian cancer (11). Given the facts that the number of microchimeric cells in parous women declines as a function of time elapsed after pregnancy, and that ovarian cancer develops at highest frequency in post-menopausal women, it is possible that fetal microchimerism may play a protective role in ovarian cancer as well.
Microchimeric fetal cells have also been shown to cluster in lung tumors in women decades after pregnancy (12). Their frequency in tumors was several-fold higher in lung tumors than in surrounding healthy lung tissue. Histochemical staining to identify the nature of the tumor-associated male microchimeric cells was not done in this study. Nevertheless, the authors speculate that the fetal cells are recruited from the bone marrow to the tumor sites where they assume their role in immune surveillance and tissue repair.
Fetal Microchimeric Cells as Cancer Stem Cells?
The role that cancer stem cells may play in the initiation and pathogenesis of cancer has been the focus of intense research in recent years. The ability to self-renew, a characteristic shared by cancer stem cells and normal stem cells residing in somatic tissues and responsible for their maintenance, have led some to suggest that tumorigenesis may initiate in normal stem cells (13). As a result of mutation or microenvironmental influences, these cells may lose regulatory controls that normally keep cell proliferation and differentiation in check, and cancer may develop. Given the stem cell-like potential of fetal microchimeric cells to differentiate into cells of multiple lineages and their persistent presence in women following pregnancy, it is intriguing to speculate that, as a consequence of genetic alterations or changes in their microenvironmental niche, these cells may act like cancer stem cells and give rise to tumors.
The results of one study suggest that fetal microchimerism may be involved in the pathogenesis or progression of cervical cancer (14). In this study, male cells were found in all cervical cancers tested. Interestingly, 24% (9 of 37) microchimeric cells were found to be cytokeratin-positive, a marker for epithelial cells. While this result suggests that circulating cells of fetal origin may have differentiated and contributed to the development of cervical tumors, the number of tumors examined was very small, and further work needs to be done to draw firm conclusions.
The overall results of the previously cited breast cancer study clearly show a protective role for fetal microchimeric cells (7). In light of the possibility that these cells may sometimes give rise to tumors, however, it is noteworthy that the incidence of fetal cells in the peripheral blood buffy coat of two breast cancer cases in this study was very high relative to the incidence in other breast tumors and in normal control women. Thus, the two outlying cases had values of 277 and 374 fetal genomes per 106 maternal genomes, whereas the median concentrations for cases and controls were 2, with ranges of (0-375) and (0-78), respectively. Perhaps these two outlier cases are rare cases where fetal cells have transformed and actually contribute to the growth of the tumor.
To investigate the tumor-generating capacity of fetal microchimeric cells, my laboratory is using a mouse model in which the fate of fetal cells that express a GFP reporter transgene can be easily tracked in female mice following pregnancy. We and others have shown that GFP-expressing fetal cells can be detected in multiple organs many months post-partum. We found the highest frequency of fetal cells in the lung. In one experiment, we crossed A/J females to males bearing a CAG/GFP transgene (CAG is a stong, ubiquitously expressed promoter). A/J mice develop lung tumors at a high frequency relative to other mouse strains. In one 8-month old A/J female, we discovered two solid lung tumor lesions in which a majority of the cells fluoresced bright green, indicating their fetal origin (Figure 1). Unfortunately, we were not able to determine the nature of these cells, but their large number and the gross appearance of the tumors suggest that they are indeed tumor cells and not immune cells recruited to the tumor site. Perhaps the microenvironment of normal lung stem cells contributes to the propensity of the A/J strain to develop lung tumors, and serves in a similar way to convert fetal cells lodged in the lung into cancer stem cells. Spurred on by this intriguing finding, we are continuing our studies and now use a luciferase reporter gene to track fetal cells, thus allowing us to use optical imaging to detect lung tumors containing fetal microchimeric cells in parous female mice.
Figure 1.
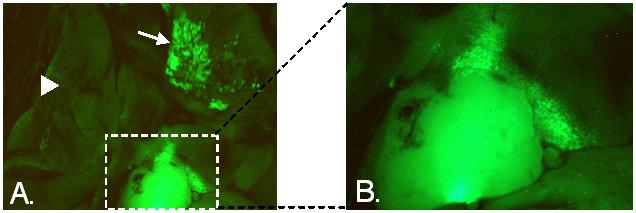
A. Lungs of A/J female mouse 8 months after she had given birth following mating with a CAG/EGFP male, observed under fluorescent light. Fetally-derived GFP-expressing cells associated with a solid tumor are within the boxed area. Image of this tumor is enlarged in B. An apparent second lesion in a different lobe of the lung is indicated by the arrow. The arrowhead points to another lobe of the lung where fetally-derived cells are absent.
Conclusion
We are only beginning to understand the role that fetal microchimeric cells play in cancer. As is the case in their involvement in non-cancerous diseases where they sometimes fight the disease and other times contribute to the disease, these cells sometimes help to suppress tumor development by taking on an immunosurveillance role, and other times they may behave like cancer stem cells and contribute to the growth of tumors. Studies using mouse models to track fetal microchimeric cells in parous females will help us understand their innate potential for each of these diametrically opposed roles, thereby assessing their involvement in human cancers and allowing for the design of new therapeutic strategies based upon this understanding.
References
- 1.Lambert NC, Lo YMD, Erickson TD, Tylee TS, Guthrie KA, Furst DE, Nelson JL. Male microchimerism in healthy women and women with scleroderma: cells or circulating DNA? A quantitative answer. Blood. 2002;100:2845–2851. doi: 10.1182/blood-2002-01-0295. [DOI] [PubMed] [Google Scholar]
- 2.O’Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, Roberts IA, Fisk NM. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JL. Pregnancy and microchimerism in autoimmune disease: protector or insurgent? Arthritis and Rheumatism. 2002;46:291–297. doi: 10.1002/art.501. [DOI] [PubMed] [Google Scholar]
- 4.Adams KM, Nelson JL. Microchimerism. An investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–1131. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 5.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 6.Gadi VK, Nelson JL. Fetal microchimerism in women with breast cancer. Cancer Res. 2007;67:9035–9038. doi: 10.1158/0008-5472.CAN-06-4209. [DOI] [PubMed] [Google Scholar]
- 7.Gadi VK, Malone KE, Guthrie KA, Porter PL, Nelson JL. Case-control study of fetal microchimerism and breast cancer. PLoS ONE. 2008;3:e1706. doi: 10.1371/journal.pone.0001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Ren X, Cao S, Li H, Hao X. Beneficial effects of fetal-maternal microchimerism on the activated haplo-identical peripheral blood stem cell treatment for cancer. Cytother. 2008;10:331–339. doi: 10.1080/14653240802061146. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore GL, Haq B, Shadduck RK, Jasthy SL, Lister J. Fetal-maternal microchimerism in normal parous females and parous female cancer patients. Expt. Hematology. 2008 doi: 10.1016/j.exphem.2008.03.020. Epub May 26. [DOI] [PubMed] [Google Scholar]
- 10.Johnson AM, O’Connell MJ, Messing EM, Reeder JE. Cecreased bladder cancer growth in parous mice. Urology. 2008 doi: 10.1016/j.urology.2008.04.028. Epub, April 4. [DOI] [PubMed] [Google Scholar]
- 11.Whiteman DC, Siskind V, Purdie DM, Green AC. Timing of pregnancy and the risk of epithelial ovarian cancer. Cancer Epidem. Biomarkers Prev. 2003;12:42–46. [PubMed] [Google Scholar]
- 12.O’Donoghue K, Sultan HA, Al-Allaf FA, Anderson JR, Wyatt-Ashmead J, Fisk NM. Microchimeric fetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod. Biomed. 2008;16:382–390. doi: 10.1016/s1472-6483(10)60600-1. Online. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 14.Cha DH, Khosrotehrani K, Kim Y, Stroh H, Bianchi DW, Johnson CL. Cervical cancer and microchimerism. Obstet. Gynecol. 2003;102:774–781. doi: 10.1016/s0029-7844(03)00615-x. [DOI] [PubMed] [Google Scholar]


